Effects of Hydrothermal Processing on Volatile and Fatty Acids Profile of Cowpeas (Vigna unguiculata), Chickpeas (Cicer arietinum) and Kidney Beans (Phaseolus vulgaris)
Abstract
1. Introduction
2. Results and Discussions
2.1. Starch, Protein, Lipid and Moisture Content of Cowpea, Chickpea and Kidney Bean
2.2. Lipid Yield and Relative Fatty Acid Composition of Cooked Cowpea, Chickpea and Kidney Bean
2.3. Untargeted Headspace Volatile Fingerprinting of Cooked Legumes
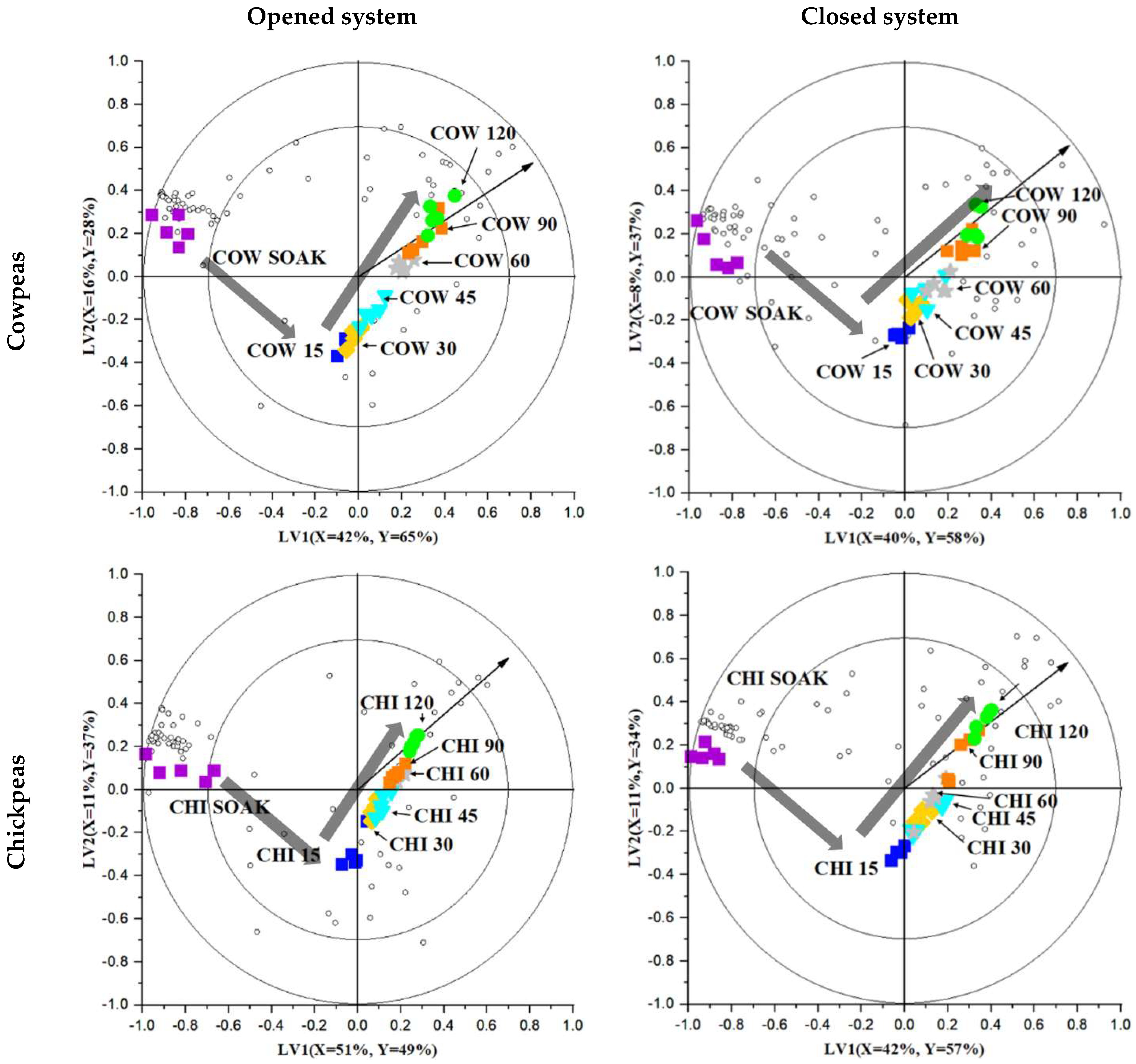
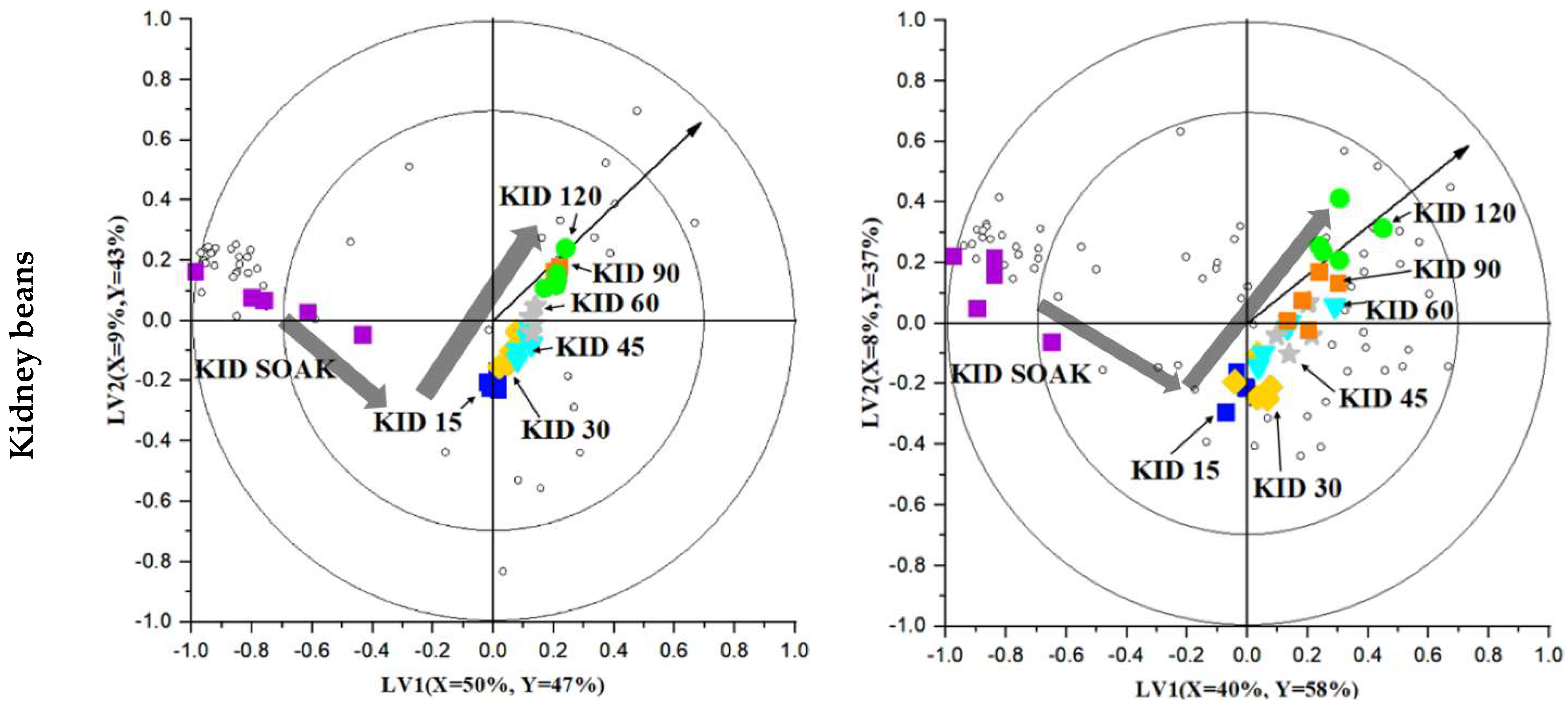
2.4. Investigating the Volatile Changes during Hydrothermal Processing Using Chemometrics
2.5. Interpretation of Volatile Compounds Changing with Processing Time and Their Associated Reaction Pathways
2.5.1. Enzyme-Related Reaction Pathways
2.5.2. Temperature-Related Reaction Pathways
3. Materials and Methods
3.1. Sample Preparation and Storage
3.2. Compositional Analyses of Uncooked Cowpeas, Chickpeas and Kidney Beans
3.3. Hydrothermal Processing of Cowpeas, Chickpeas and Kidney Beans
3.4. Determination of Fatty Acids in Cowpeas, Chickpeas and Kidney Beans Using Chromatography Flame Ionisation Detection
3.5. Determination of Volatile Compounds Using Headspace Solid-Phase Micro-Extraction Gas Chromatography-Mass Spectrometry
3.6. Pre-Processing of Headspace Volatile Chromatograms
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes—A boon to human nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M.T. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Carbonaro, M.; Grant, G.; Cappelloni, M.; Pusztai, A. Perspectives into factors limiting in vivo digestion of legume proteins: Antinutritional compounds or storage proteins? J. Agric. Food Chem. 2000, 48, 742–749. [Google Scholar] [CrossRef]
- Mohan, V.R.; Tresina, P.S.; Daffodil, E.D. Antinutritional factors in legume seeds: Characteristics and determination. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 211–220. [Google Scholar]
- Jeong, D.; Han, J.-A.; Liu, Q.; Chung, H.-J. Effect of processing, storage, and modification on in vitro starch digestion characteristics of food legumes: A review. Food Hydrocoll. 2019, 90, 367–376. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT-Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D. Nutritional quality of legume seeds as affected by some physical treatments 2. Antinutritional factors. LWT-Food Sci. Technol. 2009, 42, 1113–1118. [Google Scholar] [CrossRef]
- Aravindakshan, S.; Nguyen, T.H.A.; Kyomugasho, C.; Buvé, C.; Dewettinck, K.; Van Loey, A.; Hendrickx, M.E. The Impact of drying and rehydration on the structural properties and quality attributes of pre-cooked dried beans. Foods 2021, 10, 1665. [Google Scholar] [CrossRef]
- Bassett, A.; Kamfwa, K.; Ambachew, D.; Cichy, K. Genetic variability and genome-wide association analysis of flavor and texture in cooked beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 2021, 134, 959–978. [Google Scholar] [CrossRef]
- MacLeod, G.; Ames, J.; Betz, N.L. Soy flavor and its improvement. Crit. Rev. Food Sci. Nutr. 1988, 27, 219–400. [Google Scholar] [CrossRef]
