Effects of Extraction Methods on the Bioactivities and Nutritional Value of Virginia and Valencia-Type Peanut Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Extraction Procedure
2.3.1. Cold Press Extraction
2.3.2. Soxhlet Extraction
2.3.3. Maceration Extraction
2.4. Analytical Methods
2.4.1. Quality Characteristics
2.4.2. Pigment Quantification
2.4.3. Fatty Acid Profile
2.4.4. Phytosterol Composition
2.4.5. Tocopherols Content
2.4.6. Radical Scavenging Activity (RSA)
2.5. Statistical Analysis
3. Results and Discussions
3.1. Quality Characteristics
3.2. Chlorophyll and Carotenoid Content
3.3. Radical Scavenging Activity (RSA)
3.4. Oil Content and Fatty Acid Profile
3.5. Phytosterols Composition
3.6. Tocopherols Content
3.7. Correlation Analysis
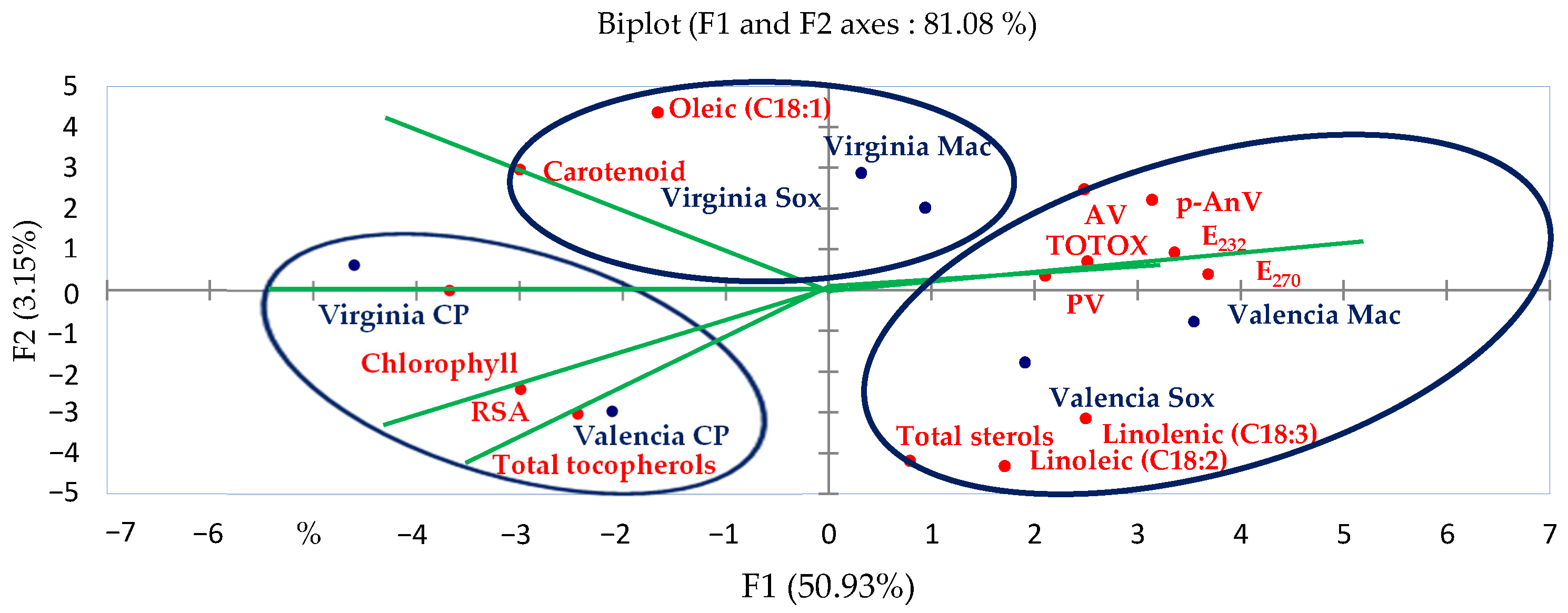
3.8. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Cold press |
| Sox | Soxhlet |
| Mac | Maceration |
| VIO | Virginia oil |
| VAO | Valencia oil |
| PO | Peanut oil |
| FA | Fatty acid |
| FAME | Fatty acid methyl ester |
| MUFA | Monounsaturated fatty acids |
| SFA | Saturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| PS | Phytosterols |
| TTC | Total tocopherol content |
| DPPH | 1,1-diphenylDiphenyl-2-picrylhydrazylPicrylhydrazyl |
| AV | Acid value |
| PV | Peroxide value |
| p-AnV | p-Anisidine value |
| PCA | Principal component analysis |
| HPLC | High-performance liquid chromatography |
| O/L | Oleic/Linoleic acid |
References
- Carrín, M.E.; Carelli, A.A. Peanut oil: Compositional data. Eur. J. Lipid Sci. Technol. 2010, 112, 697–707. [Google Scholar] [CrossRef]
- Akhtar, S.; Khalid, N.; Ahmed, I.; Shahzad, A.; Suleria, H.A.R. Physicochemical characteristics, functional properties, and nutritional benefits of peanut oil: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). Oilseeds: World Markets and Trades. Available online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (accessed on 4 November 2021).
