Proteomic Analysis of Protective Effects of Epimedium Flavonoids against Ethanol-Induced Toxicity in Retinoic Acid-Treated SH-SY5Y Cells
Abstract
:1. Introduction
2. Results
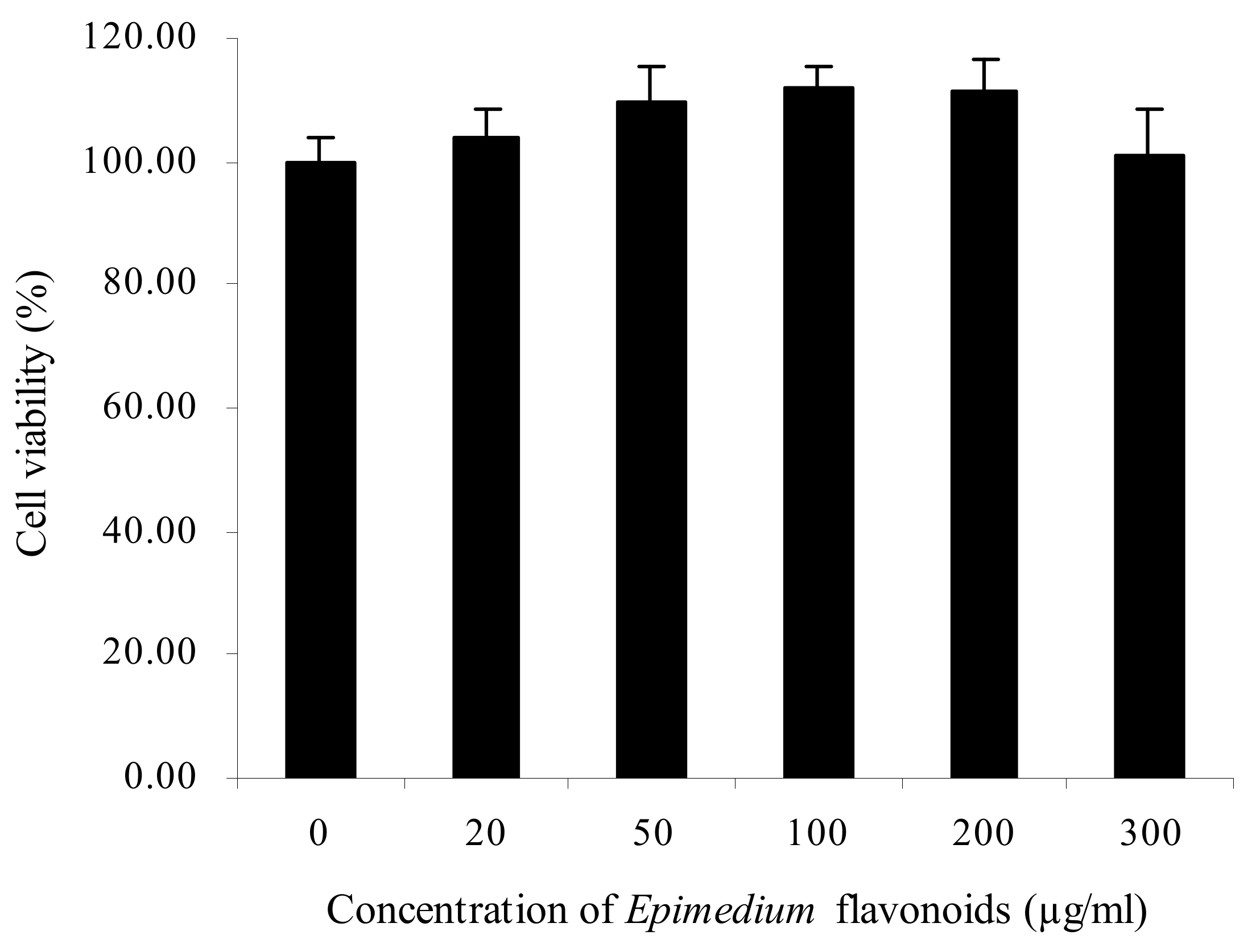
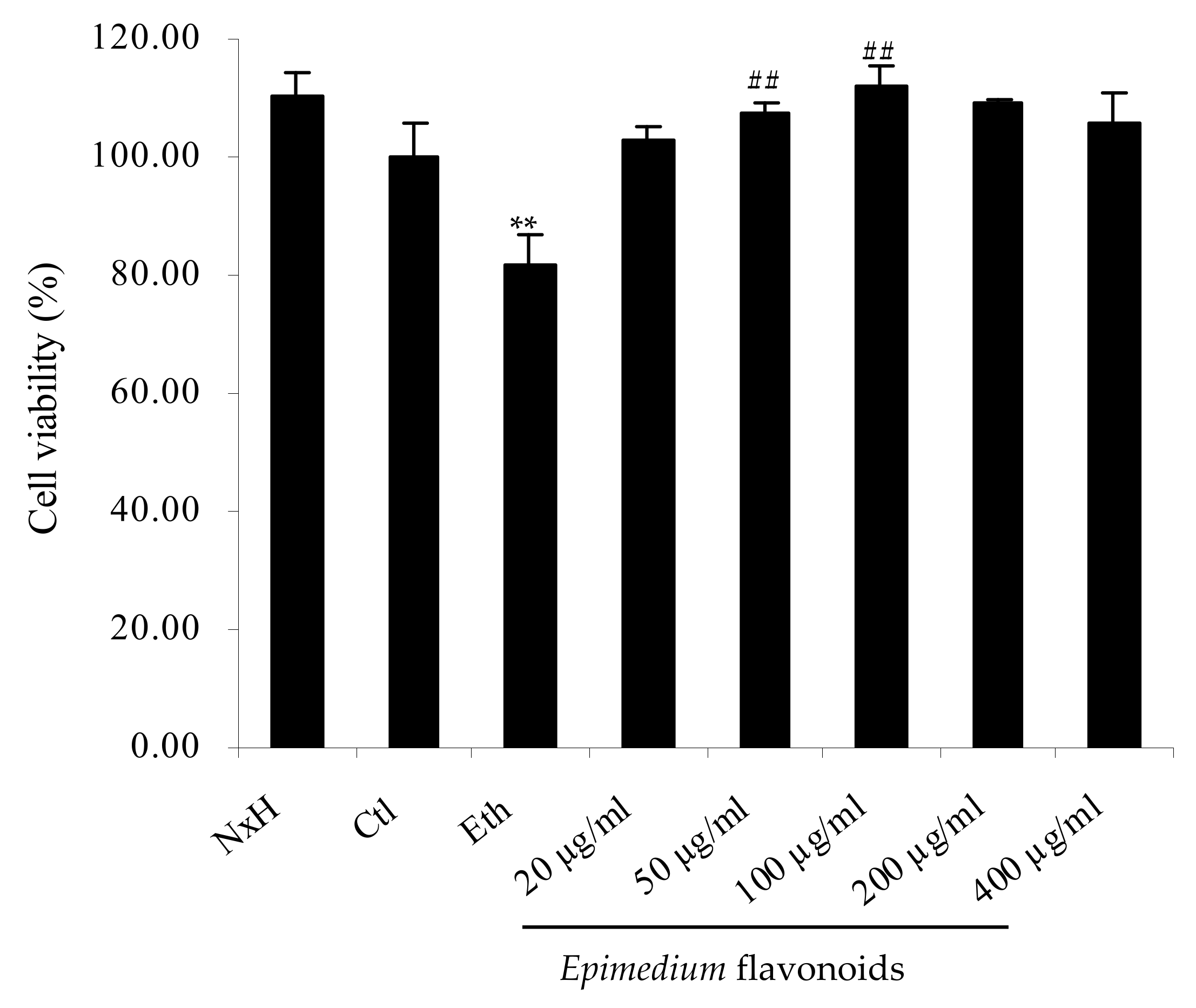
2.1. Protective Effects of Epimedium Flavonoids on RA-Treated SH-SY5Y Cells Exposed to Ethanol