- Trindler, C.; Annika Kopf-Bolanz, K.; Denkel, C. Aroma of peas, its constituents and reduction strategies–Effects from breeding to processing. Food Chem. 2022, 376, 131892. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Liu, Z.; Chang, S.K.C. Off-flavor related volatiles in soymilk as affected by soybean variety, grinding, and heat-processing methods. J. Agric. Food Chem. 2012, 60, 7457–7462. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, S.K.C. Selected odor compounds in soymilk as affected by chemical composition and lipoxygenases in five soybean materials. J. Agric. Food Chem. 2007, 55, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Soy product off-flavor generating, masking, and flavor creating. In Soy Applications in Food; CRC Press: Boca Raton, FL, USA, 2005; pp. 227–248. [Google Scholar]
- Cadwallader, K. Static headspace SPME-GC-MS volatile constituents of soymilk as affected by processing method. In Proceedings of the Soy Flavor Workshop: Sensory and Instrumental Method; University of Illinois: Champaign, IL, USA, 2004. [Google Scholar]
- Boatright, W.L.; Lei, Q. Compounds contributing to the ‘beany’ odor of aqueous solutions of soy protein isolates. J. Food Sci. 1999, 64, 667–670. [Google Scholar] [CrossRef]
- Wang, Z.H.; Dou, J.; Macura, D.; Durance, T.D.; Nakai, S. Solid phase extraction for GC analysis of beany flavours in soymilk. Food Res. Int. 1997, 30, 503–511. [Google Scholar] [CrossRef]
- Fisher, G.S.; Legendre, M.G.; Lovgren, N.V.; Schuller, W.H.; Wells, J.A. Volatile constituents of southernpea seed (Vigna unguiculata (L.) Walp.) [Cowpeas]. J. Agric. Food Chem. 1979, 27, 7–11. [Google Scholar] [CrossRef]
- Lasekan, O.; Juhari, N.; Pattiram, P. Headspace solid-phase microextraction analysis of the volatile flavour compounds of roasted chickpea (Cicer arietinum L.). Food Process. Technol. 2011, 2, 112. [Google Scholar] [CrossRef]
- Rembold, H.; Wallner, P.; Nitz, S.; Kollmannsberger, H.; Drawert, F. Volatile components of chickpea (Cicer arietinum L.) seed. J. Agric. Food Chem. 1989, 37, 659–662. [Google Scholar] [CrossRef]
- Noordraven, L.E.; Buvé, C.; Chen, C.; Hendrickx, M.E.; Van Loey, A.M. Impact of processing and storage conditions on the volatile profile of whole chickpeas (Cicer arietinum L.). ACS Food Sci. Technol. 2021, 1, 1095–1108. [Google Scholar] [CrossRef]
- Sosulski, F.; Gadan, H. Variations in lipid composition among chickpea cultivars. J. Am. Oil Chem. Soc. 1988, 65, 369–372. [Google Scholar] [CrossRef]
- Mishra, P.K.; Tripathi, J.; Gupta, S.; Variyar, P.S. Effect of cooking on aroma profile of red kidney beans (Phaseolus vulgaris) and correlation with sensory quality. Food Chem. 2017, 215, 401–409. [Google Scholar] [CrossRef]
- Oomah, B.D.; Liang, L.S.Y.; Balasubramanian, P. Volatile compounds of dry beans (Phaseolus vulgaris L.). Plant Foods Hum. Nutr. 2007, 62, 177. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Boye, J.I.; Azarnia, S.; Simpson, B.K. Volatile flavor profile of Saskatchewan grown pulses as affected by different thermal processing treatments. Int. J. Food Prop. 2016, 19, 2251–2271. [Google Scholar] [CrossRef]