- Tanji, A.; Benicha, M.; Mrabet, R. Contribution à l’étude des adventices associées aux cultures dans les sols sableux du périmètre irrigué du Loukkos: Cas du fraisier et de l’arachide. Rev. Maroc. Prot. Plantes 2015, 7, 67–80. [Google Scholar] [CrossRef]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Toomer, O.T. Nutritional chemistry of the peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef]
- Bonku, R.; Yu, J. Health aspects of peanuts as an outcome of its chemical composition. Food Sci. Hum. Wellness 2020, 9, 21–30. [Google Scholar] [CrossRef]
- Shin, E.-C.; Huang, Y.-Z.; Pegg, R.B.; Phillips, R.D.; Eitenmiller, R.R. Commercial runner peanut cultivars in the United States: Tocopherol composition. J. Agric. Food Chem. 2009, 57, 10289–10295. [Google Scholar] [CrossRef]
- Awad, A.B.; Chan, K.C.; Downie, A.C.; Fink, C.S. Peanuts as a source of β-sitosterol, a sterol with anticancer properties. Nutr. Cancer 2000, 36, 238–241. [Google Scholar] [CrossRef]
- Bezerra, F.W.F.; de Oliveira, M.S.; Bezerra, P.N.; Cunha, V.M.B.; Silva, M.P.; da Costa, W.A.; Pinto, R.H.H.; Cordeiro, R.M.; da Cruz, J.N.; Chaves Neto, A.M.J.; et al. Extraction of Bioactive Compounds. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, A.M., Isloor, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–167. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E.; Hussain, S. Comparison of cold-pressing and soxhlet extraction systems for bioactive compounds, antioxidant properties, polyphenols, fatty acids and tocopherols in eight nut oils. J. Food Sci. Technol. 2018, 55, 3163–3173. [Google Scholar] [CrossRef]
- Costa, W.A.D.; Bezerra, F.W.F.; Oliveira, M.S.D.; Andrade, E.H.D.A.; Santos, A.P.M.D.; Cunha, V.M.B.; Santos, D.C.S.D.; Banna, D.A.D.D.S.; Teixeira, E.; Carvalho Junior, R.N.D. Supercritical CO2 Extraction and Transesterification of the Residual Oil from Industrial Palm Kernel Cake with Supercritical Methanol. J. Supercrit. Fluids 2019, 147, 179–187. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Hamedi, M.; Rezaei, K.; Khodaiyan, F. Evaluation of physicochemical properties and antioxidant activities of Persian walnut oil obtained by several extraction methods. Ind. Crops Prod. 2013, 45, 133–140. [Google Scholar] [CrossRef]
- Goodrum, J.W.; Kilgo, M.B. Peanut oil extraction with SC-CO2: Solubility and kinetic functions. Trans. ASABE 1987, 30, 1865–1868. [Google Scholar] [CrossRef]
- Sharma, A.; Khare, S.K.; Gupta, M.N. Enzyme-assisted aqueous extraction of peanut oil. J. Am. Oil Chem. Soc. 2002, 79, 215–218. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.; Wang, B.; Yagoub, A.E.-G.A.; Ma, H.; Zhang, X.; Wu, M. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017, 37, 106–113. [Google Scholar] [CrossRef]
- El Bernoussi, S.; Boujemaa, I.; Harhar, H.; Belmaghraoui, W.; Matthäus, B.; Tabyaoui, M. Evaluation of oxidative stability of sweet and bitter almond oils under accelerated storage conditions. J. Stored Prod. Res. 2020, 88, 101662. [Google Scholar] [CrossRef]
- Nasri, C.; Halabi, Y.; Harhar, H.; Mohammed, F.; Bellaouchou, A.; Guenbour, A.; Tabyaoui, M. Chemical characterization of oil from four Avocado varieties cultivated in Morocco. OCL 2021, 28, 19. [Google Scholar] [CrossRef]
- Eddahhaoui, F.Z.; Boudalia, M.; Harhar, H.; Chahboun, N.; Tabyaoui, M.; Guenbour, A.; Zarrouk, A.; Bellaouchou, A. Effect of the extraction technique on the bioactive compounds and the antioxidant capacity of the Chamaerops humilis L. fruit (pulp and seeds). Chem. Data Collect. 2022, 40, 100882. [Google Scholar] [CrossRef]
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2020.
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value-Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2017.
- ISO 3656:2011; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. ISO: Geneva, Switzerland, 2011.
- ISO 6885:2016; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. ISO: Geneva, Switzerland, 2016.
