Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of the Physicochemical Properties of Fermented Beetrrot Juices
2.2. Analysis of Physicochemical Properties of Fermentated Beetrrot Powders
Thermal Properties of Powders
3. Materials and Methods
3.1. Materials
3.2. Technological Treatment
3.2.1. Juice Pressing
3.2.2. Fermentation Process
3.2.3. Spray Drying
3.3. Analytical Method
3.3.1. Dry Matter
3.3.2. Soluble Solid Content in Juices
3.3.3. pH and Total Acidity
3.3.4. Water Vapor Adsorption
3.3.5. Color Parameters
3.3.6. Morphology of Powder Particles
3.3.7. Thermal Properties
3.3.8. FT-IR Spectroscopic Analysis
3.3.9. Betalain Content
- (A)
- Spectrophotometric method
- (B)
- Chromatography method
3.4. Determination of the Number of Lactic Acid Bacteria
3.5. Statistical Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Janiszewska-Turak, E.; Hornowska, Ł.; Pobiega, K.; Gniewosz, M.; Witrowa-Rajchert, D. The influence of Lactobacillus bacteria type and kind of carrier on the properties of spray-dried microencapsules of fermented beetroot powders. Int. J. Food Sci. Technol. 2021, 56, 2166–2174. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Grzybowska, E.; Konopka, E.; Witrowa-Rajchert, D. The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products. Molecules 2021, 26, 3683. [Google Scholar] [CrossRef]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Nowacka, M.; Dadan, M.; Janowicz, M.; Wiktor, A.; Witrowa-Rajchert, D.; Mandal, R.; Pratap-Singh, A.; Janiszewska-Turak, E. Effect of nonthermal treatments on selected natural food pigments and color changes in plant material. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5097–5144. [Google Scholar] [CrossRef]
- Sionek, B. Ocena możliwości produkcji fermentowanego soku z buraka ćwikłowego z dodatkiem szczepów bakterii probiotycznych i potencjalnie probiotycznych rodzaju Lactobacillus®. Postępy Tech. Przetwórstwa Spoż. 2020, 1, 74–81. [Google Scholar]
- Ceclu, L.; Nistor, O.-V. Red Beetroot: Composition and Health Effects—A Review. J. Nutr. Med. Diet Care 2020, 5, 1510043. [Google Scholar] [CrossRef]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef] [PubMed]
