Abstract
Phytochemicals from waste materials generated by agricultural and industrial processes have become globally significant due to their accessibility and potential effectiveness with few side effects. These compounds have essential implications in both medicine and the economy. Therefore, a quantitative analysis of the phytochemical profile, sugar types, and water-soluble vitamins of dried Corchorus olitorius L.“DJMS” extract (dried Jew’s mallow stem) was carried out with HPLC. In addition, the chemical composition, TPC, chlorophyll a and b, beta-carotene, and antioxidant effect using DPPH were investigated. Furthermore, the anticancer activity of the DJMS was evaluated by SRB assay using Huh-7 and MDA-MB-231 cell lines. In the quantitative study, DJMS extract showed a high antioxidant potential (67%) due to its content of bioactive compounds such as TPC (276.37 mg 100 g−1) and chlorophyll a and b (20.31, 12.02 mg 100 g−1, respectively), as well as some vitamins and minerals such as B-complex (B12; 146.8 mg 100 g−1 and vitamin C 6.49 mg 100 g−1) and selenium (<0.2 μg kg−1). Moreover, the main sugar types found were sucrose and stachyose, which recorded 9.23 and 6.25 mg 100 g−1, respectively. Identifying phenolic and flavonoids showed that the major components were ellagic acid (4905.26 μg kg−1), ferulic acid (3628.29 μg kg−1), chlorogenic acid (3757.08 μg kg−1), luteolin—7-O-glucoside (4314.48 μg kg−1), naringin (4296.94 μg kg−1) and apigenin—6—rhamnose—8 glucoside (3078.87 μg kg−1). The dried stem extract showed significant MDA-MB-231 inhibition activity and reached 80% at a concentration of 1000 µg/mL of DJMS extract, related to the content of phytochemical components such as isoflavones like genistein (34.96 μg kg−1), which had a tremendous anticancer effect. Hence, the stem of Jew’s mallow (which is edible and characterized by its viability and low production cost) possesses the capacity to serve as a pharmaceutical agent for combating cancer owing to its abundance of bioactive components.
Keywords:
nutritional value; vitamin C; B-complex; chlorophyll; antioxidant activity; DPPH; TPC; flavonoids; HPLC; antitumor 1. Introduction
Plants have given humans and other organisms food and other health benefits for as long as they have existed [1]. Secondary metabolites, or bioactive chemicals, are present in medicinal plants [2,3,4,5,6]. Secondary metabolites are a class of substances that have been shown to have therapeutic action against several human disorders. This may account for the traditional usage of medicinal plants in treating many diseases. Quantitative assessments of the bioactive compounds in medicinal plants are essential in identifying the matching chemicals in particular plants. Thus, separating these chemicals at large concentrations from the relevant plants is possible [6,7,8]. Particularly in cancer therapy, scientific methods are used to devise treatments for patients. Patients may experience adverse effects from synthetic pharmaceuticals, however. For the treatment of diseases including cancer, Alzheimer’s, and diabetes, therefore, the development and clinical application of pharmaceuticals derived from plant materials are crucial [6,9,10,11]. According to reports, roughly 80% of the global population derives its primary medication from natural products and plant-based medicines [12]. Furthermore, approximately 25% of all medications are sourced from 500 distinct species of medicinal plants [13].
The distinctiveness of the North African and Mediterranean diets is frequently attributed to their abundance of cereal and olive oil. Vegetables contain considerable nutritional value. Mallows and other leafy green vegetables are ubiquitous in Egyptian cuisine. Jew’s mallow, or jute mallow in English, is the term Corchorus olitorius called Molokhia. This herb can grow up to 4 feet tall and is either annual or perennial. It is a well-liked green vegetable crop grown in Egypt during the summer. Fresh or dried green leaves add a pleasant flavor to soups and stews [14]. The vegetable is grown for its mucilaginous leaves, which are also used as food; its stem bark, used to make jute fiber; and its seeds, used as flavoring agents [15]. In Southeast Asia, its leaves and roots are also consumed as herbal medicine [16]. It is a genus comprising 50–60 species of annual plants that are members of the Tiliaceae family. These species’ slender, branching stems with mucilaginous leaves are utilized as vegetables in meals to foster community in jute-producing nations [17,18,19,20,21]. Molokhia leaves are edible raw or can be processed (drying, extraction, etc.) to make various goods. Additionally, they can be added to a list of items to enhance and increase their nutritional profile, functional qualities, and organoleptic quality [22,23].
Numerous studies have examined molokhia’s biochemical composition and biological activity. Molokhia leaves contain many beneficial components and are highly valued for their high content of carotenoids, vitamins A, B1, B2, B5, C, and E, folic acid, and minerals (Ca, P, K, Na, Fe) [14]. Molokhia also offers different amounts of healthy fiber and protein [16]. Its pharmacological activities come from phytochemicals such phenols, flavonoids (such as chlorogenic acid, quercetin glycosides, caffeic acid, and isorhamnetin), sterols, glycosides, tannins, saponins, lipids, and fatty acids in plant parts and seeds. These components give the plant anticancer, antioxidant, analgesic, antiviral, antipyretic, diuretic, analgesic, antibacterial, antidiabetic, cardiovascular effects, hepatoprotective, and neuroprotective properties. The leaves have been used in traditional medicine, cuisine, and skin care [6,24,25,26]. Brazilian and other folk medicines use it to treat colitis. Stomatitis, chronic bronchitis, furuncles, abscesses, contusions, gonorrhea, pain, fever, tumors, hemorrhoids, and other painful and inflammatory conditions are treated with plant leaf extract [1,27]. AL Yousef et al. and Yakoub et al. reported that dry oils of leaves and stems include many bioactive components, such as 2, 4-di-tert-butylphenol, and fatty acids, including hexadecenoic and ethyl palmitate. These bioactive substances participate in many biological processes and free radical attenuation, which causes degenerative illnesses. Thus, their bioactive molecules have several beneficial effects, including antioxidant, enzyme inhibitory, anticancer, and antibacterial properties [28,29]. Isuosuo et al. and Atalar et al. showed that C. olitorius seeds include calcium, iron, vitamin A, thiamine, riboflavin, nicotinamide, and ascorbic acid. Furthermore, this plant contains cardiac glycosides, phenolic acids, polysaccharides, sterols, and fatty acids, etc. [6,30].
Food manufacturing waste represents a significant problem because of its large quantity due to the expansion of food industry factories and technology. Despite this, it represents substantial economic wealth and is capable of employing young people and idle energies if utilized in an integrated system that includes various economic, social, environmental, and technical aspects, given its content. Waste is an essential element and can be used in multiple forms, thus preserving the environment from pollution produced by accumulating and burning these wastes.