- Espínola, F.; Vidal, A.M.; Espínola, J.M.; Moya, M.X. Processing effect and characterization of olive oils from Spanish wild olive trees (Olea europaea var. sylvestris). Molecules 2021, 26, 1304. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters-Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- ISO 12228-1:2014; Determination of Individual and Total Sterols Contents—Gas Chromatographic Method-Part 1: Animal and Vegetable Fats and Oils. ISO: Geneva, Switzerland, 2014.
- El Moudden, H.; El Idrissi, Y.; Belmaghraoui, W.; Belhoussaine, O.; El Guezzane, C.; Bouayoun, T.; Harhar, H.; Tabyaoui, M. Olive mill wastewater polyphenol-based extract as a vegetable oil shelf life extending additive. J. Food Process. Preserv. 2020, 44, e14990. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Singh, N. Impact of roasting and extraction methods on chemical properties, oxidative stability and Maillard reaction products of peanut oils. J. Food Sci. Technol. 2019, 56, 2436–2445. [Google Scholar] [CrossRef]
- Harhar, H.; Gharby, S.; Guillaume, D.; Charrouf, Z. Effect of argan kernel storage conditions on argan oil quality. Eur. J. Lipid Sci. Technol. 2010, 112, 915–920. [Google Scholar] [CrossRef]
- Khoddami, A.; Ghazali, H.M.; Yassoralipour, A.; Ramakrishnan, Y.; Ganjloo, A. Physicochemical characteristics of nigella seed (Nigella sativa L.) oil as affected by different extraction methods. J. Am. Oil Chem. Soc. 2011, 88, 533–540. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Hamdi, S.; Ferrari, G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Ind. Crops Prod. 2019, 128, 363–370. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Report of the 26th Session of the Codex Alimentarius Committee on Fats and Oils; FAO: Kuala Lumpur, Malaysia, 2019. Available online: https://www.usda.gov/ (accessed on 1 July 2022).
- Gharby, S.; Hajib, A.; Ibourki, M.; Nounah, I.; Moudden, H.E.; Elibrahimi, M.; Harhar, H. Induced changes in olive oil subjected to various chemical refining steps: A comparative study of quality indices, fatty acids, bioactive minor components, and oxidation stability kinetic parameters. Chem. Data Collect. 2021, 33, 100702. [Google Scholar] [CrossRef]
- Palanisamy, U.D.; Sivanathan, M.; Radhakrishnan, A.K.; Haleagrahara, N.; Subramaniam, T.; Chiew, G.S. An effective ostrich oil bleaching technique using peroxide value as an indicator. Molecules 2011, 16, 5709–5719. [Google Scholar] [CrossRef]
- Tasan, M.; Gecgel, U.; Demirci, M. Effects of storage and industrial oilseed extraction methods on the quality and stability characteristics of crude sunflower oil (Helianthus annuus L.). Grasas y Aceites 2011, 62, 389–398. [Google Scholar] [CrossRef]
- Malheiro, R.; Oliveira, I.; Vilas-Boas, M.; Falcão, S.; Bento, A.; Pereira, J.A. Effect of microwave heating with different exposure times on physical and chemical parameters of olive oil. Food Chem. Toxicol. 2009, 47, 92–97. [Google Scholar] [CrossRef]
- Van Hoed, V.; Barbouche, I.; De Clercq, N.; Dewettinck, K.; Slah, M.; Leber, E.; Verhé, R. Influence of filtering of cold pressed berry seed oils on their antioxidant profile and quality characteristics. Food Chem. 2011, 127, 1848–1855. [Google Scholar] [CrossRef]
- Alsufiani, H.; Ashour, W. Effectiveness of the natural antioxidant 2, 4, 4′-Trihydroxychalcone on the oxidation of sunflower oil during storage. Molecules 2021, 26, 1630. [Google Scholar] [CrossRef]
