A Network of Processes for Biorefining Burdock Seeds and Roots
Abstract
1. Introduction
2. Results
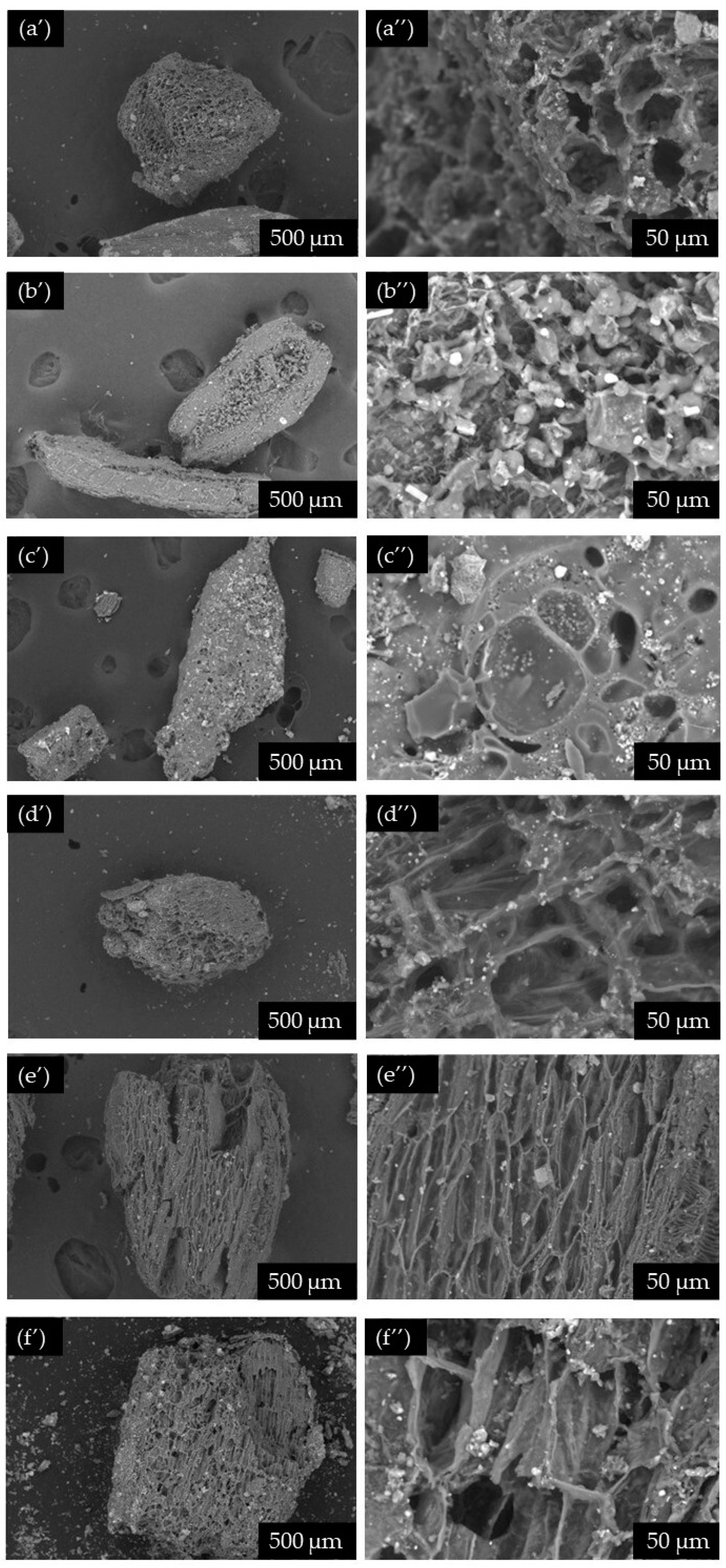
2.1. Chemical Characterisation of Raw Biomass and Extraction Residues
2.2. Study of Enzymatic Hydrolysis for Glucose Production
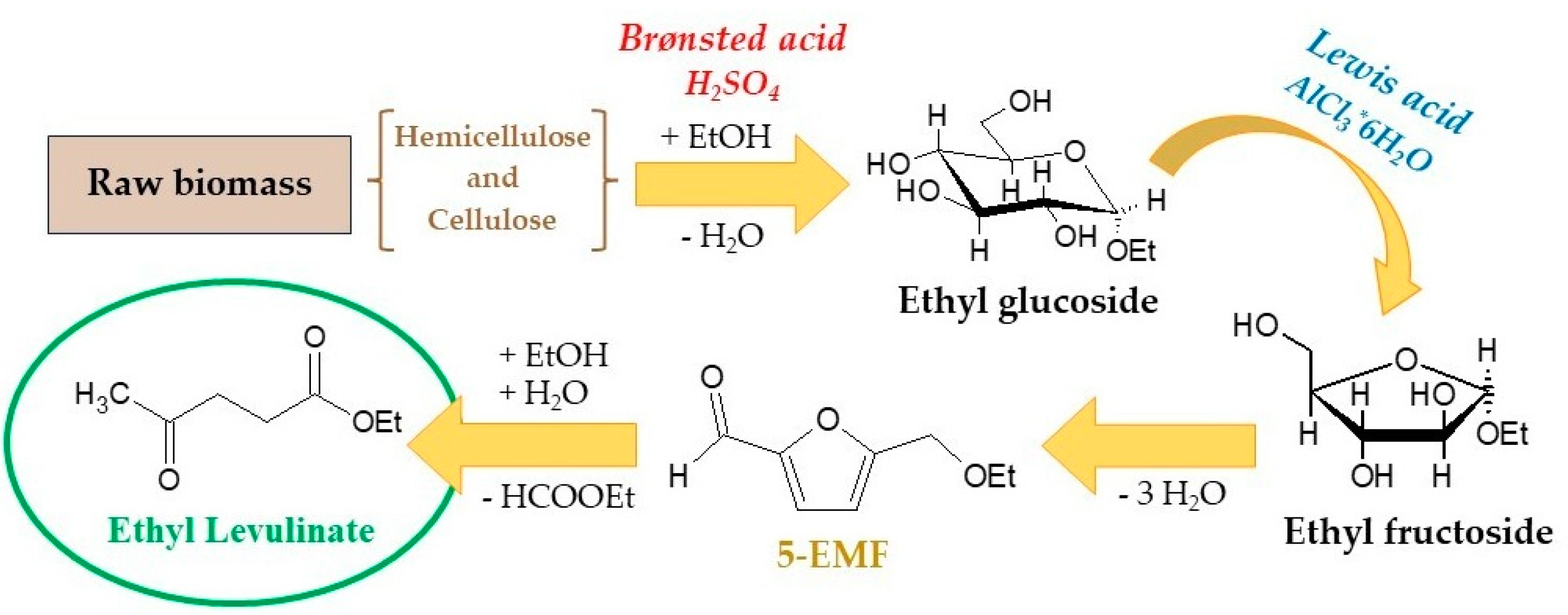
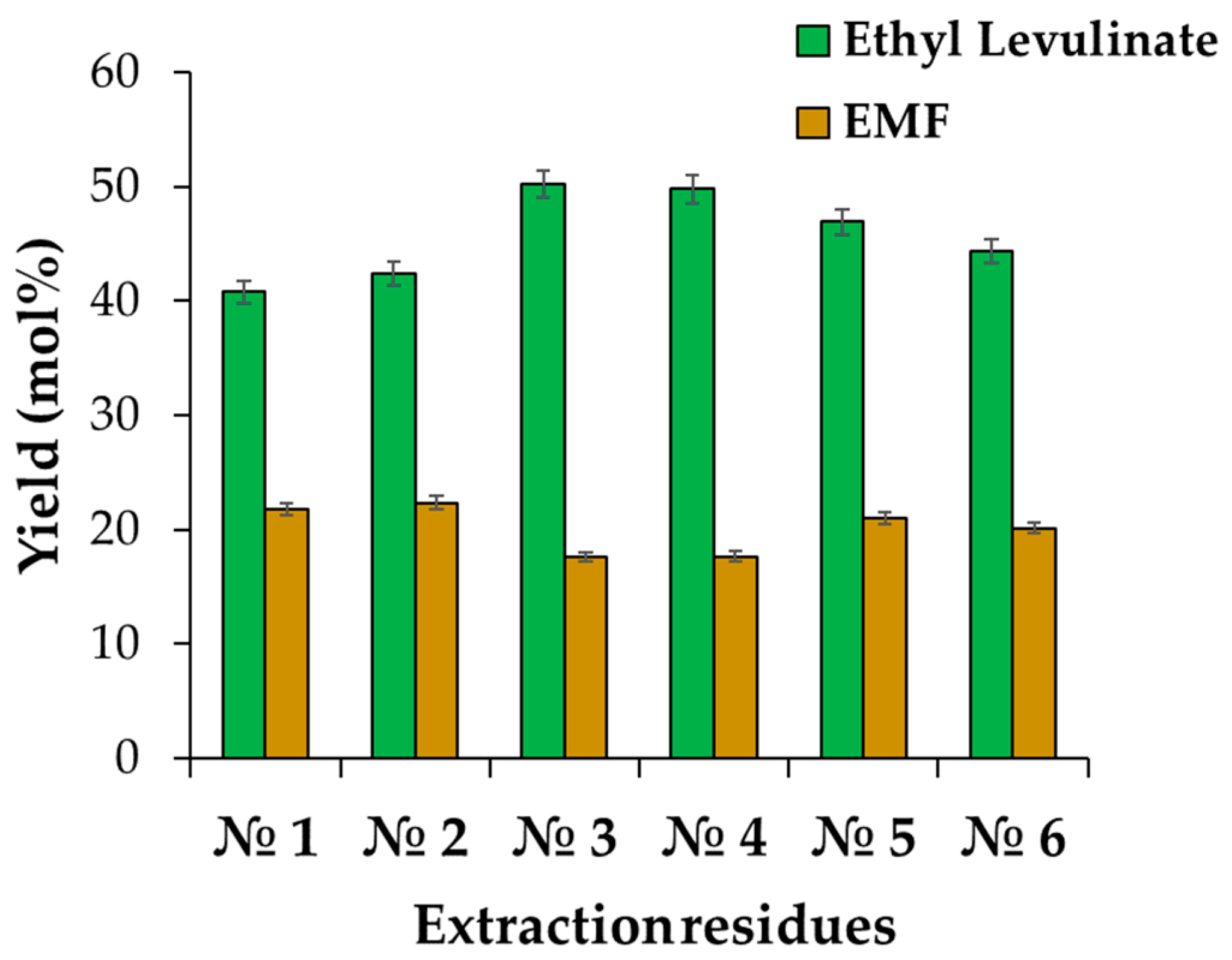
2.3. Direct Ethanolysis for the Synthesis of Ethyl Levulinate
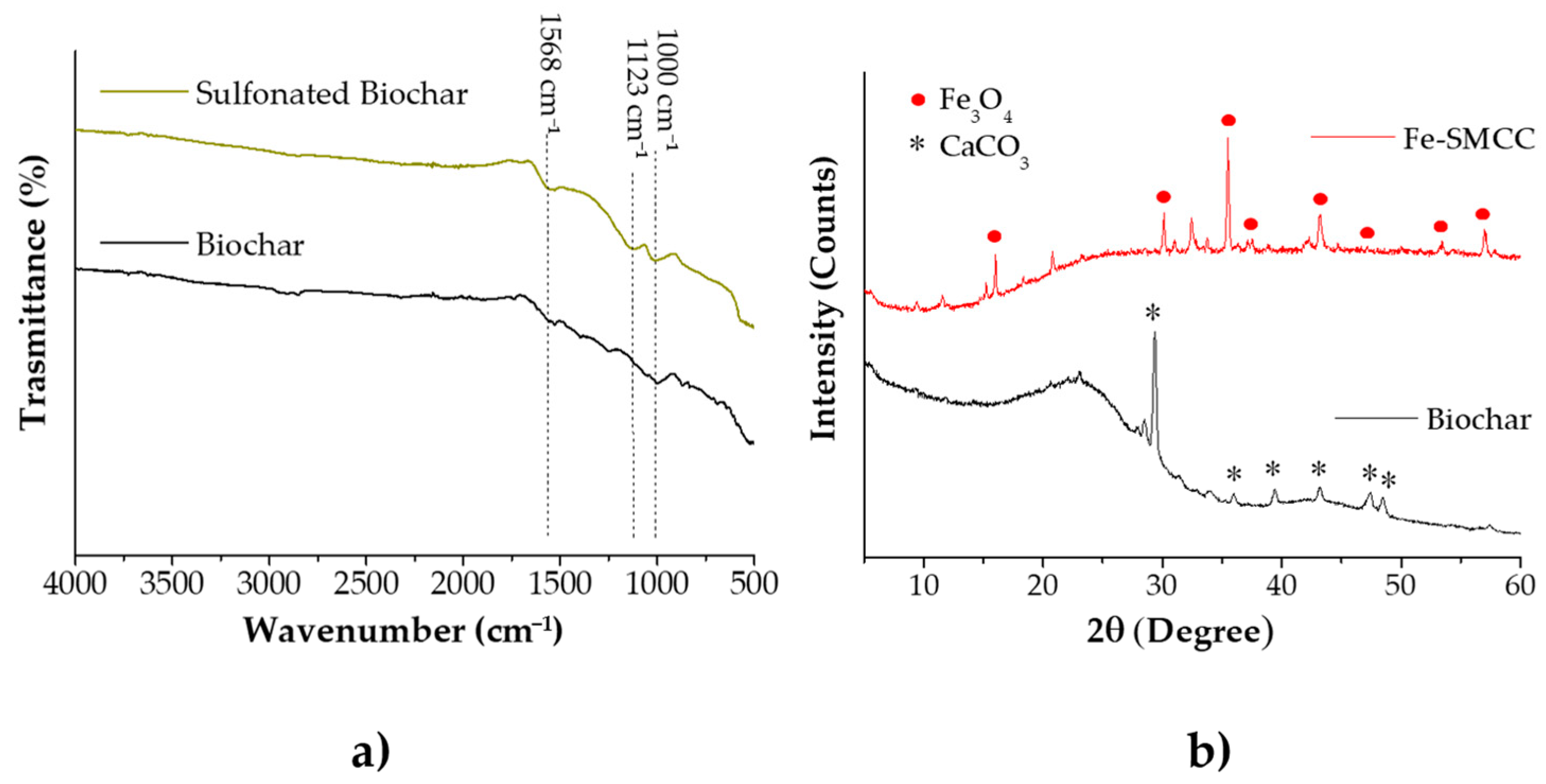
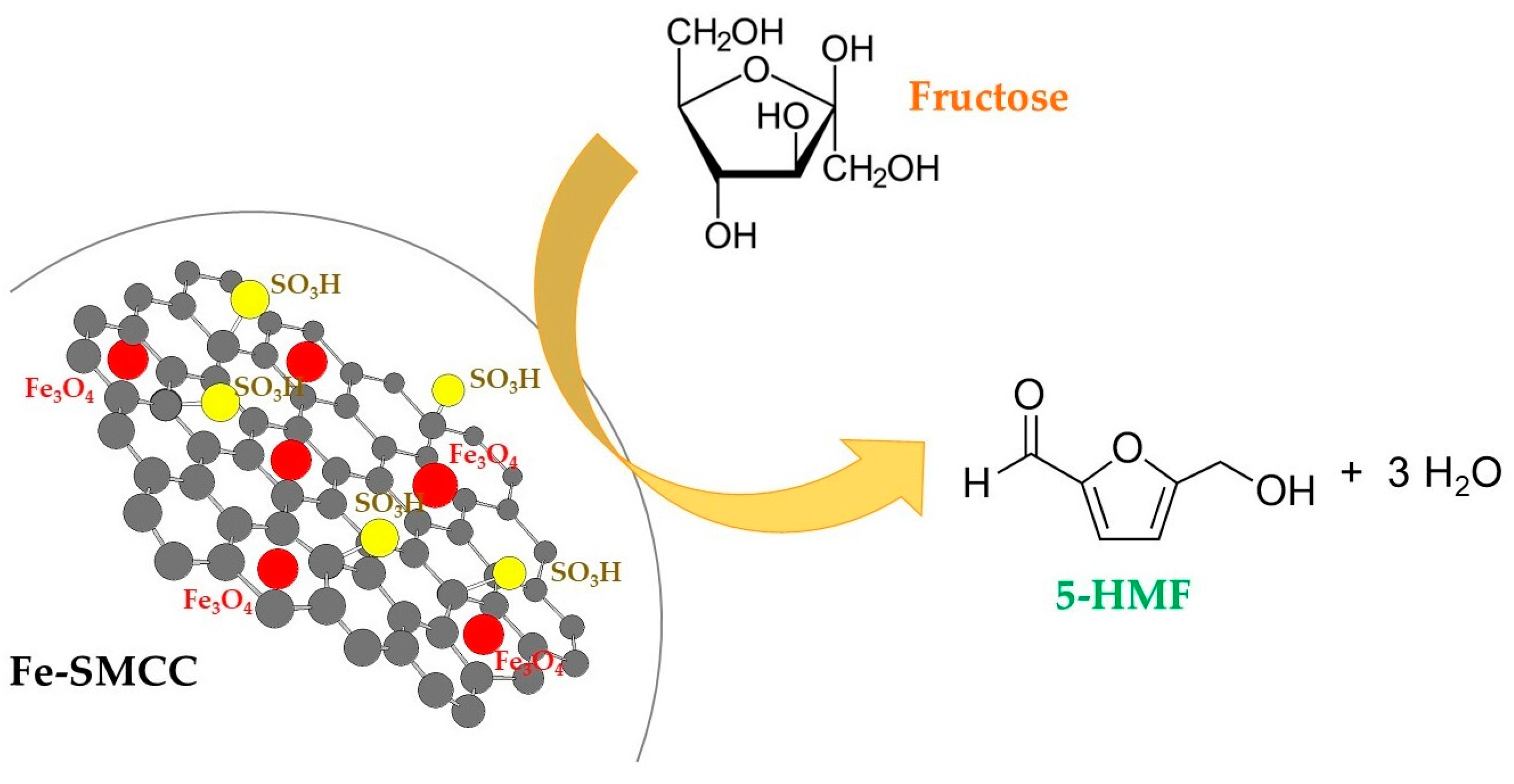
2.4. Synthesis and Characterisation of Iron Sulfonated Magnetic Carbon Catalysts
Dehydratation of Fructose for the Synthesis of 5-HMF Catalysed by Fe-SMCC
3. Discussion
Biorefinery Approach for the Integral Valorisation of Burdock Seeds and Roots
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Biomass Characterisation
4.3. Enzymatic Tests
4.4. Ethanolysis Reaction
4.5. Synthesis of Iron Sulfonated Magnetic Carbon Catalysts
Dehydratation Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Souza, A.R.C.; de Oliveira, T.L.; Fontana, P.D.; Carneiro, M.C.; Corazza, M.L.; de Messias Reason, I.J.; Bavia, L. Phytochemicals and biological activities of burdock (Arctium lappa L.) extracts: A review. Chem. Biodivers. 2022, 19, e202200615. [Google Scholar] [CrossRef]
- Petkova, N.; Hambarlyiska, I.; Tumbarski, Y.; Vrancheva, R.; Raeva, M.; Ivanov, I. Phytochemical composition and antimicrobial properties of burdock (Arctium lappa L.) roots extracts. Biointerface Res. Appl. Chem. 2022, 12, 2826–2842. [Google Scholar] [CrossRef]
- El Khatib, N.; Morel, S.; Hugon, G.; Rapior, S.; Carnac, G.; Saint, N. Identification of a sesquiterpene lactone from Arctium lappa leaves with antioxidant activity in primary human muscle cells. Molecules 2021, 26, 1328. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.M.A.; Clerici, M.T.P.S. Burdock (Arctium lappa L.) roots as a source of inulin-type fructans and other bioactive compounds: Current knowledge and future perspectives for food and non-food applications. Food Res. Int. 2021, 141, 109889. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Yu, J.; Li, Y.B.; Wang, L.; Hu, L.; Zhang, L.; Zhou, Y.H. Extraction and antioxidant activities of polysaccharides from roots of Arctium lappa L. Int. J. Biol. Macromol. 2019, 123, 531–538. [Google Scholar] [CrossRef]
- Ma, K.; Sheng, W.; Song, X.; Song, J.; Li, Y.; Huang, W.; Liu, Y. Chlorogenic Acid from Burdock Roots Ameliorates Oleic Acid-Induced Steatosis in HepG2 Cells through AMPK/ACC/CPT-1 Pathway. Molecules 2023, 28, 7257. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Dar, L.A.; Ali, T.; Kareem, O.; Rashid, R.; Khan, N.A.; Chashoo, I.A.; Bader, G.N. Arctium lappa: A review on its phytochemistry and pharmacology. In Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties; Springer: Singapore, 2022; pp. 327–348. [Google Scholar] [CrossRef]
