Abstract
Zingiber officinale Roscoe (ginger) is a plant from the Zingiberaceae family, and its extracts have been found to contain several compounds with beneficial bioactivities. Nowadays, the use of environmentally friendly and sustainable extraction methods has attracted considerable interest. The main objective of this study was to evaluate subcritical propane (scPropane), supercritical CO2 (scCO2), and supercritical CO2 with ethanol (scCO2 + EtOH) as co-solvent methods for the extraction of high value products from ginger. In addition, the reuse/recycling of the secondary biomass in a second extraction as a part of the circular economy was evaluated. Both the primary and the secondary biomass led to high yield percentages, ranging from 1.23% to 6.42%. The highest yield was observed in the scCO2 + EtOH, with biomass prior used to scCO2 extraction. All extracts presented with high similarities as far as their total phenolic contents, antioxidant capacity, and chemical composition. The most abundant compounds, identified by the two different gas chromatography-mass spectrometry (GC-MS) systems present, were a-zingiberene, β- sesquiphellandrene, a-farnesene, β-bisabolene, zingerone, gingerol, a-curcumene, and γ-muurolene. Interestingly, the reuse/recycling of the secondary biomass was found to be promising, as the extracts showed high antioxidant capacity and consisted of significant amounts of compounds with beneficial properties.
1. Introduction
Zingiber officinale Roscoe (ginger) is a plant that is widely distributed worldwide and belongs to the Zingiberaceae family. It contains compounds that present a wide range of benefits, such as antioxidant, antifungal, antibacterial, and anticancer effects [1,2,3,4]. Ginger, both fresh and dried, is used for consumption as well as medicinal applications [5]. Ginger’s extracts and essential oils have diverse chemical compositions, and they mainly include compounds belonging to terpenoids and phenolics groups which possesses significant biological activity [6]. Moreover, polysaccharides have also been reported to be components of ginger extracts [1]. The bioactive compounds present in ginger extracts/oils lead to a vast number of applications in the medicinal and cosmetic sector, as well as the food industry and the agriculture sector [7,8,9,10,11].
As a plant with significant value, ginger has attracted intensive research interest. Thus, many studies have focused on the application of different extraction methods with the aim of maximizing the extraction efficiency [1]. In the past years, new technologies for the extraction of beneficial compounds have attracted a lot of attention. Specifically, environmentally friendly, low-cost, and sustainable extraction methods have been used to extract and isolate compounds of interest [6]. Among them, super- and sub- critical extraction methods have aroused remarkable interest from the scientific community, because only small changes in the pressure and temperature can increase the selectivity of the extraction process [12]. These types of extractions have numerus advantages, with the most interesting being the decrease of the thermal degradation of the targeted compounds [12], which is a major disadvantage of the conventional extraction procedures [13,14]. The lower temperatures also lead to the reduction of the extraction cost [15].
In the literature, it is well-documented that compounds extracted from ginger possess various biological activities and can have diverse applications. However, the extraction of bioactive compounds with high recovery remains a challenge [5]. Ginger solvent extraction has been the most studied. More recently, advanced methods such as supercritical and subcritical techniques, as well as pressurized fluid and ultrasound-assisted techniques, have shown promising results for the extraction of bioactive substances [5,16]. The evaluation of these techniques for the recovery of such compounds has led to significant interest [5]. It is worth noticing that these types of extractions lead to better quality products, and that the solvent can be recycled and reused [17,18].
The aim of the present study was to explore different methods of ginger extraction, using more advanced and environmentally friendly procedures. Therefore, a variety of sub- and supercritical extractions were performed and evaluated in terms of the yield percentage, the total phenolic content, the antioxidant capacity, and the chemical composition. The gas chromatography-mass spectrometry (GC-MS) technique was also used to identify the main components. Additionally, the utilization of the residual (secondary) biomass was studied, with the purpose being the production of additional added value products. Large amounts of residual biomass, which is potentially not environmentally friendly, are produced yearly [19,20]. There is increasing interest around the utilization of this type of biomass to further isolate natural bioactive compounds [21]. To the authors’ best knowledge, this is the first study focusing on the extraction of ginger through using and comparing a variety of sub- and supercritical extractions. The main focus of the present study was to evaluate the possibility of enhancing the high value bioactive compounds’ recovery through the utilizion of the residual biomass, with a multistep extraction procedure of the secondary biomass. The recovery of those high value compounds is a key point for the valorization of the used biomass, with the possibility of their use in food, cosmetics, and other products. The main limitation regarding the reuse of biomass is the high cost. The proposed procedure is a low-cost, environmentally friendly extraction method that follows the framework of circular economy.
2. Results and Discussion
2.1. Soxhlet Extraction
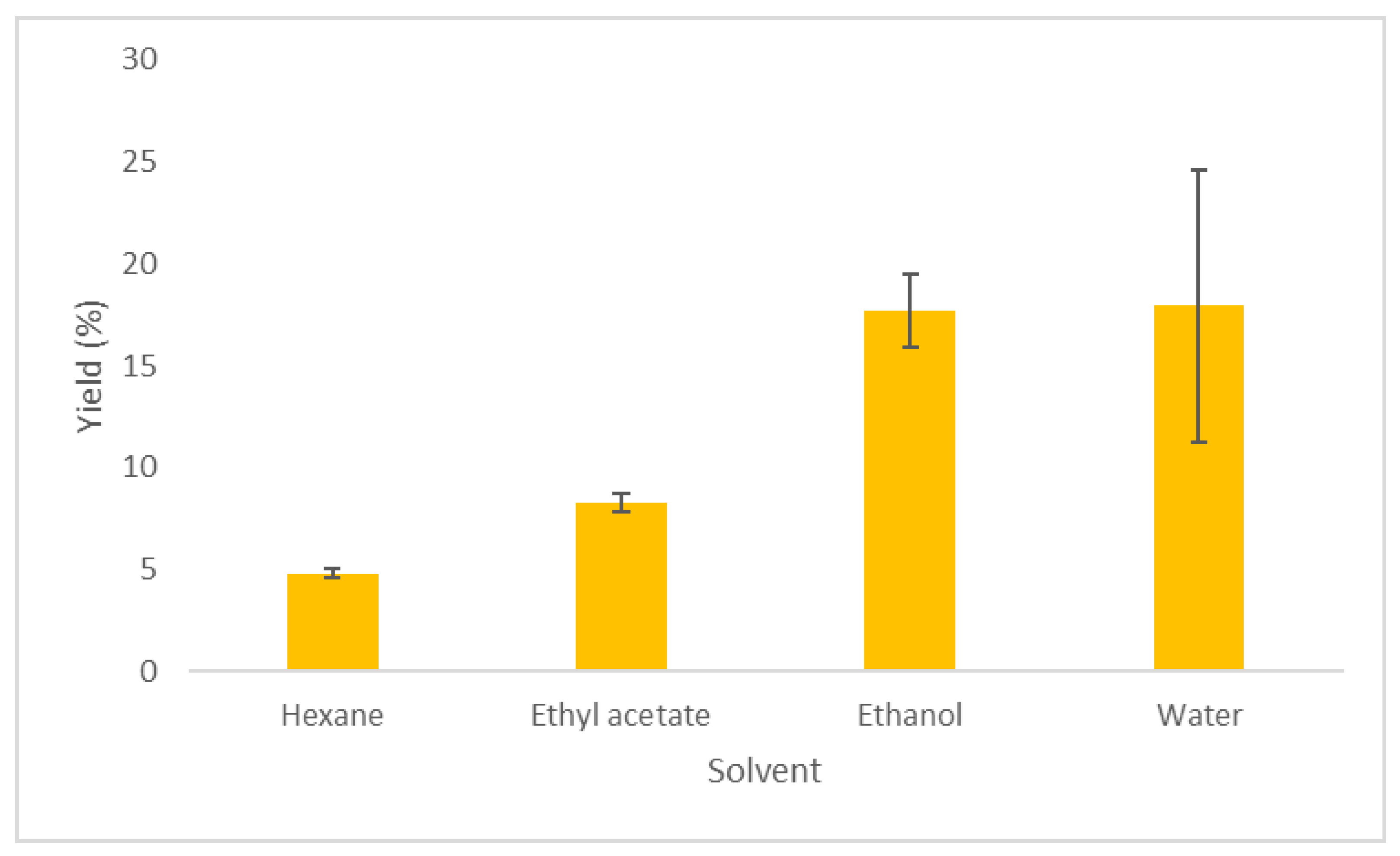
In Table 1, the extraction conditions and extraction yields using Soxhlet, in combination with different solvents, are presented. The Soxhlet extraction was performed to select the most suitable co-solvent in the supercritical extraction. The highest yield was observed using water as solvent, followed by ethanol (17.93% and 17.70%, respectively) (Table 1, Figure 1). Lower extraction yields were noticed with ethyl acetate and hexane (8.28% and 4.82%, respectively). This difference could be due to the different polarities of the used solvents (10.2 for water, 5.2 for ethanol, 4.3 for ethyl acetate, and 0.0 for hexane) [22], and their ability to solubilize the oils from the biomass.