- Myat, M.W.; Abdulkarim, S.M.; Ghazali, H.M.; Roselina, K. Physicochemical and sensory characteristics of palm olein and peanut oil blends. J. Food Agric. Environ. 2009, 7, 175–181. [Google Scholar]
- Tavakoli, H.R.; Naderi, M.; Jafari, S.M.; Naeli, M.H. Postmarketing surveillance of the oxidative stability for cooking oils, frying oils, and vanaspati supplied in the retail market. Food Sci. Nutr. 2019, 7, 1455–1465. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Roudani, A.; Boulbaroud, S.; Ibrahimi, M.; Ahmad, M.; Sultana, S.; Hadda, T.B.; Chafchaouni-Moussaoui, I. Chemical investigation of Nigella sativa L. seed oil produced in Morocco. J. Saudi Soc. Agric. Sci. 2015, 14, 172–177. [Google Scholar] [CrossRef]
- Borello, E.; Domenici, V. Determination of pigments in virgin and extra-virgin olive oils: A comparison between two near UV-Vis spectroscopic techniques. Foods 2019, 8, 18. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Falade, A.O.; Oboh, G. Thermal oxidation induces lipid peroxidation and changes in the physicochemical properties and β-carotene content of arachis oil. Int. J. Food Sci. 2015, 2015, 806524. [Google Scholar] [CrossRef]
- Can-Cauich, C.A.; Sauri-Duch, E.; Moo-Huchin, V.M.; Betancur-Ancona, D.; Cuevas-Glory, L.F. Effect of extraction method and specie on the content of bioactive compounds and antioxidant activity of pumpkin oil from Yucatan, Mexico. Food Chem. 2019, 285, 186–193. [Google Scholar] [CrossRef]
- Rasor, A.S.; Duncan, S.E. Fats and Oils—Plant Based. In Food processing: Principles and Applications, 2nd ed.; Clark, S., Jung, S., Lamsal, B., Eds.; Wiley Blackwell: Chichester, West Sussex, UK, 2014; Chapter 20; pp. 457–480. [Google Scholar]
- Bhatnagar-Panwar, M.; Bhatnagar-Mathur, P.; Bhaaskarla, V.V.; Dumbala, S.R.; Sharma, K.K. Rapid, accurate and routine HPLC method for large-scale screening of pro-vitamin A carotenoids in oilseeds. J. Plant Biochem. Biotechnol. 2015, 24, 84–92. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Tocopherols and tocotrienols in plants and their products: A review on methods of extraction, chromatographic separation, and detection. Food Res. Int. 2016, 82, 59–70. [Google Scholar] [CrossRef]
- Boujemaa, I.; El Bernoussi, S.; Harhar, H.; Tabyaoui, M. The influence of the species on the quality, chemical composition and antioxidant activity of pumpkin seed oil. OCL 2020, 27, 40. [Google Scholar] [CrossRef]
- Can-Cauich, C.A.; Sauri-Duch, E.; Cuevas-Glory, L.F.; Betancur-Ancona, D.; Ortiz-Vázquez, E.; Ríos-Soberanis, C.R.; Chel-Guerrero, L.; González-Aguilar, G.A.; Moo-Huchin, V.M. Physicochemical properties and stability of pumpkin seed oil as affected by different extraction methods and species. Int. Food Res. J. 2021, 28, 148–160. [Google Scholar] [CrossRef]
- Alhassan, K.; Agbenorhevi, J.K.; Asibuo, J.Y.; Sampson, G.O. Proximate Composition and Functional Properties of Some New Groundnut Accessions. J. Food Secur. 2017, 5, 9–12. [Google Scholar] [CrossRef][Green Version]
- Zahran, H.A.; Tawfeuk, H.Z. Physicochemical properties of new peanut (Arachis hypogaea L.) varieties. OCL 2019, 26, 19. [Google Scholar] [CrossRef]