- Zhang, X.; Herrera-Balandrano, D.D.; Huang, W.; Chai, Z.; Beta, T.; Wang, J.; Feng, J.; Li, Y. Comparison of nutritional and nutraceutical properties of burdock roots cultivated in Fengxian and Peixian of China. Foods 2021, 10, 2095. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, M.L. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends J. Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Xia, J.; Guo, Z.; Fang, S.; Gu, J.; Liang, X. Effect of drying methods on volatile compounds of burdock (Arctium lappa L.) root tea as revealed by gas chromatography mass spectrometry-based metabolomics. Foods 2021, 10, 868. [Google Scholar] [CrossRef]
- Errico, M.; Coelho, J.A.; Stateva, R.P.; Christensen, K.V.; Bahij, R.; Tronci, S. Brewer’s Spent Grain, Coffee Grounds, Burdock, and Willow–Four Examples of Biowaste and Biomass Valorization through Advanced Green Extraction Technologies. Foods 2023, 12, 1295. [Google Scholar] [CrossRef]
- Stefanov, S.M.; Fetzer, D.E.; de Souza, A.R.C.; Corazza, M.L.; Hamerski, F.; Yankov, D.S.; Stateva, R.P. Valorization by compressed fluids of Arctium lappa seeds and roots as a sustainable source of valuable compounds. J. CO2 Util. 2022, 56, 101821. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Liu, P.; Li, M.; Dong, H.; Qiao, X. Semi-preparative separation of 10 caffeoylquinic acid derivatives using high speed counter-current chromatogaphy combined with semi-preparative HPLC from the roots of burdock (Arctium lappa L.). Molecules 2018, 23, 429. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Rathour, R.K.; Devi, M.; Dahiya, P.; Sharma, N.; Kaushik, N.; Kumari, D.; Kumar, P.; Baadhe, R.R.; Walia, A.; Bhatt, A.K.; et al. Recent trends, opportunities, and challenges in sustainable management of rice straw waste biomass for green biorefinery. Energies 2023, 16, 1429. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol production from lignocellulosic biomass—Challenges and solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.; Baqar, Z.; Hussain, N.; Bilal, M.; Azam, H.M.H.; Iqbal, H.M. Application of nanomaterials for enhanced production of biodiesel, biooil, biogas, bioethanol, and biohydrogen via lignocellulosic biomass transformation. Fuel 2022, 315, 122840. [Google Scholar] [CrossRef]
- Panakkal, E.J.; Cheenkachorn, K.; Chuetor, S.; Tantayotai, P.; Raina, N.; Cheng, Y.S.; Sriariyanun, M. Optimization of deep eutectic solvent pre-treatment for bioethanol production from Napier grass. Sustain. Energy Technol. Assess. 2022, 54, 102856. [Google Scholar] [CrossRef]
- Gong, Z.; Lv, X.; Yang, J.; Luo, X.; Shuai, L. Chemocatalytic Conversion of Lignocellulosic Biomass to Ethanol: A Mini-Review. Catalysts 2022, 12, 922. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2022, 10, 437. [Google Scholar] [CrossRef]
- Becker, M.; Ahn, K.; Bacher, M.; Xu, C.; Sundberg, A.; Willför, S.; Rosenau, T.; Potthast, A. Comparative hydrolysis analysis of cellulose samples and aspects of its application in conservation science. Cellulose 2021, 28, 8719–8734. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Han, S.H.; Shin, S.J. The effect of hemicelluloses and lignin on acid hydrolysis of cellulose. Energy 2014, 77, 19–24. [Google Scholar] [CrossRef]
