Synthesis, Nanoformulations, and In Vitro Anticancer Activity of N-Substituted Side Chain Neocryptolepine Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NPA-Loaded SiO2 Nanoemulsion
2.3. Characterization
3. Results and Discussion
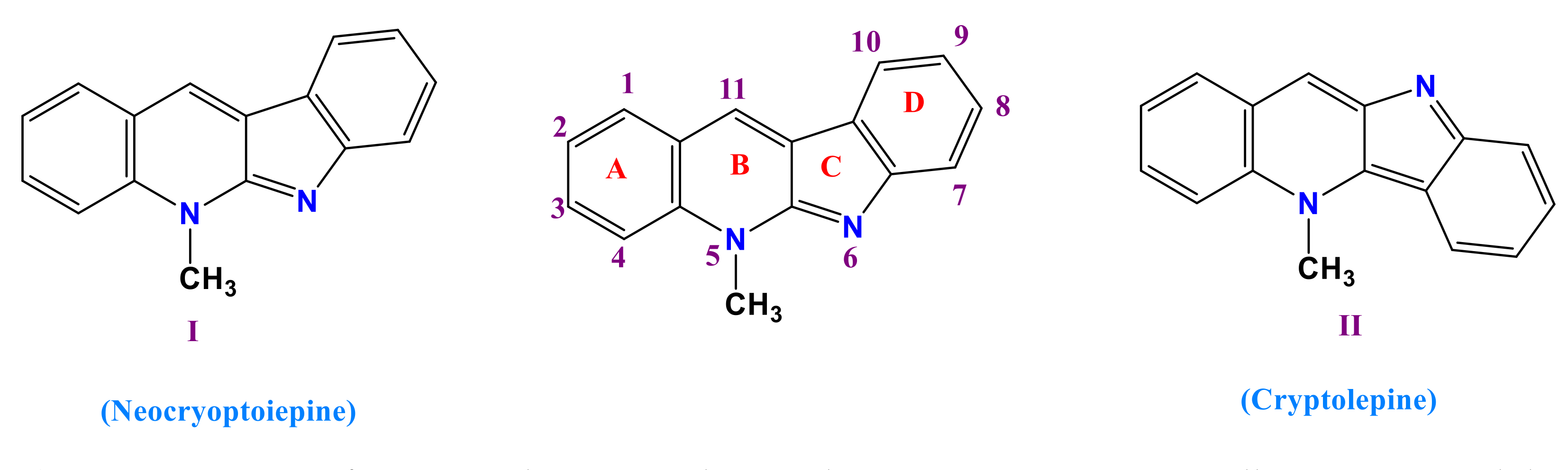
3.1. Synthesis of 11-Chloroneocryptolepine 5
3.2. Formation of Amino Neocryptolepine 7
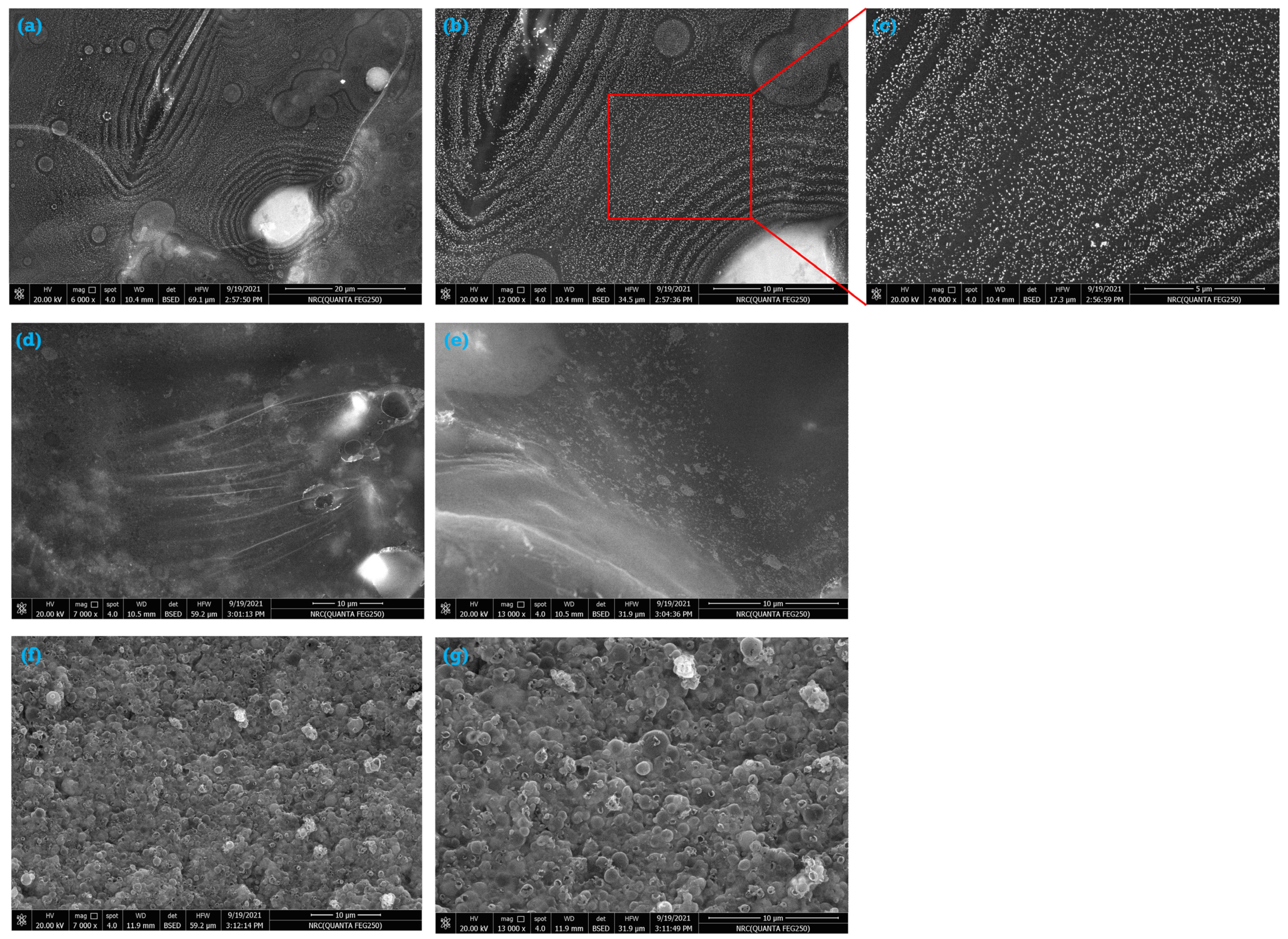
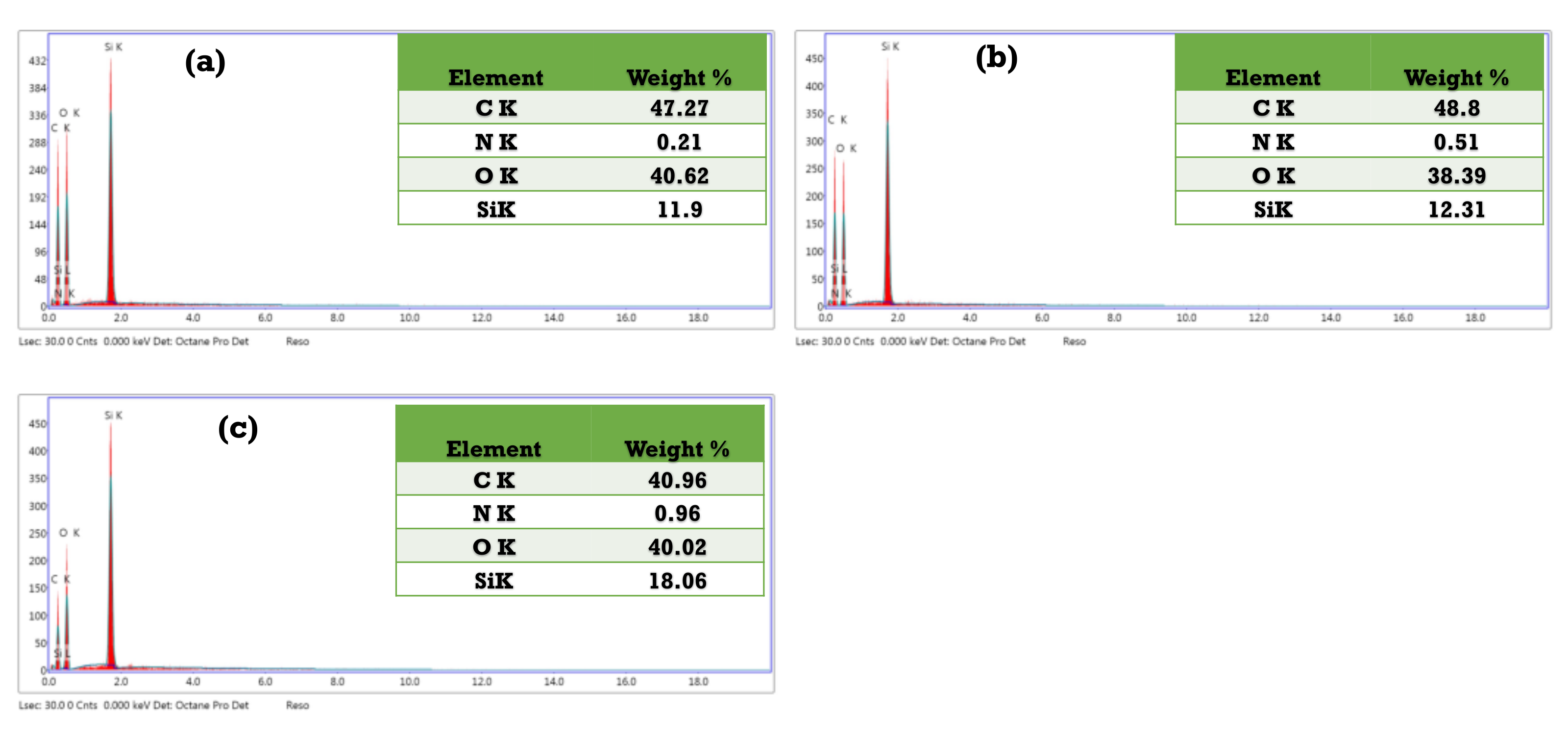
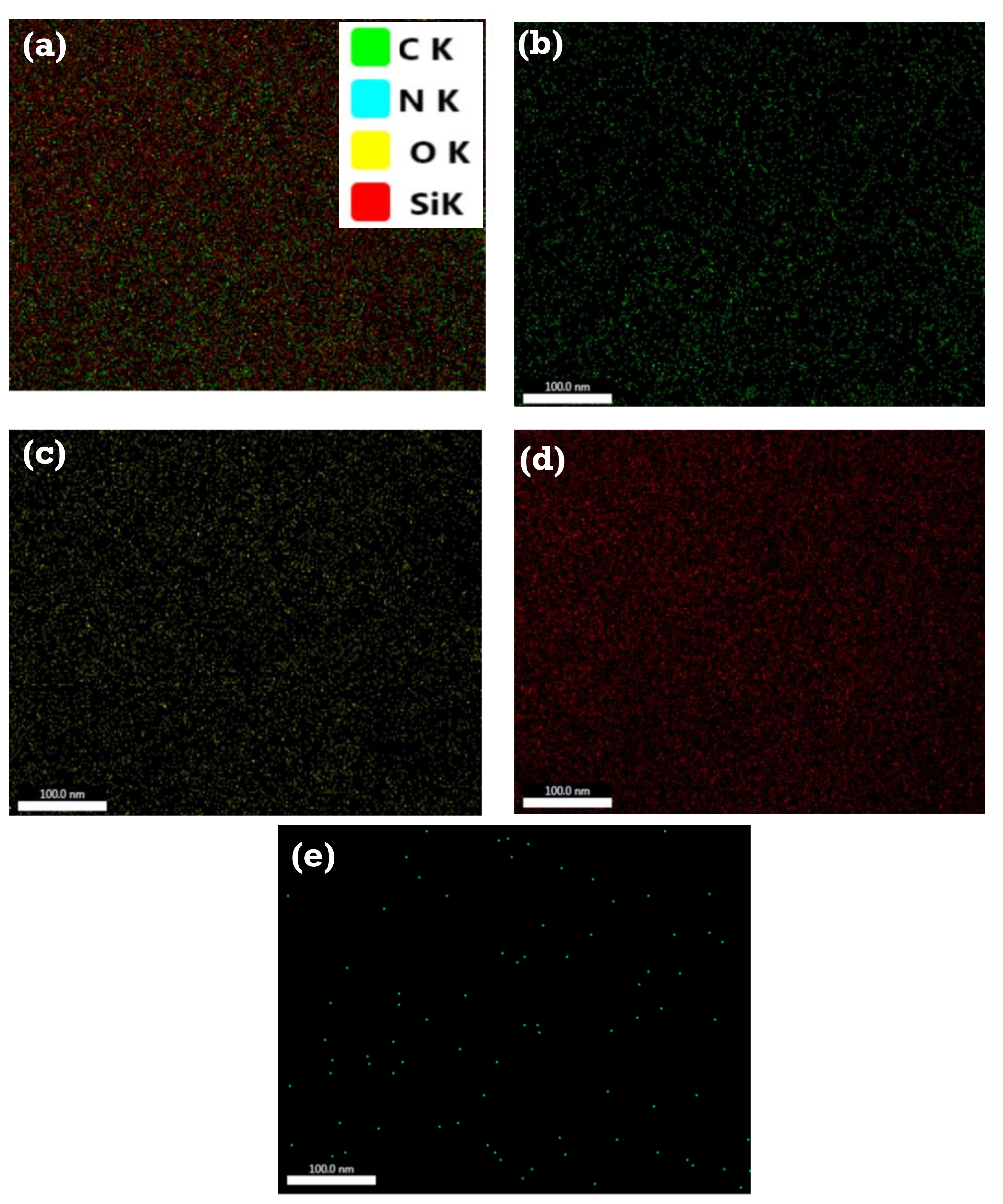
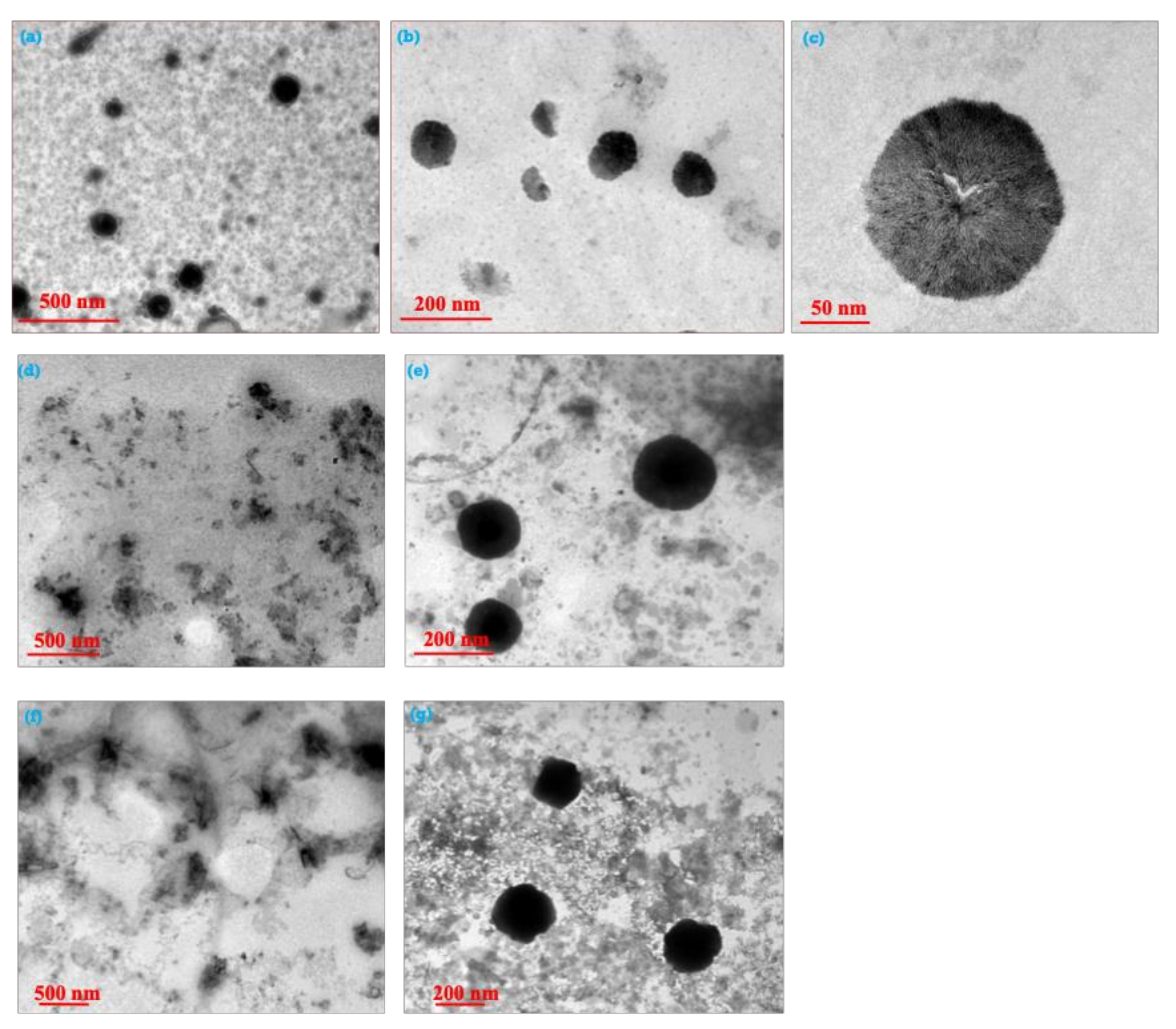
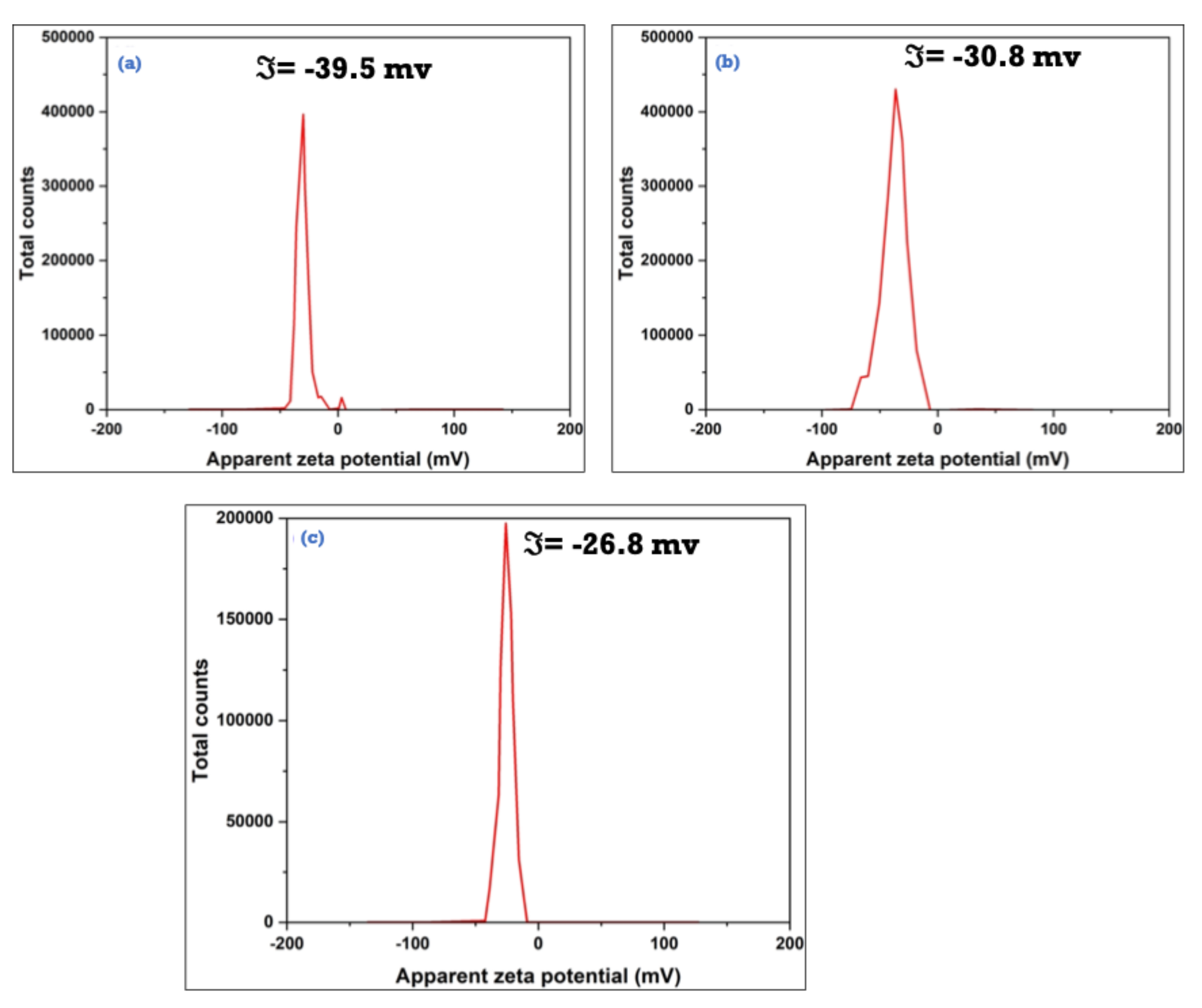
3.3. Characterization of the As-Synthesized NPA (7b)-Loaded SiO2 Nanoemulsion
3.4. Cytotoxicity of the Prepared Nanoemulsions Loaded with NPA (7b) As Drug Model
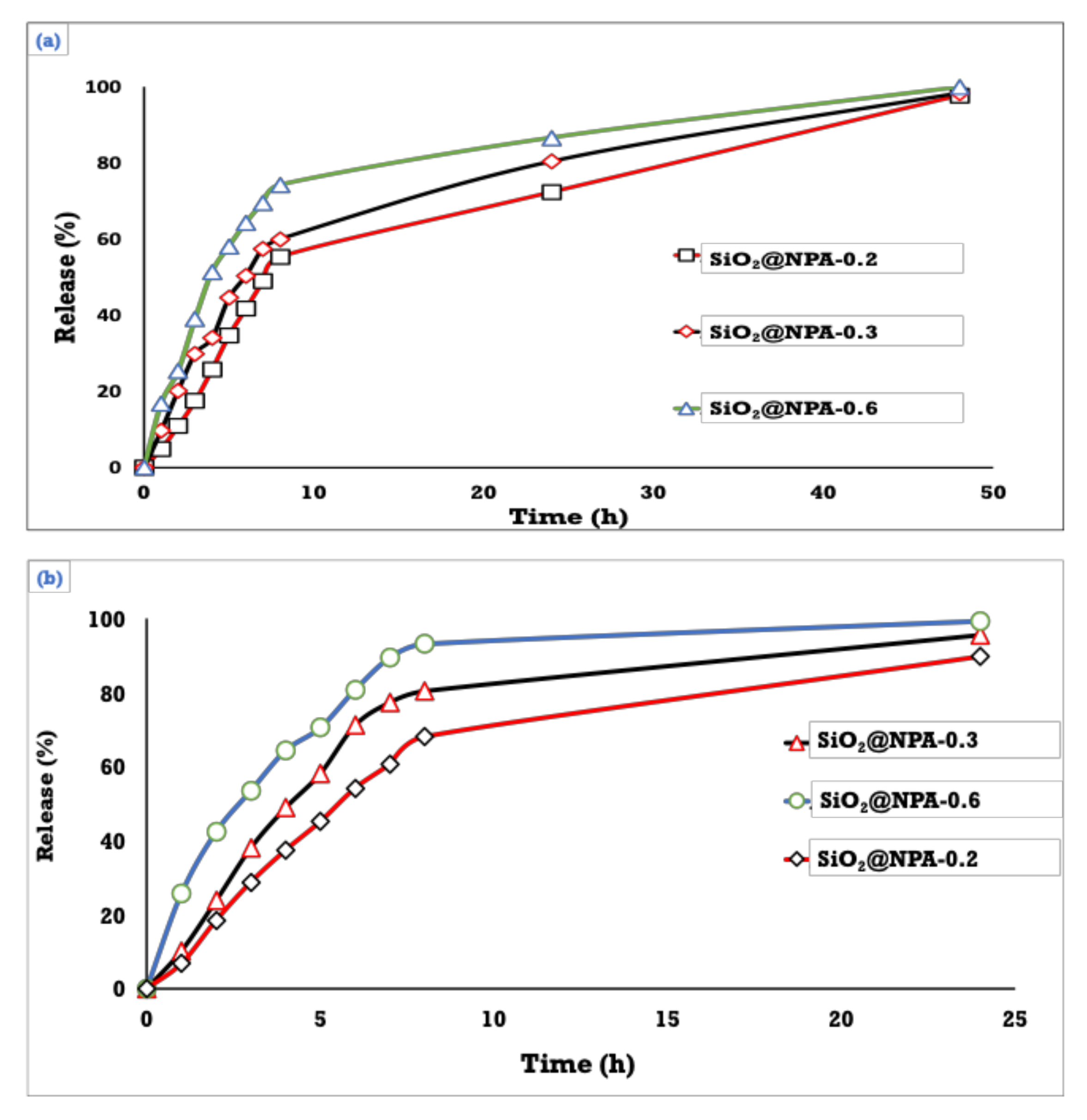
3.5. Drug Content and In Vitro Release of NPA at Two Different pH’s
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Altwaijry, N.; El-Ghlban, S.; El Sayed, I.E.-T.; El-Bahnsawye, M.; Bayomi, A.I.; Samaka, R.M.; Shaban, E.; Elmongy, E.I.; El-Masry, T.A.; Ahmed, H. In Vitro and In Vivo Antitumor Activity of Indolo [2, 3-b] Quinolines, Natural Product Analogs from Neocryptolepine Alkaloid. Molecules. 2021, 26, 754. [Google Scholar] [CrossRef] [PubMed]