- Nawade, B.; Mishra, G.P.; Radhakrishnan, T.; Dodia, S.M.; Ahmad, S.; Kumar, A.; Kumar, A.; Kundu, R. High oleic peanut breeding: Achievements, perspectives, and prospects. Trends Food Sci. Technol. 2018, 78, 107–119. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary fats and membrane function: Implications for metabolism and disease. Biol. Rev. 2005, 80, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Lopez, Y.; Smith, O.D.; Senseman, S.A.; Rooney, W.L. Genetic factors influencing high oleic acid content in Spanish market-type peanut cultivars. Crop Sci. 2001, 41, 51–56. [Google Scholar] [CrossRef]
- Grosso, N.R.; Nepote, V.; Guzmán, C.A. Chemical composition of some wild peanut species (Arachis L.) seeds. J. Agric. Food Chem. 2000, 48, 806–809. [Google Scholar] [CrossRef]
- Deme, T.; Haki, G.D.; Retta, N.; Woldegiorgis, A.; Geleta, M.; Mateos, H.; Lewandowski, P.A. Sterols as a biomarker in tracing niger and sesame seeds oils adulterated with palm oil. Heliyon 2021, 7, e06797. [Google Scholar] [CrossRef]
- Oubannin, S.; Bijla, L.; Gagour, J.; Hajir, J.; Aabd, N.A.; Salama, M.A.; Gharby, S. A comparative evaluation of proximate composition, elemental profiling and oil physicochemical properties of black cumin (Nigella sativa L.) seeds and argan (Argania spinosa L. Skeels) kernels. Chem. Data Collect. 2022, 41, 100920. [Google Scholar] [CrossRef]
- El Moudden, H.; El Idrissi, Y.; El Guezzane, C.; Belmaghraoui, W.; El Yadini, A.; Harhar, H.; Tabyaoui, M. Tradition Mills’ Picholine Olive Oil Physicochemical Characterization and Chemical Profiling across Different Cities in Morocco. Sci. World J. 2020, 2020, 1804723. [Google Scholar] [CrossRef]
- Francisco, M.L.D.; Resurreccion, A.V.A. Functional components in peanuts. Crit. Rev. Food Sci. Nutr. 2008, 48, 715–746. [Google Scholar] [CrossRef]
- Jonnala, R.S.; Dunford, N.T.; Dashiell, K.E. Tocopherol, phytosterol and phospholipid compositions of new high oleic peanut cultivars. J. Food Compos. Anal. 2006, 19, 601–605. [Google Scholar] [CrossRef]
- Maguire, L.S.; O’sullivan, S.M.; Galvin, K.; O’connor, T.P.; O’brien, N.M. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef]
- Verleyen, T.; Sosinska, U.; Ioannidou, S.; Verhé, R.; Dewettinck, K.; Huyghebaert, A.; De Greyt, W. Influence of the vegetable oil refining process on free and esterified sterols. J. Am. Oil Chem. Soc. 2002, 79, 947–953. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Effect of roasting conditions on the fat, tocopherol, and phytosterol content and antioxidant capacity of the lipid fraction from cocoa beans of different Theobroma cacao L. cultivars. Eur. J. Lipid Sci. Technol. 2014, 116, 1002–1014. [Google Scholar] [CrossRef]
- Fernández-Cuesta, Á.; Velasco, L.; Ruiz-Méndez, M.V. Novel safflower oil with high γ-tocopherol content has a high oxidative stability. Eur. J. Lipid Sci. Technol. 2014, 116, 832–836. [Google Scholar] [CrossRef]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stigmasterol: A comprehensive review. Int. J. Pharm. Sci. Res. 2011, 2, 2259. [Google Scholar]
- Gharby, S.; Harhar, H.; Guillaume, D.; Haddad, A.; Matthäus, B.; Charrouf, Z. Oxidative stability of edible argan oil: A two-year study. LWT 2011, 44, 1–8. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Matthäus, B.; Bouzoubaa, Z.; Charrouf, Z. The chemical parameters and oxidative resistance to heat treatment of refined and extra virgin Moroccan Picholine olive oil. J. Taibah Univ. Sci. 2016, 10, 100–106. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirakawa, Y.; Tomiyamaa, Y.; Mizushina, Y. Effects of microwave treatment on the oxidative stability of peanut (Arachis hypogaea) oils and the molecular species of their triacylglycerols. Eur. J. Lipid Sci. Technol. 2003, 105, 351–358. [Google Scholar] [CrossRef]