- Liu, Q.; He, W.Q.; Aguedo, M.; Xia, X.; Bai, W.B.; Dong, Y.Y.; Song, J.Q.; Richel, A.; Goffin, D. Microwave-assisted alkali hydrolysis for cellulose isolation from wheat straw: Influence of reaction conditions and non-thermal effects of microwave. Carbohydr. Polym. 2021, 253, 117170. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pre-treatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Chen, T.; Xiong, C.; Tao, Y. Enhanced hydrolysis of cellulose in ionic liquid using mesoporous ZSM-5. Molecules 2018, 23, 529. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, R.; Cheng, T.; Guo, J.; Xian, M.; Liu, H. Imidazolium-based ionic liquids for cellulose pre-treatment: Recent progresses and future perspectives. Appl. Microbiol. 2017, 101, 521–532. [Google Scholar] [CrossRef]
- Ilić, N.; Milić, M.; Beluhan, S.; Dimitrijević-Branković, S. Cellulases: From lignocellulosic biomass to improved production. Energies 2023, 16, 3598. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, O.N.; et al. Has the time finally come for green oleochemicals and biodiesel production using large-scale enzyme technologies? Current status and new developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Junior, N.L.; Bento, H.B.; de Carvalho, A.K.F.; de Souza Vandenberghe, L.P.; Soccol, C.R.; Aminabhavi, T.M.; Chandel, A.K. Process strategies to reduce cellulase enzyme loading for renewable sugar production in biorefineries. J. Chem. Eng. 2023, 451, 138690. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Khemthong, P.; Yimsukanan, C.; Narkkun, T.; Srifa, A.; Witoon, T.; Pongchaiphol, S.; Kiatphuengporn, S.; Faungnawakij, K. Advances in catalytic production of value-added biochemicals and biofuels via furfural platform derived lignocellulosic biomass. Biomass Bioenergy 2021, 148, 106033. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Ahmad, E.; Alam, M.I.; Pant, K.K.; Haider, M.A. Catalytic and mechanistic insights into the production of ethyl levulinate from biorenewable feedstocks. Green Chem. 2016, 18, 4804–4823. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. A review on catalytic synthesis of energy rich fuel additive levulinate compounds from biomass derived levulinic acid. Fuel Process. Technol. 2020, 197, 106213. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Louis, H.; Akakuru, O.U.; Joseph, I.; Enudi, O.C.; Michael, D.P. A Review on the conversion of levulinic acid and its esters to various useful chemicals. Aims Energy 2019, 7, 165–185. [Google Scholar] [CrossRef]
- Sinisi, A.; Degli Esposti, M.; Braccini, S.; Chiellini, F.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Morselli, D.; Fabbri, P. Levulinic acid-based bioplasticizers: A facile approach to enhance the thermal and mechanical properties of polyhydroxyalkanoates. Mater. Adv. 2021, 2, 7869–7880. [Google Scholar] [CrossRef]
- Xuan, W.; Hakkarainen, M.; Odelius, K. Levulinic acid as a versatile building block for plasticizer design. ACS Sustain. Chem. Eng. 2019, 7, 12552–12562. [Google Scholar] [CrossRef]