2.2. Influence of Epimedium Flavonoids on Protein Profile in RA-Treated SH-SY5Y Cells Exposed to Ethanol
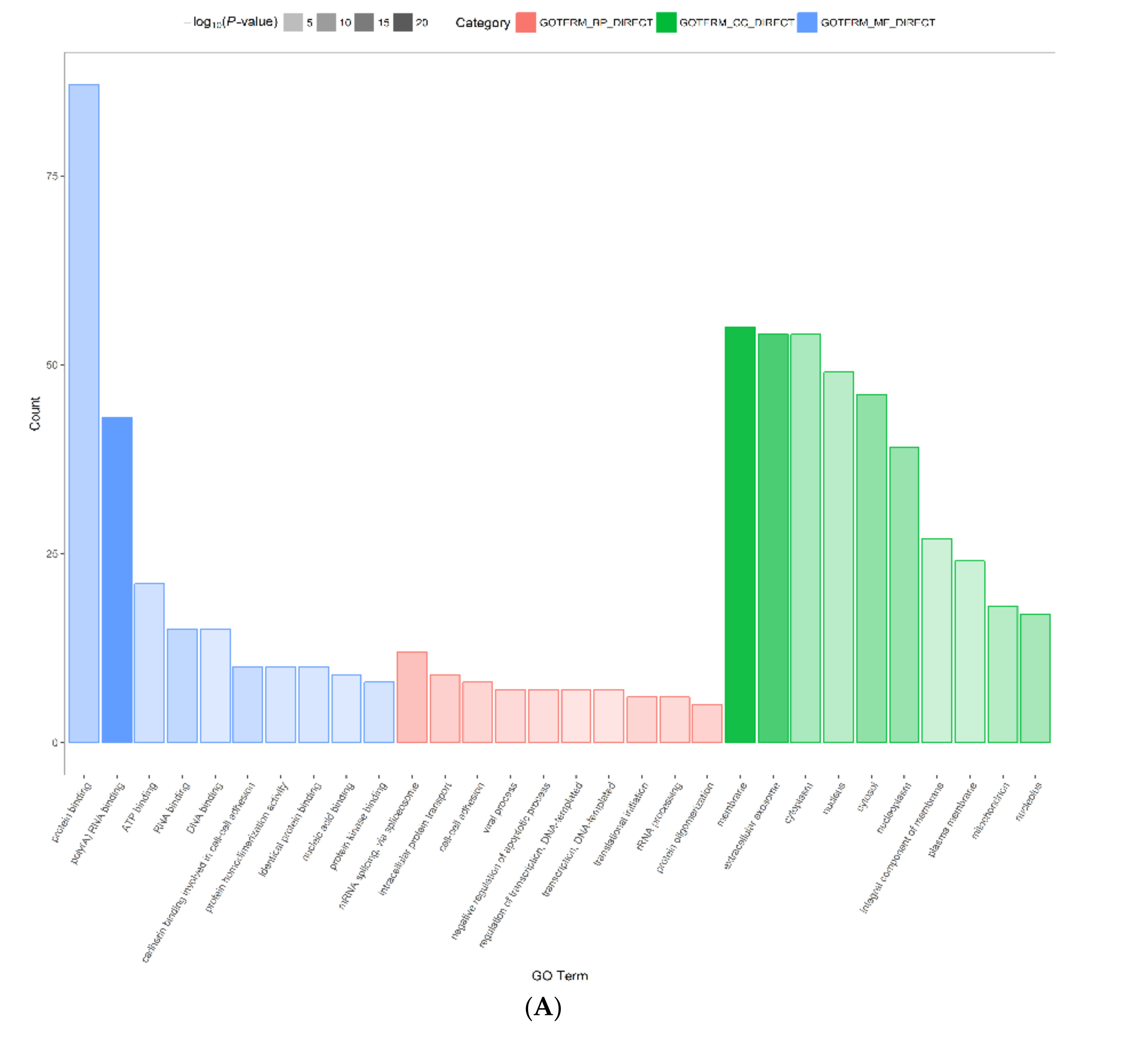
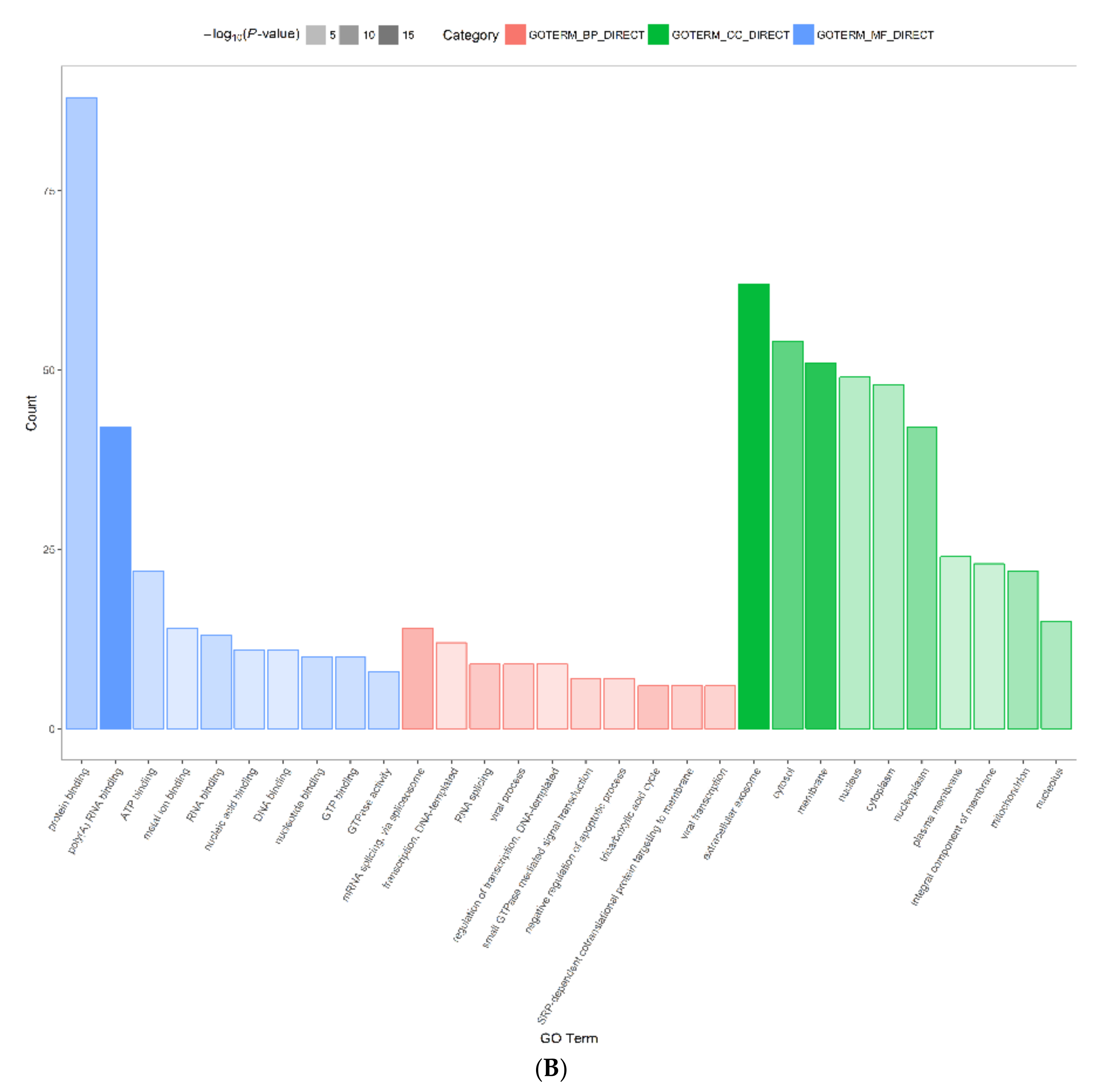
2.3. Functional Annotation and Classification of Differentially Expressed Proteins
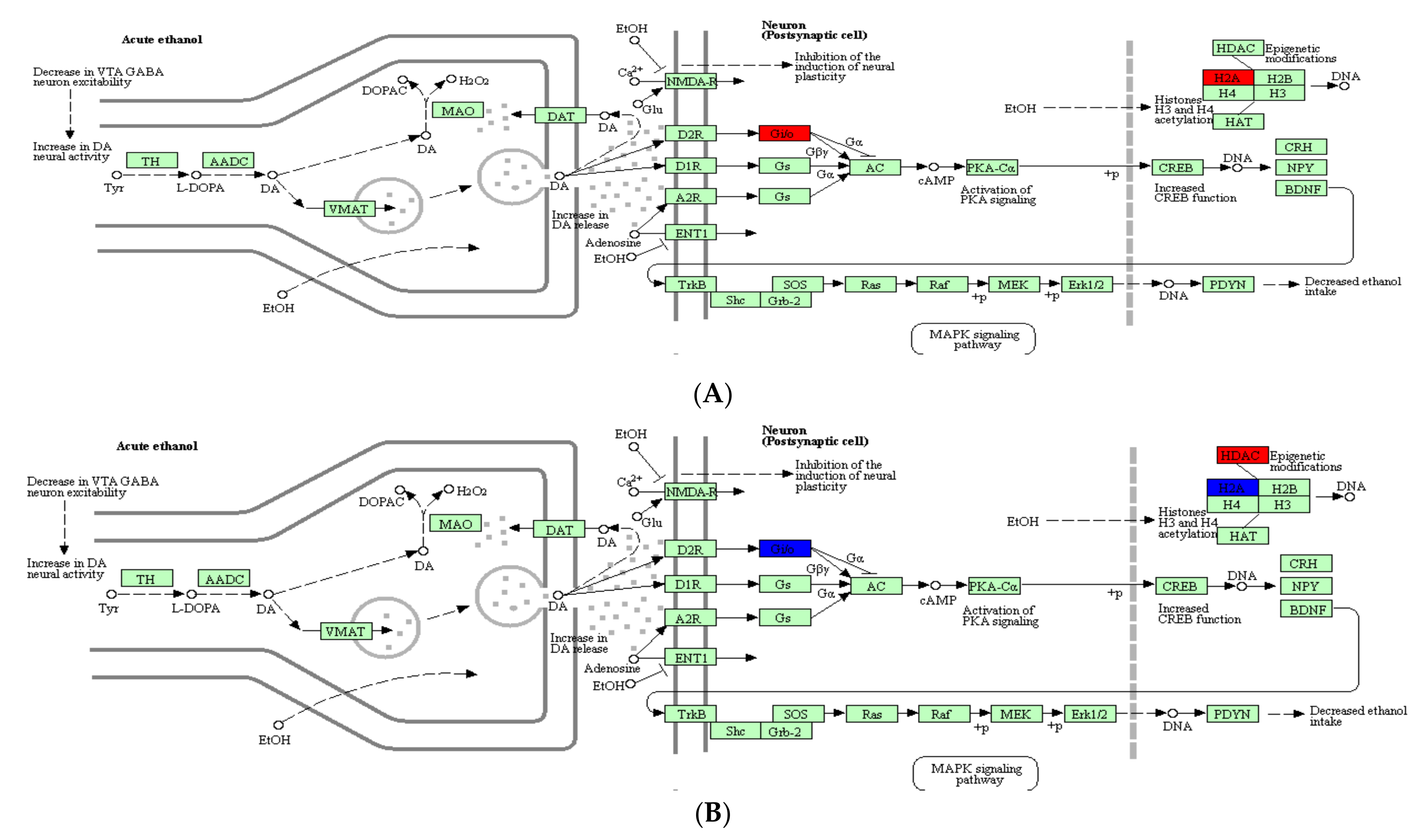
2.4. Analysis of DEPs Related to Alcoholism
2.5. Validation of Selected Proteins by Western Blot
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Epimedium Flavonoids
4.3. Qualitative and Quantitative Analyses of Epimedium Flavonoids
4.4. Cell Culture and Experimental Treatment
4.5. Cell Viability Assay
4.6. Isolation and Quantification of Proteins
4.7. Proteomic Procedure
4.8. Bioinformatic Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garaycoechea, J.I.; Crossan, G.P.; Langevin, F.; Mulderrig, L.; Louzada, S.; Yang, F.; Guilbaud, G.; Park, N.; Roerink, S.; Nik-Zainal, S.; et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature 2018, 553, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Zhang, H.F.; Lai, J.H. Alcohol dependence mediated by monoamine neurotransmitters in the central nervous system. Yi Chuan Hereditas 2014, 36, 11–20. [Google Scholar]
- World Health Organization. Global Status Report on Alcohol and Health, Geneva. 2018. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 28 January 2022).