- Shi, X.; Dean, L.O.; Davis, J.P.; Sandeep, K.P.; Sanders, T.H. The effects of different dry roast parameters on peanut quality using an industrial belt-type roaster simulator. Food Chem. 2018, 240, 974–979. [Google Scholar] [CrossRef]
- Casini, C.; Dardanelli, J.L.; Martínez, M.J.; Balzarini, M.; Borgogno, C.S.; Nassetta, M. Oil quality and sugar content of peanuts (Arachis hypogaea) grown in Argentina: Their relationship with climatic variables and seed yield. J. Agric. Food Chem. 2003, 51, 6309–6313. [Google Scholar] [CrossRef] [PubMed]
- Campos-Mondragón, M.G.; De La Barca, A.C.; Durán-Prado, A.; Campos-Reyes, L.C.; Oliart-Ros, R.M.; Ortega-García, J.; Medina-Juárez, L.A.; Angulo, O. Nutritional composition of new peanut (Arachis hypogaea L.) cultivars. Grasas y Aceites 2009, 60, 161–167. [Google Scholar] [CrossRef]

| Virginia | Valencia | |||||
|---|---|---|---|---|---|---|
| CP | Sox | Mac | CP | Sox | Mac | |
| AV (mg KOH/g) | 0.50 ± 0.05 a | 0.59 ± 0.03 a | 0.81 ± 0.02 b | 0.46 ± 0.01 a | 0.56 ± 0.05 a | 0.80 ± 0.01 b |
| PV (meq O2/kg) | 2.15 ± 0.15 a | 12.9 ± 0.10 b | 4.25 ± 0.05 c | 3.7 ± 0.10 c | 11.8 ± 0.20 d | 5.25 ± 0.15 e |
| p-AnV | 0.84 ± 0.08 a | 3.20 ± 0.02 b | 3.77 ± 0.05 c | 0.75 ± 0.05 a | 2.13 ± 0.15 d | 4.68 ± 0.65 e |
| TOTOX | 5.14 ± 0.22 a | 29.00 ± 0.17 b | 12.27 ± 0.14 c | 8.15 ± 0.14 d | 25.73 ± 0.24 e | 15.18 ± 0.36 f |
| E232 | 0.790 ± 0.002 a | 1.510 ± 0.001 b | 1.237 ± 0.001 c | 0.99 ± 0.005 d | 1.434 ± 0.005 e | 1.352 ± 0.004 f |
| E270 | 0.034 ± 0.002 a | 0.131 ± 0.001 b | 0.104 ± 0.001 c | 0.051 ± 0.001 d | 0.132 ± 0.003 b | 0.183 ± 0.001 e |
| Chlorophyll (mg/kg) | 0.72 ± 0.00 a | 0.18 ± 0.02 b | 0.27 ± 0.01 c | 0.42 ± 0.01 d | 0.15 ± 0.00 b | 0.10 ± 0.02 b |
| Carotenoid (mg/kg) | 0.51 ± 0.00 a | 0.37 ± 0.02 bc | 0.4 ± 0.00 b | 0.29 ± 0.00 cd | 0.21 ± 0.02 de | 0.18 ± 0.02 e |
| RSA (% inhibition DPPH) | 47.68 ± 0.10 a | 28.58 ± 0.26 b | 11.17 ± 0.05 c | 50.10 ± 0.15 d | 30.17 ± 0.10 e | 12.66 ± 0.20 f |
| Fatty Acids (%) | Virginia | Valencia | ||||
|---|---|---|---|---|---|---|
| CP | Sox | Mac | CP | Sox | Mac | |
| Oil yield (%) | 36.19 ± 0.89 a | 56.77 ± 1.37 b | 43.38 ± 1.14 c | 30.38 ± 0.16 d | 47.62 ± 0.24 c | 34.81 ± 1.04 ad |
| Myristic (C14:0) | ND | 0.02 ± 0.00 a | 0.03 ± 0.00 a | ND | 0.03 ± 0.00 a | 0.03 ± 0.00 a |
| Palmitic (C16:0) | 8.77 ± 0.25 a | 9.45 ± 0.01 b | 9.17 ± 0.01 c | 11.82 ± 0.02 d | 12.03 ± 0.00 e | 11.82 ± 0.02 d |
| Palmitoleic (C16:1) | 0.11 ± 0.01 a | 0.18 ± 0.00 b | 0.10 ± 0.00 ac | 0.08 ± 0.01 cd | 0.07 ± 0.00 de | 0.06 ± 0.01 e |
| Margaric (C17:0) | 0.06 ± 0.01 a | 0.03 ± 0.01 b | 0.10 ± 0.00 c | 0.05 ± 0.00 ab | 0.06 ± 0.00 a | 0.06 ± 0.00 a |
| Heptadecenoid (C17:1) | ND | 0.03 ± 0.00 b | 0.06 ± 0.01 c | ND | 0.02 ± 0.00 ab | 0.03 ± 0.01 b |
| Stearic (C18:0) | 4.38 ± 0.05 a | 3.20 ± 0.02 b | 3.50 ± 0.00 c | 3.35 ± 0.05 d | 3.36 ± 0.01 d | 3.59 ± 0.01 e |
| Oleic (C18:1) | 56.99 ± 0.01 a | 56.46 ± 0.01 b | 56.92 ± 0.03 a | 39.76 ± 0.02 c | 39.14 ± 0.01 d | 39.20 ± 0.01 d |
| Linoleic (C18:2) | 27.07 ± 0.01 a | 28.41 ± 0.01 b | 27.96 ± 0.01 c | 42.66 ± 0.01 d | 42.94 ± 0.01 e | 42.70 ± 0.01 d |
| Linolenic (C18:3) | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.07 ± 0.00 a | 0.09 ± 0.00 b | 0.09 ± 0.00 b |