- Chang, C.; Xu, G.; Jiang, X. Production of ethyl levulinate by direct conversion of wheat straw in ethanol media. Bioresour. Technol. 2012, 121, 93–99. [Google Scholar] [CrossRef]
- Le Van Mao, R.; Zhao, Q.; Dima, G.; Petraccone, D. New process for the acid-catalyzed conversion of cellulosic biomass (AC3B) into alkyl levulinates and other esters using a unique one-pot system of reaction and product extraction. Catal. Lett. 2011, 141, 271–276. [Google Scholar] [CrossRef]
- Silva, J.F.L.; Mariano, A.P.; Maciel Filho, R. Less severe reaction conditions to produce levulinic acid with reduced humins formation at the expense of lower biomass conversion: Is it economically feasible? Fuel Commun. 2021, 9, 100029. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Andas, J.; Ma, Y.K.; Phoon, B.L.; Batagarawa, S.M.; Khoerunnisa, F.; Hazwan Hussin, M.; Ng, E.P. Recent advances in heterogeneous catalysts for the synthesis of alkyl levulinate biofuel additives from renewable levulinic acid: A comprehensive review. Fuel 2022, 323, 124362. [Google Scholar] [CrossRef]
- Sajid, M.; Farooq, U.; Bary, G.; Azim, M.M.; Zhao, X. Sustainable production of levulinic acid and its derivatives for fuel additives and chemicals: Progress, challenges, and prospects. Green Chem. 2021, 23, 9198–9238. [Google Scholar] [CrossRef]
- Yamanaka, N.; Shimazu, S. Conversion of Biomass-Derived Molecules into Alkyl Levulinates Using Heterogeneous Catalysts. Reactions 2023, 4, 667–678. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Antonopoulou, G.; Braguglia, C.; Campanale, C.; Gallipoli, A.; Lyberatos, G.; Taikou, N.; Pastore, C. Lewis-Brønsted acid catalysed ethanolysis of the organic fraction of municipal solid waste for efficient production of biofuels. Bioresour. Technol. 2018, 266, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Di Bitonto, L.; Locaputo, V.; D’Ambrosio, V.; Pastore, C. Direct Lewis-Brønsted acid ethanolysis of sewage sludge for production of liquid fuels. Appl. Energy 2020, 259, 114163. [Google Scholar] [CrossRef]
- Zuo, M.; Lin, L.; Zeng, X. The synthesis of potential biofuel 5-ethoxymethylfurfural: A review. Fuel 2023, 343, 127863. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R. 5-Ethoxymethylfurfural—A remarkable biofuel candidate. Biomass Biofuels Biochem. 2020, 355–375. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Lam, M.K.; Setiabudi, H.D.; Vo, D.V.N. State-of-the-Art of the Synthesis and Applications of Sulfonated Carbon-Based Catalysts for Biodiesel Production: A Review. Energy Technol. 2021, 9, 2100303. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Markova, M.E.; Lugovoy, Y.V.; Kosivtsov, Y.Y.; Matveeva, V.G.; Sulman, M.G. Plant-Biomass-Derived Carbon Materials as Catalyst Support, A Brief Review. Catalysts 2023, 13, 655. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, H.H.; Kim, S.G.; Lee, S.J. Fabrication and characteristics of sulfonated carbon foam via high internal phase emulsions with polypyrrole-modified carbon nanotubes. Polymer 2023, 281, 126144. [Google Scholar] [CrossRef]