- Ilango, S.; Antony, U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and Chemical Properties of Chokeberry Juice Fermented by Novel Lactic Acid Bacteria with Potential Probiotic Properties during Fermentation at 4 °C for 4 Weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef]
- Aljahani, A.H. Microbiological and physicochemical quality of vegetable pickles. J. Saudi Soc. Agric. Sci. 2020, 19, 415–421. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy vs. non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Di Marco, S.; Bacceli, M.; Di Serio, M.G.; Di Loreto, G.; Cellini, M.; Simone, N. Lactiplantibacillus plantarum Used as Single, Multiple, and Mixed Starter Combined with Candida boidinii for Table Olive Fermentations: Chemical, Textural, and Sensorial Characterization of Final Products. Fermentation 2021, 7, 239. [Google Scholar] [CrossRef]
- Abouloifa, H.; Khodaei, N.; Rokni, Y.; Karboune, S.; Brasca, M.; D’Hallewin, G.; Salah, R.B.; Saalaoui, E.; Asehraou, A. The prebiotics (Fructo-oligosaccharides and Xylo-oligosaccharides) modulate the probiotic properties of Lactiplantibacillus and Levilactobacillus strains isolated from traditional fermented olive. World J. Microbiol. Biotechnol. 2020, 36, 185. [Google Scholar] [CrossRef]
- Değirmencioğlu, N.; Gurbuz, O.; Şahan, Y. The Monitoring, Via an In vitro Digestion System, of the Bioactive Content of Vegetable Juice Fermented with Saccharomyces cerevisiae and Saccharomyces boulardii. J. Food Process. Preserv. 2016, 40, 798–811. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Kołakowska, W.; Pobiega, K.; Gramza-Michałowska, A. Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. Appl. Sci. 2021, 11, 7864. [Google Scholar] [CrossRef]
- Janiszewska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids microencapsulation by spray drying method and supercritical micronization. Food Res. Int. 2017, 99, 891–901. [Google Scholar] [CrossRef]
- Devaki, C.; Premavalli, K. Fermented Vegetable Beverages. In Fermented Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 321–367. [Google Scholar]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Mousavi Khaneghah, A.; Sant’Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Astuti, D.; Salengke, S.; Laga, A.; Mariyati Bilangd, M.; Mochtar, H.; Warisf, A. Characteristics of pH, Total Acid, Total Soluble Solid on Tomato Juice by Ohmic Heating Technology. Int. J. Sci. Basic Appl. Res. IJSBAR 2018, 39, 21–28. [Google Scholar]