- Naidoo, T.; Gerrano, A.; Mellem, J. The effect of processing on in vitro protein and starch digestibility and predictive glycaemic index of five Vigna unguiculata (cowpea) cultivars. Ann. Univ. Dunarea De Jos Galati Fascicle VI: Food Technol. 2017, 41, 31–41. [Google Scholar]
- Xu, Y.; Cartier, A.; Obielodan, M.; Jordan, K.; Hairston, T.; Shannon, A.; Sismour, E. Nutritional and anti-nutritional composition, and in vitro protein digestibility of Kabuli chickpea (Cicer arietinum L.) as affected by differential processing methods. J. Food Meas. Charact. 2016, 10, 625–633. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Cui, S.W.; Li, Y.; Xu, S.; Wu, Y.; Wang, J.; Bai, Z.; et al. Nutrients, phytochemicals and antioxidant activities of 26 kidney bean cultivars. Food Chem. Toxicol. 2017, 108, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Thomas, M.; Bhardwaj, H.L. Chemical composition, functional properties and microstructural characteristics of three kabuli chickpea (Cicer arietinum L.) as affected by different cooking methods. Int. J. Food Sci. Technol. 2014, 49, 1215–1223. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.-S.A.; El-Fishawy, F.A.; El-Geddawy, M.A.; Kurz, T.; El-Rify, M.N. The changes in the lipid composition of mung bean seeds as affected by processing methods. Int. J. Food Eng. 2007, 3. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry, 4th ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Prinyawiwatkul, W.; Beuchat, L.R.; McWatters, K.H.; Phillips, R.D. Changes in fatty acid, simple sugar, and oligosaccharide content of cowpea (Vigna unguiculata) flour as a result of soaking, boiling, and fermentation with Rhizopus microsporus var. oligosporus. Food Chem. 1996, 57, 405–413. [Google Scholar] [CrossRef]
- Caprioli, G.; Giusti, F.; Ballini, R.; Sagratini, G.; Vila-Donat, P.; Vittori, S.; Fiorini, D. Lipid nutritional value of legumes: Evaluation of different extraction methods and determination of fatty acid composition. Food Chem. 2016, 192, 965–971. [Google Scholar] [CrossRef]
- Yaylayan, V.A. Recent advances in the chemistry of Strecker degradation and Amadori rearrangement: Implications to aroma and color formation. Food Sci. Technol. Res. 2003, 9, 1–6. [Google Scholar] [CrossRef]
- Stark, W.; Forss, D. A compound responsible for mushroom flavour in dairy products. J. Dairy Res. 1964, 31, 253–259. [Google Scholar] [CrossRef]
- Gomes, J.; Jadrić, S.; Winterhalter, M.; Brkić, S. Alcohol dehydrogenase isoenzymes in chickpea cotyledons. Phytochemistry 1982, 21, 1219–1224. [Google Scholar] [CrossRef]
- Khrisanapant, P.; Kebede, B.; Leong, S.Y.; Oey, I. A comprehensive characterisation of volatile and fatty acid profiles of legume seeds. Foods 2019, 8, 651. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Sun, G.-M.; Liu, Y.-G.; Lv, L.-L.; Yang, W.-X.; Zhao, W.-F.; Wei, C.-B. Aroma volatile compounds from two fresh pineapple varieties in China. Int. J. Mol. Sci. 2012, 13, 7383–7392. [Google Scholar] [CrossRef]
- Dotson, G.S.; Maier, A.; Parker, A.; Haber, L.T. Immediately Dangerous to Life or Health (IDLH) Value Profile: Furan (CAS no. 110-00-9); US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2016.