- Nongbe, M.C.; Ekou, T.; Ekou, L.; Yao, K.B.; Le Grognec, E.; Felpin, F.X. Biodiesel production from palm oil using sulfonated graphene catalyst. Renew. Energy 2017, 106, 135–141. [Google Scholar] [CrossRef]
- Militello, M.P.; Martínez, M.V.; Tamborini, L.; Acevedo, D.F.; Barbero, C.A. Towards Photothermal Acid Catalysts Using Eco-Sustainable Sulfonated Carbon Nanoparticles—Part I: Synthesis, Characterization and Catalytic Activity towards Fischer Esterification. Catalysts 2023, 13, 1341. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Biomass-derived sulfonated polycyclic aromatic carbon catalysts for biodiesel production by esterification reaction. Biofuels Bioprod. Biorefining 2023, 17, 1343–1367. [Google Scholar] [CrossRef]
- Parida, S.; Singh, M.; Pradhan, S. Biomass wastes: A potential catalyst source for biodiesel production. Bioresour. Technol. Rep. 2022, 18, 101081. [Google Scholar] [CrossRef]
- Zou, R.; Qian, M.; Wang, C.; Mateo, W.; Wang, Y.; Dai, L.; Li, X.; Zhao, Y.; Huo, E.; Wang, L.; et al. Biochar: From by-products of agro-industrial lignocellulosic waste to tailored carbon-based catalysts for biomass thermochemical conversions. J. Chem. Eng. 2022, 441, 135972. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Slak, J.; Pomeroy, B.; Kostyniuk, A.; Grilc, M.; Likozar, B. A review of bio-refining process intensification in catalytic conversion reactions, separations and purifications of hydroxymethylfurfural (HMF) and furfural. Chem. Eng. J. 2022, 429, 132325. [Google Scholar] [CrossRef]
- Kong, Q.S.; Li, X.L.; Xu, H.J.; Fu, Y. Conversion of 5-hydroxymethylfurfural to chemicals: A review of catalytic routes and product applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Li, Q.; Xia, H. High-Yield Synthesis of 5-Hydroxymethylfurfural from Untreated Wheat Straw Catalyzed by FePO4 and Organic Acid in a Biphasic System. Energy Fuels 2023, 37, 12953–12965. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Y.; Qin, H.; Qi, Z. Advances of Ionic Liquids and Deep Eutectic Solvents in Green Processes of Biomass-Derived 5-Hydroxymethylfurfural. ChemSusChem 2022, 15, e202102635. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Ji, Z.; Cheng, Y.; Zheng, L.; Wang, L.; Li, X. Experiments and Kinetic Modeling of Fructose Dehydration to 5-Hydroxymethylfurfural with Hydrochloric Acid in Acetone—Water Solvent. Ind. Eng. Chem. Res. 2022, 61, 13877–13885. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; Pastore, C.; di Bitonto, L.; Angelini, A.; Quaranta, E. Conversion of fructose into 5-HMF: A study on the behaviour of heterogeneous cerium-based catalysts and their stability in aqueous media under mild conditions. RSC Adv. 2015, 5, 26941–26948. [Google Scholar] [CrossRef]
- Yan, P.; Wang, H.; Liao, Y.; Wang, C. Zeolite catalysts for the valorization of biomass into platform compounds and biochemicals/biofuels: A review. Renew. Sust. Energy Rev. 2023, 178, 113219. [Google Scholar] [CrossRef]