- Coy-Barrera, E. Chapter 17—Analysis of Betalains (Betacyanins and Betaxanthins). In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–619. [Google Scholar]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytotherapy Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Tawfike, A.F.; Donia, M.S.; Ehrlich, A.; Wessjohann, L.A. Influence of pickling process on Allium cepa and Citrus limon metabolome as determined via mass spectrometry-based metabolomics. Molecules 2019, 24, 928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyżowska, A.; Siemianowska, K.; Śniadowska, M.; Nowak, A. Bioactive Compounds and Microbial Quality of Stored Fermented Red Beetroots and Red Beetroot Juice. Pol. J. Food Nutr. Sci. 2020, 70, 35–44. [Google Scholar] [CrossRef]
- Broeckx, G.; Kiekens, S.; Jokicevic, K.; Byl, E.; Henkens, T.; Vandenheuvel, D.; Lebeer, S.; Kiekens, F. Effects of initial cell concentration, growth phase, and process parameters on the viability of Lactobacillus rhamnosus GG after spray drying. Dry. Technol. 2020, 38, 1474–1492. [Google Scholar] [CrossRef]
- Lian, W.-C.; Hsiao, H.-C.; Chou, C.-C. Survival of bifidobacteria after spray-drying. Int. J. Food Microbiol. 2002, 74, 79–86. [Google Scholar] [CrossRef]
- Hao, F.; Fu, N.; Ndiaye, H.; Woo, M.W.; Jeantet, R.; Chen, X.D. Thermotolerance, Survival, and Stability of Lactic Acid Bacteria After Spray Drying as Affected by the Increase of Growth Temperature. Food Bioprocess Technol. 2021, 14, 120–132. [Google Scholar] [CrossRef]
- Noguerol, A.T.; Igual, M.; Pagán, M.J. Comparison of biopreservatives obtained from a starter culture of Pediococcus acidilactici by different techniques. Food Biosci. 2021, 42, 101114. [Google Scholar] [CrossRef]
- Moreira, M.T.C.; Martins, E.; Perrone, T.; de Freitas, R.; Queiroz, L.S.; de Carvalho, A.F. Challenges associated with spray drying of lactic acid bacteria: Understanding cell viability loss. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3267–3283. [Google Scholar] [CrossRef]
- Dumitru, M.; Vodnar, D.C.; Elemer, S.; Ciurescu, G.; Habeanu, M.; Sorescu, I.; Georgescu, S.E.; Dudu, A. Evaluation of Non-Encapsulated and Microencapsulated Lactic Acid Bacteria. Appl. Sci. 2021, 11, 9867. [Google Scholar] [CrossRef]