| Arachidic C20:0 | 1.47 ± 0.00 a | 1.27 ± 0.00 b | 1.22 ± 0.01 c | 1.32 ± 0.01 d | 1.33 ± 0.01 d | 1.43 ± 0.01 e |
| Eicosenoic (C20:1) | 1.07 ± 0.01 a | 0.90 ± 0.00 b | 0.88 ± 0.00 c | 0.87 ± 0.00 c | 0.93 ± 0.00 d | 0.97 ± 0.00 e |
| ƩSFA | 14.69 ± 0.01 a | 13.95 ± 0.00 b | 13.99 ± 0.01 b | 16.55 ± 0.01 c | 16.79 ± 0.01 d | 16.9 ± 0.01 e |
| ƩMUFA | 58.18 ± 0.01 a | 57.54 ± 0.01 b | 57.90 ± 0.02 c | 40.71 ± 0.03 d | 40.13 ± 0.02 e | 40.24 ± 0.01 f |
| ƩPUFA | 27.13 ± 0.01 a | 28.46 ± 0.01 b | 28.02 ± 0.01 c | 42.73 ± 0.01 d | 43.02 ± 0.01 e | 42.79 ± 0.00 f |
| ƩUFA | 85.31 ± 0.02 a | 86.00 ± 0.01 b | 85.92 ± 0.01 c | 83.44 ± 0.01 d | 83.16 ± 0.01 e | 83.03 ± 0.01 f |
| O/L ratio | 2.11 ± 0.00 a | 1.99 ± 0.00 b | 2.03 ± 0.01 c | 0.93 ± 0.00 d | 0.91 ± 0.00 e | 0.92 ± 0.00 de |
| Sterols (mg/kg) | Virginia | Valencia | ||||
|---|---|---|---|---|---|---|
| CP | Sox | Mac | CP | Sox | Mac | |
| Total sterols | 1970.60 ± 4.2 a | 1904.16 ± 3.16 b | 1929.73 ± 3.96 c | 2816.34 ± 4.74 d | 2272.24 ± 3.74 e | 2676.88 ± 3.75 f |
| Cholesterol | 7.24 ± 0.00 a | 6.84 ± 0.01 b | 4.19 ± 0.01 c | 5.49 ± 0.01 d | 6.26 ± 0.00 e | 6.61 ± 0.01 f |
| Campesterol | 271.65 ± 0.36 a | 261.08 ± 0.26 b | 256.69 ± 0.3 c | 429.71 ± 0.35 d | 347.53 ± 0.34 e | 410.04 ± 0.36 f |
| Stigmasterol | 178.50 ± 0.25 a | 190.94 ± 0.08 b | 186.15 ± 0.14 c | 208.23 ± 0.21 d | 166.86 ± 0.19 e | 193.39 ± 0.14 f |
| β-Sitosterol | 1277.41 ± 1.81 a | 1205.25 ± 1.17 b | 1241.69 ± 2.01 c | 1839.42 ± 2.23 d | 1473.07 ± 1.94 e | 1740.68 ± 1.89 f |
| Δ-5-avenosterol | 170.08 ± 0.07 a | 169.63 ± 0.09 a | 163.53 ± 0.08 b | 250.62 ± 0.08 c | 208.29 ± 0.13 d | 236.62 ± 0.10 e |
| Δ-7-stigmasterol | 2.73 ± 0.01 a | 1.94 ± 0.01 b | 1.08 ± 0.01 c | 2.56 ± 0.02 d | 2.03 ± 0.03 b | 3.65 ± 0.01 e |
| Δ-7-avenosterol | 10.76 ± 0.01 a | 8.48 ± 0.01 b | 7.22 ± 0.01 c | 15.74 ± 0.06 d | 14.33 ± 0.01 e | 16.78 ± 0.02 f |
| Tocopherol (mg/kg) | Virginia | Valencia | ||||
|---|---|---|---|---|---|---|
| CP | Sox | Mac | CP | Sox | Mac | |
| Total tocopherols | 719.36 ± 2.39 a | 367.26 ± 1.96 b | 40.29 ± 2.02 c | 818.66 ± 1.79 d | 651.10 ± 2.08 e | 74.09 ± 1.56 f |
| α-tocopherol | 319.60 ± 1.04 a | 123.29 ± 0.62 b | 5.65 ± 0.36 c | 392.28 ± 0.73 d | 314.70 ± 0.81 e | 13.86 ± 0.52 f |
| β-tocopherol | 11.41 ± 0.11 a | 12.85 ± 0.09 b | 2.56 ± 0.16 b | 10.87 ± 0.17 a | 11.38 ± 0.07 a | 5.24 ± 0.15 c |
| γ-tocopherol | 343.68 ± 1.26 a | 214.62 ± 0.76 b | 30.91 ± 1.09 c | 371.71 ± 0.32 d | 309.03 ± 0.77 e | 54.84 ± 0.74 f |
| δ-tocopherol | 26.16 ± 0.34 a | 12.21 ± 0.07 b | 0.00 ± 0.00 c | 29.78 ± 0.13 d | 15.71 ± 0.16 e | 0.00 ± 0.00 c |
| Variables | Total Sterols | Total Tocopherols | Oleic (C18:1) | Linoleic (C18:2) | Linolenic (C18:3) | AV | PV | p-AnV | TOTOX | E232 | E270 | Chlorophyll | Carotenoid | RSA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total sterols | 1 | |||||||||||||
| Total tocopherols | 0.204 | 1 | ||||||||||||
| Oleic (C18:1) | −0.885 | −0.215 | 1 | |||||||||||
| Linoleic (C18:2) | 0.884 | 0.205 | −0.999 | 1 | ||||||||||
| Linolenic (C18:3) | 0.621 | −0.049 | −0.879 | 0.871 | 1 | |||||||||
| AV | −0.106 | −0.975 | 0.088 | −0.082 | 0.181 | 1 | ||||||||
| PV | −0.253 | −0.036 | −0.083 | 0.102 | 0.244 | −0.057 | 1 | |||||||
| p-AnV | −0.076 | −0.952 | 0.006 | 0.004 | 0.278 | 0.926 | 0.252 | 1 | ||||||
| TOTOX | −0.252 | −0.193 | −0.077 | 0.096 | 0.277 | 0.101 | 0.987 | 0.406 | 1 | |||||
