Effect of Calcium Compound Type and Dosage on the Properties of Acid Rennet Goat’s Milk Gels
Abstract
1. Introduction
2. Results and Discussion
2.1. Quality of Goat’s Milk
2.2. Effect of Calcium Dose and Calcium Compound on the pH Value of Goat’s Milk after Pasteurization
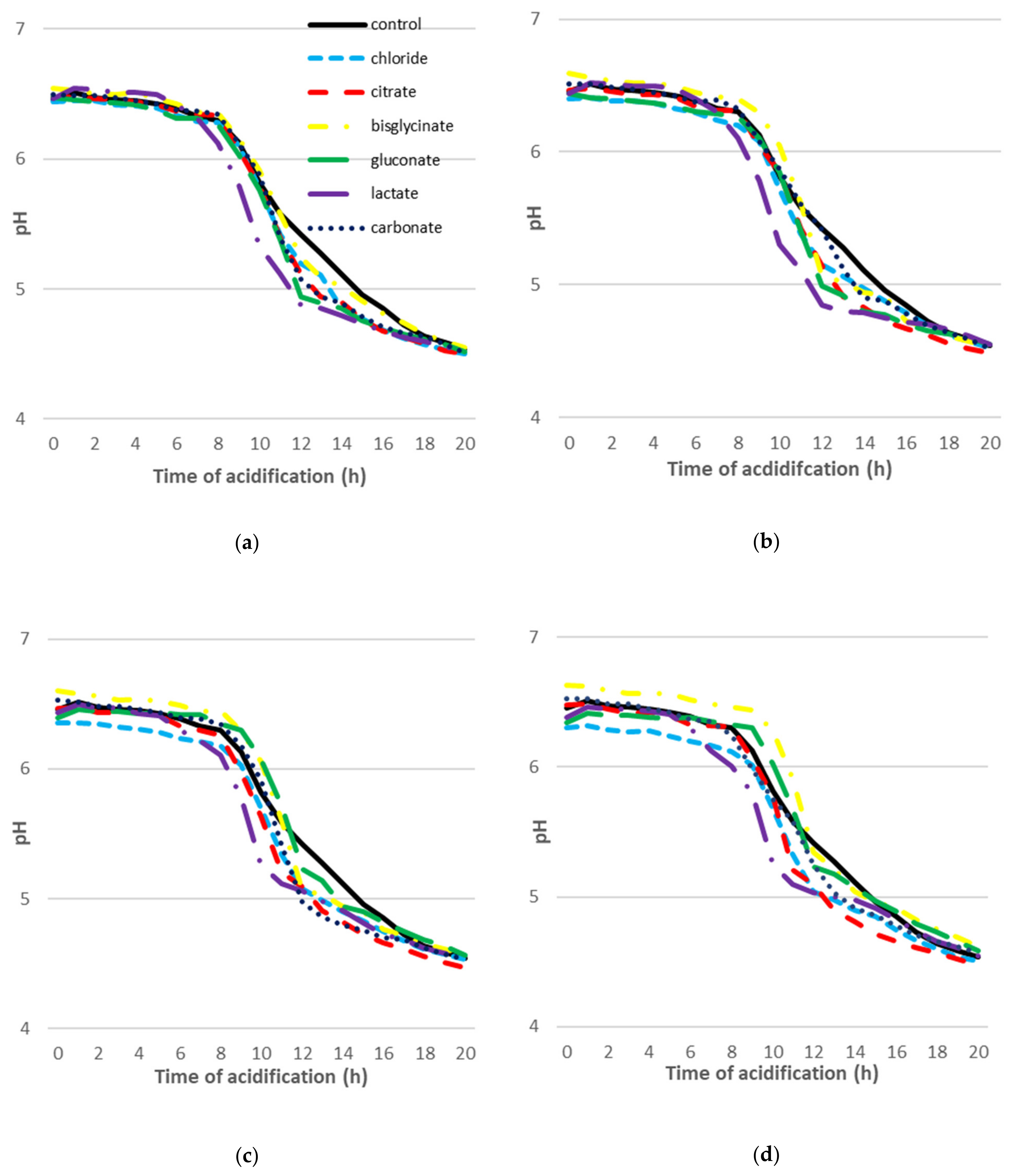
2.3. The Dynamics of Acidification of Goat’s Milk with the Addition of Calcium Compounds
2.4. The Effect of Calcium Addition on the Properties of Acid Rennet Goat’s Milk Gels
2.4.1. The pH Value of Goat’s Milk Gels
2.4.2. Syneresis of Goat’s Milk Gels
2.4.3. Color of Goat’s Milk Gels
2.4.4. Texture of Goat’s Milk Gels
2.4.5. The Organoleptic Evaluation of Goat’s Milk Gels
3. Materials and Methods
3.1. Materials
3.2. Raw Goat’s Milk Analysis
3.3. Acid Rennet Gels Production
3.4. The Dynamics of Milk Acidification
3.5. pH of Acid Rennet Gel
3.6. Syneresis (%) of Acid Rennet Gels
3.7. Color of Acid Rennet Gels
3.8. Texture Analysis of Acid Rennet Gels
3.9. Qualitative Organoleptic Evaluation of Acid Rennet Gels
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kanahara, M.; Kai, H.; Okamura, T.; Wada, T.; Suda, K.; Imaizumi, T.; Sagawa, K. Usefulness of high-concentration calcium chloride solution for correction of activated partial thromboplastin time (APTT) in patients with high-hematocrit value. Thromb. Res. 2008, 121, 781–785. [Google Scholar] [CrossRef]
- Szeleszczuk, Ł.; Kuras, M. Znaczenie wapnia w metabolizmie człowieka i czynniki wpływające na jego biodostępność w diecie. The role of calcium in human metabolism and factors affecting its bioavailability. Biul. Wydziału Farm. WUM 2014, 3, 16–22. (In Polish) [Google Scholar]
- Aguayo, E.; Requejo-Jackman, C.; Stanley, R.; Woolf, A. Hot water treatment in combination with calcium ascorbate dips increases bioactive compounds and helps to maintain fresh-cut apple quality. Postharvest Biol. Technol. 2015, 110, 158–165. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Sun, X.; Chen, F.; Lai, S.; Yang, H. Calcium permeation property and firmness change of cherry tomatoes under ultrasound combined with calcium lactate treatment. Ultrason. Sonochemistry 2020, 60, 104784. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, H. Effects of calcium ion on gel properties and gelation of tilapia (Oreochromisniloticus) protein isolates processed with pH shift method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Siemianowski, K.; Szpendowski, J. Możliwość zwiększania zawartości wapnia w serach twarogowych w świetle dotychczasowych badań. Possibilities of tvarog cheeses enrichment with calcium in the light of hitherto existing research. Nauk. Inżynierskie I Technol. 2012, 4, 83–98. (In Polish) [Google Scholar]
- Ziarno, M.; Zaręba, D.; Piskorz, J. Wzbogacanie maślanki w wapń, magnez oraz białka serwatkowe. Fortifying buttermilk with calcium, magnesium, and whey proteins. Żywność Nauka Technol. Jakość 2009, 2, 14–27. (In Polish) [Google Scholar]
- Augustin, M.A.; Clarke, P.T. Effects of added salts on the heat stability of recombined concentrated milk. J. Dairy Res. 1990, 57, 213–226. [Google Scholar] [CrossRef]
- Szpendowski, J.; Kłobukowski, J.; Prokop, E. Wpływ dodatku chlorku wapnia i ogrzewania mleka na skład chemiczny serów twarogowych. The effect of calcium chloride added to milk and milk heating on the chemical composition of cottage cheeses. Żywność Nauka Technol. Jakość 2005, 3, 36–45. (In Polish) [Google Scholar]
- Danków, R.; Pikul, J. Przydatność technologiczna mleka koziego do przetwórstwa. Technological suitability of goat milk for processing. Nauka Przyr. Technol. 2011, 5, 7. (In Polish) [Google Scholar]
- Tarapata, J.; Smoczyński, M.; Maciejczyk, M.; Żulewska, J. Effect of calcium chloride addition on properties of acid-rennet gels. Int. Dairy J. 2020, 106, 104707. [Google Scholar] [CrossRef]
- Sørensen, I.; Le, T.T.; Larsen, L.B.; Wiking, L. Rennet coagulation and calcium distribution of raw milk reverse osmosis retentate. Int. Dairy J. 2019, 95, 71–77. [Google Scholar] [CrossRef]
- Lucey, J.A. Acid coagulation of milk. In Advanced Dairy Chemistry; McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer: New York, NY, USA, 2016; pp. 309–328. [Google Scholar]
- Jaworski, J.; Kuncewicz, A. Właściwości fizykochemiczne mleka. Physicochemical properties of milk. In Mleczarstwo; Ziajka, S., Ed.; UWM: Olsztyn, Poland, 2007; pp. 53–99. (In Polish) [Google Scholar]
- Gastaldi, E.; Pellegrini, A.; Lagaude, B.; Tarodo de la Fuente, B. Functions of Added Calcium in Acid Milk Coagulation. J. Food Sci. 1994, 59, 310–312. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1170/2009 of 30 November 2009 amending Directive 2002/46/EC of the European Parliament and of Council and Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards the lists of vitamin and minerals and their forms that can be added to foods, including food supplements (Text with EEA relevance). Off. J. Eur. Union 2009, L314, 36–42.