- Szulc, K.; Lenart, A. Water vapour adsorption properties of agglomerated baby food powders. J. Food Eng. 2012, 109, 135–141. [Google Scholar] [CrossRef]
- Da Silva Carvalho, A.G.; da Costa Machado, M.T.; de Freitas Queiroz Barros, H.D.; Cazarin, C.B.B.; Maróstica Junior, M.R.; Hubinger, M.D. Anthocyanins from jussara (Euterpe edulis Martius) extract carried by calcium alginate beads pre-prepared using ionic gelation. Powder Technol. 2019, 345, 283–291. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Bai, J. Effect of spray-drying temperature on physicochemical, antioxidant and antimicrobial properties of pectin/sodium alginate microencapsulated carvacrol. Food Hydrocoll. 2020, 100, 105420. [Google Scholar] [CrossRef]
- Zhang, C.; Khoo, S.L.A.; Chen, X.D.; Quek, S.Y. Microencapsulation of fermented noni juice via micro-fluidic-jet spray drying: Evaluation of powder properties and functionalities. Powder Technol. 2020, 361, 995–1005. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Characterization of beet root extract (Beta vulgaris) encapsulated with maltodextrin and inulin. Molecules 2020, 25, 5498. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Ghalenoei, M.G.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Walton, D.E.; Mumford, C.J. The Morphology of Spray-Dried Particles. Chem. Eng. Res. Des. 1999, 77, 442–460. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of Microencapsulation by Spray Drying and Freeze Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Czyżowska, A.; Klewicka, E.; Libudzisz, Z. The influence of lactic acid fermentation process of red beet juice on the stability of biologically active colorants. Eur. Food Res. Technol. 2006, 223, 110–116. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Conrad, J.; Klaiber, I.; Beifuss, U.; Carle, R. Structural investigations on betacyanin pigments by LC NMR and 2D NMR spectroscopy. Phytochemistry 2004, 65, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Małecka, B. Metody analizy termicznej połączone z analizą produktów gazowych (TG-DSC-MS). LAB Lab. Apar. Bad. 2012, 17, 6–16. [Google Scholar]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Macêdo, R.; de Moura, O.; de Souza, A.; Macêdo, A. Comparative studies on some analytical methods: Thermal decomposition of powder milk. J. Therm. Anal. Calorim. 1997, 49, 857–862. [Google Scholar] [CrossRef]