- Moro, S.; Chipman, J.K.; Wegener, J.W.; Hamberger, C.; Dekant, W.; Mally, A. Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment. Mol. Nutr. Food Res. 2012, 56, 1197–1211. [Google Scholar] [CrossRef]
- Palmers, S.; Grauwet, T.; Celus, M.; Wibowo, S.; Kebede, B.T.; Hendrickx, M.E.; Van Loey, A. A kinetic study of furan formation during storage of shelf-stable fruit juices. J. Food Eng. 2015, 165, 74–81. [Google Scholar] [CrossRef]
- Culleré, L.; Fernández de Simón, B.; Cadahía, E.; Ferreira, V.; Hernández-Orte, P.; Cacho, J. Characterization by gas chromatography–olfactometry of the most odor-active compounds in extracts prepared from acacia, chestnut, cherry, ash and oak woods. LWT-Food Sci. Technol. 2013, 53, 240–248. [Google Scholar] [CrossRef]
- Azarnia, S.; Boye, J.I. Flavour compounds in legumes: Chemical and sensory aspects. In Progress in Food Science and Technology, Eds.Greco, A.J., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; Volume 1, pp. 1–34. [Google Scholar]
- Chigwedere, C.M.; Tadele, W.W.; Yi, J.; Wibowo, S.; Kebede, B.T.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Insight into the evolution of flavor compounds during cooking of common beans utilizing a headspace untargeted fingerprinting approach. Food Chem. 2019, 275, 224–238. [Google Scholar] [CrossRef]
- Grauwet, T.; Kebede, B.T.; Delgado, R.M.; Lemmens, L.; Manzoni, F.; Vervoort, L.; Hendrickx, M.; Stephen Elmore, J.; Van Loey, A. Evaluating the potential of high pressure high temperature and thermal processing on volatile compounds, nutritional and structural properties of orange and yellow carrots. Eur. Food Res. Technol. 2015, 240, 183–198. [Google Scholar] [CrossRef]
- Vogel, J.T.; Tan, B.-C.; McCarty, D.R.; Klee, H.J. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J. Biol. Chem. 2008, 283, 11364–11373. [Google Scholar] [CrossRef]
- Heatherbell, D.A.; Wrolstad, R.E.; Libbey, L.M. Carrot volatiles. J. Food Sci. 1971, 36, 219–224. [Google Scholar] [CrossRef]
- Shamaila, M.; Durance, T.; Girard, B. Water blanching effects on headspace volatiles and sensory attributes of carrots. J. Food Sci. 1996, 61, 1191–1195. [Google Scholar] [CrossRef]
- Mosciano, G. Organoleptic characteristics of flavor materials. Perfum. Flavorist 1993, 18, 43. [Google Scholar]
- Megazyme. Total Starch Assay Procedure; Megazyme: Bray, Ireland, 2019. [Google Scholar]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric Finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International. Method 930.04 Moisture in Plants; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International. Method 963.22 Methyl Esters of Fatty Acids in Oils and Fats; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Kebede, B.T.; Grauwet, T.; Tabilo-Munizaga, G.; Palmers, S.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Headspace components that discriminate between thermal and high pressure high temperature treated green vegetables: Identification and linkage to possible process-induced chemical changes. Food Chem. 2013, 141, 1603–1613. [Google Scholar] [CrossRef]
- Liu, F.; Grauwet, T.; Kebede, B.T.; Van Loey, A.; Liao, X.; Hendrickx, M. Comparing the Effects of high hydrostatic pressure and thermal processing on blanched and unblanched mango (Mangifera indica L.) nectar: Using headspace fingerprinting as an untargeted approach. Food Bioprocess Technol. 2014, 7, 3000–3011. [Google Scholar] [CrossRef]
- Arcena, M.R.; Kebede, B.; Leong, S.Y.; Silcock, P.; Oey, I. Feasibility of using integrated fingerprinting, profiling and chemometrics approach to understand (bio) chemical changes throughout commercial red winemaking: A case study on Merlot. Food Res. Int. 2020, 127, 108767. [Google Scholar] [CrossRef]
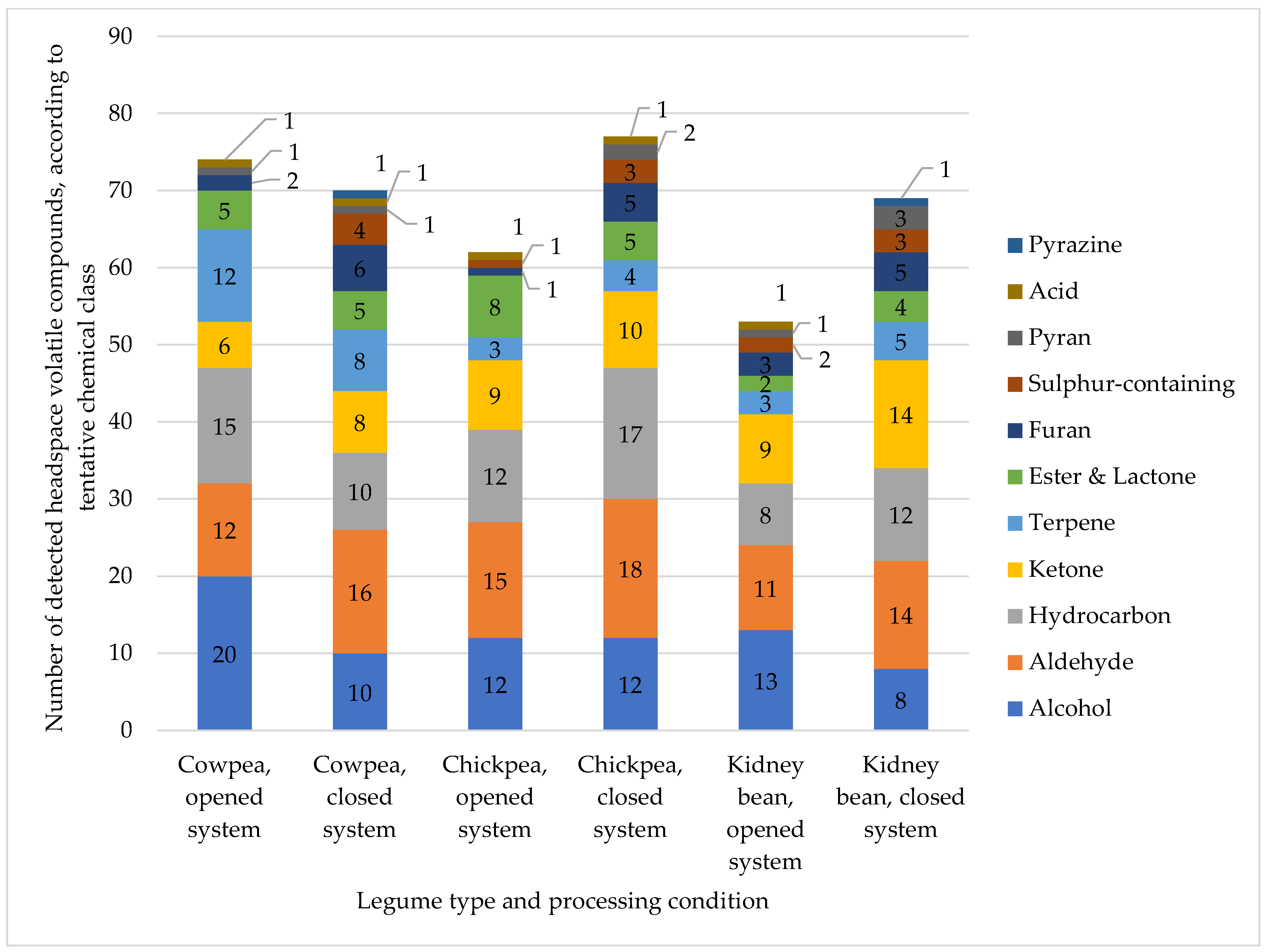
 represents cowpeas;
represents cowpeas;  represents chickpeas;
represents chickpeas;  represents kidney beans. (A): 2(E),4(E)-heptadienal, opened and closed system, (B): 1-octen-3-ol, opened and closed system, (C): α-terpinyl acetate, opened and closed system.