- Nuthakki, V.K.; Mudududdla, R.; Bharate, S.B. Role of basic aminoalkyl chains in the lead optimization of Indoloquinoline alkaloids. Eur. J. Med. Chem. 2021, 227, 113938. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Świtalska, M.; Wang, L.; Shaban, E.; Hossain, M.I.; El Sayed, I.E.T.; Wietrzyk, J.; Inokuchi, T. Structural modifications of nature-inspired indoloquinolines: A mini review of their potential antiproliferative activity. Molecules 2019, 24, 2121. [Google Scholar] [CrossRef] [PubMed]
- Emam, S.M.; El Sayed, I.E.T.; Ayad, M.I.; Hathout, H.M.R. Synthesis, characterization and anticancer activity of new Schiff bases bearing neocryptolepine. J. Mol. Struct. 2017, 1146, 600–619. [Google Scholar] [CrossRef]
- Okada, M.; Mei, Z.-W.; Hossain, M.I.; Wang, L.; Tominaga, T.; Takebayashi, T.; Murakami, M.; Yasuda, M.; Shigehiro, T.; Kasai, T. Synthesis and in vitro cancer cell growth inhibition evaluation of 11-amino-modified 5-Me-indolo [2, 3-b] quinolines and their COMPARE analyses. Med. Chem. Res. 2016, 25, 879–892. [Google Scholar] [CrossRef]
- Lu, W.-J.; Wicht, K.J.; Wang, L.; Imai, K.; Mei, Z.-W.; Kaiser, M.; El Sayed, I.E.T.; Egan, T.J.; Inokuchi, T. Synthesis and antimalarial testing of neocryptolepine analogues: Addition of ester function in SAR study of 2, 11-disubstituted indolo [2, 3-b] quinolines. Eur. J. Med. Chem. 2013, 64, 498–511. [Google Scholar] [CrossRef]