- Do Carmo, E.L.; Teodoro, R.A.R.; Félix, P.H.C.; Fernandes, R.V.D.B.; de Oliveira, R.; Veiga, T.R.L.A.; Borges, S.; Botrel, D.A. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2018, 249, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Siemons, I.; Politiek, R.; Boom, R.; Van der Sman, R.; Schutyser, M. Dextrose equivalence of maltodextrins determines particle morphology development during single sessile droplet drying. Food Res. Int. 2020, 131, 108988. [Google Scholar] [CrossRef]
- Kushwaha, R.; Kumar, V.; Vyas, G.; Kaur, J. Optimization of different variable for eco-friendly extraction of betalains and phytochemicals from beetroot pomace. Waste Biomass Valorization 2018, 9, 1485–1494. [Google Scholar] [CrossRef]
- Aztatzi-Rugerio, L.; Granados-Balbuena, S.Y.; Zainos-Cuapio, Y.; Ocaranza-Sánchez, E.; Rojas-López, M. Analysis of the degradation of betanin obtained from beetroot using Fourier transform infrared spectroscopy. J. Food Sci. Technol. 2019, 56, 3677–3686. [Google Scholar] [CrossRef]
- Sengupta, D.; Mondal, B.; Mukherjee, K. Visible light absorption and photo-sensitizing properties of spinach leaves and beetroot extracted natural dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 85–92. [Google Scholar] [CrossRef]
- Rybak, K.; Parniakov, O.; Samborska, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Energy and quality aspects of freeze-drying preceded by traditional and novel pre-treatment methods as exemplified by red bell pepper. Sustainability 2021, 13, 2035. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The quality of red bell pepper subjected to freeze-drying preceded by traditional and novel pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Sokołowska, B.; Woźniak, Ł.; Skąpska, S.; Porębska, I.; Nasiłowska, J.; Rzoska, S.J. Evaluation of quality changes of beetroot juice after high hydrostatic pressure processing. High Press. Res. 2017, 37, 214–222. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef]
- Miguel, M. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 2005, 222, 586–593. [Google Scholar] [CrossRef]