Table 1.
Experimental conditions and extraction yields of Zingiber officinale Roscoe acquired under 6 h of Soxhlet extraction using different solvents.
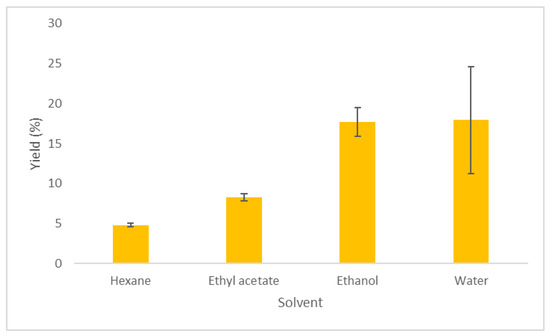
Figure 1.
Extraction yield % of Zingiber officinale Roscoe using Soxhlet extraction with different solvents.
Similar to our results, Al-Areer et al. found that, [24] when performing Soxhlet extractions at 90 °C for 9 h, the highest extraction yield was observed when ethanol was used as solvent, followed by ethyl acetate and hexane. A different trend was observed by Lemma and Egza, [25] who noticed a higher yield in the hexane than the ethanolic extract when Soxhlet extractions were performed for about 4 h at the boiling point of each solvent.
Moreover, the antioxidant capacity, as well as the concentration of total phenols of the extracts, were evaluated. The results are shown in Table 2. The highest antioxidant activities were observed in the ethyl acetate and ethanolic extracts. Regarding the total phenolic content, the ethanolic extract presented the highest concentration.

Table 2.
Antioxidant capacity and total phenolic content (mg of gallic acid equivalents (GAE) per 100 g of sample) of primary biomass of Zingiber officinale Roscoe extracts acquired under Soxhlet extraction using different solvents.
The differences in the extraction yields, total phenolic contents, and antioxidant activity may occur because different solvents can extract different active compounds [26]. Water, even though it presented the higher extraction yield, showed the lowest total phenolic content and antioxidant activity. This can be due to the high temperature used in that case (100.5 °C), leading to the thermal decomposition of high bioactive compounds (Table 2). It is well-known that the high temperatures used in the Soxhlet extraction process (up to the boiling point of each solvent), along with the long extraction time, can lead to the thermal degradation of some compounds [27]. More specifically, the continuous evaporation and extraction of the target compounds caused by the solvents, the high extraction kinetics, and the prolonged extraction time can promote the decomposition of thermolabile target compounds [28]. Thermolabile target compounds can consist of polyphenols that present high antioxidant capacity [29].
Μoreover, it is reported in the literature that many factors can affect antioxidant activities. Besides the amount and strength of the antioxidant compounds, the ability of the antioxidants to transfer a hydrogen to a free radical such as DPPH· can also be affected by the environment where the reaction takes place; for example, in different types of solvents [30].
2.2. Sub- and Supercritical Extraction
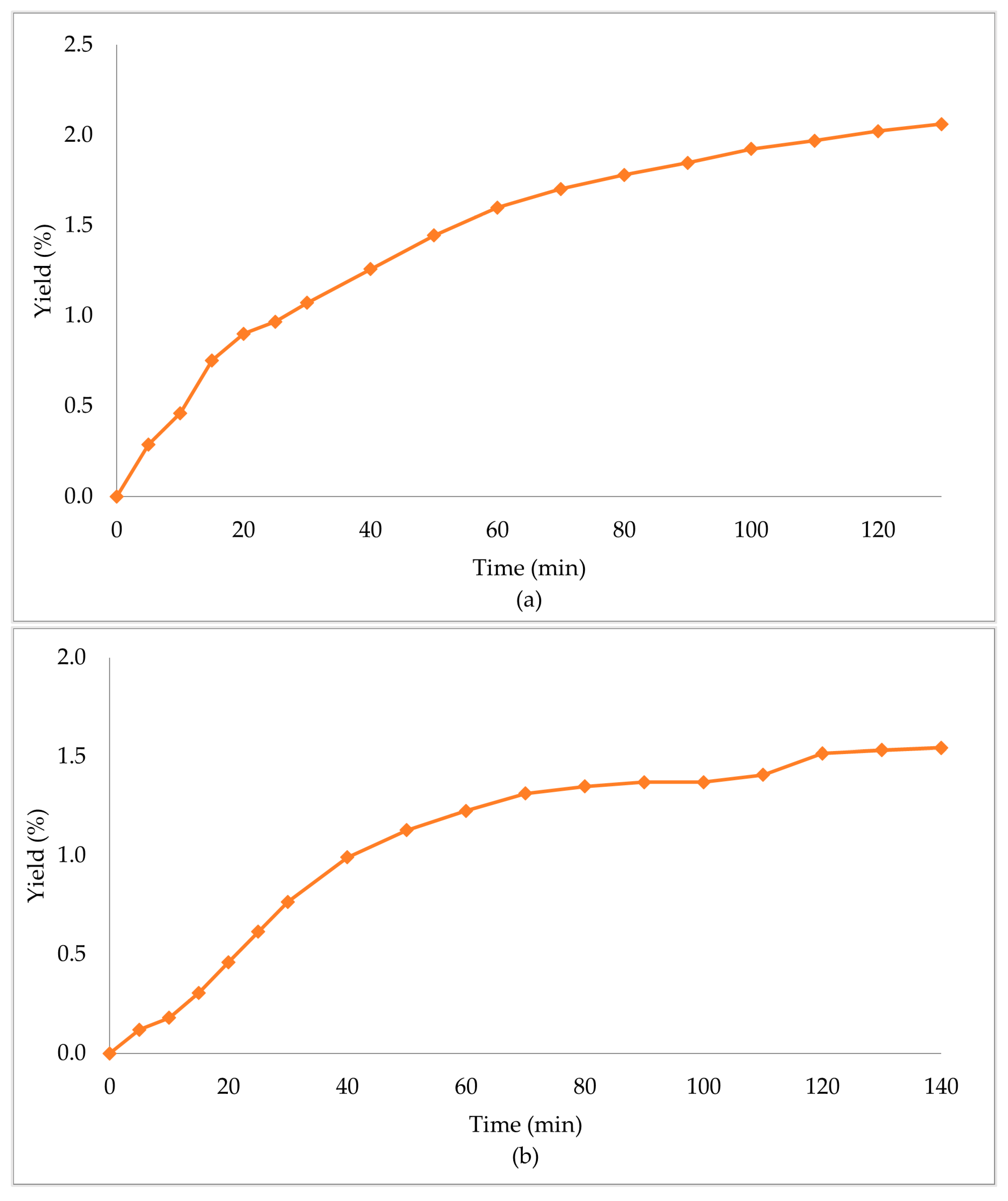
In Table 3, the extraction conditions for the sub- and supercritical extractions are presented. The primary biomass extraction resulted in high yield percentages (Table 4, Figure 2). The supercritical CO2 extraction with ethanol as the co-solvent (scCO2 + EtOH) gave an extraction yield of 6.06%, which was the highest of the three obtained, followed by subcritical propane (scPropane) and supercritical CO2 (scCO2), with yield percentages of 2.06% and 1.54%, respectively. It is worth noticing that scCO2 + EtOH led to higher yields in less time (half the time in comparison with the other methods). Considering the increase in the yield percentage, and the decrease of the time needed, the addition of ethanol had a positive effect in the overall extraction.