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Williams, A.N.; Johnson, C.; Ou, X.M. The effects of antidepressant drug on ethanol-induced cell death. Drug Discov. Ther. 2007, 1, 130–135. [Google Scholar]
- Gilpin, N.W.; Koob, G.E. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol Res. Health 2008, 31, 185–195. [Google Scholar] [PubMed]
- Hoertel, N.; Le, S.Y.; Schuster, J.P.; Limosin, F. Gender differences in firesetting: Results from the national epidemiologic survey on alcohol and related conditions (NESARC). Psychiatry Res. 2011, 190, 352–358. [Google Scholar] [CrossRef]
- Agostini, J.F.; Toé, H.C.Z.D.; Vieira, K.M.; Baldin, S.L.; Costa, N.L.F.; Cruz, C.U.; Longo, L.; Machado, M.M.; da Silveira, T.R.; Schuck, P.F.; et al. Cholinergic system and oxidative stress changes in the brain of a zebrafish model chronically exposed to ethanol. Neurotox. Res. 2018, 33, 749–758. [Google Scholar] [CrossRef]
- Getachew, B.; Csoka, A.B.; Garden, A.R.; Copeland, R.L.; Tizabi, Y. Sodium butyrate protects against ethanol-induced toxicity in SH-SY5Y cell line. Neurotox. Res. 2021, 39, 2186–2193. [Google Scholar] [CrossRef]
- Liu, Q.; Lawrence, A.J.; Liang, J.H. Traditional Chinese medicine for treatment of alcoholism: From ancient to modern. Am. J. Chin. Med. 2011, 39, 1–13. [Google Scholar] [CrossRef]
- Yang, X.H. Application of Three Chinese Medicinal Materials to Prevention and Treatment of Alcohol Dependence or Intoxication and Their Quality Control. Ph.D. Dissertation, Xi’an Jiaotong University, Xi’an, China, 2018. [Google Scholar]
- Keung, W.M. Anti-dipsotropic isoflavones: The potential therapeutic agents for alcohol dependence. Med. Res. Rev. 2003, 23, 669–696. [Google Scholar] [CrossRef]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lien, E.J.; Lien, L.L. Chemical and pharmacological investigations of Epimedium species: A survey. Prog. Drug Res. 2003, 60, 1–57. [Google Scholar] [PubMed]
- Zhang, H.F.; Niu, L.L.; Yang, X.H.; Li, L. Analysis of water-soluble polysaccharides in an edible medicinal plant Epimedium: Method development, validation and application. J. AOAC Int. 2014, 97, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Yang, X.H. Asian medicine: Protect rare plants. Nature 2012, 482, 35. [Google Scholar] [CrossRef] [Green Version]
- Shindel, A.W.; Xin, Z.; Lin, G.; Fandel, T.M.; Huang, Y.C.; Banie, L.; Breyer, B.N.; Garcia, M.M.; Lin, C.S.; Lue, T.F. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. J. Sex. Med. 2010, 7, 1518–1528. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.G.; Ye, S.F.; Huang, J.M.; Tian, Y.; Xu, Y.; Wu, M.; Wang, J.; Wu, S.; Cai, J. How do Chinese medicines that tonify the kidney inhibit dopaminerginc neuron apoptosis. Neural Regen. Res. 2013, 8, 2820–2826. [Google Scholar]
- Le, L.; Jiang, B.P.; Xu, J.; Hu, K.P.; Chen, S.L. Research strategy of traditional Chinese medicine proteomics. Zhongguo Zhong Yao Za Zhi 2016, 41, 4096–4102. [Google Scholar]
- Yue, Q.X.; Cao, Z.W.; Guan, S.H.; Liu, X.H.; Tao, L.; Wu, W.Y.; Li, Y.X.; Yang, P.Y.; Liu, X.; Guo, D.A. Proteomics characterization of the cytotoxicity mechanism of ganoderic acid D and computer-automated estimation of the possible drug target network. Mol. Cell. Proteom. 2008, 7, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.L.; Hung, Y.C.; Wang, P.W.; Chen, S.T.; Hsu, T.K.; Sintupisut, N.; Cheng, C.S.; Lyu, P.C. Functional proteomic and structural insights into molecular targets related to the growth inhibitory effect of tanshinone IIA on HeLa cells. Proteomics 2010, 10, 914–929. [Google Scholar] [CrossRef]
- Chou, H.C.; Lu, Y.C.; Cheng, C.S.; Chen, Y.W.; Lyu, P.C.; Lin, C.W.; Timms, J.F.; Chan, H.L. Proteomic and redox-proteomic analysis of berberine-induced cytotoxicity in breast cancer cells. J. Proteom. 2012, 75, 3158–3176. [Google Scholar] [CrossRef]
- Feng, L.X.; Jing, C.J.; Tang, K.L.; Tao, L.; Cao, Z.W.; Wu, W.Y.; Guan, S.H.; Jiang, B.H.; Yang, M.; Liu, X.; et al. Clarifying the signal network of salvianolic acid B using proteomic assay and bioinformatic analysis. Proteomics 2011, 11, 1473–1485. [Google Scholar] [CrossRef]