represents kidney beans. (A): 2(E),4(E)-heptadienal, opened and closed system, (B): 1-octen-3-ol, opened and closed system, (C): α-terpinyl acetate, opened and closed system.
 represents cowpeas;
represents cowpeas;  represents chickpeas;
represents chickpeas;  represents kidney beans. (A): 2(E),4(E)-heptadienal, opened and closed system, (B): 1-octen-3-ol, opened and closed system, (C): α-terpinyl acetate, opened and closed system.
represents kidney beans. (A): 2(E),4(E)-heptadienal, opened and closed system, (B): 1-octen-3-ol, opened and closed system, (C): α-terpinyl acetate, opened and closed system.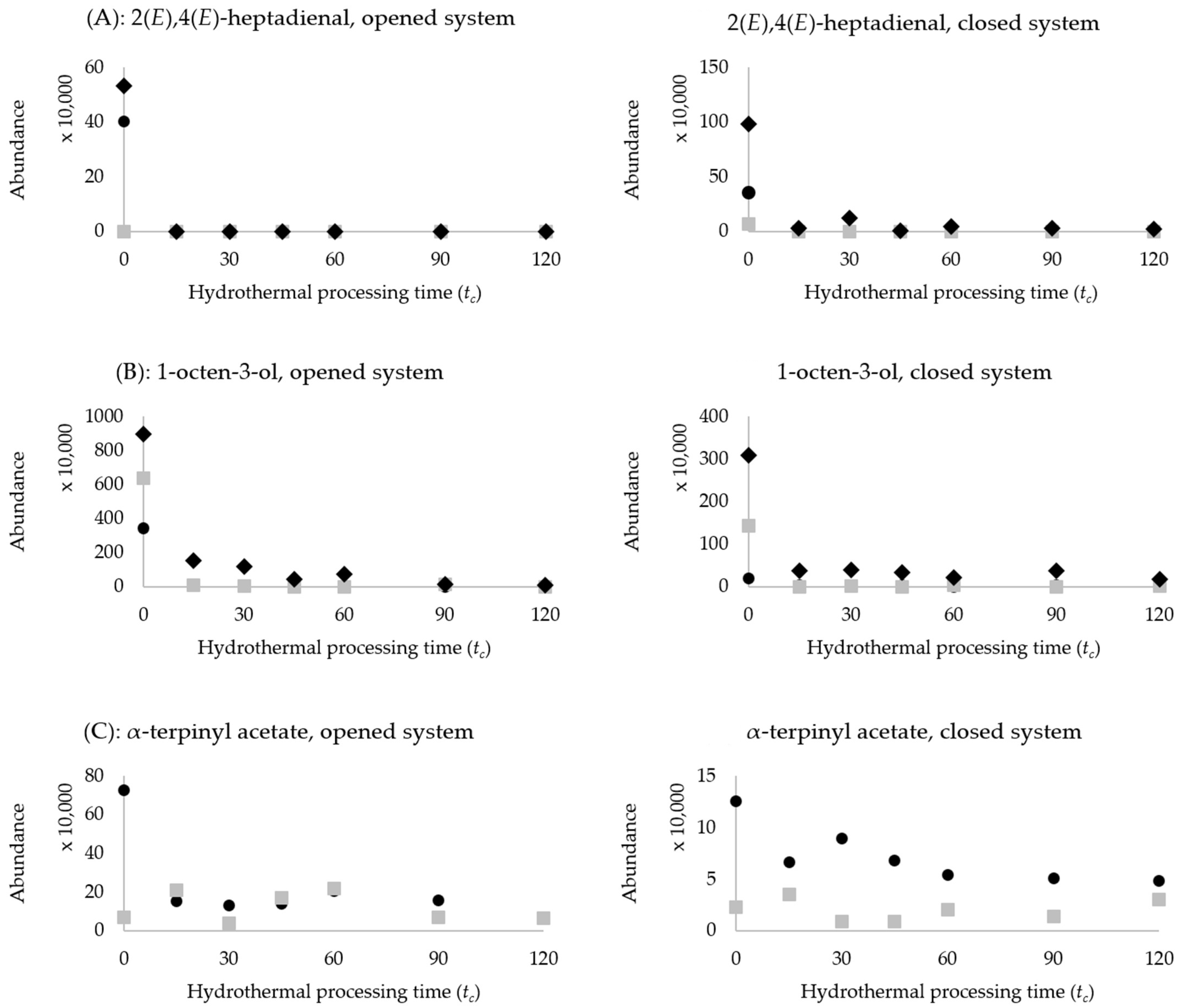
 represents cowpeas;
represents cowpeas;  represents chickpeas;
represents chickpeas;  represents kidney beans. (A): 2-ethyl-furan, opened and closed system, (B): Dimethyl sulfide, closed system, (C): Pyridine, closed system, (D): 2H-benzopyran, opened and closed system.
represents kidney beans. (A): 2-ethyl-furan, opened and closed system, (B): Dimethyl sulfide, closed system, (C): Pyridine, closed system, (D): 2H-benzopyran, opened and closed system.
 represents cowpeas;
represents cowpeas;  represents chickpeas;
represents chickpeas;  represents kidney beans. (A): 2-ethyl-furan, opened and closed system, (B): Dimethyl sulfide, closed system, (C): Pyridine, closed system, (D): 2H-benzopyran, opened and closed system.