Without a doubt, one of the deadliest threats facing humanity today is cancer. Egypt had 134,632 cancer cases in 2020, with liver (27,895), breast (22,038), bladder (10,655), non-Hodgkin lymphoma (7305), lung (6538), leukemia (5231), and prostate (4767) having the highest incidence. Egypt had 89,042 cancer deaths in 2020, which is considered the highest mortality rate [31]. To our knowledge, no research addresses the chemical composition (carbohydrates, protein, etc.), bioactive profile identification, antioxidant activity, and antiproliferative activities of dried Jew’s mallow stems. Therefore, our study aims to shed light on the diverse array of compounds in botanical components. Thus, the stem of mallow could be considered a hidden treasure and promising cure for different types of cancer, such as liver (Huh-7) and breast (MDA-MB-231).
2. Results and Discussion
2.1. Chemical Composition
Technically identified as Corchorus olitorius L., mallow is a blossoming plant which has long been used in traditional medical and culinary applications. Diverse parts of the mallow plant have been used for various purposes, but we are interested in the chemical composition of dried mallow stems. Figure 1 displays the primary significance of the nutrient composition and estimated energy value, presented in terms of dry weight. The obtained results show that mallow has a high content of protein, carbohydrate, fat, and ash (20.22, 57.00, 2.86 and 17.84%) in dried stems compared to dried leaves, which ranged between 10.44 and 36.73% for protein, 46.07% for carbohydrate, 1.7 for fat and 7 and 16.13% for ash [31,32,33]. Fat was the least prevalent, with a macronutrient abundance of less than 3%. Therefore, the results reflected the finding that dried stems had a high nutritional value, which could be considered a great supplement, especially protein carbohydrates, as a source of energy and building tissues for different consumer categories, especially children and athletes. Each 100 g of dried stems gives 334.62 Kcal, which agrees with those findings reported by Abbasi et al. [34].
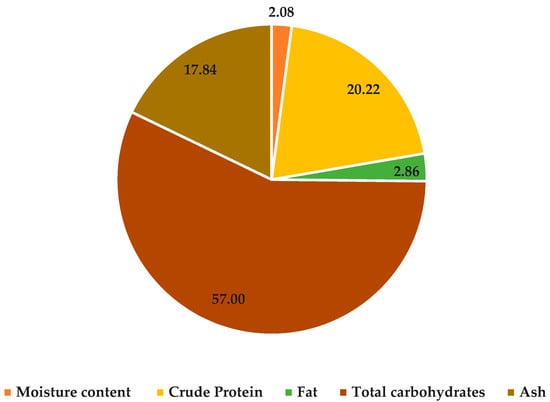
Figure 1.
Chemical composition of dried Jew’s mallow stems (g 100 g−1).
2.2. Sugar Content
Regarding sugar composition, DJMS contained stachyose, sucrose, maltose, glucose, rhamnose, galacturonic, xylose, galactose, mannose, fructose, arabinose, mannitol, and ribose as the main sugars (Table 1). The present study describes the sugar composition in dried mallow stems for the first time. Sucrose and stachyose were the most abundant sugars (9.227 and 6.254 g 100 g−1 of dry weight). Elsayed et al. [35] reported that the types of sugars are analyzed through chromatography; the polysaccharides in the eight Aloe species contained 18 saccharides, glucuronic acid, stachyose, galacturonic acid, sucrose, glucose, xylose, galactose, rhamnose, mannose, arabinose, fructose, polyol, mannitol, and sorbitol. The quantitative distribution of these components varied across the different species. Moreover, the polysaccharides produced by alloxan were shown to have antihyperglycemic effects in diabetic rats and an ability to block alpha-glucosidase.

Table 1.
Identification of sugar types and vitamin and mineral contents of dried Jew’s mallow stems.
2.3. Mineral Content
Otherwise, DJMS is considered a source of minerals because it has a high ash content (17.84 g 100 g−1). The main elements found in it are Fe, Ca, Mg, P, K, and Se, as shown in Table 1. It could be a great resource for treating malnutrition diseases such as anemia by supplementing different functional foods. Also, some of these elements significantly affect anticancers such as Se. There is evidence suggesting that selenium compounds may possess chemopreventive properties. Research is underway to see if these compounds can also impact established malignancies. In addition, Radomska et al., Frajese et al., and Croci et al. [36,37,38] reported that K ascorbate has been proposed to function as a potassium intracellular transporter and can potentially impede the cell cycle in cancerous cells.
2.4. Vitamin Content
The DJMS content of water-soluble vitamins is shown in Table 1. The values of vitamin B complex in dried mallow stems were recorded as 5.62, 67.52, 9.31, 25.68, and 146.80 mg 100 kg−1 for B1, B2, B6, B9, and B12, respectively. Meanwhile, vitamin C (ascorbic acid) recorded a low content, reaching 6.49 mg 100 g−1 compared to leaves containing 258 mg 100 g−1 FW, as documented by Ragasa et al. [39]. Stems of the Jew’s mallow could be considered a source of water-soluble vitamins such as vitamins B and C, especially vitamin B complex, identified for the first time in dried Jew’s mallow stems. Several studies have suggested that vitamin B supplements reduce cancer risks; therefore, one of our research objectives is determining vitamin B [40,41]. In addition, vitamin C is among the most potent antioxidant properties and may be an effective anticancer agent. The metabolomic and epigenetic profiles of cancer cells may be altered by vitamin C, and the elimination of cancer stem cells may result from the vitamin’s activation of ten-eleven translocation (TET) proteins and downregulation of pluripotency factors [42].
2.5. Bioactive Profile
The extent of the bioactive profile of the DJMS methanol was assessed. The concentration of total phenolic compounds (TPC) was higher in the dried stems (276.37 mg GAE 100 g−1), while Beghdad et al. [43] reported that the TPC in stem extract obtained from M. sylvestris L. was lower (217.30 mg GAE 100 g−1) than leaves (2412.30 mg GAE 100 g−1). Meanwhile, Mouas et al. [44] found that TPC in leaves of C. olitorius L. was lower (200 mg GAE 100 g−1) than in our study. Also, Ben Yakoub et al. [45] reported that the ethanolic extract of C. olitorius leaves showed a low TPC (9.20 mg GAE 100 g−1 DW). Other compounds and different types of phenols can explain this variation.