- Takagaki, A. Production of 5-hydroxymethylfurfural from glucose in water by using transition metal-oxide nanosheet aggregates. Catalysts 2019, 9, 818. [Google Scholar] [CrossRef]
- Sampath, G.; Kannan, S. Fructose dehydration to 5-hydroxymethylfurfural: Remarkable solvent influence on recyclability of Amberlyst-15 catalyst and regeneration studies. Catal. Comm. 2013, 37, 41–44. [Google Scholar] [CrossRef]
- Licursi, D.; Galletti, A.M.R.; Bertini, B.; Ardemani, L.; Scotti, N.; Di Fidio, N.; Fulignati, S.; Antonetti, C. Design approach for the sustainable synthesis of sulfonated biomass-derived hydrochars and pyrochars for the production of 5-(hydroxymethyl) furfural. Sustain. Chem. Pharm. 2023, 35, 101216. [Google Scholar] [CrossRef]
- Xiong, X.; Iris, K.M.; Chen, S.S.; Tsang, D.C.; Cao, L.; Song, H.; Kwon, E.E.; Ok, Y.S.; Zhang, S.; Poon, C.S. Sulfonated biochar as acid catalyst for sugar hydrolysis and dehydration. Catal. Today 2018, 314, 52–61. [Google Scholar] [CrossRef]
- Gao, W.; Wan, Y.; Dou, Y.; Zhao, D. Synthesis of partially graphitic ordered mesoporous carbons with high surface areas. Adv. Energy Mat. 2011, 1, 115–123. [Google Scholar] [CrossRef]
- di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- da Luz Corrêa, A.P.; Bastos, R.R.C.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Preparation of sulfonated carbon-based catalysts from murumuru kernel shell and their performance in the esterification reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef] [PubMed]
- Ngaosuwan, K.; Goodwin, J.G., Jr.; Prasertdham, P. A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Renew. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Landin-Rodriguez, L.E.; Leyva-Ramos, S.; Medellin-Castillo, N.A. Modification of corncob with citric acid to enhance its capacity for adsorbing cadmium (II) from water solution. Chem. Eng. J. 2012, 180, 113–120. [Google Scholar] [CrossRef]
- Nabeshima, E.H.; Moro, T.M.A.; Campelo, P.H.; Sant’Ana, A.S.; Clerici, M.T.P.S. Tubers and roots as a source of prebiotic fibers. Adv. Food Nutr. Res. 2020, 94, 267–293. [Google Scholar] [CrossRef] [PubMed]
- Errico, M.; Ramírez-Márquez, C.; Torres Ortega, C.E.; Rong, B.-G.; Segovia-Hernandez, J.G. Design and control of an alternative distillation sequence for bioethanol purification. J. Chem. Technol. Biotechnol. 2015, 90, 2180–2185. [Google Scholar] [CrossRef]
- Ishiguro, Y.; Onodera, S.; Benkeblia, N.; Shiomi, N. Variation of total FOS, total IOS, inulin and their related-metabolizing enzymes in burdock roots (Arctium lappa L.) stored under different temperatures. Postharvest Biol. Technol. 2010, 56, 232–238. [Google Scholar] [CrossRef]
- Angelini, A.; Scelsi, E.; Ancona, V.; Aimola, G.; Pastore, C. Performic acid pre-treatment of poplar biomasses grown on a contaminated area for enhanced enzymatic digestibility: A viable route to obtain fine-products and recovery of contaminants. J. Clean. Prod. 2022, 369, 133346. [Google Scholar] [CrossRef]