Table 3.
Extraction yields of Zingiber officinale Roscoe acquired under subcritical and supercritical extraction using different extraction conditions.

Table 4.
Total phenolic content of Zingiber officinale Roscoe extracts acquired under different extraction conditions (mg GAE 100 g−1).
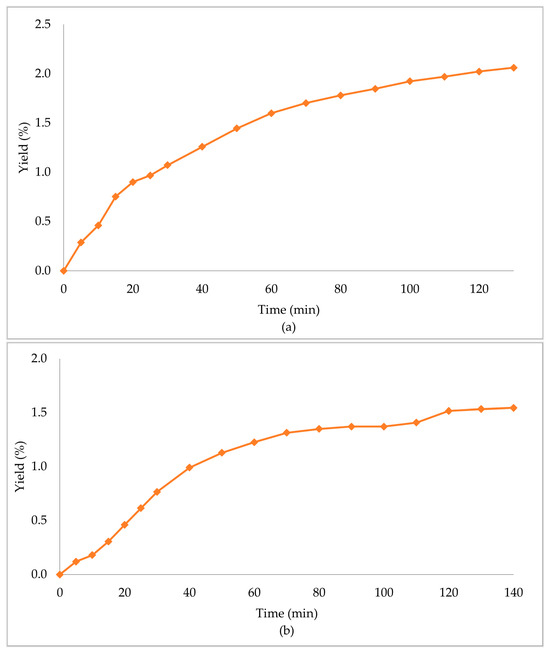
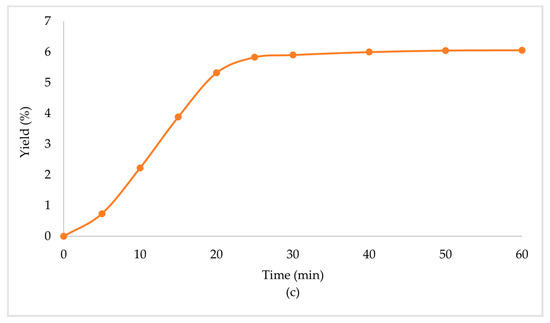
Figure 2.
Extraction yield % of Zingiber officinale Roscoe extracts acquired under (a) scPropane extraction, (b) scCO2 and (c) scCO2 +EtOH.
Ethanol, when used as a co-solvent, solubilized the CO2 and led to a decrease of the viscosity of the mixture of solvents (CO2 + EtOH), and an increase of density [31,32]. The combination of the solvents accelerated the extraction process, led to less usage of CO, and, and enhanced the extraction yield. Additionally, the polar mixture of solvents led to an increase of the extracted amount of polar and soluble compounds [22]. In general, the usage of co-solvents may improve the extraction performance due to the enhanced transport of solute [33].
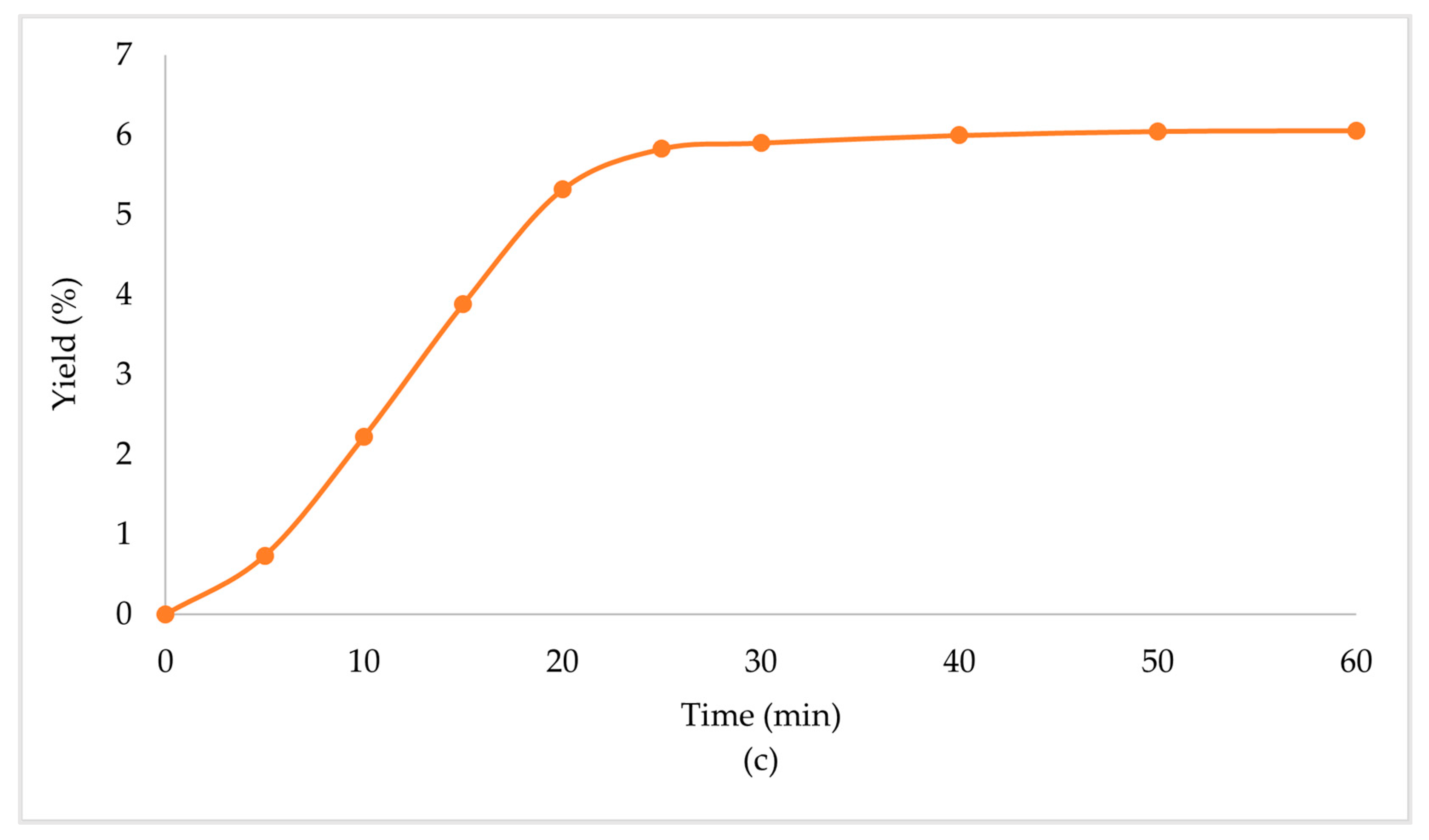