Meanwhile, our study showed that the content of dried Jew’s mallow stem was low in chlorophyll (20.31 and 12.02 mg 100 g−1 for chlorophyll a and b, respectively) and Beta-carotene (1.5 mg 100 g−1). Upadhyay [46] documented that plant pigments are secondary metabolites that inhibit the proliferation of cancer cells by halting their growth and cell division. These substances hinder biological activities in cancer cells, including signaling pathways, cell cycle regulation, apoptosis, and autophagy. In addition to their anticancer properties, these substances also help regulate excessive blood pressure, obesity, hyperglycemia, and hypercholesterolemia, and address cardiovascular issues.
2.6. Identification of Phenolic, Flavonoid and Isoflavone Compounds
Phenolic compounds, a broad family of secondary plant metabolites, are recognized as antioxidants and other dietary reducing agents crucial for the quality of plant-based diets. They maintain the body’s tissues against oxidative stress-related diseases like cancer, inflammation, and coronary heart disease [1]. The phenolic and flavonoid compounds of dried C. olitorius methanolic (Table 2) revealed that the stem extract contained high amounts of phenolic compounds, for example, ellagic acid, pyrogallol, chlorogenic acid, ferulic acid, and catechin. Moreover, it had a high number of flavonoids such as apigenin -6-rhamnose-8-glucoside, apigenin-6-arabinose-8-glactoside, naringin, Luteolin 7-glucoside, rosmarinic and quercetin-3-glucoside.

Table 2.
Identifying phenolic, flavonoid and isoflavone compounds (µg 100 g−1) of dried Jew’s mallow stems.
Our results agreed with Khan et al. [47], who reported that Corchorus species include chlorogenic acid and quercetin-3-glucoside. Cynarin, chlorogenic acid, quercetin-3-glucoside, and biochanin A are documented to be medicinally critical natural compounds and are found in high concentration in the leaf, stem, and root extracts of the plant, exhibiting significant anticancer, antidiabetic, antibacterial, and anti-inflammatory activities [48]. Chlorogenic acid and related compounds are generally recognized as antioxidants [49]. Otherwise, the predominant isoflavone in the methanolic extract of DJMS was isorhamnetin (5502.00 µg 100 g−1) and genistein (59.91 µg 100 g−1), as shown in Table 2. The antioxidant properties of isoflavones are generally recognized for their potential benefits to human health. Physical activity can create a disparity between reactive oxygen species (ROS) and antioxidants [50], which indicates that genistein may lower the likelihood of tumor formation [51].
2.7. Antioxidant Capacity %
The antioxidant activity achieved a substantial proportion of 67% due to the abundant presence of phenolic compounds and other dietary reducing agents. The findings of our study are consistent with those of Karimi et al. [35], indicating a direct relationship between the number of phenolic compounds and their antioxidant capacity. For example, Lan [49] found that the level of chlorogenic acid in Flos Lonicerae extracts correlates with their antioxidant properties. Higher levels of chlorogenic acid enhance the effectiveness of scavenging the DPPH radical, as indicated by the latest results. Moreover, flavonoids are a widespread category of phenolic chemicals that exhibit antioxidant activity in laboratory settings, primarily due to derivatives of naringenin [52]. Naringenin is a prominent flavonoid compound mainly derived from grapefruit and oranges. Naringenin exhibits strong antioxidant properties and can reduce cholesterol levels, prevent cancer, and reduce inflammation [53,54]. Furthermore, it is commonly accepted that isoflavones, such as genistein, positively affect human health due to their antioxidant properties [50]. Genistein, a common soy isoflavone, is an antioxidant that directly donates hydrogen atoms from the hydroxyl group linked to the benzene ring [55,56].
2.8. Anticancer Effects of Dried Mallow Stem
To investigate the anticancer effects of dried Jew’s mallow stems, two types of human cancer cell lines (liver “Huh-7” and breast “MDA-MB-231”) were treated with the dried mallow stems. There was evidence that the mallow stem extract suppressed the viability of MDA-MB- 231breast cells by 80% at 1000 µg mL−1; meanwhile, it suppressed the viability of Huh-7liver cells by 40% at the same concentration. Also, there was a significant difference between the non-treated cells (p < 0.05) and at all low concentrations between 0.01 and 1000 µL/mL. Therefore, the IC50 of MDA-MB-231 is >100 µg mL−1; meanwhile, the IC50 for Huh-7 is >1000 µg mL−1 (Figure 2). Accordingly, the results reported here agree with those previously reported regarding the highly antiproliferative activity of C. Olitorius leaf extract against Caco-2 cell lines and had a low effect on sk-ov-3 cancer cell lines after treatment at the same concentration (250 µg mL−1) [6]. In another study, it was stated that the compounds in the Ocimum basilicum and Impatiens walleriana extracts suppressed cell viability by more than 78% [57]. The anticancer effect may be correlated to the higher content of bioactive compounds, which increase the antioxidant effect, rather than the low concentration of dried mallow stem extract. According to Omoruyi et al. [58], TPC inhibits cancer cell growth, invasion, adhesion, and tube formation due to the substantial content of the bioactive compound of C. olitorius, which appears to have considerable antioxidant and antiproliferative activities. Caffeic acids naturally exhibit biological activities such as antioxidant, antimutagenic, anticancer, and anti-obesity properties [55]. In addition, Sarkar and Li [59] showed that genistein, the primary isoflavone in soy, effectively suppresses cancer development in animal models. Therefore, these results suggest that the powder of mallow stem may be preferable as a natural and safe breast cancer therapeutic agent. Moreover, Frajese et al. [37] showed that potassium enhanced the antitumor properties of vitamin C in specific breast cancer cell lines. Furthermore, plant pigments such as carotenoids, anthocyanins, betalains, chlorophyll, and lycopene hinder biological activities in cancer cells, including signaling pathways, cell cycle regulation, apoptosis, and autophagy [46].
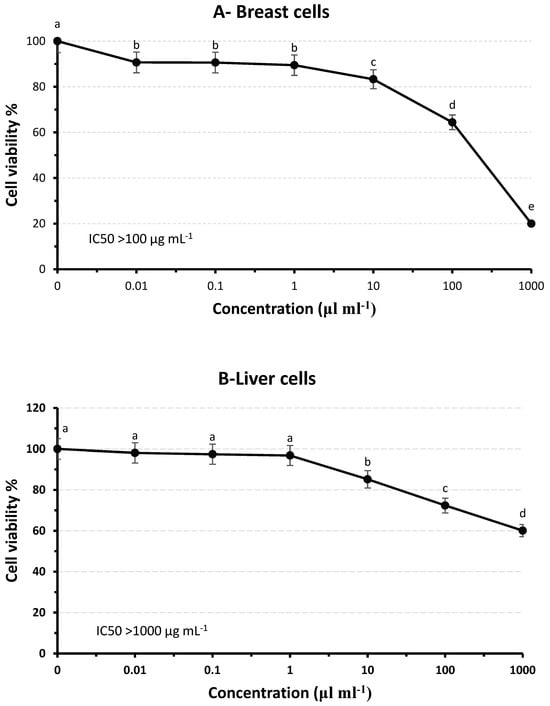
Figure 2.