represents kidney beans. (A): 2-ethyl-furan, opened and closed system, (B): Dimethyl sulfide, closed system, (C): Pyridine, closed system, (D): 2H-benzopyran, opened and closed system.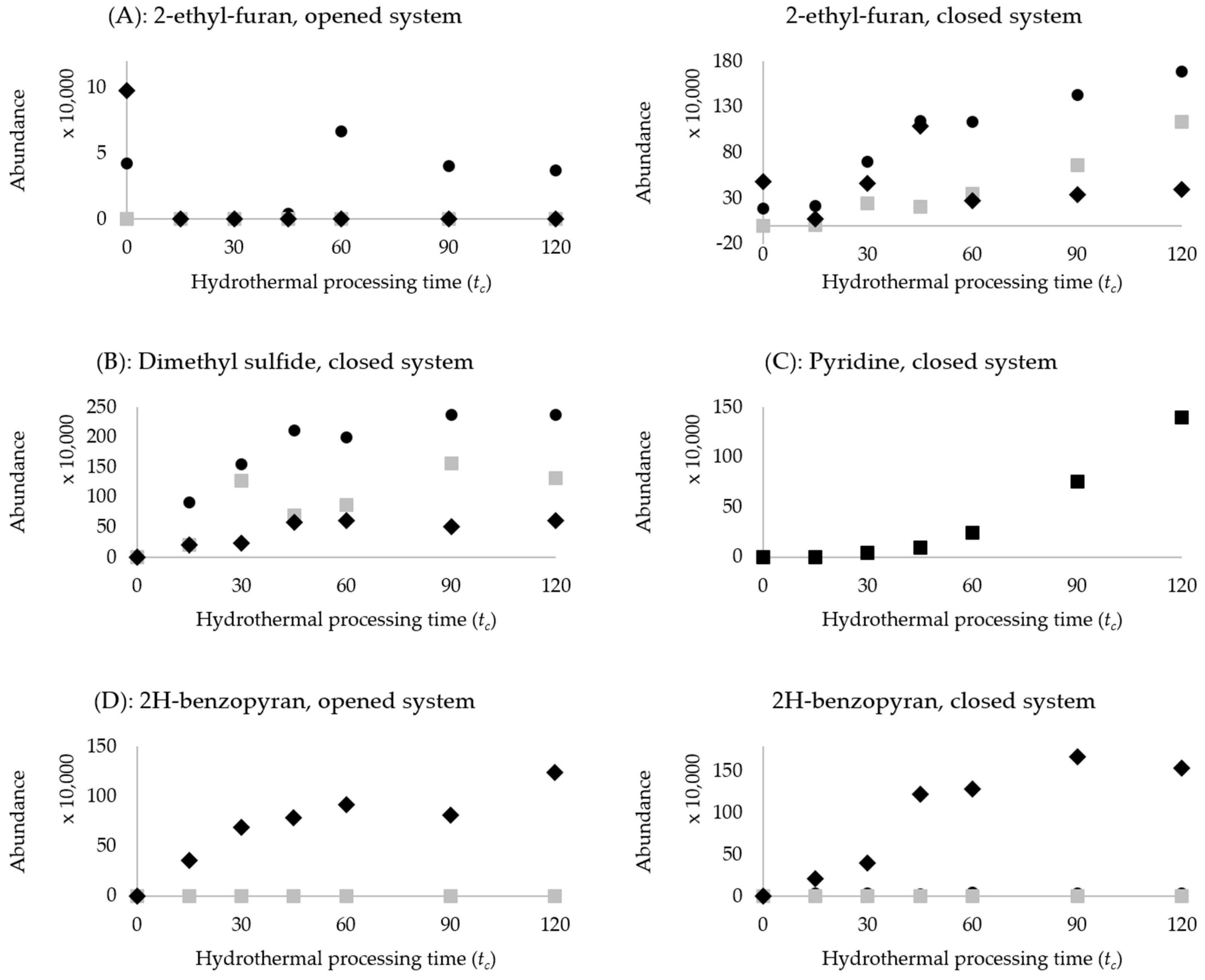
| tc (min) | Lipid Yield (g/100 g DW) | C16:0 (g/100 g Lipid) | C18:0 (g/100 g Lipid) | C18:1 (g/100 g Lipid) | C18:2 (g/100 g Lipid) | C18:3 (g/100 g Lipid) | SFAs (g/100 g Lipid) | MUFAs (g/100 g Lipid) | PUFAs (g/100 g Lipid) | ω-6/ω-3 Ratio | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cowpea | 0 | 3.31 ± 0.18 c | 27.43 ± 0.19 a | 4.41 ± 0.24 a | 5.48 ± 0.18 a | 36.51 ± 0.23 a | 24.99 ± 0.21 ab | 31.84 ± 0.15 b | 5.48 ± 0.18 a | 61.50 ± 0.17 a | 1.46 ± 0.02 a |
| 15 | 2.81 ± 0.06 b | 26.95 ± 0.65 ab | 4.21 ± 0.12 a | 6.06 ± 0.11 a | 36.57 ± 0.41 ab | 24.72 ± 0.29 a | 31.17 ± 0.74 ab | 6.06 ± 0.11 a | 61.29 ± 0.65 a | 1.48 ± 0.01 abc | |
| 30 | 2.54 ± 0.10 ab | 26.16 ± 0.39 b | 4.04 ± 0.05 a | 5.85 ± 0.30 a | 36.67 ± 0.34 ab | 24.94 ± 0.23 a | 30.21 ± 0.37 a | 5.85 ± 0.30 a | 61.61 ± 0.55 ab | 1.47 ± 0.01 ab | |
| 45 | 2.43 ± 0.08 a | 27.04 ± 0.66 ab | 4.03 ± 0.18 a | 6.18 ± 0.71 a | 37.79 ± 0.42 c | 24.73 ± 0.15 a | 31.07 ± 0.79 ab | 6.18 ± 0.71 a | 62.51 ± 0.53 abc | 1.53 ± 0.01 d | |
| 60 | 2.39 ± 0.05 a | 27.04 ± 0.06 ab | 3.99 ± 0.22 a | 6.01 ± 0.26 a | 37.41 ± 0.13 abc | 25.57 ± 0.29 b | 31.03 ± 0.25 ab | 6.01 ± 0.26 a | 62.97 ± 0.38 c | 1.46 ± 0.01 a | |
| 90 | 2.41 ± 0.22 a | 26.84 ± 0.39 ab | 4.05 ± 0.15 a | 6.10 ± 0.17 a | 37.49 ± 0.68 bc | 24.92 ± 0.37 a | 30.89 ± 0.45 ab | 6.10 ± 0.17 a | 62.41 ± 0.96 abc | 1.50 ± 0.02 bcd | |
| 120 | 2.66 ± 0.2 ab | 26.60 ± 0.40 ab | 4.36 ± 0.38 a | 5.65 ± 0.27 a | 37.90 ± 0.39 c | 24.99 ± 0.30 ab | 30.95 ± 0.19 ab | 5.65 ± 0.27 a | 62.90 ± 0.44 bc | 1.52 ± 0.03 cd | |
| Chickpea | 0 | 8.17 ± 0.25 a | 10.30 ± 0.05 a | 1.98 ± 0.03 d | 37.47 ± 0.20 a | 46.32 ± 0.20 e | 2.12 ± 0.02 a | 12.28 ± 0.07 b | 37.47 ± 0.2 a | 48.45 ± 0.20 e | 21.81 ± 0.18 d |
| 15 | 8.73 ± 1.44 a | 10.28 ± 0.03 a | 1.74 ± 0.04 bc | 38.31 ± 0.10 b | 45.65 ± 0.13 d | 2.14 ± 0.01 ab | 12.03 ± 0.02 a | 38.31 ± 0.1 b | 47.78 ± 0.13 d | 21.35 ± 0.11 cd | |
| 30 | 8.23 ± 0.96 a | 10.29 ± 0.05 a | 1.74 ± 0.03 bc | 38.88 ± 0.04 cd | 45.07 ± 0.03 c | 2.12 ± 0.03 a | 12.04 ± 0.04 a | 38.88 ± 0.04 cd | 47.18 ± 0.02 bc | 21.30 ± 0.33 cd | |
| 45 | 8.48 ± 0.32 a | 10.71 ± 0.12 c | 1.82 ± 0.050 c | 39.31 ± 0.14 d | 44.13 ± 0.30 a | 2.13 ± 0.05 a | 12.54 ± 0.17 c | 39.31 ± 0.14 d | 46.26 ± 0.29 a | 20.75 ± 0.56 bc | |
| 60 | 8.33 ± 0.70 a | 10.42 ± 0.05 ab | 1.70 ± 0.040 ab | 39.30 ± 0.19 d | 44.49 ± 0.16 ab | 2.27 ± 0.05 c | 12.11 ± 0.03 ab | 39.30 ± 0.19 d | 46.77 ± 0.20 ab | 19.59 ± 0.36 a | |
| 90 | 7.93 ± 1.15 a | 10.43 ± 0.02 ab | 1.66 ± 0.02 ab | 38.82 ± 0.17 cd | 44.93 ± 0.10 bc | 2.25 ± 0.05 bc | 12.09 ± 0.03 ab | 38.82 ± 0.17 cd | 47.19 ± 0.15 bc | 19.96 ± 0.43 ab | |