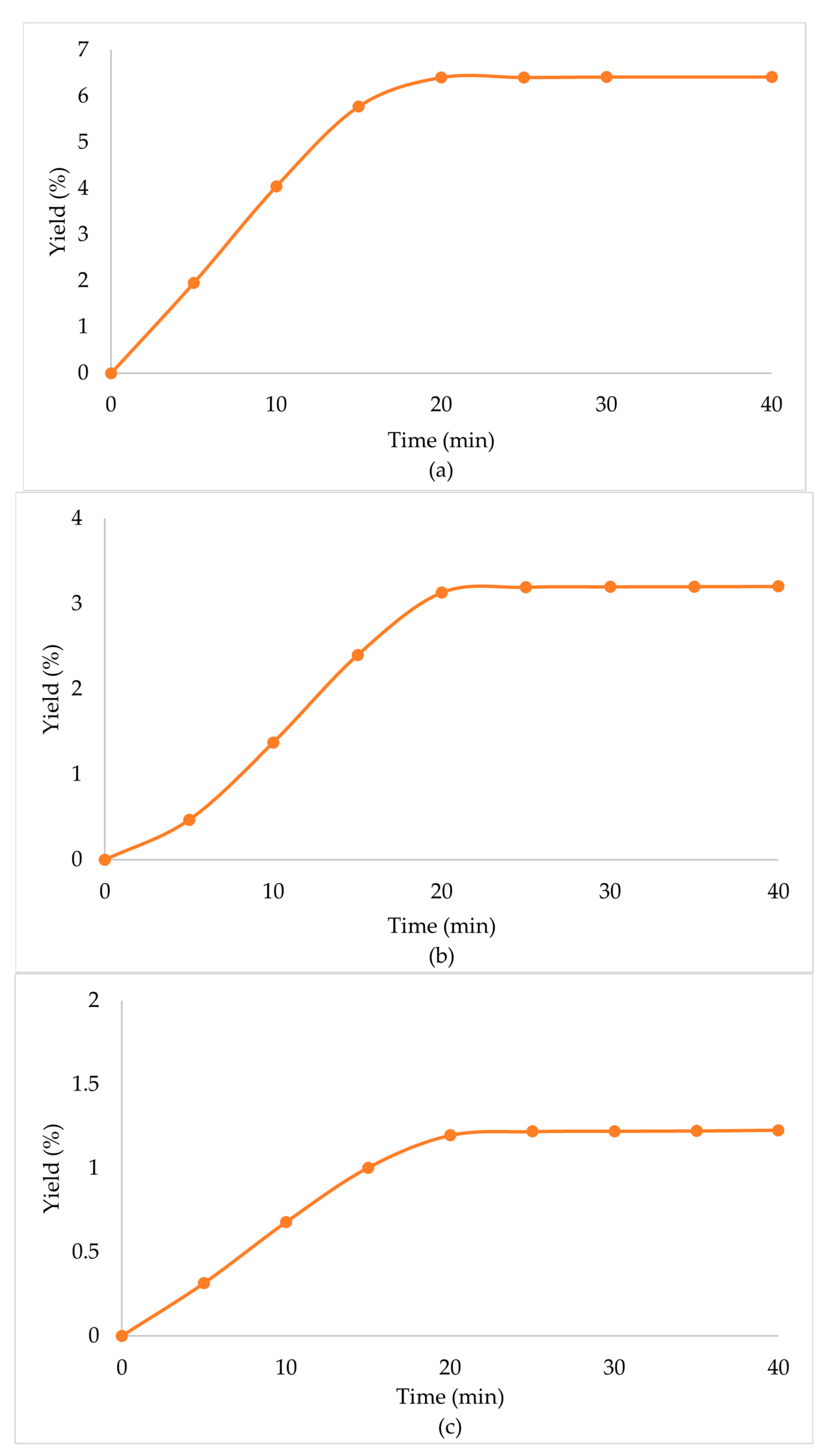
Due to the enhanced performance of the scCO2 + EtOH extraction, when a secondary biomass was used, high extraction yields were observed in all cases (Table 4, Figure 3). Specifically, in the case of the scCO2 + EtOH extraction of the secondary biomass that was used once before in scCO2 extraction, the extraction yield was significantly higher than that of the primary biomass. CO2 behaves as a nonpolar solvent and presents with a low extraction performance for polar compounds [34]. The co-solvent (EtOH) that was used in the secondary biomass extraction had a positive influence on the extraction procedure, since it lowered the viscosity of the solvents, increased the density, and altered the overall polarity of the mixture of solvents, which led to the enhancement of the extraction yield.
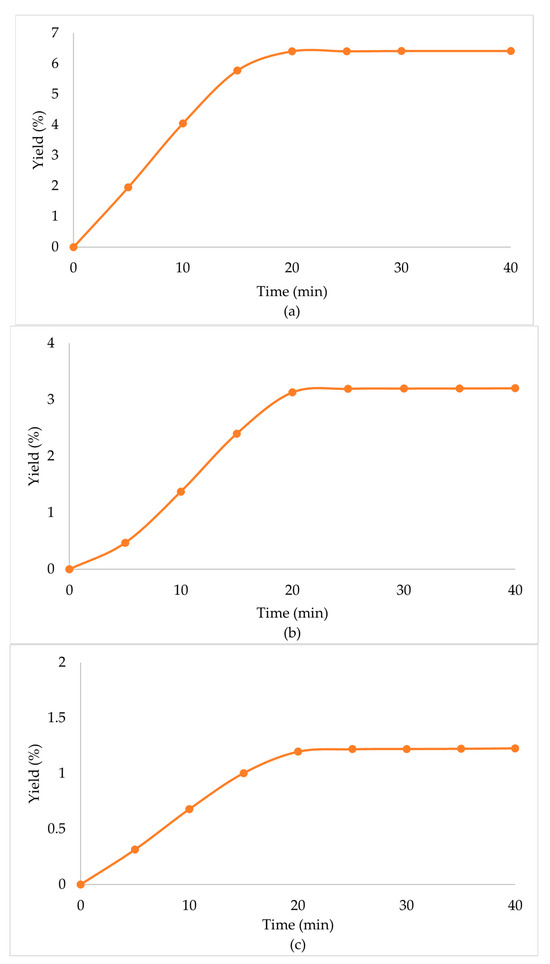
Figure 3.
Extraction yield % of Zingiber officinale Roscoe extracts acquired under scCO2 +EtOH, with secondary biomass, previously used at (a) scCO2 once, (b) scCO2 + EtOH once and (c) scCO2 + EtOH twice.