- Kowalska, M.; Ambroziak, A.; Aljewicz, M.; Cichosz, G. Wzbogacone w wapń i magnez produkty mleczarskie. Fortification of dairy products by calcium and magnesium. Postępy Tech. Przetwórstwa Spożywczego 2012, 1, 93–98. (In Polish) [Google Scholar]
- Chudy, S.; Bilska, A.; Kowalski, R.; Teichert, J. Colour of milk and milk products in CIE L*a*b* space. Med. Weter. 2020, 76, 77–81. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Lagriffoul, G.; Paccard, P.; Guillet, I.; Chilliard, Y. Composition of goat and sheep milk products: An update. Small Rumin. Res. 2008, 79, 57–72. [Google Scholar] [CrossRef]
- Palacios, C.; Cormick, G.; Hofmeyr, G.J.; Garcia-Casal, M.N.; Peña-Rosas, J.P.; Betrán, A.P. Calcium-fortified foods in public health programs: Considerations for implementation. Ann. N. Y. Acad. Sci. 2020, 1485, 3–21. [Google Scholar] [CrossRef]
- Barłowska, J.; Wolanciuk, A.; Kędzierska-Matysek, M.; Litwińczuk, Z. Wpływ sezonu produkcji na podstawowy skład chemiczny oraz zawartość makro- i mikroelementów w mleku krowim i kozim. Effect of production season on basic chemical composition and content of macro- and microelements in cow’s and goat’s milk. Żywność Nauka Technol. Jakość 2013, 6, 69–78. (In Polish) [Google Scholar]
- Kędzierska-Matysek, M.; Barłowska, J.; Litwińczuk, Z.; Koperska, N. Content of macro- and microelements in goat milk in relation to the lactation stage and region of production. J. Elem. 2015, 20, 107–114. [Google Scholar] [CrossRef]
- Currò, S.; De Marchi, M.; Claps, S.; Salzano, A.; De Palo, P.; Manuelian, C.L.; Neglia, G. Differences in the Detailed Milk Mineral Composition of Italian Local and Saanen Goat Breeds. Animals 2019, 9, 412. [Google Scholar] [CrossRef]
- Pandya, A.J.; Ghodke, K.M. Goat and sheep products rather than cheeses and yoghurts. Small Rumin. Res. 2007, 68, 193–206. [Google Scholar] [CrossRef]
- Barszczewski, J. Stan trwałych użytków zielonych i ich wykorzystanie w kraju. Status of permanent grasslands and their use domestically. Woda-Środowisko-Obsz. Wiej. Rozpr. Nauk. I Monogr. 2015, 40, 16–35. (In Polish) [Google Scholar]
- Barłowska, J.; Litwińczuk, Z.; Domaradzki, P.; Pastuszka, R.; Wójcik-Saganek, A. Wpływ sezonu na skład chemiczny i profil kwasów tłuszczowych mleka krowiego i koziego produkowanego w gospodarstwach ekologicznych. Impact of season on chemical composition and fatty acid profile of cow’s and goat’s milk produced in organic farms. Żywność Nauka Technol. Jakość 2016, 1, 45–56. (In Polish) [Google Scholar] [CrossRef]
- Barłowska, M.; Litwińczuk, Z.; Wolanciuk, A.; Pastuszka, R. Skład chemiczny, jakość cytologiczna i przydatność technologiczna mleka krów trzech ras o umaszczeniu czerwono-białym żywionych systemem TMR. The chemical composition, cytological quality and technological suitability of the milk of three breeds of red and white cows fed in a TMR system. Rocz. Nauk. Pol. Tow. Zootech. 2014, 10, 115–124. (In Polish) [Google Scholar]
- Król, J.; Brodziak, A.; Kędzierska-Matysek, M.; Brodziak, A.; Zaborska, A.; Litwińczuk, A. The effect of selected factors on yield and protein and mineral retention in traditionally produced tvarog. J. Elem. 2018, 23, 959–969. [Google Scholar] [CrossRef]
- Mulawka, E.; Dmytrów, I.; Mituniewicz-Małek, A.; Godula, K. Rodzaj kultury starterowej a wybrane cechy fizykochemiczne sera twarogowego w czasie przechowywania. Type of starter culture and selected physicochemical characteristics of curd cheese (tvarog) during storage. Żywność Nauka Technol. Jakość 2019, 26, 95–110. (In Polish) [Google Scholar] [CrossRef]
- Żylińska, J.; Siemianowski, K.; Bohdziewicz, K.; Pawlikowska, K.; Kołakowski, P.; Szpendowski, J.; Bardowski, J. Kultury starterowe do produkcji twarogów kwasowych—Rola i oczekiwania. Postępy Mikrobiol. 2014, 53, 288–298. [Google Scholar]
- Pastuszka, R.; Barłowska, J.; Litwińczuk, Z. Walory odżywcze i prozdrowotne mleka koziego. Nutritional value and health-promoting properties of goat milk. Med. Weter. 2015, 71, 480–485. (In Polish) [Google Scholar]
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Strzałkowska, N.; Jóźwik, A.; Bagnicka, E.; Krzyżewski, J.; Horbańczuk, K.; Pyzel, B.; Horbańczuk, J.O. Chemical composition, physical traits and fatty acid profile of goat milk as related to the stage of lactation. Anim. Sci. Pap. Rep. 2009, 27, 311–320. [Google Scholar]
- Park, Y.W.; Haenlein, G.F.W. Goat milk, its products and nutrition. In Handbook of Food Products Manufacturing; Hui, Y.H., Ed.; John Wiley & Sons: New York, NY, USA, 2007; pp. 447–486. [Google Scholar]