| 120 | 7.93 ± 0.27 a | 10.49 ± 0.04 b | 1.64 ± 0.02 a | 38.43 ± 0.29 bc | 45.31 ± 0.27 cd | 2.23 ± 0.05 abc | 12.13 ± 0.05 ab | 38.43 ± 0.29 bc | 47.54 ± 0.32 cd | 20.37 ± 0.36 abc | |
| Kidney bean | 0 | 3.36 ± 0.19 a | 18.72 ± 0.75 a | 3.14 ± 0.53 a | 14.47 ± 3.41 a | 29.25 ± 2.00 a | 33.93 ± 1.66 a | 21.86 ± 0.6 a | 14.47 ± 3.41 a | 63.19 ± 3.59 ab | 0.86 ± 0.03 a |
| 15 | 3.10 ± 0.32 a | 18.59 ± 3.19 a | 3.43 ± 0.79 a | 12.49 ± 0.90 a | 28.11 ± 2.28 a | 36.85 ± 2.26 a | 22.03 ± 3.88 a | 12.49 ± 0.9 a | 64.96 ± 4.50 ab | 0.76 ± 0.02 a | |
| 30 | 3.00 ± 0.16 a | 18.66 ± 1.74 a | 2.98 ± 0.67 a | 15.94 ± 4.53 a | 27.01 ± 1.92 a | 34.23 ± 2.87 a | 21.64 ± 2.40 a | 15.94 ± 4.53 a | 61.24 ± 4.79 ab | 0.79 ± 0.01 a | |
| 45 | 3.32 ± 0.17 a | 18.30 ± 0.52 a | 2.78 ± 0.37 a | 14.12 ± 0.74 a | 28.54 ± 0.31 a | 35.61 ± 0.52 a | 21.08 ± 0.63 a | 14.12 ± 0.74 a | 64.15 ± 0.65 ab | 0.80 ± 0.01 a | |
| 60 | 3.71 ± 0.43 a | 20.07 ± 1.03 a | 3.73 ± 1.12 a | 17.46 ± 3.52 a | 24.94 ± 3.63 a | 32.95 ± 2.40 a | 23.80 ± 1.82 a | 17.46 ± 3.52 a | 57.89 ± 4.60 a | 0.76 ± 0.11 a | |
| 90 | 3.30 ± 0.48 a | 17.42 ± 0.13 a | 2.25 ± 0.40 a | 12.40 ± 0.41 a | 30.25 ± 1.17 a | 37.13 ± 1.17 a | 19.67 ± 0.45 a | 12.40 ± 0.41 a | 67.38 ± 0.73 b | 0.82 ± 0.05 a | |
| 120 | 3.07 ± 0.36 a | 17.28 ± 0.72 a | 4.11 ± 1.01 a | 13.65 ± 2.43 a | 28.84 ± 1.81 a | 35.69 ± 2.01 a | 21.40 ± 1.65 a | 13.65 ± 2.43 a | 64.53 ± 3.72 ab | 0.81 ± 0.02 a |
| VID Coefficient | Tentative Identity | RIobserved | RIliterature |
|---|---|---|---|
| Cowpea | |||
| Positive VID | |||
| Aldehyde (1) | |||
| 0.602 | Benzaldehyde | 1554 | 1543 |
| Hydrocarbon (1) | |||
| 0.620 | Branched hydrocarbon | 988 | N/A |
| Terpene (1) | |||
| 0.763 | α-Muurolene | 1726 | 1734 |
| Negative VID | |||
| Alcohol (14) | |||
| −0.754 | 1-Heptanol | 1467 | 1462 |
| −0.711 | 1-Pentanol | 1257 | 1255 |
| −0.706 | 1-Nonanal | 1419 | 1412 |
| −0.705 | 1-Hexanol | 1365 | 1357 |
| −0.704 | 2-Ethyl-1-hexanol | 1499 | 1490 |
| −0.700 | 1-Penten-3-ol | 1156 | 1174 |
| −0.699 | 3(Z)-Hexen-1-ol | 1400 | 1381 |
| −0.695 | 1-Octanol | 1562 | 1549 |
| −0.689 | 1-Octen-3-ol | 1461 | 1461 |
| −0.675 | Ethanol | 912 | 939 |
| −0.666 | 3-Octanol | 1405 | N/A |
| −0.658 | 2(Z)-Penten-1-ol | 1333 | 1318 |
| −0.652 | 1-Heptanol | 1467 | 1462 |
| −0.650 | 3-Methyl-1-butanol | 1210 | 1214 |
| Aldehyde (8) | |||
| −0.745 | 2(E)-Nonenal | 1547 | 1550 |
| −0.741 | 5-Ethylcyclopent-1-enecarboxaldehyde | 1451 | 1416 |
| −0.712 | Nonanal | 1419 | N/A |
| −0.699 | 2(E)-Hexenal | 1236 | 1238 |
| −0.680 | Pentadecanal | 1945 | 2024 |
| −0.649 | Hexanal | 1076 | 1078 |
| −0.640 | 2(E),4(E)-Heptadienal | 1491 | 1471 |
| −0.623 | 2-Butenal | 1603 | N/A |
| Ester and Lactone (2) | |||
| −0.693 | α-Terpinyl acetate | 1698 | 1704 |
| −0.600 | Hexyl acetate | 1289 | 1259 |
| Ketone (2) | |||
| −0.693 | 3-Hydroxy-2-butanone (Acetoin) | 1309 | 1295 |
| −0.665 | 3-Octen-2-one | 1434 | 1414 |
| Terpene (5) | |||
| −0.727 | Linalool | 1552 | 1551 |
| −0.707 | Estragole | 1677 | 1687 |