When the biomass, prior used in scCO2 + EtOH, was extracted in a multistep extraction twice with scCO2 + EtOH, the extraction yield was lower than the primary biomass, but still significant. Mainly, this was observed due to the high amounts of polar compounds that the mixture of solvents obtained through the first extraction, and when only CO2 was left in the chamber, the capability to extract polar compounds was minimized. In continuation, when the extraction procedure was repeated, and the mixture of solvents renewed, their capability to extract those compounds improved once again and the extraction yield remained significant.
High yield percentages for ginger have been reported by Mesomo et al., [15] and Mesomo et al., [11] using CO2 and Propane as solvents, in various pressures and temperatures. For CO2, the yield percentages ranged from 0.22% to 3.21%, and for Propane from 1.98% to 2.70%. For CO2, the pressure led to a positive effect on the yield, and for propane, both pressure and temperature. Similarly, Salea et al., [35] using scCO2 in various conditions, calculated a range of yields from 1.55% to 2.95%. That range was attributed to the variety of pressure and temperature conditions used in each extraction.
Zancan et al. [36] performed scCO2 and scCO2 + EtOH, and did not observe significant differences in the yield percentages between the two methods, but ethanol favored the extraction of gingerols and shogaols.
2.3. Total Phenolic Contents and Antioxidant Capacity
Table 4 presents the total phenolic contents acquired through using different extraction conditions and solvents, as well as both primary and secondary biomass. The total phenolic contents were expressed as mg of gallic acid equivalent per 100 g of sample. In both the primary and secondary biomass, similar total phenolic contents were observed, with the highest being in scCO2 + EtOH using secondary biomass, which was formerly used in the same type of extraction.
Stoilova et al., [37] found a total phenolic content of 871 mg/g dry extract acquired through high pressure CO2 extraction.
A similar trend can be observed for the antioxidant capacity of the extracts (Table 5, Figures S2 and S3). Overall, according to the IC50 values, the scCO2 + EtOH extract presented the highest antioxidant activity, and the scCO2 extract the lowest.

Table 5.
Antioxidant capacity of Zingiber officinale Roscoe extracts acquired under different extraction conditions.
The antioxidant properties of ginger extracts obtained by different extraction methods have been widely studied. Its antioxidant properties are due to compounds such as gingerols, flavonoids, and phenolic acids [38].
Mesomo et al., [15] studying different conditions of supercritical extraction, observed the highest antioxidant capacity with scCO2. Zancan et al. [36] observed that, when no gingerols and shogaols were yet obtained by the extraction the antioxidant, activity was much lower. Stoilova et al. [37] calculated the IC50 for inhibition of DPPH to be 0.64 μg mL−1.
2.4. GC-MS Analysis
Ginger includes both volatiles such as geraniol, borneol, terpineol, curcumene, zingiberol, α-farnesene, α-sesquiphellandrene, α- β-bisabolene, β-elemene etc., as well as non-volatile compounds such as gingerols, shogaols, zingerone, and paradols [7]. The composition of ginger extracts and oils differ significantly. The bioactive compounds of ginger essential oils are mainly monoterpenes and sesquiterpene hydrocarbons, and their chemical composition depends on the nature (fresh/dry) and place of origin of the ginger rhizome, as well as the extraction method employed [10]. They are also composed of oxygenated hydrocarbon compounds including aldehydes, phenols, esters, oxide ethers, alcohols, and ketones [10].
The chemical composition of the extracts/oils was determined by GC-MS analysis, and all the compounds identified have been compiled and presented in Table 6 along with their area percentage. The extracts obtained with all the tested systems were found to have quite similar chemical compositions, and the main substances were a-zingiberene, β-sesquiphellandrene, a-farnesene, β-bisabolene, zingerone, gingerol, a-curcumene, and γ-muurolene. Similar compounds have also been identified in the literature [11,38,39,40]. In the case of scPropane, some more compounds, i.e., monoterpenes and sesquiterpenes, were identified but only in traces. When a primary biomass was used, Soxhlet with ethanol extracted less compounds in comparison with scPropane, scCO2, and scCO2 + EtOH, signifying the increased efficiency of such advanced extraction techniques.

Table 6.
Chemical composition of ginger extracts (Type of biomass: Primary) using different extraction methods (+:presence/% of total area).
Our results regarding the main components are in agreement with the bibliography [11]. Based on literature data, higher essential oil and β-zingiberene contents were obtained for the dried ginger rhizome than that of the fresh ones. Moreover, the drying method has been found to play a significant role in essential oil’s yield and its chemical composition. Temperatures lower than 70 °C can increase the yield of ginger oil without having any effect on the transformation of 6-gingerol to 6-shogaol [41].
It is noteworthy that most of the above-mentioned compounds were identified after the reuse/recycling of the secondary biomass, highlighting the possibility to extract the maximum value from the used biomass. In Table 7, the identified compounds of the secondary biomass extractions are presented with their area percentage. After the first extraction of the secondary biomass, the compounds identified were less in comparison with the primary biomass’s identified compounds, but their area percentage was higher.

Table 7.
Chemical composition of ginger extracts (Type of biomass: Secondary) using different and sequential extraction methods (+: presence/% of total area).
The main identified compounds (a-zingiberene, β-sesquiphellandrene, a-farnesene, β-bisabolene, zingerone, gingerol, and a-curcumene) that can be found in almost all of the studied extracts have been previously reported to possess significant antioxidant properties. This is in agreement with our results [42,43,44,45,46,47]. Badrunanto et al., [45] when studying the antioxidant components of Indonesian ginger essential oil, observed that, amongst others, a-zingiberene, β-sesquiphellandrene, a-farnesene, β-bisabolene, and a-curcumene, presented a high correlation with the antioxidant activity of the oil. Similarly, Misharina et al. [46] highlighted the antioxidant activity of zingiberene, β-sesquiphellandrene and β-bisabolene. Gingerol analogues have been associated with ginger extracts’ antioxidant activity [42,43,47]. Specifically, Danwilai et al. [42] studied the antioxidant activity of ginger extract oral supplements in cancer patients who were receiving adjuvant chemotherapy. These supplements contained 20 mg day−1 6-gingerol, and results found a significant increase regarding antioxidant activity, and a decrease of oxidative marker levels. Moreover, Wang et al. [43] observed that 10-gingerol presented with about 34.2% DPPH radical scavenging activity, and 6-gingerol about 16.3%. Furthermore, they noticed that the antioxidant activity of those ginger compounds contributed to the antimicrobial activity against Acinobacter baummannii infections. 6-gingerol, 8-gingerol, and 10-gingerol’s antioxidant activity was studied by Dugasani et al. [47], who observed that the DPPH scavenging potential was in the order of 10-gingerol > 8-gingerol > 6-gingerol. Rajan et al. [44] studied zingerone’s antioxidant activity using a DPPH free radical method, and observed a dose dependent increase of the compound’s antioxidant activity.
In general, it is well reported in the literature that the components of ginger extracts/essential oils have significant bioactivities and health-promoting properties, and thus can have applications in various sectors [7,8,9,10,11]. Based on literature data, ginger extracts/oils containing most of the bioactive compounds found in the present have been reported to have significant pharmacological, medicinal, and cosmetic applications, as they have been found to possess antimicrobial and antiseptic activity, anti-carcinogenic potential, neuroprotective activity, anti-obese activity, anti-diabetic effect, and analgesic activity as well as provide cardiovascular protection [8,11]. Another significant application is in the food industry, as the bioactive compounds of ginger can provide oxidative and storage stability, sensorial properties, preservation, oxidative resistance, and anti-bacterial activity in consuming products [7]. As well, another notable factor is its application in agriculture for the control of plant diseases which minimizes simultaneously the possible negative effects on the environment, animals, and human health [9,10].