- Shaban, E.; Wicht, K.J.; Wang, N.; Mei, Z.-W.; Hayashi, I.; El Gokha, A.A.A.; Kaiser, M.; El Sayed, I.E.; Egan, T.J.; Inokuchi, T. Synthesis and antimalarial activity of some neocryptolepine analogues carrying a multifunctional linear and branched carbon-side chains. Heterocycles 2015, 89, 1055–1064. [Google Scholar]
- El Bardicy, S.; El Sayed, I.; Yousif, F.; Van der Veken, P.; Haemers, A.; Augustyns, K.; Pieters, L. Schistosomicidal and molluscicidal activities of aminoalkylamino substituted neo-and norneocryptolepine derivatives. Pharm. Biol. 2012, 50, 134–140. [Google Scholar] [CrossRef][Green Version]
- El Sayed, I.; Van der Veken, P.; Steert, K.; Dhooghe, L.; Hostyn, S.; Van Baelen, G.; Lemiere, G.; Maes, B.U.W.; Cos, P.; Maes, L.; et al. Synthesis and antiplasmodial activity of aminoalkylamino-substituted neocryptolepine derivatives. J. Med. Chem. 2009, 52, 2979–2988. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Odawara, T.; Misumi, R.; Mei, Z.; Peng, W.; El-Sayed, I.E.; Inokuchi, T. Improved Synthesis and Reaction of 11-Chloroneocryptolepines, Strategic Scaffold for Antimalaria Agent, and Their 6-Methyl Congener from Indole-3-carboxylate. J. Heterocycl. Chem. 2014, 51, 1106–1114. [Google Scholar] [CrossRef]