- Pastore, C.; D’Ambrosio, V. Intensification of Processes for the Production of Ethyl Levulinate Using AlCl3·6H2O. Energies 2021, 14, 1273. [Google Scholar] [CrossRef]
- Wood, T.M.; Bhat, K.M. Methods for Measuring Cellulase Activities. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 160, pp. 87–112. [Google Scholar] [CrossRef]


| Samples | Seeds | Roots | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw Biomass | Extraction Residues | Raw Biomass | Extraction Residues | |||||
| № 1 | № 2 | № 3 | № 4 | № 5 | № 6 | |||
| Solvent | - | scCO2 + EtOH | Propane | Propane | Propane | - | scCO2 + MeOH/H2O | scCO2 + MeOH/H2O |
| Extraction Conditions | - | 40 °C 200 bar | 40 °C 60 bar | 80 °C 200 bar | Mix of T and P | - | 40 °C, 200 bar | 80 °C, 200 bar |
| Ref. in Article J CO2 Util. [13] | - | Run 2 | Run 4 | Run 8 | - | - | Runs 15–17 | Runs 18–20 |
| Moisture content, % | 10.2 ± 0.4 | 4.6 ± 0.2 | 4.5 ± 0.2 | 3.4 ± 0.1 | 3.9 ± 0.1 | 9.7 ± 0.3 | 6.2 ± 0.1 | 6.0 ± 0.1 |
| Total Solids composition (wt%) | ||||||||
| Total Lipids | 17.4 ± 0.6 | 6.7 ± 0.3 | 8.3 ± 0.3 | 6.2 ± 0.2 | 9.5 ± 0.4 | 0.3 | - | - |
| Proteins | 29.7 ± 1.1 | 17 ± 0.6 | 18.6 ± 0.7 | 14.7 ± 0.5 | 12.7 ± 0.5 | 21.6 ± 0.8 | 15.6 ± 0.6 | 14.4 ± 0.4 |
| EHS * | 18.4 ± 0.8 | 19.1 ± 0.7 | 21 ± 0.8 | 20.4 ± 0.7 | 21.9 ± 0.8 | 18.1 ± 0.6 | 17.9 ± 0.6 | 18.8 ± 0.7 |
| Cellulose | 13.5 ± 0.6 | 24.7 ± 1.0 | 26.7 ± 1.1 | 26.5 ± 0.6 | 24.9 ± 0.8 | 14.1 ± 0.6 | 19.2 ± 0.7 | 21.9 ± 1.0 |
| Lignin | 11.6 ± 0.5 | 19.5 ± 0.8 | 16.5 ± 0.6 | 21.2 ± 0.6 | 19.6 ± 0.8 | 23.1 ± 0.9 | 28.7 ± 1.2 | 26.5 ± 1.1 |
| Ashes | 4.7 ± 0.1 | 5 ± 0.1 | 4.9 ± 0.1 | 4.3 ± 0.1 | 4.2 ± 0.1 | 16.5 ± 0.6 | 12.6 ± 0.4 | 13.1 ± 0.4 |
| Substrate | Total Carbohydrate Content (wt%) | Enzymatic Degradation Yield (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | 24 | 48 | 72 | |||||
| FPU/gsubstrate | 5 | 30 | 5 | 30 | 5 | 30 | ||
| Cellulose | 100 | 71.6 ± 2.8 | 84.5 ± 0.9 | 80.8 ± 0.8 | 100 | 88.2 ± 1.9 | 100 | |
| Burdock seeds | 31.9 ± 1.4 | 40.1 ± 1.3 | 88.2 ± 2.2 | 46.0 ± 1.8 | 93.8 ± 1.7 | 49.6 ± 1.0 | 100 | |
| № 1 | 43.8 ± 1.7 | 43.5 ± 0.9 | 81.2 ± 1.6 | 45.2 ± 1.6 | 94.1 ± 1.4 | 46.5 ± 1.4 | 96.7 ± 1.8 | |
| № 2 | 47.7 ± 1.9 | 40.9 ± 1.1 | 81.7 ± 1.4 | 44.0 ± 1.1 | 83.9 ± 1.5 | 47.1 ± 1.3 | 89.3 ± 1.2 | |
| № 3 | 46.9 ± 1.3 | 25.7 ± 0.7 | 48.4 ± 0.6 | 28.3 ± 0.8 | 51.7 ± 0.8 | 29.5 ± 0.7 | 53.7 ± 0.8 | |
| № 4 | 46.8 ± 1.6 | 28.5 ± 0.9 | 51.9 ± 0.8 | 27.8 ± 0.9 | 54.8 ± 0.7 | 30.2 ± 0.8 | 56.0 ± 1.4 | |
| Burdock roots | 32.2 ± 1.2 | 58.5 ± 1.4 | 88.3 ± 1.2 | 68.4 ± 1.4 | 95.6 ± 2.1 | 68.5 ± 1.9 | 100 | |
| № 5 | 37.1 ± 1.3 | 73.2 ± 1.8 | 82.3 ± 1.4 | 87.7 ± 1.6 | 87.3 ± 0.9 | 94.5 ± 2.1 | 100 | |
| № 6 | 40.7 ± 1.7 | 75.5 ± 1.6 | 98.4 ± 2.5 | 79.5 ± 1.1 | 100 | 86.6 ± 1.6 | 100 | |
| Samples | Extraction Residues | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seeds | Roots | |||||||||
| Elemental Composition (wt%) | C | O | Ca | S | Fe | C | O | Ca | S | Fe |
| Biochar | 83.0 | 14.2 | 0.96 | - | - | 69.3 | 24.8 | 1.6 | 0.32 | - |