- Wu, P.; He, F.C.; Jiang, Y. Label-free methods in quantitative proteomics. Prog. Biochem. Biophys. 2013, 40, 281–292. [Google Scholar] [CrossRef]
- Lu, J.; Liu, L.; Pang, X.; Zhang, S.; Jia, Z.; Ma, C.; Zhao, L.; Lv, J. Comparative proteomics of milk fat globule membrane in goat colostrum and mature milk. Food Chem. 2016, 209, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Oscar-Berman, M.; Marinković, K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007, 17, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Giancola, P.R.; Josephs, R.A.; Parrott, D.J.; Duke, A.A. Alcohol myopia revisited: Clarifying aggression and other acts of disinhibition through a distorted lens. Perspect. Psychol. Sci. 2010, 5, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.F.; Yang, T.S.; Li, Z.Z.; Wang, Y. Simultaneous extraction of epimedin A, B, C and icariin from Herba Epimedii by ultrasonic technique. Ultrason. Sonochem. 2008, 15, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Li, L.; Xue, Y.B.; Zhou, X.X.; Tang, J.H. Flavonoids from Epimedium pubescens: Extraction and mechanism, antioxidant capacity and effects on CAT and GSH-Px of Drosophila melanogaster. PeerJ 2020, 8, e8361. [Google Scholar] [CrossRef] [Green Version]
- Wernicke, C.; Hellmann, J.; Finckh, U.; Rommelspacher, H. Chronic ethanol exposure changes dopamine D2 receptor splicing during retinoic acid-induced differentiation of human SH-SY5Y cells. Pharmacol. Rep. 2010, 62, 649–663. [Google Scholar] [CrossRef]
- Xie, H.; Hu, L.; Li, G. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar]
- Sha, D.; Li, L.; Ye, L.; Liu, R.; Xu, Y. Icariin inhibits neurotoxicity of beta-amyloid by upregulating cocaine-regulated and amphetamine-regulated transcripts. Neuroreport 2009, 20, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.R.; Ram, P.T.; Iyengar, R. G protein pathways. Science 2002, 296, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Kostenis, E.; Pfeil, E.M.; Annala, S. Heterotrimeric G (q) proteins as therapeutic targets? J. Biol. Chem. 2020, 295, 5206–5215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.P.; Cui, H.M.; Wang, W.; Zhao, B.; Lai, J.H. The region-specific activation of Ca2+/calmodulin dependent protein kinase II and extracellular signal-regulated kinases in hippocampus following chronic alcohol exposure. Brain Res. Bull. 2012, 89, 191–196. [Google Scholar] [CrossRef] [PubMed]
- The State Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Du, M.; Gao, J.; Zhan, B.; Mao, X. Label-free quantitative proteomic analysis of milk fat globule membrane proteins of yak and cow and identification of proteins associated with glucose and lipid metabolism. Food Chem. 2019, 275, 59–68. [Google Scholar] [CrossRef]
- Dennis, G.J.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Lempicki, R.; Sherman, B.T. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, H.; Li, L.; Zhou, X.; Liu, Y.; Lai, J. Proteomic Analysis of Protective Effects of Epimedium Flavonoids against Ethanol-Induced Toxicity in Retinoic Acid-Treated SH-SY5Y Cells. Molecules 2022, 27, 1026. https://doi.org/10.3390/molecules27031026
Yang X, Zhang H, Li L, Zhou X, Liu Y, Lai J. Proteomic Analysis of Protective Effects of Epimedium Flavonoids against Ethanol-Induced Toxicity in Retinoic Acid-Treated SH-SY5Y Cells. Molecules. 2022; 27(3):1026. https://doi.org/10.3390/molecules27031026
Chicago/Turabian StyleYang, Xiaohua, Huafeng Zhang, Lu Li, Xuexue Zhou, Yichao Liu, and Jianghua Lai. 2022. "Proteomic Analysis of Protective Effects of Epimedium Flavonoids against Ethanol-Induced Toxicity in Retinoic Acid-Treated SH-SY5Y Cells" Molecules 27, no. 3: 1026. https://doi.org/10.3390/molecules27031026
APA StyleYang, X., Zhang, H., Li, L., Zhou, X., Liu, Y., & Lai, J. (2022). Proteomic Analysis of Protective Effects of Epimedium Flavonoids against Ethanol-Induced Toxicity in Retinoic Acid-Treated SH-SY5Y Cells. Molecules, 27(3), 1026. https://doi.org/10.3390/molecules27031026