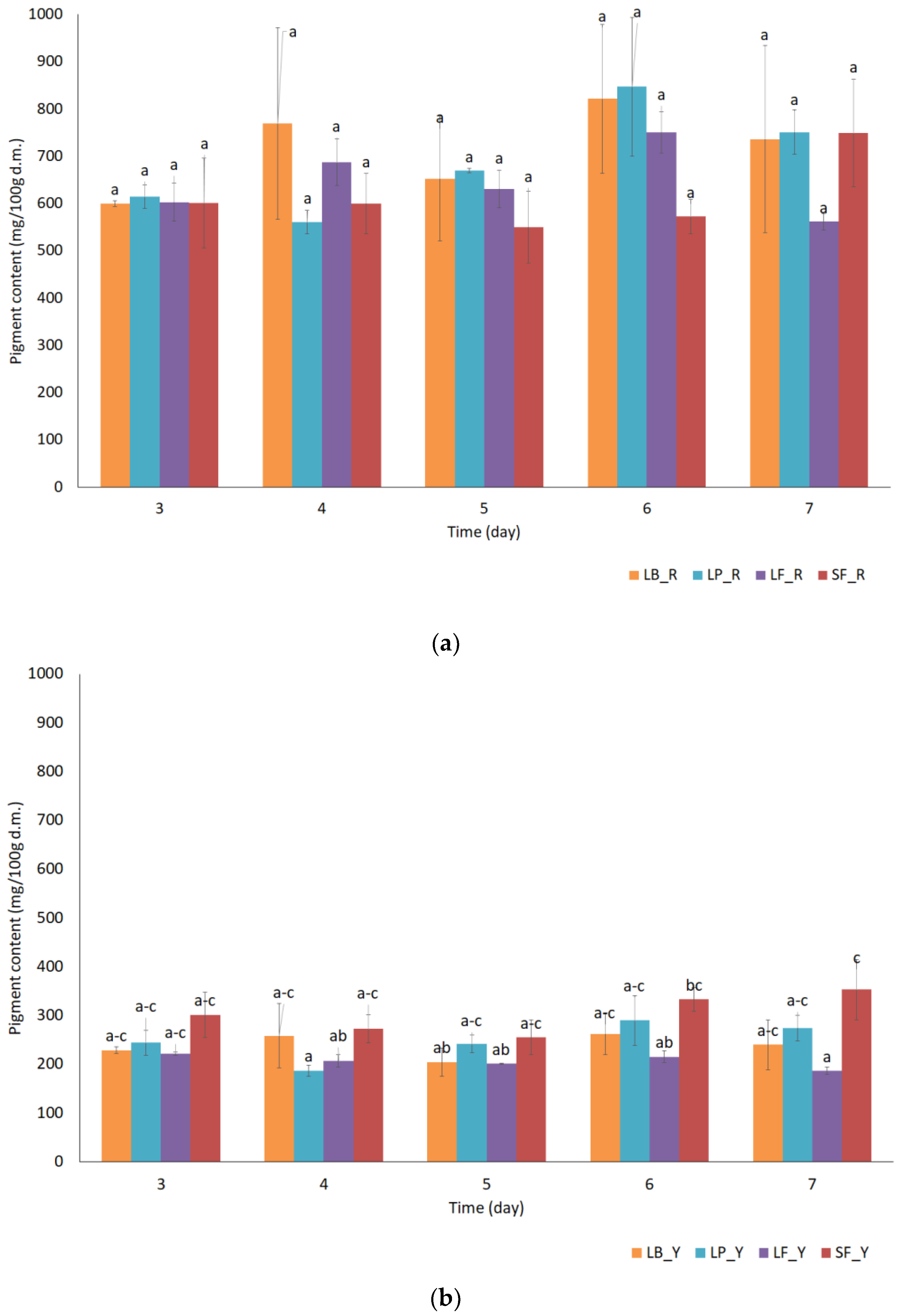

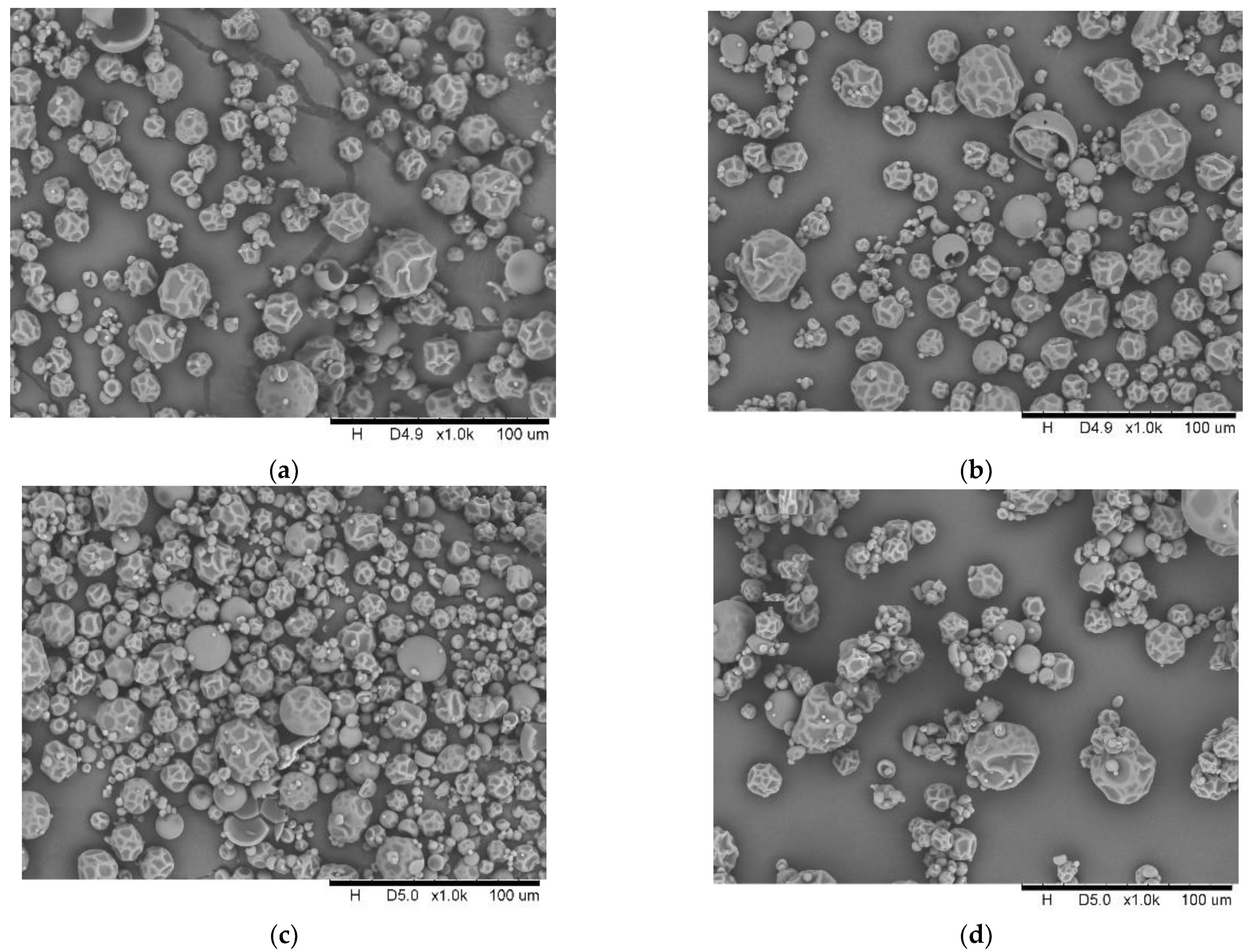

| Sample Name | Extract (° Brix) | pH (-) | Dry Matter d.m. (%) | Total Acidity (g Lactic Acid /100 g Product) | Color Coefficients | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| B_0 | 7.35 ± 0.07 cd | 5.8 ± 0.05 g | 4.3 ± 0.1 a | 0.35 ± 0.04 ab | 5.27 ± 0.04 i | 7.37 ± 0.16 h | 0.17 ± 0.01 bd |
| LB_3 | 7.60 ± 0.00 ef | 4.2 ± 0.05 e | 5.9 ± 0.1 a | 1.09 ± 0.30 ef | 4.09 ± 0.04 h | 4.88 ± 0.01 d | 0.45 ± 0.12 eg |
| LB_4 | 6.95 ± 0.07 a | 4.1 ± 0.05 d | 4.7 ± 1.2 a | 0.84 ± 0.01 bf | 1.31 ± 0.07 b | 6.90 ± 0.07 g | 0.07 ± 0.04 ac |
| LB_5 | 7.80 ± 0.00 g | 4.0 ± 0.05 c | 5.7 ± 0.2 a | 0.86 ± 0.09 cf | 4.02 ± 0.05 h | 8.00 ± 0.01 i | 0.36 ± 0.08 df |
| LB_6 | 7.35 ± 0.07 cd | 4.0 ± 0.05 c | 4.2 ± 0.7 a | 0.72 ± 0.03 ae | 3.07 ± 0.08 e | 7.01 ± 0.08 gh | 0.68 ± 0.10 gj |
| LB_7 | 7.70 ± 0.00 fg | 3.8 ± 0.05 a | 4.3 ± 1.1 a | 0.89 ± 0.03 cf | 2.74 ± 0.04 d | 7.17 ± 0.16 gh | 0.51 ± 0.10 eh |
| LF_3 | 7.80 ± 0.00 g | 4.0 ± 0.05 c | 6.1 ± 0.6 a | 1.64 ± 0.23 g | 4.13 ± 0.04 g | 4.07 ± 0.13 c | 0.16 ± 0.15 bd |
| LF_4 | 7.60 ± 0.00 ef | 4.0 ± 0.05 c | 5.2 ± 0.1 a | 0.86 ± 0.07 cf | 1.24 ± 0.05 b | 5.61 ± 0.01 e | −0.08 ± 0.08 ab |
| LF_5 | 7.65 ± 0.07 eg | 3.9 ± 0.05 b | 5.1 ± 0.3 a | 1.05 ± 0.14 df | 4.26 ± 0.04 g | 9.79 ± 0.08 l | 0.81 ± 0.12 ik |
| LF_6 | 7.60 ± 0.00 ef | 3.9 ± 0.05 b | 4.9 ± 0.2 a | 0.80 ± 0.06 bf | 3.74 ± 0.06 f | 8.81 ± 0.06 j | 0.78 ± 0.08 hk |
| LF_7 | 7.60 ± 0.00 ef | 3.9 ± 0.05 b | 5.4 ± 0.2 a | 0.70 ± 0.10 ae | 3.85 ± 0.08 f | 10.47 ± 0.10 m | 0.94 ± 0.03 jk |
| LP_3 | 7.55 ± 0.07 ef | 3.9 ± 0.05 b | 5.6 ± 0.1 a | 1.59 ± 0.26 g | 5.16 ± 0.02 i | 9.90 ± 0.13 l | 1.38 ± 0.11 l |
| LP_4 | 7.30 ± 0.00 bc | 3.9 ± 0.05 b | 5.6 ± 0.1 a | 1.19 ± 0.00 eg | 2.12 ± 0.05 c | 10.53 ± 0.09 m | 0.52 ± 0.05 eh |
| LP_5 | 7.70 ± 0.00 fg | 3.8 ± 0.05 a | 5.0 ± 0.2 a | 1.29 ± 0.09 fg | 5.74 ± 0.05 j | 15.85 ± 0.07 o | 1.95 ± 0.05 m |
| LP_6 | 7.15 ± 0.07 b | 3.9 ± 0.05 b | 4.0 ± 0.6 a | 1.19 ± 0.16 eg | 3.71 ± 0.03 f | 9.39 ± 0.08 k | 0.95 ± 0.04 k |
| LP_7 | 7.50 ± 0.00 de | 3.8 ± 0.05 a | 4.3 ± 0.2 a | 1.27 ± 0.09 fg | 3.75 ± 0.07 f | 11.04 ± 0.23 n | 1.04 ± 0.06 k |
| SF_3 | 7.70 ± 0.10 fg | 5.9 ± 0.05 h | 5.2 ± 0.7 a | 0.56 ± 0.06 ad | 3.75 ± 0.02 f | 2.11 ± 0.07 a | −0.16 ± 0.06 a |