Antiproliferative activity of dried Jew’s mallow stems against MDA-MB-231 (A) and Huh-7 (B) cancer cells. The experimental values (means and SD for n = 3) with the small letters are significantly different (p ≤ 0.05).
3. Materials and Methods
3.1. Materials and Chemicals
Fresh Jew’s mallow (Corchorus olitorius Seady) was purchased from the local market in Giza, Egypt. The species we work on is Corchorus olitorius Seady, and it has already been identified by Elwakil et al. [60] under the accession numbers for RbcL and MatK gene in the database of the National Library of Medicine.
The accession numbers for the RbcL gene, “gene bank: LC732566” [60] and for the MatK gene, “gene bank: LC732050” [60]. Also, botanical identification was conducted by Dr. Rim Hamdy, a botanist in the Botany Department, Faculty of Science, Cairo University, Giza, Egypt, and a voucher specimen was retained at the Herbarium of the Botany Department (CAI), Faculty of Science.
Folin–Ciocalteu, 2,2-diphenyl-1-picryl hydrazyl (DPPH), gallic acid standard, sulfuric acid, sodium hydroxide, boric acid, hydrochloric acid, copper sulfate, potassium sulfate, 2,6-dichlorophenol, ascorbic acid, all chemical, reagent, solvent and standards which were used in identification by HPLC were acquired from Sigma-Aldrich, St. Louis, MO, USA.
3.2. Estimation of the Chemical Constituents of Dried Stems
The plants were washed and the leaves were separated from the stems. The stems were dried using a conventional dryer at 50 °C for 20 h and ground using the grinder to a fine powder (Touch Elzenoky 40,510 King Kitchen Machine, 1000 Watt, Elzenoky Company, Cairo, Egypt) and sieved with a 40-mesh sieve as shown in Figure 3. The dried samples were stored in polypropylene packages at −18 °C for further analysis. The dried stems’ moisture, ash, fiber, and fat percentages were estimated according to AOAC methods [60]. The protein content was ascertained utilizing the Kjeldahl method. The percentage of protein was determined by multiplying the nitrogen value by a factor of 5.5 [61]. In addition, mineral contents, including Fe, Ca, Mg, P, K and Se were determined according to the method described by AOAC [60], using a Pye Unicom SP1900 atomic absorption spectrometry (Perkin Elmer model 4100ZL; PerkinElmer, Shelton, CT, USA). Carbohydrate was calculated by difference.
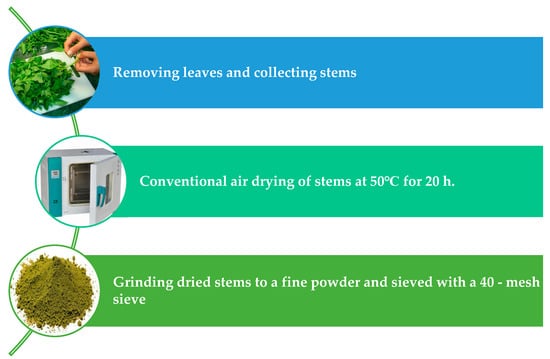
Figure 3.
Preparation steps of dried Jew’s mallow stems (DJMS).
3.3. Estimation of Chlorophyll a, b and β-Carotene
The β-carotene and chlorophyll a and b contents were determined using the method described by Youssef et al. [23]. A 200 mg DJMS was vigorously shaken with 10 mL of acetone-hexane combination (4:6) for 5 minutes and filtered through filter paper. The extract volume was precisely modified to 10 mL using a volumetric flask. The extract’s absorbance was measured at 453, 505, 645, and 663 nm wavelength using a spectrophotometer (6505 UV/VIS, Jenway L.T.D., Felsted, Dunmow, UK). β-carotene and chlorophyll a and b contents were determined using the following formulas:
β-carotene (mg 100 mL−1) = 0.216 × A663 − 1.220 × A645 − 0.304 × A505 + 0.452 × A453
Chlorophyll a (mg 100 mL−1) = 0.999 × A 663 − 0.0989 × A645
Chlorophyll b (mg 100 mL−1) = −0.328 × A663 + 1.77 × A645, further expressed in mg per 100 g dry weight.
3.4. Estimation of Total Phenolic Compounds (TPC)
Methanolic DJMS extract was prepared by maceration of the stem powder (5 mg 100 mL−1 methanol 80%). The resulting extract was agitated in a shaker at 300 rpm for 1 h and filtered using Watman No. 1 filter paper. The analysis of all extracts was conducted three times. The TPC of the extract was measured using the Folin–Ciocalteu reagent method as described by El-Beltagi et al. [62], with some modifications. The absorbance was determined at a wavelength of 765 nm utilizing a spectrophotometer. The results were expressed as mg GAE 100 g−1 of the sample’s dry weight (DW).
3.5. Estimation of Free Radical-Scavenging Activity
The extract solution’s ability to scavenge free radicals was determined following the methodology previously detailed by Yakoub et al. [29], with some modifications. An extract solution (0.1 mL) was added to 3.9 mL DPPH (0.0024 mg 100 mL−1 methanol). The solution was stored in a dark environment at room temperature, and the absorbance was quantified at a wavelength of 517 nm after a 30-min incubation period using a UV-visible spectrophotometer. The quantification of free radical scavenging activity was determined as the percentage of inhibition, as defined by the following equation:
In that order, A control and A sample represent the absorbance of the DPPH methanolic solution and the DJMS extract.
3.6. High-Performance Liquid Chromatography (HPLC) Methods
All HPLC quantification analyses of samples were analyzed in ISO/IEC17025: a 2017 accredited laboratory.
3.6.1. Estimation of Sugar Compounds
The sample was homogenized using a high-speed homogenizer with deionized water for 3 min, divided into 3 intervals, at a temperature of 60 °C. Subsequently, the homogenized sample was filtered through a membrane with a pore size of 0.22 µm. A portion of 1.5 mL of these solutions was transferred into vials for the analysis. The refractive index detector-coupled Agilent (Series 1200) chromatographic system has a quaternary pump, degasser, and auto-injector. Agilent collected chromatographic data. As previously explained, the samples acquired were analyzed under the conditions specified by Zielinski et al. [63]. The method was verified using the steps followed by Coelho [64]. The technique provided values for recovery (90–106%) and limits of detection (0.03–4.4 g 100 g−1) and quantification (0.08–1.99 g 100 g−1).