- Mayer, H.; Fiechter, G. Physical and chemical characteristics of sheep and goat milk in Austria. Int. Dairy J. 2012, 24, 57–63. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1662/2006 of 6 November 2006 amending Regulation (EC) No 853/2004 of the European Parliament and of the Council laying down specific hygiene rules for food of animal origin (Text with EEA relevance). Off. J. Eur. Union 2006, L320, 1–10.
- Znamirowska, A.; Szajnar, K.; Pawlos, M. Organic magnesium salts fortification in fermented goat’s milk. Int. J. Food Prop. 2019, 22, 1615–1625. [Google Scholar] [CrossRef]
- Znamirowska, A.; Kalicka, D.; Pawlos, M.; Szajnar, K. Quality of yoghurts from goat’s milk enriched with magnesium chloride. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 369–372. [Google Scholar] [CrossRef][Green Version]
- Sikora, J.; Kawęcka, A. Jakość produktu tradycyjnego z mleka koziego—Sera podkarpackiego białego. Quality of the white podkarpacki cheese, a traditional goat milk product. Wiadomości Zootech. 2015, 53, 10–15. (In Polish) [Google Scholar]
- Litwińczuk, A.; Kędzierska-Matysek, M.; Barłowska, J. Wydajność i jakość mleka kóz o różnych genotypach alfa-s1-kazeiny z rejonu Wielkopolski i Podkarpacia. Productivity and quality of milk from goats of different alpha-s1-casein genotypes from Wielkopolska and Podkarpacie regions. Med. Weter. 2007, 63, 192–195. (In Polish) [Google Scholar]
- Danków, R.; Cais-Sokolińska, D.; Pikul, J.; Wójtowski, J. Jakość cytologiczna mleka koziego. Cytological quality of goat’s milk. Med. Weter. 2003, 59, 77–80. (In Polish) [Google Scholar]
- Brodziak, A.; Król, J.; Barłowska, J.; Litwińczuk, Z. Effect of production season on protein fraction content in milk of various breeds of goats in Poland. Int. J. Dairy Technol. 2014, 67, 410–419. [Google Scholar] [CrossRef]
- Ziarno, M.; Semeniuk, E.; Kycia, K. Wpływ dodatku soli wapnia na stabilność mleka przeznaczonego do produkcji sera typu cottage cheese. The impact of the calcium salts addition on the stability of milk used in the cottage cheese production. Żywność Nauka Technol. Jakość 2004, 2, 81–91. (In Polish) [Google Scholar]
- Znamirowska, A.; Buniowska, M.; Kuźniar, P. Wzbogacanie mleczanem magnezu i wapnia mlecznych napojów fermentowanych przez Bifidobacterium animalis ssp. Lactis Bb-12. Fortification of fermented milk beverages by Bifidobacterium animalis ssp. lactis bb-12 with magnesium and calcium lactate. Zesz. Probl. Postępów Nauk Rol. 2018, 592, 107–117. (In Polish) [Google Scholar] [CrossRef]
- Robles-Rodríguez, C.E.; Szymańska, E.; Huppertz, T.; Özkan, L. Dynamic modeling of milk acidification: An empirical approach. Food Bioprod. Process. 2021, 128, 41–51. [Google Scholar] [CrossRef]
- Li, X.Y.; Cheng, M.; Li, J.; Zhao, X.; Qin, Y.S.; Chen, D.; Wang, J.M.; Wang, C.F. Change in the structural and functional properties of goat milk protein due to pH and heat. J. Dairy Sci. 2020, 103, 1337–1351. [Google Scholar] [CrossRef]
- Lucey, J.A. Formation, structural properties, and rheology of acid-coagulated milk gels. In Cheese: Chemistry, Physics and Microbiology, Volume 1, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 179–197. [Google Scholar] [CrossRef]
- Azizi, R.; Farahnaky, A. Ultrasound assisted-viscosifying of kappa carrageenan without heating. Food Hydrocoll. 2016, 61, 85–91. [Google Scholar] [CrossRef]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocoll. 2016, 54 (Part B), 284–292. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Yang, H. Effects of sucrose addition on the rheology and microstructure of k-carrageenan gel. Food Hydrocoll. 2018, 75, 164–173. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Van der Linden, E.; Foegeding, E.A. Gelation: Principles, Models and Applications to Proteins, Chapter 2. In Modern Biopolymer Science; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 29–91. [Google Scholar] [CrossRef]
- Lucey, J.A. Cultured dairy products: An overview of their gelation and texture properties. Int. J. Dairy Technol. 2004, 57, 77–84. [Google Scholar] [CrossRef]
- Delgado, K.F.; Da Silva Frasao, B.; Costa, M.P. Different Alternatives to Improve Rheological and Textural Characteristics of Fermented Goat Products—A Review. Rheol. Open Access 2017, 1, 1–6. [Google Scholar]
- Ziarno, M.; Więcławski, S. Wpływ dodatku mleczanu wapnia na rozwój bakterii fermentacji mlekowej w bulionie MRS i w mleku. The Influence of the Calcium Lactate Addition on the Growth of the Lactic Acid Bacteria in the MRS Broth and Milk. Żywność Nauka Technol. Jakość 2006, 4, 110–119. (In Polish) [Google Scholar]