3. Materials and Methods
3.1. Materials
Ethyl acetate (99.5%) was obtained from NEON comercial (Suzano, SP, Brazil), ethanol (99.5%) from ACS CIENTIFICA (Sumare, SP, Brazil), hexane (98.5%) from exodo cientifica (Sumare, SP, Brazil), CO2 (99.95%) and propane (99.5%) from White Martins S.A. (Curitiba, PR, Brazil), Folin-Ciocalteu’s reagent from CARLOS ERBA REAGENTS (Val-de-Reuil, France), Sodium carbonate anhydrous (>99.5%) from Fluka (Buchs, Switzerland), 1,1-Diphenyl-2-picrylhydrazyl Free Radical (DPPH) from TCI EUROPE N.V. (Zwijndrecht, Belgium), and gallic acid from Sigma Aldrich (Darmstadt, Germany). The Soxhlet extractor was purchased from Qualividros (Passos, MG, Brazil).
3.2. Sample Preparation
Very fresh Zingiber officinale Roscoe rhizomes were purchased from the local market at Curitiba, Brazil. After being transferred to the laboratory, the rhizomes were washed carefully with water and cut into smaller sized pieces to dry evenly. After being dried at 30 °C with air circulation until the moisture content became less than 10% (Table 8), the samples were grounded in a blender and stored in plastic bags until use.

Table 8.
Moisture content of Zingiber officinale Roscoe before and after drying.
3.3. Soxhlet Extraction
Soxhlet extraction was performed according to the AOAC [48] method. Briefly, 5 g of biomass and 150 mL of solvent were used in each extraction. The total duration was 6 h, using different temperatures depending on the solvent (Table 1). Specifically, the extractions took place at each solvent’s boiling point. The solvents used were ethyl acetate, ethanol, hexane, and water and, in each case, the procedure was repeated three times. A rotary vacuum evaporator (IKA RV 10 combined with IKA HB 10-IKA, Campinas, SP, Brazil) was used to concentrate the Soxhlet extracts, and they were then dried in an air circulation oven at 40 °C for about 24 h. The extraction yields were calculated with the Equation (1):
Yield (%) = [mass of dried extract (g)/initial mass of biomass (g)] × 100
3.4. Sub- and Supercritical Extraction
Sub- and supercritical extractions were performed at an extraction unit (inner volume 80 cm3, length 16 cm and ∅ = 2.52 cm) which consisted of the extractor and a syringe pump. Additionally, the extraction system was equipped with pressure and temperature sensors in order to monitor the conditions. A thermostatic bath was attached to the system to better control the temperature. Furthermore, a needle valve controlled the flow inside the extractor, which was directly proportional to the pressure. The extractor was loaded with 10–15 g of biomass. Ιn the cases a co-solvent was used, its mass was 20–25 g. The temperature was set at 60 °C. For the scPropane extraction, the pressure was set at 100 bar, and for the scCO2 and scCO2 +EtOH extraction the pressure was set at 150 bar. The CO2 or the propane was loaded in the vessel until the desired pressure was achieved. In the beginning of each extraction process, a 30 min static extraction was performed, and after that a dynamic extraction was carried out with a flow rate of approximately 1–2 mL min−1. The dynamic extraction ended when no more extract was collected in the sampling tubes. The extraction yields were calculated by the Equation (1).
Extraction of the used primary biomass was also performed to evaluate the possibility of obtaining compounds of interest even with secondary biomass. Specifically, biomass prior used in scCO2 extraction was extracted a second time using scCO2 + EtOH. Moreover, a multistep approach was performed for the primary biomass that was used in scCO2 + EtOH, which then was extracted again twice with scCO2 + EtOH.
3.5. Total Phenolic Contents and Antioxidant Capacity
The total phenolic contents were determined using the Folin-Ciocalteu method as described by Box, 1983 [49]. Briefly, 5 mL of 10 μg mL−1 extract diluted in ethanol was added in glass bottles with 0.25 mL of Folin-Ciocalteu Reagent. After gentle stirring and 2 min of waiting, 0.75 mL of Na2CO3 200 g L−1 was added and the solution was left in the dark for 1 h. The absorbance was measured at 765 nm in a Hitachi U2000 Spectrophotometer (Hitachi, Ltd., Santa Clara, CA, USA). The results were calculated using a calibration curve of gallic acid (0.5–10 mg L−1), and expressed as mg of gallic acid equivalents per 100 g of sample.
The free radical quenching ability of the extracts was determined using the DPPH reagent, as described by Mensor et al. [50]. Briefly, 2.5 mL of extract diluted in ethanol in concentrations ranging from 5 to 250 μg mL−1 (apart from the extract from Soxhlet that water was used as solvent, where the concentrations were ranging between 5 and 1000 μg mL−1, because of the low antioxidant capacity it presented) and DPPH in a final concentration of 0.3 mM were mixed. After 30 min in the dark, the absorbance was measured at 518 nm (Hitachi U2000 Spectrophotometer). The antioxidant capacity (AC) was calculated using the equation:
where abssample is the absorbance of the solution consisting of the extract, DPPH, and ethanol, absblank the absorbance of the extract and ethanol, and abscontrol the absorbance of DPPH and ethanol.
AC (%) = 100 − {[(abssample − absblank) × 100]/abscontrol}
After the results were plotted, the concentration (μg mL−1) of each extract needed to inhibit 50% (IC50) of radicals’ production was calculated by linear regression.
3.6. Statistics
Significant differences in the yield percentages, total phenolic contents and IC50 values were assessed by post-hoc multiple comparison tests (Bonferroni test, p < 0.05, ANOVA) using the IBM SPSS statistics Inc. (Version: 28.0.1.0 (142)) software package.
3.7. GC-MS Analysis
Analysis of the samples (injection volume 1 μL) were performed using two GC-MS systems. The first system was composed of an Agilent 6890 Series GC system and an Agilent 5973 Network mass selective detector (MSD) (Agilent Technologies, Santa Clara, CA, USA). The second system was an Agilent 5975B inert MSD integrated to an Agilent 6890N Network GC (Agilent Technologies, Santa Clara, CA, USA). More information about the analytical columns, the temperature programs, and the conditions adopted in the MSD are reported in Supplementary Materials. Enhanced Data Analysis software was used for the analysis of the chromatograms, and NIST MS Search 2.0 software was used for compound identification.
4. Conclusions
In this work, compressed solvents technology was investigated for the extraction of high value products from Zingiber officinale Roscoe. Soxhlet extraction was used in preliminary experiments for the selection of the most appropriate co-solvent for scCO2. Using Soxhlet extraction, the highest yields were observed when water and ethanol were used, i.e., 17.93% and 17.7%, respectively. Ethanol was selected as the co-solvent for the supercritical extraction due to its high yield percentage, total phenolic content, and antioxidant capacity. When compressed solvents were used, the most efficient was scCO2 + ethanol, reaching about 6% extraction yield. High antioxidant activity was also observed for the ginger extracts in all cases.
The proposed procedure for the extraction of the secondary biomass used CO2 and ethanol as co-solvents, resulting in high extraction yields (reaching 6.42%) and an accelerated extraction time. Except the high extraction yields, another advantage of the reusage of the biomass is that, even after two extractions, high value bioactive compounds, such as zingerone and gingerol, were detected, and the extracts presented high antioxidant capacity with IC50 values up to 132.39 μg mL−1.