- El-Gokha, A.A.; Boshta, N.M.; Hussein, M.K.A. Synthesis and structure-activity relationships of novel neocryptolepine derivatives. Chem. Res. Chin. Univ. 2017, 33, 373–377. [Google Scholar] [CrossRef]
- Wang, N.; Wicht, K.J.; Shaban, E.; Ngoc, T.A.; Wang, M.-Q.; Hayashi, I.; Hossain, M.I.; Takemasa, Y.; Kaiser, M.; El Sayed, I.E.T.; et al. Synthesis and evaluation of artesunate–indoloquinoline hybrids as antimalarial drug candidates. Medchemcomm 2014, 5, 927–931. [Google Scholar] [CrossRef]
- Lu, W.-J.; Świtalska, M.; Wang, L.; Yonezawa, M.; El-Sayed, I.E.-T.; Wietrzyk, J.; Inokuchi, T. In vitro antiproliferative activity of 11-aminoalkylamino-substituted 5 H-indolo [2, 3-b] quinolines; improving activity of neocryptolepines by installation of ester substituent. Med. Chem. Res. 2013, 22, 4492–4504. [Google Scholar] [CrossRef]
- Ahmed, A.A.S.; Awad, H.M.; El-Sayed, I.E.-T.; El Gokha, A.A. Synthesis and antiproliferative activity of new hybrids bearing neocryptolepine, acridine and α-aminophosphonate scaffolds. J. Iran. Chem. Soc. 2020, 17, 1211–1221. [Google Scholar] [CrossRef]