- Jacob, M.; Jaros, D.; Rohm, H. Recent advances in milk clotting enzymes. Int. J. Dairy Technol. 2011, 64, 14–33. [Google Scholar] [CrossRef]
- Stelios, K.; Emmanuel, A. Characteristics of set type yoghurt made from caprine or ovine milk and mixtures of the two. Int. J. Food Sci. Technol. 2004, 39, 319–324. [Google Scholar] [CrossRef]
- Moschopoulou, E.; Sakkas, L.; Zoidou, E.; Theodorou, G.; Sgouridou, E.; Kalathaki, C.; Moatsou, G.; Chatzigeorgiou, A.; Politis, I.; Moatsou, G. Effect of Milk Kind and Storage on the Biochemical, Textural and Biofunctional Characteristics of Set-Type Yoghurt. Int. Dairy J. 2018, 77, 47–55. [Google Scholar] [CrossRef]
- Dmytrów, I. Wpływ probiotycznych bakterii kwasu mlekowego na stabilność przechowalniczą kwasowych serów twarogowych. Effect of lactic acid probiotic bacteria on storage stability of acid curd cheeses (tvarog). Żywność Nauka Technol. Jakość 2015, 5, 49–60. (In Polish) [Google Scholar] [CrossRef]
- Liu, X.T.; Zhang, H.; Wang, F.; Luo, J.; Guo, H.Y.; Ren, F.Z. Rheological and structural properties of differently acidified and renneted milk gels. J. Dairy Sci. 2014, 97, 3292–3299. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated Anthocyanins from Edible Sources and Their Applications in Food Systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Augustin, M.A. Mineral Salts and Their Effect on Milk Functionality. Aust. J. Dairy Technol. 2000, 55, 61–64. [Google Scholar]
- Rożnowski, J. Ocena barwy produktów spożywczych. Evaluation of food color. Laboratorium 2006, 5, 36–44. (In Polish) [Google Scholar]
- Domagała, J. Instrumental Texture, Syneresis and Microstructure of Yoghurts Prepared from Goat, Cow and Sheep Milk. Int. J. Food Prop. 2009, 12, 605–615. [Google Scholar] [CrossRef]
- Domagała, J.; Wszołek, M. Wpływ sposobu zagęszczania oraz rodzaju szczepionki na teksturę i podatność na synerezę jogurtu i biojogurtów z mleka koziego. Effect of concentration method and starter culture type on the texture and susceptibility to syneresis of yoghurt and bio-yoghurts made of goat’s milk. Zywnosc Nauka Technol. Jakosc 2008, 15, 118–126. (In Polish) [Google Scholar]
- Costa, M.P.; Balthazar, C.F.; Rodrigues, B.L.; Lazaro, C.A.; Silva, A.C.; Cruz, A.G.; Conte Junior, C.A. Determination of biogenic amines by high-performance liquidchromatography (HPLC-DAD) in probiotic cow’s and goat’s fermented milks and acceptance. Food Sci. Nutr. 2015, 3, 172–178. [Google Scholar] [CrossRef]
- Widodo, W.; Taufiq, T.T.; Anindita, N.S. Fermented goat milk and cow milk produced by different starters of lactic acid bacteria: Quality studies. J. Agric. Sci. Technol. A 2013, 3, 904–911. [Google Scholar]
- Donato, L.; Guyomarc’h, F. Formation and Properties of the Whey Protein/Kappa-Casein Complexes in Heated Skim Milk—A Review. Dairy Sci. Technol. 2013, 89, 3–29. [Google Scholar] [CrossRef]
- Siemianowski, K.; Bohdziewicz, K.; Szpendowski, J.; Kołakowski, P.; Żylińska, J.; Bardowski, J. Wpływ zwiększenia zawartości suchej masy w surowcu na teksturę i mikrostrukturę twarogu kwasowego. The effect of increased dry matter content of raw material on the texture and microstructure of acid tvorog. Acta Agrophysica 2015, 2, 183–192. (In Polish) [Google Scholar]
- Foegeding, E.A.; Drake, M.A. Invited review: Sensory and mechanical properties of cheese texture. J. Dairy Sci. 2007, 90, 1611–1624. [Google Scholar] [CrossRef]
- Sołowiej, B.; Gustaw, W. Wpływ chlorku wapnia na właściwości fizykochemiczne analogów serów topionych na bazie białek mleka i tłuszczu mlecznego. Effect of calcium chloride on physicochemical properties of processed cheese analogues based on milk proteins and milk fat. Żywność Nauka Technol. Jakość 2013, 1, 137–150. (In Polish) [Google Scholar]
- Pawlos, M.; Znamirowska, A.; Szajnar, K.; Kalicka, D. The influence of the dose of calcium bisglycinate on physicochemical properties, sensory analysis and texture profile of kefirs during 21 days of cold storage. Acta Sci. Pol. Technol. Aliment. 2016, 15, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ziarno, M.; Zaręba, D. The effect of the addition of microbial transglutaminase before the fermentation process on the quality characteristics of three types of yogurt. Food Sci. Biotechnol. 2019, 29, 109–119. [Google Scholar] [CrossRef]
- Singh, H.; Waungana, A. Influence of heat treatment of milk on cheesemaking properties. Int. Dairy J. 2001, 11, 543–551. [Google Scholar] [CrossRef]
- Vasbinder, A.J. Casein-Whey Protein Interactions in Heated Milk. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 22 November 2002. Available online: https://dspace.library.uu.nl/bitstream/handle/1874/765/full.pdf;jsessionid=1B5A8D719D80C744291B4B439C2C2467?sequence=1 (accessed on 26 July 2021).