The extracts obtained with all the tested methods presented similar chemical compositions, and the most abundant substances were a-zingiberene, β-sesquiphellandrene, a-farnesene, β-bisabolene, zingerone, gingerol, a-curcumene, and γ-muurolene. All these compounds present beneficial properties and can have real applications in various sectors, such as the food and pharmaceutical industries. After the first reuse, high value compounds were identified as well, similar to those of the primary biomass. Taking into consideration the high yield percentages and the antioxidant capacity, as well as the chemical composition, it is safe to conclude that the addition of ethanol as a co-solvent in the scCO2 extraction had a positive effect both in the extraction of the primary biomass and in the secondary biomass extraction. Based on the results, the reuse of a secondary biomass (raw material) presents high significance. Indeed, the maximum utilization of biomass can contribute to the achievement of the goals of circular economy. Furthermore, although not tested in this work, the residual biomass after the secondary extraction could potentially be used as a raw fertilizing/pest repelling compound, enhancing the soils that are employed in agriculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29040871/s1, Supplementary Materials S1. GC-MS Analysis, Figure S1: Antioxidant capacity (%) of Zingiber officinale Roscoe extracts (μg mL−1) acquired under Soxhlet extraction using (a) ethyl acetate, (b) ethanol, (c) hexane and (d) water as solvents. Figure S2: Antioxidant capacity (%) of Zingiber officinale Roscoe extracts (μg mL−1) acquired under (a) scPropane extraction, (b) scCO2 and (c) scCO2 +EtOH. Figure S3: Antioxidant capacity (%) of Zingiber officinale Roscoe extracts (μg mL−1) acquired under scCO2 +EtOH, with biomass previously used at (a) scCO2 once, (b) scCO2 +EtOH once and (c) scCO2 +EtOH twice.
Author Contributions
Conceptualization, M.A., M.P. and M.L.C.; methodology, A.S., M.G.F.B., M.A., M.P. and M.L.C.; software, A.S., M.G.F.B., M.A., M.P. and M.L.C.; validation, A.S., M.G.F.B., M.A., M.P. and M.L.C.; formal analysis, A.S., M.A., M.P. and M.L.C.; investigation, A.S., M.G.F.B., M.A., M.P. and M.L.C.; resources, M.A., M.P. and M.L.C.; data curation, A.S., M.A., M.P. and M.L.C.; writing—original draft preparation, A.S., M.A. and M.P.; writing—review and editing, A.S., M.A., M.P. and M.L.C.; visualization, A.S. and M.A.; supervision, M.A., M.P. and M.L.C.; project administration, M.A. and M.P. funding acquisition, M.A. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 778168.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the Laboratory of Instrumental Analysis of the University of Patras as well as the Laboratory of Photo-Catalytic Processes and Environmental Chemistry of Institute of Nanoscience and Nanotechnology, NCSR Demokritos, for GC-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hu, W.; Yu, A.; Wang, S.; Bai, Q.; Tang, H.; Yang, B.; Wang, M.; Kuang, H. Extraction, Purification, Structural Characteristics, Biological Activities, and Applications of the Polysaccharides from Zingiber officinale Roscoe. (Ginger): A Review. Molecules 2023, 28, 3855. [Google Scholar] [CrossRef]
- Ballester, P.; Cerdá, B.; Arcusa, R.; García-Muñoz, A.M.; Marhuenda, J.; Zafrilla, P. Antioxidant Activity in Extracts from Zingiberaceae Family: Cardamom, Turmeric, and Ginger. Molecules 2023, 28, 4024. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Ficker, C.; Smith, M.L.; Akpagana, K.; Gbeassor, M.; Zhang, J.; Durst, T.; Assabgui, R.; Arnason, J.T. Bioassay-guided isolation and identification of antifungal compounds from ginger. Phytother. Res. 2003, 17, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Garza-Cadena, C.; Ortega-Rivera, D.M.; Machorro-García, G.; Gonzalez-Zermeño, E.M.; Homma-Dueñas, D.; Plata-Gryl, M.; Castro-Muñoz, R. A comprehensive review on Ginger (Zingiber officinale) as a potential source of nutraceuticals for food formulations: Towards the polishing of gingerol and other present biomolecules. Food Chem. 2023, 413, 135629. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; Labib, R.M.; Sobeh, M.; Farag, M.A. Gingerols and shogaols: A multi-faceted review of their extraction, formulation, and analysis in drugs and biofluids to maximize their nutraceutical and pharmaceutical applications. Food Chem. X 2023, 20, 100947. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, M.N.; Nazir, A.; Fallico, B. Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications. Antioxidants 2023, 12, 2015. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Saeed, Z.; Pervaiz, M.; Ali, F.; Younas, U.; Bashir, R.; Bukhari, S.M.; Khan, R.R.M.; Jelani, S.; Sikandar, R. Evaluation of essential oil extracted from ginger, cinnamon and lemon for therapeutic and biological activities. Biocatal. Agric. Biotechnol. 2022, 44, 102470. [Google Scholar] [CrossRef]
- Gunasena, M.T.; Rafi, A.; Zobir, S.A.M.; Hussein, M.Z.; Ali, A.; Kutawa, A.B.; Wahab, M.A.A.; Sulaiman, M.R.; Adzmi, F.; Ahmad, K. Phytochemicals Profiling, Antimicrobial Activity and Mechanism of Action of Essential Oil Extracted from Ginger (Zingiber officinale Roscoe cv. Bentong) against Burkholderia glumae Causative Agent of Bacterial Panicle Blight Disease of Rice. Plants 2022, 11, 1466. [Google Scholar] [CrossRef]
- Abdullahi, A.; Ahmad, K.; Ismail, I.S.; Asib, N.; Ahmed, O.H.; Abubakar, A.I.; Siddiqui, Y.; Ismail, M.R. Potential of Using Ginger Essential Oils-Based Nanotechnology to Control Tropical Plant Diseases. Plant Pathol. J. 2020, 36, 515–535. [Google Scholar] [CrossRef]
- Mesomo, M.C.; Corazza, M.L.; Ndiaye, P.M.; Santa, O.R.D.; Cardozo, L.; Scheer, A.d.P. Supercritical CO2 extracts and essential oil of ginger (Zingiber officinale R.): Chemical composition and antibacterial activity. J. Supercrit. Fluids 2013, 80, 44–49. [Google Scholar] [CrossRef]
- Pronyk, C.; Mazza, G. Design and scale-up of pressurized fluid extractors for food and bioproducts. J. Food Eng. 2009, 95, 215–226. [Google Scholar] [CrossRef]
- Ding, S.; An, K.; Zhao, C.; Li, Y.; Guo, Y.; Wang, Z. Effect of drying methods on volatiles of Chinese ginger (Zingiber officinale Roscoe). Food Bioprod. Process. 2012, 90, 515–524. [Google Scholar] [CrossRef]
- Sikora, E.; Cieślik, E.; Leszczyńska, T.; Filipiak-Florkiewicz, A.; Pisulewski, P.M. The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chem. 2008, 107, 55–59. [Google Scholar] [CrossRef]