| SF_4 | 8.10 ± 0.00 h | 5.9 ± 0.05 h | 5.3 ± 0.5 a | 0.41 ± 0.07 ac | 0.81 ± 0.03 a | 3.14 ± 0.07 b | −0.04 ± 0.09 ab |
| SF_5 | 8.00 ± 0.00 h | 5.9 ± 0.05 h | 5.7 ± 0.8 a | 0.27 ± 0.03 a | 3.18 ± 0.02 e | 3.44 ± 0.13 b | −0.02 ± 0.07 ab |
| SF_6 | 7.70 ± 0.00 fg | 5.0 ± 0.05 f | 4.6 ± 0.2 a | 0.35 ± 0.03 ab | 3.10 ± 0.03 e | 5.07 ± 0.13 d | 0.58 ± 0.00 ei |
| SF_7 | 8.00 ± 0.00 h | 5.1 ± 0.05 f | 4.3 ± 0.6 a | 0.24 ± 0.02 a | 2.86 ± 0.11 d | 6.24 ± 0.30 f | 0.31 ± 0.16 ce |
| Sample Name | Dry Matter (%) | Water Activity (-) | Mass Increment after 24 h (%) | Total Acidity (g Lactic Acid /100 g Product) | Lightness L* | Redness a* | Yellowness b* |
|---|---|---|---|---|---|---|---|
| LB_powder | 94.5 ± 0.5 b | 0.35 ± 0.01 a | 20.8 ± 1.0 b | 2.99 ± 0.12 a | 43.04 ± 0.01 a | 39.14 ± 0.05 b | −18.45 ± 0.00 a |
| LF_powder | 94.2 ± 0.3 b | 0.37 ± 0.01 a | 16.6 ± 0.5 a | 2.85 ± 0.26 a | 51.29 ± 0.26 c | 34.69 ± 0.25 a | 2.64 ± 0.02 c |
| LP_powder | 94.6 ± 0.1 c | 0.35 ± 0.00 a | 16.5 ± 0.7 a | 2.86 ± 0.09 a | 48.97 ± 0.06 b | 39.18 ± 0.20 b | −18.47 ± 0.12 a |
| SF_powder | 90.2 ± 0.1 a | 0.52 ± 0.01 b | 19.5 ± 0.8 b | 2.96 ± 0.39 a | 49.22 ± 0.01 b | 38.86 ± 0.01 b | −15.30 ± 0.01 b |
| Betaxanthin mg/100 g d.m. | Sum | Betacyanin mg/100 g d.m. | Sum | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | |||
| Fresh beetroot juice | 183.7 ± 1.1 | 4.8 ± 0.1 | 6.0 ± 0.1 | 6.1 ± 0.2 | 3.5 ± 0.1 | 204.2 ± 1.4 d | 2.0 ± 0.0 | 1639.0 ± 30.2 | 3.0 ± 0.2 | 104.3 ± 0.0 | 1.2 ± 0.1 | 0 | 5.0 ± 0.1 | 3.7 ± 0.2 | 0 | 1758.3 ± 29.8 d |
| SF_5 | 1.4 ± 0.1 | 0 | 0 | 0 | 0 | 1.4 ± 0.1 c | 0 | 2.0 ± 0.1 | 0 | 0.3 ± 0.0 | 8.4 ± 0.3 | 0 | 0 | 0 | 0 | 10.8 ± 0.4 a |
| LB_5 | 0.3 ± 0.0 | 0 | 0 | 0 | 0 | 0.3 ± 0.0 b | 1.6 ± 0.0 | 79.8 ± 1.6 | 1.6 ± 0.5 | 27.1 ± 0.6 | 1.7 ± 0.1 | 0 | 0 | 1.0 ± 0.1 | 0 | 112.7 ± 2.2 b |
| LP_5 | 0.1 ± 0.0 | 0 | 0 | 0 | 0 | 0.1 ± 0.0 a | 1.2 ± 0.0 | 69.4 ± 4.9 | 1.1 ± 0.1 | 26.0 ± 1.1 | 0.6 ± 0.0 | 0 | 0 | 0.9 ± 0.1 | 0 | 99.3 ± 6.0 c |
| LF_5 | 0.1 ± 0.0 | 0 | 0 | 0 | 0 | 0.1 ± 0.0 a | 1.1 ± 0.1 | 65.3 ± 4.8 | 1.2 ± 0.0 | 23.5 ± 1.5 | 1.0 ± 0.1 | 0 | 0 | 0.8 ± 0.0 | 0 | 92.8 ± 6.5 c |
| SF_powder | 2.3 ± 0.1 | 0 | 0 | 0 | 0 | 2.3 ± 0.1 B | 9.4 ± 0.1 | 335.2 ± 1.0 | 6.6 ± 0.0 | 98.1 ± 2.2 | 0.9 ± 0.1 | 1.4 ± 0.1 | 1.1 ± 0.2 | 8.2 ± 0.3 | 13.7 ± 0.3 | 474.6 ± 1.9 A |