- Bonjean, K.; De Pauw-Gillet, M.-C.; Defresne, M.-P.; Colson, P.; Houssier, C.; Dassonneville, L.; Bailly, C.; Greimers, R.; Wright, C.; Quetin-Leclercq, J.; et al. The DNA intercalating alkaloid cryptolepine interferes with topoisomerase II and inhibits primarily DNA synthesis in B16 melanoma cells. Biochemistry 1998, 37, 5136–5146. [Google Scholar] [CrossRef]
- Riechert-Krause, F.; Weisz, K. Indoloquinolines as DNA binding ligands. Heterocycl. Commun. 2013, 19, 145–166. [Google Scholar] [CrossRef]
- Mendonça Munhoz, A.; Santanelli di Pompeo, F.; De Mezerville, R. Nanotechnology, nanosurfaces and silicone gel breast implants: Current aspects. Case Rep. Plast. Surg. Hand Surg. 2017, 4, 99–113. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Engvall, K.; Hakkarainen, M.; Kotarba, A. Recent progress on parylene C polymer for biomedical applications: A review. Prog. Org. Coat. 2020, 140, 105493. [Google Scholar] [CrossRef]
- Bhowmik, D.; Gopinath, H.; Kumar, B.P.; Duraivel, S.; Kumar, K.P.S. Controlled release drug delivery systems. Pharma Innov. 2012, 1, 24–32. [Google Scholar]
- Jia, L. Nanoparticle formulation increases oral bioavailability of poorly soluble drugs: Approaches, experimental evidences and theory. Curr. Nanosci. 2005, 1, 237–243. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2020, 81, 101081. [Google Scholar] [CrossRef]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions—Advances in Formulation, Characterization and Applications in Drug Delivery; Intech Open: London, UK, 2014; Volume 3. [Google Scholar]
- Kumar, S.L.H.; Singh, V. Nanoemulsification—A novel targeted drug delivery tool. J. Drug Deliv. Ther. 2012, 2, 40–45. [Google Scholar] [CrossRef]
- Mahdi, Z.H.; Maraie, N.K. Overview on Nanoemulsion as a recently developed approach in Drug Nanoformulation. Res. J. Pharm. Technol. 2019, 12, 5554–5560. [Google Scholar] [CrossRef]
- Taskar, P.; Tatke, A.; Majumdar, S. Advances in the use of prodrugs for drug delivery to the eye. Expert Opin. Drug Deliv. 2017, 14, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Gamal, W.; Fahmy, R.H.; Mohamed, M.I. Development of novel amisulpride-loaded liquid self-nanoemulsifying drug delivery systems via dual tackling of its solubility and intestinal permeability. Drug Dev. Ind. Pharm. 2017, 43, 1530–1538. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; Uttam, K.M.; Sengupta, P.; Rakesh, K.T. Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Benita, S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur. J. Pharm. Biopharm. 2004, 58, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Walia, M.K.; Kumar, S.L. Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: A review. Pharmacophore 2013, 4, 15–25. [Google Scholar]