- Sheehan, J.J.; Patel, A.D.; Drake, M.A.; McSweeney, P.L.H. Effect of partial or total substitution of bovine for caprine milk on the compositional, volatile, non-volatile and sensory characteristics of semi-hard cheeses. Int. Dairy J. 2009, 19, 498–509. [Google Scholar] [CrossRef]
- Szwocer, J.; Wituszyńska, B.; Obrusiewicz, T.; Najdeker, M.; Januszewska, H. Próby zastosowania ultrafiltracji w produkcji serków twarogowych z mleka koziego. Attempts to use ultrafiltration in the production of acid-curd cheese from goat’s milk. Postępy Tech. Przetwórstwa Spożywczego 2001, 1, 10–16. (In Polish) [Google Scholar]
- Ratu, R.N.; Usturoi, M.G.; Avarvarei, B.V. Quality of Raw Cow Milk Utilised in Cheese Processing. Sci. Pap. Anim. Sci. Ser. Lucr. Ştiinţifice Ser. Zooteh. 2015, 63, 128–130. [Google Scholar]
- Szajnar, K.; Pawlos, M.; Znamirowska, A. The Effect of the Addition of Chokeberry Fiber on the Quality of Sheep’s Milk Fermented by Lactobacillus rhamnosus and Lact. Acidophilus. Int. J. Food Sci. 2021, 2021, 7928745. [Google Scholar] [CrossRef] [PubMed]
- Pawlos, M.; Znamirowska, A.; Kluz, M.; Szajnar, K.; Kowalczyk, M. Low-lactose fermented goat milks with Bifidobacterium animalis ssp. lactis Bb-12. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 751–755. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Liu, Q.; Pang, X.; Zhao, X.; Yang, H. Sanitising efficacy of lactic acid combined with low-concentration sodium hypochlorite on Listeria innocua in organic broccoli sprouts. Int. J. Food Microbiol. 2019, 295, 41–48. [Google Scholar] [CrossRef] [PubMed]
| Properties | Mean ± SD 1 |
|---|---|
| Protein, g 100 g−1 | 2.71 ± 0.31 |
| Fat, g 100 g−1 | 2.79 ± 0.49 |
| Lactose, g 100 g−1 | 4.70 ± 0.24 |
| Total solids, g 100 g−1 | 10.40 ± 0.73 |
| Density, g mL−1 | 1.028 ± 0.01 |
| Freezing point, °C | −0.573 ± 0.020 |
| pH | 6.68 ± 0.08 |
| TBC 2, log CFU mL−1 | 5.63 ± 0.06 |
| SCC 3, log cells mL−1 | 6.01 ± 0.05 |
| Calcium Compound | Calcium Dose, mg Ca 100 g−1 of Milk | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Chloride | 6.57e ± 0.01 | 6.53d ± 0.01 | 6.49c ± 0.01 | 6.42b ± 0.01 | 6.36a ± 0.01 |
| Citrate | 6.57a ± 0.02 | 6.57a ± 0.01 | 6.57a ± 0.01 | 6.58a ± 0.01 | 6.58a ± 0.01 |
| Bisglycinate | 6.57a ± 0.01 | 6.67b ± 0.01 | 6.68b ± 0.01 | 6.71c ± 0.01 | 6.73c ± 0.01 |
| Gluconate | 6.57c ± 0.01 | 6.57c ± 0.01 | 6.53b ± 0.02 | 6.51b ± 0.01 | 6.46a ± 0.01 |
| Lactate | 6.57c ± 0.01 | 6.57c ± 0.01 | 6.55bc ± 0.01 | 6.53b ± 0.01 | 6.48a ± 0.01 |
| Carbonate | 6.57a ± 0.01 | 6.60b ± 0.01 | 6.62b ± 0.01 | 6.63b ± 0.02 | 6.62b ± 0.01 |
| Calcium Compound | Calcium Dose, mg Ca 100 g−1 of Milk | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Chloride | 4.63b ± 0.01 | 4.60a ± 0.01 | 4.60a ± 0.01 | 4.59a ± 0.01 | 4.59a ± 0.01 |
| Citrate | 4.61a ± 0.02 | 4.63b ± 0.01 | 4.64b ± 0.01 | 4.65c ± 0.01 | 4.65c ± 0.01 |
| Bisglycinate | 4.62a ± 0.01 | 4.63ab ± 0.01 | 4.63ab ± 0.01 | 4.64b ± 0.01 | 4.64b ± 0.01 |
| Gluconate | 4.63b ± 0.01 | 4.63b ± 0.00 | 4.63b ± 0.01 | 4.61ab ± 0.01 | 4.59a ± 0.01 |
| Lactate | 4.63a ± 0.01 | 4.64a ± 0.00 | 4.62a ± 0.01 | 4.62a ± 0.02 | 4.62a ± 0.01 |
| Carbonate | 4.62a ± 0.02 | 4.66b ± 0.00 | 4.67c ± 0.00 | 4.69d ± 0.01 | 4.70d ± 0.00 |
| Properties | Calcium Compound p Values | Calcium Dose p Values | Calcium Compound × Calcium Dose p Values | |
|---|---|---|---|---|
| pH | ↑ 0.0000 | ns 0.6258 | ↑ 0.0000 | |
| Color | L* | ↑ 0.0000 | ↑ 0.0183 | ↑ 0.0000 |
| a* | ↑ 0.0000 | ns 0.1101 | ↑ 0.0000 | |
| b* | ↑ 0.0000 | ns 0.2207 | ↑ 0.0000 | |
| C | ↑ 0.0000 | ↑ 0.0000 | ↑ 0.0000 | |
| h° | ↑ 0.0000 | ns 0.7426 | ↑ 0.0000 | |
| Syneresis | ↑ 0.0000 | ↑ 0.0165 | ↑ 0.0000 | |
| Hardness | ↑ 0.0000 | ns 0.5999 | ns 0.3173 | |
| Cohesiveness | ↑ 0.0164 | ns 0.0927 | ↑ 0.0000 | |