| LB_powder | 0.3 ± 0.0 | 0 | 0 | 0 | 0 | 0.3 ± 0.0 A | 7.5 ± 0.2 | 394.5 ± 1.7 | 6.3 ± 0.3 | 135.5 ± 0.4 | 5.4 ± 0.5 | 1.7 ± 0.1 | 1.8 ± 0.1 | 6.3 ± 0.2 | 1.7 ± 0.1 | 560.5 ± 1.7 C |
| LP_powder | 0.3 ± 0.0 | 0 | 0 | 0 | 0 | 0.3 ± 0.0 A | 6.9 ± 0.2 | 408.4 ± 1.6 | 7.0 ± 0.3 | 156.3 ± 0.6 | 3.2 ± 0.5 | 0.7 ± 0.1 | 1.4 ± 0.1 | 7.2 ± 0.1 | 1.8 ± 0.0 | 592.9 ± 1.0 D |
| LF_powder | 0.3 ± 0.0 | 0 | 0 | 0 | 0 | 0.3 ± 0.0 A | 5.5 ± 0.2 | 375.0 ± 0.9 | 6.4 ± 0.0 | 139.3 ± 2.9 | 6.3 ± 0.2 | 1.8 ± 0.0 | 2.1 ± 0.0 | 6.0 ± 0.1 | 2.2 ± 0.0 | 544.5 ± 4.0 B |
| Sample Name | Step 1 | Step 2 | Step 3 | Sum [%] | Decomposition Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. Range (°C) | Mass Loss (%) | Temp. Range (°C) | Mass Loss (%) | Temp. Range (°C) | Mass Loss (%) | 1 | 2 | ||||
| LB_powder | 30–120 | 0 | 120–420 | 56.7 | 420–600 | 1.6 | 58.3 | 63.8 | 160.2 | 202.9 | 286.5 |
| LF_powder | 30–120 | 2.9 | 120–420 | 57.5 | 420–600 | 1.3 | 61.7 | 62.9 | 160.5 | 207.2 | 287.6 |
| LP_powder | 30–120 | 3.5 | 120–420 | 56.2 | 420–600 | 0.9 | 60.6 | 60.4 | 160.5 | 206.5 | 289.2 |
| SF_powder | 30–120 | 4.9 | 120–420 | 52.5 | 420–600 | 5.7 | 63.1 | 42.7 | 167.5 | - | 272.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska-Turak, E.; Walczak, M.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Witrowa-Rajchert, D. Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules 2022, 27, 1008. https://doi.org/10.3390/molecules27031008
Janiszewska-Turak E, Walczak M, Rybak K, Pobiega K, Gniewosz M, Woźniak Ł, Witrowa-Rajchert D. Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules. 2022; 27(3):1008. https://doi.org/10.3390/molecules27031008
Chicago/Turabian StyleJaniszewska-Turak, Emilia, Maciej Walczak, Katarzyna Rybak, Katarzyna Pobiega, Małgorzata Gniewosz, Łukasz Woźniak, and Dorota Witrowa-Rajchert. 2022. "Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder" Molecules 27, no. 3: 1008. https://doi.org/10.3390/molecules27031008
APA StyleJaniszewska-Turak, E., Walczak, M., Rybak, K., Pobiega, K., Gniewosz, M., Woźniak, Ł., & Witrowa-Rajchert, D. (2022). Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules, 27(3), 1008. https://doi.org/10.3390/molecules27031008