- Mesomo, M.C.; Scheer, A.d.P.; Perez, E.; Ndiaye, P.M.; Corazza, M.L. Ginger (Zingiber officinale R.) extracts obtained using supercritical CO2 and compressed propane: Kinetics and antioxidant activity evaluation. J. Supercrit. Fluids 2012, 71, 102–109. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of Non-conventional Extraction Methods: Toward a Sustainable and Green Production of Valuable Compounds from Mushrooms. Food Eng. Rev. 2015, 8, 214–234. [Google Scholar] [CrossRef]
- Riera, E.; Golás, Y.; Blanco, A.; Gallego, J.; Blasco, M.; Mulet, A. Mass transfer enhancement in supercritical fluids extraction by means of power ultrasound. Ultrason. Sonochemistry 2004, 11, 241–244. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, S.-B.; Park, K.-A.; Hong, I.-K. Characterization of extraction and separation of rice bran oil rich in EFA using SFE process. Sep. Purif. Technol. 1999, 15, 1–8. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Liang, J.; Lu, Q.; Lerner, R.; Sun, X.; Zeng, H.; Liu, Y. Agricultural Wastes. Water Environ. Res. 2011, 83, 1439–1466. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crop. Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Guedes, A.R.; de Souza, A.R.C.; Barbi, R.C.T.; Escobar, E.L.N.; Zanoello, É.F.; Corazza, M.L. Extraction of Synadenium grantii Hook f. using conventional solvents and supercritical CO2 + ethanol. J. Supercrit. Fluids 2020, 160, 104796. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. Security Requirements for Cryptographic Modules; Federal Information Processing Standards Publications (FIPS PUBS) 140-2, Change Notice 2 December 03, 2002; Department of Commerce: Washington, DC, USA, 2001. [CrossRef]
- Al-Areer, N.W.; Al Azzam, K.M.; Al Omari, R.H.; Al-Deeb, I.; Bekbayeva, L.; Negim, E.-S. Quantitative analysis of total phenolic and flavonoid compounds in different extracts from ginger plant (Zingiber officinale) and evaluation of their anticancer effect against colorectal cancer cell lines. Pharmacia 2023, 70, 905–919. [Google Scholar] [CrossRef]
- Lemma, T.S.; Egza, T.F. Extraction and Characterization of Essential Oil from Ginger Rhizome Collected from Arba Minch Market. Int. J. Eng. Trends Technol. 2019, 67, 41–45. [Google Scholar] [CrossRef]
- Fragkouli, R.; Antonopoulou, M.; Asimakis, E.; Spyrou, A.; Kosma, C.; Zotos, A.; Tsiamis, G.; Patakas, A.; Triantafyllidis, V. Mediterranean Plants as Potential Source of Biopesticides: An Overview of Current Research and Future Trends. Metabolites 2023, 13, 967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2021, 119, 579–591. [Google Scholar] [CrossRef]
- Bahrin, N.; Muhammad, N.; Abdullah, N.; Talip, B.H.A.; Jusoh, S.; Theng, S.W. Effect of Processing Temperature on Antioxidant Activity of Ficus carica Leaves Extract. J. Sci. Technol. 2018, 10, 2. Available online: https://publisher.uthm.edu.my/ojs/index.php/JST/article/view/3005 (accessed on 6 February 2024). [CrossRef]
- Thavamoney, N.; Sivanadian, L.; Tee, L.H.; Khoo, H.E.; Prasad, K.N.; Kong, K.W. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J. Food Sci. Technol. 2018, 55, 2523–2532. [Google Scholar] [CrossRef]
- Araújo, M.N.; Azevedo, A.Q.P.L.; Hamerski, F.; Voll, F.A.P.; Corazza, M.L. Enhanced extraction of spent coffee grounds oil using high-pressure CO2 plus ethanol solvents. Ind. Crop. Prod. 2019, 141, 111723. [Google Scholar] [CrossRef]
- Juchen, P.T.; Araujo, M.N.; Hamerski, F.; Corazza, M.L.; Voll, F.A.P. Extraction of parboiled rice bran oil with supercritical CO2 and ethanol as co-solvent: Kinetics and characterization. Ind. Crop. Prod. 2019, 139, 111506. [Google Scholar] [CrossRef]
- Araus, K.A.; del Valle, J.M.; Robert, P.S.; de la Fuente, J.C. Effect of triolein addition on the solubility of capsanthin in supercritical carbon dioxide. J. Chem. Thermodyn. 2012, 51, 190–194. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Salea, R.; Veriansyah, B.; Tjandrawinata, R.R. Optimization and scale-up process for supercritical fluids extraction of ginger oil from Zingiber officinale var. Amarum. J. Supercrit. Fluids 2017, 120, 285–294. [Google Scholar] [CrossRef]
- Zancan, K.C.; Marques, M.O.; Petenate, A.J.; Meireles, M.A. Extraction of ginger (Zingiber officinale Roscoe) oleoresin with CO2 and co-solvents: A study of the antioxidant action of the extracts. J. Supercrit. Fluids 2002, 24, 57–76. [Google Scholar] [CrossRef]
- Stoilova, I.; Krastanov, A.; Stoyanova, A.; Denev, P.; Gargova, S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007, 102, 764–770. [Google Scholar] [CrossRef]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A Comparative Study on Chemical Composition and Antioxidant Activity of Ginger (Zingiber officinale) and Cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.I.; Park, H.W.; Yang, J.H.; Shin, T.-Y.; Kim, Y.-C.; Baek, N.-I.; Kim, S.-H.; Choi, S.U.; Kwon, B.-M.; et al. Cytotoxic components from the dried rhizomes of Zingiber officinale Roscoe. Arch. Pharmacal Res. 2008, 31, 415–418. [Google Scholar] [CrossRef]
- Agarwal, M.; Walia, S.; Dhingra, S.; Khambay, B.P.S. Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Manag. Sci. 2001, 57, 289–300. [Google Scholar] [CrossRef]
- Huang, T.-C.; Chung, C.-C.; Wang, H.-Y.; Law, C.-L.; Chen, H.-H. Formation of 6-Shogaol of Ginger Oil Under Different Drying Conditions. Dry. Technol. 2011, 29, 1884–1889. [Google Scholar] [CrossRef]
- Danwilai, K.; Konmun, J.; Sripanidkulchai, B.-O.; Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer Manag. Res. 2017, 9, 11–18. [Google Scholar] [CrossRef]
- Wang, H.-M.; Chen, C.-Y.; Chen, H.-A.; Huang, W.-C.; Lin, W.-R.; Chen, T.-C.; Lin, C.-Y.; Chien, H.-J.; Lu, P.-L.; Lin, C.-M.; et al. Zingiber officinale (ginger) compounds have tetracycline-resistance modifying effects against clinical extensively drug-resistant Acinetobacter baumannii. Phytother. Res. 2010, 24, 1825–1830. [Google Scholar] [CrossRef]
- Rajan, I.; Narayanan, N.; Rabindran, R.; Jayasree, P.R.; Kumar, P.R.M. Zingerone Protects Against Stannous Chloride-Induced and Hydrogen Peroxide-Induced Oxidative DNA Damage In Vitro. Biol. Trace Elem. Res. 2013, 155, 455–459. [Google Scholar] [CrossRef]
- Badrunanto; Wahyuni, W.T.; Farid, M.; Batubara, I.; Yamauchi, K. Antioxidant components of the three different varieties of Indonesian ginger essential oil: In vitro and computational studies. Food Chem. Adv. 2023, 4, 100558. [Google Scholar] [CrossRef]
- Misharina, T.A.; Terenina, M.B.; Krikunova, N.I. Antioxidant properties of essential oils. Appl. Biochem. Microbiol. 2009, 45, 642–647. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; Method 925.10; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Box, J. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).