| Springiness | ↑ 0.0000 | ns 0.1509 | ↑ 0.0480 | |
| Adhesiveness | ↑ 0.0000 | ns 0.7660 | ns 0.5509 | |
| Consistency | ns 0.5890 | ↑ 0.0000 | ns 0.9713 | |
| Milky-creamy taste | ns 0.7675 | ↑ 0.0000 | ns 0.5449 | |
| Salty taste | ns 0.9283 | ↑ 0.0000 | ns 0.9362 | |
| Sour taste | ns 0.5309 | ↑ 0.0000 | ns 0.3527 | |
| Goatish taste | ns 0.0523 | ↑ 0.0081 | ns 0.9691 | |
| Off-taste | ns 0.7274 | ↑ 0.0000 | ns 0.2153 | |
| Fermentation odor | ns 0.8597 | ↑ 0.0000 | ns 0.9983 | |
| Goatish odor | ns 0.9995 | ↑ 0.0007 | ns 0.9999 | |
| Off-odor | ns 0.9998 | ↑ 0.0000 | ns 0.3556 | |
| Calcium Compound | Calcium Dose, mg Ca 100 g−1 of Milk | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Chloride | 28.14a ± 2.73 | 30.37a ± 1.27 | 30.04a ± 2.32 | 31.05a ± 0.86 | 34.09b ± 0.70 |
| Citrate | 29.66b ± 1.72 | 26.16ab ± 0.31 | 26.81ab ± 0.51 | 24.92a ± 0.74 | 24.90a ± 0.12 |
| Bisglycinate | 29.63c ± 2.43 | 26.90bc ± 3.55 | 23.59b ± 1.57 | 22.50ab ± 3.56 | 21.78a ± 2.31 |
| Gluconate | 31.63a ± 0.21 | 31.08ab ± 0.95 | 34.16ab ± 3.35 | 37.69b ± 1.04 | 38.03b ± 2.06 |
| Lactate | 28.05a ± 2.70 | 30.76ab ± 1.05 | 30.50ab ± 1.14 | 31.70b ± 2.41 | 34.73b ± 2.11 |
| Carbonate | 32.27b ± 1.87 | 31.04b ± 2.13 | 22.40a ± 2.93 | 21.88a ± 1.45 | 21.99a ± 0.83 |
| Calcium Compound | Color | Calcium Dose, mg Ca 100 g−1 of Milk | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | ||
| Chloride | L* | 92.04a ± 0.27 | 92.07a ± 0.05 | 92.36a ± 0.40 | 92.34a ± 0.30 | 92.33a ± 0.04 |
| a* | −1.51c ± 0.05 | −1.47b ± 0.01 | −1.47b ± 0.04 | −1.48b ± 0.06 | −1.42a ± 0.01 | |
| b* | 7.00c ± 0.06 | 6.89b ± 0.05 | 6.89b ± 0.14 | 6.83a ± 0.09 | 6.87b ± 0.03 | |
| C | 7.17b ± 0.07 | 6.95a ± 0.05 | 6.95a ± 0.14 | 6.99a ± 0.08 | 7.00a ± 0.03 | |
| h° | 102.21b ± 0.39 | 102.44b ± 0.12 | 102.46b ± 0.50 | 102.44b ± 0.62 | 101.44a ± 0.09 | |
| Citrate | L* | 90.44a ± 0.25 | 90.86ab ± 0.53 | 90.82b ± 0.33 | 90.71b ± 0.19 | 90.87b ± 0.37 |
| a* | −1.50a ± 0.05 | −1.51a ± 0.02 | −1.53a ± 0.04 | −1.55a ± 0.03 | −1.55a ± 0.03 | |
| b* | 7.00a ± 0.06 | 7.04a ± 0.05 | 7.05a ± 0.09 | 7.00a ± 0.09 | 6.99a ± 0.12 | |
| C | 7.07a ± 0.07 | 7.31c ± 0.05 | 7.50d ± 0.08 | 7.18b ± 0.08 | 7.00a ± 0.07 | |
| h° | 101.12a ± 0.39 | 101.26a ± 0.22 | 101.71a ± 0.36 | 101.76a ± 0.36 | 101.89a ± 0.41 | |
| Bisglycinate | L* | 90.42ab ± 0.27 | 90.39a ± 0.03 | 90.65b ± 0.06 | 90.72b ± 0.14 | 91.72c ± 0.12 |
| a* | −1.52a ± 0.05 | −1.56a ± 0.01 | −1.55a ± 0.00 | −1.56a ± 0.03 | −1.55a ± 0.04 | |
| b* | 7.01a ± 0.06 | 7.01a ± 0.04 | 7.06a ± 0.04 | 7.00a ± 0.05 | 6.98a ± 0.19 | |
| C | 6.97a ± 0.07 | 7.06b ± 0.04 | 7.02ab ± 0.04 | 7.08b ± 0.06 | 6.99ab ± 0.22 | |
| h° | 101.21a ± 0.39 | 101.39a ± 0.08 | 101.77b ± 0.05 | 101.96bc ± 0.45 | 102.17c ± 0.35 | |
| Gluconate | L* | 92.14d ± 0.27 | 91.62c ± 0.04 | 91.39b ± 0.06 | 91.22b ± 0.08 | 90.92a ± 0.14 |
| a* | −1.50a ± 0.05 | −1.48a ± 0.02 | −1.49a ± 0.01 | −1.51a ± 0.02 | −1.52a ± 0.02 | |
| b* | 6.99a ± 0.06 | 7.09ab ± 0.02 | 7.11b ± 0.04 | 7.22c ± 0.05 | 7.23c ± 0.05 | |
| C | 6.97a ± 0.07 | 7.09ab ± 0.02 | 7.19b ± 0.10 | 7.17b ± 0.05 | 7.17b ± 0.05 | |
| h° | 100.21a ± 0.39 | 100.93a ± 0.12 | 100.92a ± 1.40 | 100.90a ± 0.05 | 100.88a ± 0.12 | |
| Lactate | L* | 92.02b ± 0.27 | 90.03a ± 0.27 | 90.08a ± 0.06 | 90.03a ± 0.03 | 90.03a ± 0.04 |
| a* | −1.51a ± 0.05 | −1.55a ± 0.01 | −1.55a ± 0.00 | −1.54a ± 0.01 | −1.56a ± 0.01 | |
| b* | 7.00a ± 0.06 | 6.98a ± 0.07 | 6.97a ± 0.03 | 7.02a ± 0.05 | 7.01a ± 0.04 | |
| C | 7.17b ± 0.07 | 7.05a ± 0.07 | 7.05a ± 0.03 | 7.13b ± 0.01 | 7.12b ± 0.03 | |
| h° | 102.21a ± 0.39 | 102.74b ± 0.14 | 102.79b ± 0.07 | 102.82b ± 0.15 | 102.83b ± 0.03 | |
| Carbonate | L* | 92.06d ± 0.27 | 91.04c ± 0.21 | 90.84b ± 0.01 | 90.67a ± 0.08 | 90.53a ± 0.26 |