3.6.2. Estimation of Vitamin B Complex Content
Vitamin B complexes were fractionated according to the method defined by Antakli et al. [65], with a slight modification using a variable wavelength detector (VWD) instead of a fluorescence detector with the VWD set at 280 nm. This method was validated by following some details, including LOD, LOQ and recovery range (97–100%) [65].
3.6.3. Estimation of Vitamin C Content
Chromatographic measurements were made using an HPLC system (model Series 1200, Agilent Technologies, Waldbronn, Germany) with a column compartment ODS C18 (250 × 4.6 mm ID, five μm particle size) set at 25 °C. A half gram of the sample and 300 μm of 0.56% (w/v) meta-phosphoric acid solution were added to the special centrifuge and filtration tube, shaken for 30 s, and centrifuged at 10 °C (10 min, 3000× g). The supernatant was filtrated with a 13 mm 0.45 μm Teflon filter disc into a vial for analysis by HPLC. The experiment involves performing an isocratic chromatographic separation using a mobile phase of deionized water/acetic acid (0.1%, v/v) and MeOH in a relative proportion of 95:5 (v/v). The eluent flowed at a rate of 0.7 mL min−1. Vitamin C was identified by comparing the retention duration of the sample peak with that of the reference ascorbic acid, using a wavelength of 254 nm [66]. The following parameters were determined: limits of detection and quantification (LOQ < 5 μg 100 mL−1 and LOD < 2 μg 100 mL−1), and recovery (95%) as performed in the same method [66].
3.6.4. Estimation of Phenolic and Flavonoid Compounds
A high-performance liquid chromatography system with a variable wavelength detector (model Series 1200, Agilent Technologies, Waldbronn, Germany) was used. Further, the HPLC was equipped with an autosampler, quaternary pump degasser, and column compartment set at 35 °C. The analyses used a stainless-steel column (4 × 250 mm, i.d.) filled with a C18 reverse phase (BDS 5 μm, Labio, Czech Republic). Samples were prepared using the Schieber et al. method [67] to determine the phenolic acids and flavonoids. The validation parameters are according to the guide for validation set out by da Silva Padilha [68]. The method showed recovery (92–104%) and limits of detection (0.06–0.95 µg 100 g−1) and quantification (0.05–1.51 µg 100 g−1).
In an ultrasonic bath, DJMS sample weights (100 mg) were extracted in 45 min with 10 mL methanol. The specimens were centrifugated for 7 min at 4200 revolutions per minute. Before analysis, the liquid portion was passed through a Chromafil AO-45/25 polyamide filter and collected in a vial. The HPLC procedure commenced with a linear gradient at a fluid flow of 1 mL min−1, utilizing a mobile phase consisting of water/acetic acid (98:2 v/v, referred to as solvent A) and acetonitrile/methanol (50:50 v/v, referred to as solvent B). The gradient began with 5% solvent B and progressively increased to 30% at 25 min, 40% at 35 min, 52% at 40 min, 70% at 50 min, and finally reached 100% at 55 min. A 5-min wash in both solvents restored the starting conditions. All chromatograms were displayed at 280 nm and flavonoids at 330 to determine phenolic acids. All components were discovered and measured by comparing the peak areas with external standards.
3.6.5. Estimation of Isoflavone Compounds
Extraction was carried out as described by Kaufman et al. [69]. The validation parameters are according to the guide for validation set out by da Silva Padilha [68], and showed recovery (97–102%) and limits of detection (0.06–0.13 µg 100 g−1) and quantification (0.06–0.29 µg 100 g−1). Half a gram of the samples was prepared and added to a test tube containing 4 mL of 80% MeOH. These samples were vortexed, and the supernatant was collected and placed in 15 mL CorexTM centrifuge tubes. The previous step was repeated three times for each sample. Tubes were centrifuged for 20 min at 27,000× g at 22 °C in a Superspeed refrigerated centrifuge. The supernatant, which contained the isoflavonoids, was then air-dried. Next, 1 mL of 80% MeOH was added to the air-dried test tubes. The tubes were then vortexed, covered, and kept in a refrigerator at 4 °C until high-performance liquid chromatography analysis. HPLC was performed using a hypersil BDS C18 column (4.6 × 250 mm in size) at a 254 nm wavelength.
3.7. Cytotoxicity Assay
Huh-7: liver cancer and MDA-MB-231: breast cancer (transitional cell carcinoma) were obtained from Nawah Scientific Inc. (Mokatam, Cairo, Egypt). Cells were maintained in McCoy’s media supplemented with 100 mg mL−1 of streptomycin, 100 units mL−1 of penicillin, and 10% of heat-inactivated fetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37 °C. Cell viability was evaluated using the SRB test. The 100 μL aliquots of cell suspension containing 5 × 103 cells were placed in 96-well plates and cultured in a complete medium for 24 h. The cells were subjected to an additional 100 μL of medium-containing DJMS extract at different doses, ranging from 0.01 to 1000 μg/mL. After 72 h of DIMS extract treatment, the cells were immobilized by replacing the medium with 150 μL of 10% TCA and incubating them at 4 °C for 1 h. The TCA solution was eliminated, and the cells were rinsed five times with distilled water. A total of 70 microliter portions of a 0.4% weight/volume solution of SRB were added and placed in a dark environment at room temperature for 10 min. The plates were rinsed three times with a 1% acetic acid solution and left to dry naturally overnight. Next, 150 μL of TRIS (10 mM) solution was introduced to dissolve the SRB stain bound to the protein. The solution’s absorbance was subsequently measured at a wavelength of 540 nm using a BMG LABTECH®-FLUO star Omega microplate reader (Ortenberg, Germany) [70,71]. The cytotoxicity % was calculated using the provided equation and corrected absorbance.
Cytotoxicity % = (100 × (control − sample)
3.8. Statistical Analysis
Costat Version 6.45 was used for all statistical analysis (CoHort Software Version 6.45, Monterey, CA, USA). One-way analysis of variance (ANOVA) was used as the primary statistical analysis method. A Shapiro–Wilk test was used to verify that each experiment’s normality distributions were correct. A Duncan’s multiple range test was performed with a 5% significant point. A Bartlett’s test was employed to determine whether the variation within sample groups was homogeneous. Systat Software Inc., Erkrath, Germany, used SigmaPlot Version 12 to create the graphs and figures.