- Majeed, A.; Bashir, R.; Farooq, S.; Maqbool, M. Preparation, characterization and applications of nanoemulsions: An insight. J. Drug Deliv. Ther. 2019, 9, 520–527. [Google Scholar] [CrossRef]
- Laxmi, M.; Bhardwaj, A.; Mehta, S.; Mehta, A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif. Cells Nanomed. Biotechnol. 2015, 43, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1305–1317. [Google Scholar] [CrossRef]
- Sokolsky-Papkov, M.; Kabanov, A. Synthesis of well-defined gold nanoparticles using pluronic: The role of radicals and surfactants in nanoparticles formation. Polymers 2019, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Budiputra, D.K.; Mauludin, R. Curcumin nanoemulsion for transdermal application: Formulation and evaluation. Drug Dev. Ind. Pharm. 2015, 41, 560–566. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, L.; Xing, Y.; Zhao, Y.; Wang, Z.; Han, J. Enhanced oral bioavailability of celecoxib nanocrystalline solid dispersion based on wet media milling technique: Formulation, optimization and in vitro/in vivo evaluation. Pharmaceutics 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sayed, I.E.-T.; Ullah, S.; Al-Hartomy, O.A.; Hasanein, A.M.; Ahmed, A.A.S.; Kahilo, K.A.; El-Naggar, M.E. Synthesis, Nanoformulations, and In Vitro Anticancer Activity of N-Substituted Side Chain Neocryptolepine Scaffolds. Molecules 2022, 27, 1024. https://doi.org/10.3390/molecules27031024
El Sayed IE-T, Ullah S, Al-Hartomy OA, Hasanein AM, Ahmed AAS, Kahilo KA, El-Naggar ME. Synthesis, Nanoformulations, and In Vitro Anticancer Activity of N-Substituted Side Chain Neocryptolepine Scaffolds. Molecules. 2022; 27(3):1024. https://doi.org/10.3390/molecules27031024
Chicago/Turabian StyleEl Sayed, Ibrahim El-Tantawy, Sami Ullah, Omar A. Al-Hartomy, Asmaa Mohammed Hasanein, Abdullah A. S. Ahmed, Khaled A. Kahilo, and Mehrez E. El-Naggar. 2022. "Synthesis, Nanoformulations, and In Vitro Anticancer Activity of N-Substituted Side Chain Neocryptolepine Scaffolds" Molecules 27, no. 3: 1024. https://doi.org/10.3390/molecules27031024
APA StyleEl Sayed, I. E.-T., Ullah, S., Al-Hartomy, O. A., Hasanein, A. M., Ahmed, A. A. S., Kahilo, K. A., & El-Naggar, M. E. (2022). Synthesis, Nanoformulations, and In Vitro Anticancer Activity of N-Substituted Side Chain Neocryptolepine Scaffolds. Molecules, 27(3), 1024. https://doi.org/10.3390/molecules27031024