| a* | −1.51b ± 0.05 | −1.48b ± 0.03 | −1.43a ± 0.01 | −1.43a ± 0.01 | −1.44ab ± 0.10 | |
| b* | 7.00a ± 0.06 | 7.01a ± 0.03 | 6.99a ± 0.05 | 6.99a ± 0.08 | 7.00a ± 0.27 | |
| C | 7.17a ± 0.07 | 7.15a ± 0.03 | 7.11a ± 0.01 | 7.11a ± 0.08 | 7.11a ± 0.28 | |
| h° | 102.21b ± 0.39 | 102.27b ± 0.16 | 102.10b ± 0.07 | 101.95a ± 0.07 | 101.82a ± 0.28 | |
| Properties | Calcium Compound | Calcium Dose, mg Ca 100 g−1 of Milk | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | ||
| Hardness, N | Chloride | 2.99a ± 0.05 | 3.15a ± 0.35 | 3.37a ± 0.24 | 2.90a ± 0.28 | 3.22a ± 0.39 |
| Citrate | 2.65a ± 0.04 | 2.79ab ± 0.09 | 3.19b ± 0.17 | 2.83ab ± 0.45 | 2.80ab ± 0.22 | |
| Bisglycinate | 2.35b ± 0.30 | 1.97ab ± 0.45 | 1.67ab ± 0.32 | 1.41a ± 0.64 | 1.53ab ± 0.81 | |
| Gluconate | 2.65a ± 0.04 | 2.88a ± 0.09 | 3.18b ± 0.18 | 3.38b ± 0.33 | 3.61b ± 0.01 | |
| Lactate | 2.99a ± 0.05 | 2.71a ± 0.44 | 3.55a ± 0.58 | 3.37a ± 0.51 | 3.06a ± 0.14 | |
| Carbonate | 2.65a ± 0.04 | 2.72b ± 0.01 | 2.84b ± 0.47 | 2.28a ± 0.05 | 3.02a ± 0.31 | |
| Cohesiveness | Chloride | 0.25a ± 0.01 | 0.37b ± 0.02 | 0.37b ± 0.01 | 0.36b ± 0.03 | 0.40b ± 0.01 |
| Citrate | 0.19a ± 0.17 | 0.37b ± 0.03 | 0.16a ± 0.14 | 0.42c ± 0.05 | 0.16a ± 0.14 | |
| Bisglycinate | 0.43a ± 0.08 | 0.49a ± 0.01 | 0.51a ± 0.09 | 0.45a ± 0.06 | 0.53a ± 0.05 | |
| Gluconate | 0.29a ± 0.10 | 0.26a ± 0.09 | 0.47b ± 0.03 | 0.43b ± 0.03 | 0.34ab ± 0.01 | |
| Lactate | 0.25a ± 0.01 | 0.33a ± 0.00 | 0.76b ± 0.00 | 0.71b ± 0.06 | 0.71b ± 0.03 | |
| Carbonate | 0.26a ± 0.11 | 0.25a ± 0.09 | 0.36a ± 0.05 | 0.26a ± 0.16 | 0.37a ± 0.05 | |
| Springiness, mm | Chloride | 6.47a ± 0.10 | 7.66ab ± 1.06 | 8.14b ± 0.14 | 8.34b ± 0.10 | 8.68b ± 0.15 |
| Citrate | 6.75a ± 0.08 | 6.80a ± 0.55 | 7.17ab ± 0.51 | 7.54b ± 0.47 | 7.74b ± 0.41 | |
| Bisglycinate | 14.69a ± 0.63 | 14.51a ± 0.41 | 14.51a ± 0.47 | 14.87a ± 0.46 | 14.90a ± 0.47 | |
| Gluconate | 14.42a ± 0.58 | 14.43a ± 0.89 | 15.25ab ± 0.79 | 15.74ab ± 1.01 | 16.95b ± 0.40 | |
| Lactate | 6.47a ± 0.10 | 8.17b ± 0.29 | 8.65bc ± 1.43 | 9.67c ± 0.38 | 14.89d ± 0.08 | |
| Carbonate | 8.75a ± 0.09 | 8.93a ± 0.05 | 9.26a ± 0.56 | 8.72a ± 0.89 | 8.76a ± 0.28 | |
| Adhesiveness, mJ | Chloride | 0.15a ± 0.09 | 0.12a ± 0.03 | 0.10a ± 0.01 | 0.10a ± 0.01 | 0.11a ± 0.01 |
| Citrate | 0.17b ± 0.05 | 0.08a ± 0.03 | 0.17b ± 0.09 | 0.12ab ± 0.04 | 0.08a ± 0.03 | |
| Bisglycinate | 0.10a ± 0.08 | 0.14a ± 0.13 | 0.10a ± 0.10 | 0.14a ± 0.08 | 0.07a ± 0.08 | |
| Gluconate | 0.02a ± 0.03 | 0.02a ± 0.03 | 0.02a ± 0.00 | 0.05a ± 0.05 | 0.03a ± 0.00 | |
| Lactate | 0.15b ± 0.09 | 0.07ab ± 0.06 | 0.00a ± 0.00 | 0.00a ± 0.00 | 0.10b ± 0.00 | |
| Carbonate | 0.23a ± 0.06 | 0.23a ± 0.06 | 0.24a ± 0.07 | 0.21a ± 0.01 | 0.21a ± 0.02 | |
| Calcium Compound | Properties | Calcium Dose, mg Ca 100 g−1 of Milk | ||
|---|---|---|---|---|
| 0 | 10 | 20 | ||
| Chloride | Consistency | 2.83a ± 0.40 | 2.83a ± 0.94 | 3.67a ± 0.81 |
| Milky-creamy taste | 3.00a ± 1.54 | 3.67a ± 0.82 | 2.83a ± 1.33 | |
| Salty taste | 1.83a ± 0.34 | 2.00a ± 0.55 | 2.50a ± 0.52 | |
| Sour taste | 5.33b ± 0.31 | 3.83a ± 0.83 | 4.33ab ± 0.23 | |
| Goatish taste | 5.67a ± 1.87 | 3.50a ± 0.64 | 4.17a ± 0.83 | |
| Off-taste | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.17a ± 0.11 | |
| Fermentation odor | 4.83b ± 0.66 | 3.50ab ± 1.05 | 2.50a ± 0.84 | |
| Goatish odor | 2.25a ± 0.91 | 1.67a ± 0.21 | 1.83a ± 0.33 | |
| Off-odor | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.00a ± 0.00 | |
| Citrate | Consistency | 2.83a ± 0.40 | 3.33ab ± 0.63 | 4.17b ± 0.23 |
| Milky-creamy taste | 3.00a ± 0.54 | 3.67a ± 0.43 | 3.00a ± 0.79 | |
| Salty taste | 1.83a ± 0.34 | 2.33a ± 0.21 | 1.83a ± 0.28 | |
| Sour taste | 5.33b ± 0.31 | 3.67a ± 0.27 | 3.83a ± 0.58 | |