4. Conclusions
The stem of C. olitorius is highly regarded for its substantial nutritional content, which includes abundant dietary fiber, protein, vitamins (B-complex), and minerals (iron). Moreover, it contains various major phytoconstituents, namely phenolic, flavonoid, and isoflavone compounds (ellagic acid, ferulic acid, chlorogenic acid, luteolin—7-O-glucoside, naringin, apigenin—6—rhamnose—8 glucoside, isorhamnetin, and genistein), vitamin C, and chlorophyll a and b, which give it high potential as a natural antioxidant. The data presented here align with earlier findings of C. olitorius leaves regarding their biological activity. Overall, the dried stems are characterized by being edible and available in large quantities as a cheap source of bioactive compounds. Moreover, the utilization of food industry waste consists of sustainability and environmental protection policies. Therefore, according to the results of this study on the phytoconstituents and biological activities of C. olitorius dried stem, it shows promise as a viable alternative ingredient in the pharmaceutical and nutraceutical industries, especially for cancer. Otherwise, additional in vitro and in vivo studies are necessary to comprehend how the natural chemical may serve as a possible anticancer medication.
Author Contributions
Conceptualization, M.R.A. and H.H.I.; methodology, M.R.A., H.H.I. and A.A.S.-E.; software, M.R.A. and H.H.I.; validation, M.R.A., H.H.I. and A.A.S.-E.; investigation, H.H.I., A.A.S.-E. and M.R.A.; resources, M.R.A., H.H.I. and A.A.S.-E.; data curation, M.R.A. and H.H.I.; writing—original draft preparation, M.R.A., H.H.I. and A.A.S.-E.; writing—review and editing, M.R.A. and H.H.I.; visualization, M.R.A. and H.H.I.; supervision, M.R.A. and H.H.I.; project administration M.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biswas, A.; Dey, S.; Huang, S.; Deng, Y.; Birhanie, Z.M.; Zhang, J.; Akhter, D.; Liu, L.; Li, D. A Comprehensive Review of C. capsularis and C. olitorius: A Source of Nutrition, Essential Phytoconstituents and Pharmacological Activities. Antioxidants 2022, 11, 1358. [Google Scholar] [CrossRef]
- Elmastas, M.; Ozturk, L.; Gokce, I.; Erenler, R.; Aboul-Enein, H.Y. Determination of antioxidant activity of marshmallow flower (Althaea officinalis L.). Anal. Lett. 2004, 37, 1859–1869. [Google Scholar] [CrossRef]
- Ökten, S.; Cakmak, O.; Erenler, R.; Şahin, Ö.Y.; Tekin, Ş. Simple and convenient preparation of novel 6, 8-disubstituted quinoline derivatives and their promising anticancer activities. Turk. J. Chem. 2013, 37, 896–908. [Google Scholar] [CrossRef]
- Elmastaş, M.; Telci, İ.; Akşit, H.; Erenler, R. Comparison of total phenolic contents and antioxidant capacities in mint genotypes used as spices/Baharat olarak kullanılan nane genotiplerinin toplam fenolik içerikleri ve antioksidan kapasitelerinin karşılaştırılması. Turk. J. Biochem. 2015, 40, 456–462. [Google Scholar] [CrossRef]
- Erenler, R.; Telci, I.; Ulutas, M.; Demirtas, I.; Gul, F.; Elmastas, M.; Kayir, O. Chemical Constituents, Quantitative Analysis and Antioxidant Activities of Echinacea purpurea (L.) Moench and Echinacea pallida (Nutt.) Nutt. J. Food Biochem. 2015, 39, 622–630. [Google Scholar] [CrossRef]
- Atalar, M.N.; Erenler, R.; Turkan, F.; Alma, M.H.; Demirtas, I.; Baran, A.; Saltan, F.Z. Phytochemical analysis and biological activity of Corchorus olitorius L.: Quantitative analysis of bioactive compounds byLC–MS/MS, antibacterial, enzyme inhibition, and cytotoxic activities. Eur. J. Integr. Med. 2023, 62, 102290. [Google Scholar] [CrossRef]
- Erenler, R.; Meral, B.; Sen, O.; Elmastas, M.; Aydin, A.; Eminagaoglu, O.; Topcu, G. Bioassay-guided isolation, identification of compounds from Origanum rotundifolium and investigation of their antiproliferative and antioxidant activities. Pharm. Biol. 2017, 55, 1646–1653. [Google Scholar] [CrossRef]
- Erenler, R.; Telci, I.; Elmastaş, M.; Akşit, H.; Gül, F.; Tufekcy, A.R.; Kayir, Ö. Quantification of flavonoids isolated from Mentha spicata in selected clones of Turkish mint landraces. Turk. J. Chem. 2018, 42, 1695–1705. [Google Scholar] [CrossRef]
- Elmastas, M.; Celik, S.M.; Genc, N.; Aksit, H.; Erenler, R.; Gulcin, İ. Antioxidant activity of an Anatolian herbal tea—Origanum minutiflorum: Isolation and characterization of its secondary metabolites. Int. J. Food Prop. 2018, 21, 374–384. [Google Scholar] [CrossRef]
- Karan, T.; Erenler, R. Fatty acid constituents and anticancer activity of Cladophora fracta (OF Müller ex Vahl) Kützing. Trop. J. Pharm. Res. 2018, 17, 1977–1982. [Google Scholar] [CrossRef]
- Erenler, R.; Genç, N.; Elmastaş, M.; Eminağaoğlu, Ö. Evaluation of antioxidant capacity with total phenolic content of Galanthus krasnovii (Amaryllidaceae). Turk. J. Biodivers. 2019, 2, 13–17. [Google Scholar] [CrossRef]
- Beyene, B.; Beyene, B.; Deribe, H. Review on application and management of medicinal plants for the livelihood of the local community. J. Resour. Dev. Manag. 2016, 22, 33–39. [Google Scholar]
- Shityakov, S.; Bigdelian, E.; Hussein, A.A.; Hussain, M.B.; Tripathi, Y.C.; Khan, M.U.; Shariati, M.A. Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019, 176, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, I.M.; El-Araby, S.M. Effect of organic manure source and biofertilizer type on growth, productivity and chemical composition of Jew’s Mallow (Corchorus olitorious L.) plants. J Agric. Env. Sci. Alex. Univ. Egypt 2003, 2, 88–105. [Google Scholar]
- Zakaria, Z.A.; Somchit, M.N.; Zaiton, H.; Mat Jais, A.M.; Sulaiman, M.R.; Farah, W.O.; Nazaratulmawarina, R.; Fatimah, C.A. The in vitro antibacterial activity of Corchorus olitorius extracts. Int. J. Pharmacol. 2006, 2, 213–215. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Magda, M.H.; Shafeek, M.R.; Aisha, H.A. Growth, yield and leaf content of Jews mallow plant (Corchorus olitorius) by soil fertilizer with different level of compost manure and chemical fertilizer. Middle East J. Agric. Res. 2014, 3, 543–548. [Google Scholar]