| E232 | −0.102 | −0.515 | −0.177 | 0.200 | 0.393 | 0.453 | 0.843 | 0.705 | 0.914 | 1 | ||||
| E270 | 0.138 | −0.680 | −0.351 | 0.360 | 0.626 | 0.671 | 0.549 | 0.870 | 0.664 | 0.869 | 1 | |||
| Chlorophyll | −0.188 | 0.577 | 0.415 | −0.437 | −0.573 | −0.567 | −0.652 | −0.767 | −0.744 | −0.939 | −0.924 | 1 | ||
| Carotenoid | −0.705 | 0.162 | 0.892 | −0.900 | −0.903 | −0.245 | −0.362 | −0.401 | −0.408 | −0.580 | −0.719 | 0.778 | 1 | |
| RSA | 0.167 | 0.953 | −0.045 | 0.034 | −0.261 | −0.963 | −0.206 | −0.961 | −0.356 | −0.664 | −0.794 | 0.730 | 0.348 | 1 |
| Variables | Total Sterols | Total Tocopherols | Oleic (C18:1) | Linoleic (C18:2) | Linolenic (C18:3) | AV | PV | p-AnV | TOTOX | E232 | E270 | Chlorophyll | Carotenoid | RSA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total sterols | 0 | |||||||||||||
| Total tocopherols | 0.698 | 0 | ||||||||||||
| Oleic (C18:1) | 0.019 | 0.683 | 0 | |||||||||||
| Linoleic (C18:2) | 0.019 | 0.697 | <0.001 | 0 | ||||||||||
| Linolenic (C18:3) | 0.188 | 0.926 | 0.021 | 0.024 | 0 | |||||||||
| AV | 0.841 | 0.001 | 0.869 | 0.878 | 0.732 | 0 | ||||||||
| PV | 0.629 | 0.947 | 0.875 | 0.848 | 0.641 | 0.915 | 0 | |||||||
| p-AnV | 0.885 | 0.003 | 0.991 | 0.994 | 0.593 | 0.008 | 0.630 | 0 | ||||||
| TOTOX | 0.629 | 0.714 | 0.885 | 0.856 | 0.595 | 0.848 | 0.000 | 0.425 | 0 | |||||
| E232 | 0.848 | 0.296 | 0.737 | 0.704 | 0.440 | 0.367 | 0.035 | 0.118 | 0.011 | 0 | ||||
| E270 | 0.794 | 0.137 | 0.495 | 0.483 | 0.184 | 0.145 | 0.259 | 0.024 | 0.150 | 0.025 | 0 | |||
| Chlorophyll | 0.721 | 0.230 | 0.413 | 0.386 | 0.235 | 0.241 | 0.161 | 0.075 | 0.090 | 0.006 | 0.009 | 0 | ||
| Carotenoid | 0.118 | 0.759 | 0.017 | 0.014 | 0.014 | 0.640 | 0.481 | 0.431 | 0.422 | 0.227 | 0.107 | 0.069 | 0 | |
| RSA | 0.751 | 0.003 | 0.933 | 0.948 | 0.618 | 0.002 | 0.695 | 0.002 | 0.489 | 0.151 | 0.059 | 0.100 | 0.499 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrissi, Z.L.E.; El Moudden, H.; Mghazli, N.; Bouyahya, A.; Guezzane, C.E.; Alshahrani, M.M.; Al Awadh, A.A.; Goh, K.W.; Ming, L.C.; Harhar, H.; et al. Effects of Extraction Methods on the Bioactivities and Nutritional Value of Virginia and Valencia-Type Peanut Oil. Molecules 2022, 27, 7709. https://doi.org/10.3390/molecules27227709
Idrissi ZLE, El Moudden H, Mghazli N, Bouyahya A, Guezzane CE, Alshahrani MM, Al Awadh AA, Goh KW, Ming LC, Harhar H, et al. Effects of Extraction Methods on the Bioactivities and Nutritional Value of Virginia and Valencia-Type Peanut Oil. Molecules. 2022; 27(22):7709. https://doi.org/10.3390/molecules27227709
Chicago/Turabian StyleIdrissi, Zineb Lakhlifi El, Hamza El Moudden, Najoua Mghazli, Abdelhakim Bouyahya, Chakir El Guezzane, Mohammed Merae Alshahrani, Ahmed Abdullah Al Awadh, Khang Wen Goh, Long Chiau Ming, Hicham Harhar, and et al. 2022. "Effects of Extraction Methods on the Bioactivities and Nutritional Value of Virginia and Valencia-Type Peanut Oil" Molecules 27, no. 22: 7709. https://doi.org/10.3390/molecules27227709
APA StyleIdrissi, Z. L. E., El Moudden, H., Mghazli, N., Bouyahya, A., Guezzane, C. E., Alshahrani, M. M., Al Awadh, A. A., Goh, K. W., Ming, L. C., Harhar, H., & Tabyaoui, M. (2022). Effects of Extraction Methods on the Bioactivities and Nutritional Value of Virginia and Valencia-Type Peanut Oil. Molecules, 27(22), 7709. https://doi.org/10.3390/molecules27227709