| Goatish taste | 5.67b ± 0.87 | 3.67a ± 0.37 | 3.67a ± 0.63 | |
| Off-taste | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.17a ± 0.11 | |
| Fermentation odor | 4.83c ± 0.66 | 3.50b ± 0.55 | 2.33a ± 0.52 | |
| Goatish odor | 2.25a ± 0.91 | 1.67a ± 0.21 | 1.67a ± 0.23 | |
| Off-odor | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.00a ± 0.00 | |
| Bisglycinate | Consistency | 2.83b ± 0.40 | 1.83a ± 0.58 | 1.67a ± 0.21 |
| Milky-creamy taste | 3.00a ± 1.54 | 3.00a ± 0.53 | 2.67a ± 0.75 | |
| Salty taste | 1.83a ± 0.34 | 1.83a ± 0.17 | 2.00a ± 0.26 | |
| Sour taste | 5.33b ± 0.31 | 4.00a ± 0.63 | 3.67a ± 0.63 | |
| Goatish taste | 5.67b ± 0.87 | 4.33a ± 0.63 | 3.67a ± 0.75 | |
| Off-taste | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.17a ± 0.41 | |
| Fermentation odor | 4.83c ± 0.66 | 3.50b ± 0.35 | 1.83a ± 0.37 | |
| Goatish odor | 2.25a ± 0.51 | 1.67a ± 0.21 | 1.67a ± 0.23 | |
| Off-odor | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.00a ± 0.00 | |
| Gluconate | Consistency | 2.83a ± 0.40 | 3.67b ± 0.34 | 4.67c ± 0.34 |
| Milky-creamy taste | 3.00a ± 1.54 | 2.83a ± 1.37 | 2.67a ± 1.37 | |
| Salty taste | 1.83a ± 0.34 | 2.07a ± 0.60 | 2.00a ± 0.26 | |
| Sour taste | 5.33b ± 0.31 | 3.83a ± 0.72 | 3.83a ± 0.98 | |
| Goatish taste | 5.67b ± 0.87 | 3.17a ± 0.94 | 3.00a ± 0.60 | |
| Off-taste | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.00a ± 0.11 | |
| Fermentation odor | 4.83c ± 0.66 | 3.17b ± 0.17 | 1.67a ± 0.82 | |
| Goatish odor | 2.25a ± 0.91 | 1.67a ± 0.21 | 1.67a ± 0.33 | |
| Off-odor | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.00a ± 0.00 | |
| Lactate | Consistency | 2.83a ± 0.40 | 3.00a ± 0.41 | 3.17a ± 0.47 |
| Milky-creamy taste | 3.00a ± 0.54 | 3.00a ± 0.89 | 3.17a ± 0.14 | |
| Salty taste | 1.83a ± 0.34 | 1.83a ± 0.38 | 1.50a ± 0.55 | |
| Sour taste | 5.33b ± 0.31 | 3.83a ± 0.47 | 3.50a ± 0.64 | |
| Goatish taste | 5.67b ± 0.87 | 3.50a ± 0.64 | 3.67a ± 0.21 | |
| Off-taste | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.00a ± 0.00 | |
| Fermentation odor | 4.83c ± 0.66 | 3.50b ± 0.25 | 2.33a ± 0.51 | |
| Goatish odor | 2.25b ± 0.31 | 1.67ab ± 0,21 | 1.23a ± 0.28 | |
| Off-odor | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.00a ± 0.00 | |
| Carbonate | Consistency | 2.83a ± 0.40 | 2.87a ± 0.82 | 3.33a ± 0.75 |
| Milky-creamy taste | 3.00a ± 0.54 | 2.93a ± 0.37 | 3.00a ± 0.10 | |
| Salty taste | 1.83a ± 0.34 | 1.77a ± 0.73 | 1.83a ± 0.28 | |
| Sour taste | 5.33a ± 0.31 | 5.83a ± 0.48 | 4.80a ± 0.84 | |
| Goatish taste | 5.67a ± 0.87 | 4.17a ± 0.79 | 4.17a ± 0.98 | |
| Off-taste | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.17a ± 0.11 | |
| Fermentation odor | 4.83c ± 0.66 | 3.33b ± 0.21 | 1.83a ± 0.17 | |
| Goatish odor | 2.25a ± 0.91 | 1.87a ± 0.21 | 1.83a ± 0.33 | |
| Off-odor | 1.00a ± 0.00 | 1.00a ± 0.00 | 1.00a ± 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlos, M.; Znamirowska, A.; Szajnar, K. Effect of Calcium Compound Type and Dosage on the Properties of Acid Rennet Goat’s Milk Gels. Molecules 2021, 26, 5563. https://doi.org/10.3390/molecules26185563
Pawlos M, Znamirowska A, Szajnar K. Effect of Calcium Compound Type and Dosage on the Properties of Acid Rennet Goat’s Milk Gels. Molecules. 2021; 26(18):5563. https://doi.org/10.3390/molecules26185563
Chicago/Turabian StylePawlos, Małgorzata, Agata Znamirowska, and Katarzyna Szajnar. 2021. "Effect of Calcium Compound Type and Dosage on the Properties of Acid Rennet Goat’s Milk Gels" Molecules 26, no. 18: 5563. https://doi.org/10.3390/molecules26185563
APA StylePawlos, M., Znamirowska, A., & Szajnar, K. (2021). Effect of Calcium Compound Type and Dosage on the Properties of Acid Rennet Goat’s Milk Gels. Molecules, 26(18), 5563. https://doi.org/10.3390/molecules26185563