- Choudhary, S.B.; Sharma, H.K.; Karmakar, P.G.; Kumar, A.A.; Saha, A.R.; Hazra, P.; Mahapatra, B.S. Nutritional profile of cultivated and wild jute (‘Corchorus’) species. Australian J. Crop Sci. 2013, 7, 1973–1982. [Google Scholar]
- Choudhary, S.B.; Sharma, H.K.; Anil Kumar, A.; Maruthi, R.T.; Karmakar, P.G. The genus Corchorus L. (Malvaceae) in India: Species distribution and ethnobotany. Genet. Resour. Crop Evol. 2017, 64, 1675–1686. [Google Scholar] [CrossRef]
- Oboh, G.; Raddatz, H.; Henle, T. Characterization of the antioxidant properties of hydrophilic and lipophilic extracts of Jute (Corchorus olitorius) leaf. Int. J. Food Sci. Nutr. 2009, 60, 124–134. [Google Scholar] [CrossRef]
- Giro, A.; Ferrante, A. Yield and quality of Corchorus olitorius baby leaf grown in a floating system. J. Hortic. Sci. Biotechnol. 2016, 91, 603–610. [Google Scholar] [CrossRef]
- Ghellam, M.; Fatena, B.; Koca, İ. Physical and chemical characterization of Corchorus olitorius leaves dried by different drying techniques. Discov. Food 2022, 2, 14. [Google Scholar] [CrossRef]
- Morsy, N.E.; Rayan, A.M.; Youssef, K.M. Physicochemical properties, antioxidant activity, phytochemicals and sensory evaluation of rice-based extrudates containing dried Corchorus olitorius L. leaves. J. Food Process. Technol. 2015, 6, 1000408. [Google Scholar] [CrossRef]
- Youssef, K.M.; Mokhtar, S.; Morsy, N. Effect of hot air drying variables on phytochemicals and antioxidant capacity of Jew’s mallow (Corchorus olitorius L.) leaves. Suez Canal Univ. J. Food Sci. 2014, 2, 11–18. [Google Scholar] [CrossRef]
- Taiwo, B.J.; Taiwo, G.O.; Olubiyi, O.O.; Fatokun, A.A. Polyphenolic compounds with anti-tumour potential from Corchorus olitorius (L.) Tiliaceae, a Nigerian leaf veget. Bioorganic Med. Chem. Lett. 2016, 26, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Soykut, G.; Becer, E.; Calis, I.; Yucecan, S.; Vatansever, S. Apoptotic effects of Corchorus olitorius L. leaf extracts in colon adenocarcinoma cell lines. Prog. Nutr. 2018, 20, 689–698. [Google Scholar] [CrossRef]
- Abuzaid, H.; Amin, E.; Moawad, A.; Abdelmohsen, U.R.; Hetta, M.; Mohammed, R. Liquid chromatography high-resolution mass spectrometry analysis, phytochemical and biological study of two aizoaceae plants: A new kaempferol derivative from Trianthema portulacastrum L. Pharmacogn. Res. 2020, 12, 212–218. [Google Scholar] [CrossRef]
- Ndlovu, J.; Afolayan, A.J. Nutritional analysis of the South African wild vegetable Corchorus olitorius L. Asian J. Plant Sci. 2008, 7, 615–618. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Amina, M.; Ahamad, S.R. Comparative study on the chemical composition of Corchorus olitoriusl leaf and stem dry oils. Biomed. Res. 2017, 28, 4581–4587. [Google Scholar]
- Yakoub, A.R.B.; Abdehedi, O.; Jridi, M.; Elfalleh, W.; Nasri, M.; Ferchichi, A. Flavonoids, phenols, antioxidant, and antimicrobial activities in various extracts from Tossa jute leave (Corchorus olitorus L.). Ind. Crops Prod. 2018, 118, 206–213. [Google Scholar] [CrossRef]
- Isuosuo, C.; Akaneme, F.; Abu, N. Nutritional evaluation of the seeds of Corchorus olitorius: A neglected and underutilized species in Nigeria. Pak. J. Nutr. 2019, 18, 692–703. [Google Scholar] [CrossRef]
- Labib, A.S.; Somaia, A.; Helmy, O. Quality indices of Jew’s mallow and spinach during frozen storage. Plant Foods Hum. Nutr. 1997, 50, 333–347. [Google Scholar] [CrossRef]
- Haridy, A.G.; Abbas, H.S.; Mousa, A.A. Growth and Yield of Some Jew’s Mallow (Corchorus olitorius L.) Ecotypes as Affected by Planting Dates and Foliar Application of Gibberellic and Humic Acids. Assiut J. Agric. Sci. 2019, 50, 107–124. [Google Scholar] [CrossRef]
- Abdalla, M.M.; Attia, M.; Yousef, M.I.; Abd el-Aal, M.H. Effect of cooking on nutritive value of Jew’s mallow (Corchorus olitorius L.) and mallow (Malva parviflora L.) leaves. Alex. J. Food Sci. Technol. 2016, 13, 1–10. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Shah, M.H.; Khan, M.A. Nutritional Contents of Wild Edible Vegetables. In Wild Edible Vegetables of Lesser Himalayas; Ethnobotanical and Nutraceutical Aspects; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 141–167. [Google Scholar] [CrossRef]
- Elsayed, A.; Ezzat, S.; Khalil, M.; Seham, S. Chemical composition and evaluation of possible alpha-glucosidase inhibitory activity of eight Aloe species. J. Med. Plants Res. 2016, 10, 167–178. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef] [PubMed]
- Frajese, G.V.; Benvenuto, M.; Fantini, M.; Ambrosin, E.; Sacchetti, P.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Bei, R. Potassium increases the antitumor effects of ascorbic acid in breast cancer cell lines in vitro. Oncol. Lett. 2016, 11, 4224–4234. [Google Scholar] [CrossRef] [PubMed]
- Croci, S.; Bruni, L.; Bussolati, S.; Castaldo, M.; Dondi, M. Potassium bicarbonate and D-ribose effects on A72 canine and HTB-126 human cancer cell line proliferation in vitro. Cancer Cell Int. 2011, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Chen, T.S.; Ma, C.Y.; Meng, Y.B.; Zhang, Y.F.; Chen, Y.W.; Zhou, Y.H. Effect of vitamin B supplementation on cancer incidence, death due to cancer, and total mortality: A PRISMA-compliant cumulative meta-analysis of randomized controlled trials. Medicine 2016, 95, e3485. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Hafez, H.A.; Kamel, M.A.; Ghamry, H.I.; Shukry, M.; Farag, M.A. Dietary Vitamin B Complex: Orchestration in Human Nutrition throughout Life with Sex Differences. Nutrients 2022, 14, 3940. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Vivar, J.A.; Tan, M.G.S.; Shen, C.C. Chemical constituents of Corchorus olitorius L. Int. J. Pharmacognf. Phytochem. Res. 2016, 8, 2085–2089. [Google Scholar]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and Antioxidant Effects of Vitamin C in Cancer in Correspondence to Its Dietary and Pharmacological Concentrations. Oxidative Med. Cell. Longev. 2019, 24, 7286737. [Google Scholar] [CrossRef]