| Sulfonated biochar | 76.8 | 19.7 | - | 2.34 | - | 68.2 | 26.8 | - | 2.64 | - |
| Fe-SMCC | 65.6 | 21.4 | - | 1.27 | 5.51 | 70.3 | 16.3 | 0.86 | 1.03 | 4.18 |
| Catalysts | Total Acid Density (mmolSO3H/g) |
|---|---|
| Fe-SMCC № 1 | 0.86 ± 0.02 |
| Fe-SMCC № 2 | 0.98 ± 0.03 |
| Fe-SMCC № 3 | 0.95 ± 0.02 |
| Fe-SMCC № 4 | 1.36 ± 0.04 |
| Fe-SMCC № 5 | 1.32 ± 0.04 |
| Fe-SMCC № 6 | 0.78 ± 0.02 |
| E | Solvent | Catalyst | Fructose Conversion (mol%) | 5-HMF | Formic Acid | Levulinic Acid | ||
|---|---|---|---|---|---|---|---|---|
| Yield (mol%) | Selectivity (%) | R | Yield (mol%) | Yield (mol%) | ||||
| 1 | MIBK | In the absence of a catalyst | 28.8 ± 1.2 | 6.6 ± 0.3 | 22.9 ± 2.0 | 2.2 | - | - |
| 2 | Amberlyst-15 | 91.8 ± 2.3 | 14.5 ± 0.5 | 15.8 ± 0.8 | 2.4 | 32.3 ± 1.6 | 28.6 ± 1.4 | |
| 3 | Fe-SMCC № 1 | 53.6 ± 1.7 | 18.7 ± 0.3 | 34.9 ± 1.7 | 2.4 | - | - | |
| 4 | Fe-SMCC № 2 | 57.0 ± 0.5 | 21.1 ± 0.8 | 37.0 ± 1.7 | 2.8 | - | - | |
| 5 | Fe-SMCC № 3 | 55.0 ± 0.7 | 23.1 ± 0.8 | 42.0 ± 2.0 | 2.8 | - | - | |
| 6 | Fe-SMCC № 4 | 58.3 ± 0.5 | 21.9 ± 1.6 | 37.6 ± 3.1 | 2.9 | - | - | |
| 7 | Fe-SMCC № 5 | 52.5 ± 1.9 | 16.2 ± 0.6 | 30.9 ± 2.3 | 2.6 | - | - | |
| 8 | Fe-SMCC № 6 | 62.5 ± 1.4 | 18.8 ± 0.4 | 30.1 ± 1.3 | 2.5 | - | - | |
| 9 | GVL | Fe-SMCC № 1 | 69.0 ± 1.4 | 28.9 ± 1.7 | 41.9 ± 3.3 | - | - | - |
| 10 | Fe-SMCC № 2 | 75.3 ± 1.6 | 31.4 ± 1.3 | 41.7 ± 2.6 | - | - | - | |
| 11 | Fe-SMCC № 3 | 55.5 ± 1.2 | 41.9 ± 0.8 | 55.5 ± 1.9 | - | - | - | |
| 12 | Fe-SMCC № 4 | 74.2 ± 1.6 | 35.5 ± 1.4 | 47.8 ± 2.9 | - | - | - | |
| 13 | Fe-SMCC № 5 | 76.0 ± 1.9 | 30.5 ± 0.9 | 40.1 ± 2.2 | - | - | - | |
| 14 | Fe-SMCC № 6 | 77.1 ± 1.8 | 31.5 ± 1.1 | 40.9 ± 2.4 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Bitonto, L.; Scelsi, E.; Errico, M.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Corazza, M.L.; Shigueyuki Kanda, L.R.; Hájek, M.; Stateva, R.P.; et al. A Network of Processes for Biorefining Burdock Seeds and Roots. Molecules 2024, 29, 937. https://doi.org/10.3390/molecules29050937
di Bitonto L, Scelsi E, Errico M, Reynel-Ávila HE, Mendoza-Castillo DI, Bonilla-Petriciolet A, Corazza ML, Shigueyuki Kanda LR, Hájek M, Stateva RP, et al. A Network of Processes for Biorefining Burdock Seeds and Roots. Molecules. 2024; 29(5):937. https://doi.org/10.3390/molecules29050937
Chicago/Turabian Styledi Bitonto, Luigi, Enrico Scelsi, Massimiliano Errico, Hilda Elizabeth Reynel-Ávila, Didilia Ileana Mendoza-Castillo, Adrián Bonilla-Petriciolet, Marcos Lucio Corazza, Luis Ricardo Shigueyuki Kanda, Martin Hájek, Roumiana P. Stateva, and et al. 2024. "A Network of Processes for Biorefining Burdock Seeds and Roots" Molecules 29, no. 5: 937. https://doi.org/10.3390/molecules29050937
APA Styledi Bitonto, L., Scelsi, E., Errico, M., Reynel-Ávila, H. E., Mendoza-Castillo, D. I., Bonilla-Petriciolet, A., Corazza, M. L., Shigueyuki Kanda, L. R., Hájek, M., Stateva, R. P., & Pastore, C. (2024). A Network of Processes for Biorefining Burdock Seeds and Roots. Molecules, 29(5), 937. https://doi.org/10.3390/molecules29050937