- Beghdad, M.C.; Chahid, B.; Fatima, B.; Fatima-Zohra, S.; Meriem, B.; Farid, C. Antioxidant activity, phenolic and flavonoid content in leaves, flowers, stems and seeds of mallow (Malva sylvestris L.) from North Western of Algeria. Afr. J. Biotechnol. 2014, 13, 486–491. [Google Scholar] [CrossRef]
- Mouas, T.N.; Kabouche, Z.; Benabid, N.; Bendal, M. Investigations on Bioactive Compounds and In Vitro Biological Potent of Corchorus olitorius. L. from Algerian Cultivar. Proceedings 2021, 65. [Google Scholar] [CrossRef]
- Ben Yakoub, A.R.; Abdehedi, O.; Jridi, M.; Elfalleh, W.; Bkhairia, I.; Nasri, M.; Ferchichi, A. Bioactive polysaccharides and their soluble fraction from Tossa jute (Corchorus olitorius L.) leaves. Food Biosci. 2020, 37, 100741. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Plant pigments as dietary anticancer agents. Int. J. Green Pharm. (IJGP) 2018, 12, S93–S107. [Google Scholar]
- Khan, M.; Bano, S.; Javed, M.A.; Mueed, A. A comprehensive review on the chemistry and pharmacology of Corchorus species—A source of cardiac glycosides, triterpenoids, ionones, flavonoids, coumarins, steroids and some other compounds. J. Sci. Ind. Res. 2006, 65, 283–298. [Google Scholar]
- Chen, C.C.; Agrawal, D.C.; Lee, M.R.; Lee, R.J.; Kuo, C.L.; Wu, C.R.; Tsay, H.S. Influence of LED light spectra on in v intro somatic embryogenesis and LC–MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb.: Amedicinal herb. Bot. Stud. 2016, 57, 9. [Google Scholar] [CrossRef] [PubMed]
- Lan, W. Effect of chlorogenic acid on antioxidant activity of Flos Lonicerae extracts. J. Zhejiang Univ. Sci. B 2007, 8, 673–679. [Google Scholar]
- Yoon, G.A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Muthugounder, S.; Presser, N.; Viswanathan, S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv. Exp. Med. Biol. 2004, 546, 121–165. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Alruwad, M.I.; Sabry, M.M.; Gendy, A.M.; El-Dine, R.S.; El Hefnawy, H.M. In Vitro Cytotoxic Potential of Selected Jordanian Flora and Their Associated Phytochemical Analysis. Plants 2023, 12, 1626. [Google Scholar] [CrossRef]
- Ahmad, A.; Prakash, R.; Khan, M.S.; Altwaijry, N.; Asghar, M.N.; Raza, S.S.; Khan, R. Enhanced Antioxidant Effects of Naringenin Nanoparticles Synthesized using the High-Energy Ball Milling Method. ACS Omega 2022, 7, 34476–34484. [Google Scholar] [CrossRef]
- Sawa, T.; Nakao, M.; Akaike, T.; Ono, K.; Maeda, H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: Implications for the anti-tumor-promoter effect of vegetables. J. Agric. Food Chem. 1999, 47, 397–402. [Google Scholar] [CrossRef]
- Sierens, J.; Hartley, J.A.; Campbell, M.J.; Leathem, A.J.; Woodside, J.V. In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog. Carcinog. Mutagen. 2002, 22, 227–234. [Google Scholar] [CrossRef]
- Hanachi, P.; Fakhrnezhad, F.R.; Zarringhalami, R.; Orhan, I.E. Cytotoxicity of Ocimum basilicum and Impatiens walleriana Extracts on AGS and SKOV-3 cancer cell lines by flow cytometry analysis. Int. J. Cancer Manag. 2021, 14, e102610. [Google Scholar] [CrossRef]
- Omoruyi, S.I.; Kangwa, T.S.; Ibrakaw, A.S.; Cupido, C.N.; Marnewick, J.L.; Ekpo, O.E.; Hussein, A.A. Cytotoxic activities of selected plants of the family Amaryllidaceaeon brain tumour cell lines. S. Afr. J. Bot. 2021, 136, 118–125. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Elwakil, H.D.M.; Gomaa, S.E.; FM Zaitoun, A.; El-kader, A.; Bassant, G.M.; Abdelsalam, N.R. Morphological, Biochemical and Barcoding Analysis of Different Egyptian Jew’s mallow (Corchorus olitorius L.) Landraces. J. Adv. Agric. Res. 2023, 28, 582–596. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 19th ed.; AOAC: Arlington, VA, USA, 2010. [Google Scholar]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical Characterization and Utilization of Dried Red Beetroot (Beta vulgaris) Peels Extract in Maintaining the Quality of Nile Tilapia Fish Fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, A.A.F.; Braga, C.M.; Demiate, I.M.; Beltrane, F.L.; Nogueira, A.; Wosiacki, G. Development and optimization of a HPLC-RI method for the determination of major sugars in apple juice and evaluation of the effect of the ripening stage. Food Sci. Technol. 2014, 34, 38–43. [Google Scholar] [CrossRef]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
- Antakli, S.; Sarkees, N.; Sarraf, T. Determination of water-soluble vitamins B1, B2, B3, B6, B9, B12 and C on C18 column with particle size 3um in some manufactured food products by HPLC with UV-DAD/FLD detection. Int. J. Pharm. Pharm. Sci. 2015, 7, 219–224. [Google Scholar]
- Romeu-Nadal, M.; Morera-Pons, S.; Castellote, A.I.; Lopez-Sabater, M.C. Rapid high-performance liquid chromatographic method for Vitamin C determination in human milk versus an enzymatic method. J. Chromatogr. B 2006, 830, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- da Silva Padilha, C.V.; Miskinis, G.A.; de Souza, M.E.A.O.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; dos Santos Lima, M. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A comparative survey of leguminous plants as sources of the isoflavones; genistyein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 11, 77–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).