| −0.649 | Terpinen-4-ol | 1615 | 1611 |
| −0.634 | Eucalyptol | 1225 | 1213 |
| −0.622 | Carvone | 1736 | 1737 |
| Chickpea | |||
| Positive VID | |||
| Hydrocarbon (1) | |||
| 0.696 | Branched hydrocarbon | 1010 | N/A |
| Terpene (1) | |||
| 0.646 | γ-Terpinene | 1267 | 1255 |
| Negative VID | |||
| Acid (1) | |||
| −0.687 | Hexanoic acid | 1816 | 1865 |
| Alcohol (11) | |||
| −0.895 | Ethanol | 912 | 939 |
| −0.815 | 1-Octanol | 1562 | 1549 |
| −0.803 | 1-Hexanol | 1365 | 1357 |
| −0.801 | 1-Heptanol | 1467 | 1462 |
| −0.801 | 1-Penten-3-ol | 1156 | 1174 |
| −0.801 | 1-Pentanol | 1257 | 1255 |
| −0.799 | 1-Octen-3-ol | 1461 | 1461 |
| −0.791 | 3(Z)-Hexen-1-ol | 1400 | 1381 |
| −0.780 | 2(Z)-Penten-1-ol | 1333 | 1318 |
| −0.716 | 4-Ethylcyclohexanol | 1520 | N/A |
| −0.678 | 3-Hydroxy-2-butanone (Acetoin) | 1309 | 1295 |
| Aldehyde (14) | |||
| −0.820 | Nonanal | 1419 | 1412 |
| −0.800 | 2(E)-Decenal | 1652 | 1655 |
| −0.795 | 2(Z)-Heptenal | 1352 | 1349 |
| −0.793 | 5-Ethylcyclopent-1-enecarboxaldehyde | 1451 | 1416 |
| −0.786 | Heptanal | 1196 | 1202 |
| −0.782 | 2(E)-Octenal | 1458 | 1466 |
| −0.776 | 2(E),4(E)-Heptadienal | 1491 | 1503 |
| −0.774 | Pentanal | 963 | 985 |
| −0.758 | Hexanal | 1076 | 1068 |
| −0.740 | Octanal | 1311 | 1307 |
| −0.726 | 2-Undecenal | 1741 | N/A |
| −0.700 | 2-Ethyl-4-pentenal | 1324 | N/A |
| −0.695 | 2,4-Decadienal | 1790 | 1767 |
| −0.667 | 3-Methyl-1-butanal | 904 | 924 |
| Ester and Lactone (3) | |||
| −0.792 | Hexanoic acid, ethenyl ester | 1667 | N/A |
| −0.752 | Hexahydro-2,5-methano-2H-furo [3,2-b]pyran | 1263 | N/A |
| −0.660 | Dihydro-5-pentyl-2(3H)-furanone | 1957 | 2005 |
| Furan (1) | |||
| −0.732 | 2-Pentyl-furan | 1247 | 1224 |
| Hydrocarbon (1) | |||
| −0.681 | 2-Methoxy-2-propenyl-benzene | 1805 | N/A |
| −0.656 | 1-Ethyl-1-methyl-cyclopentane | 1430 | N/A |
| Ketone (4) | |||
| −0.709 | 2,5-Octanedione | 1344 | N/A |
| −0.695 | 3-Octen-2-one | 1434 | 1414 |
| −0.692 | 2-Octanone | 1306 | 1323 |
| −0.686 | 6-Methyl-3-heptanone | 1273 | N/A |
| Kidney bean | |||
| Positive VID | |||
| Pyran (1) | |||
| 0.740 | 2H-1-benzopyran | 1553 | N/A |
| Negative VID | |||
| Acid (1) | |||
| −0.712 | Hexanoic acid | 1816 | 1833 |
| Alcohol (10) | |||
| −0.863 | 1-Octen-3-ol | 1461 | 1461 |
| −0.819 | 1-Penten-3-ol | 1156 | 1174 |
| −0.817 | 1-Hexanol | 1365 | 1357 |
| −0.815 | 3(Z)-Hexen-1-ol | 1400 | 1381 |
| −0.794 | 1-Heptanol | 1467 | 1462 |
| −0.779 | 3-Methyl-1-butanol | 1209 | 1214 |
| −0.765 | 1-Butoxy-2-propanol | 1358 | N/A |
| −0.734 | 2(Z)-Penten-1-ol | 1333 | N/A |
| −0.728 | 1-Pentanol | 1257 | 1255 |
| −0.721 | Ethanol | 912 | 939 |
| Aldehyde (9) | |||
| −0.815 | 2(E)-Hexenal | 1236 | 1238 |
| −0.790 | Hexanal | 1076 | 1078 |
| −0.786 | 2(E),4(E)-Heptadienal | 1520 | 1471 |
| −0.786 | 2(E)-Nonenal | 1547 | 1550 |
| −0.786 | 2(E)-Pentenal | 1134 | 1146 |
| −0.784 | 2(Z)-Heptenal | 1352 | 1349 |
| −0.779 | 3-Methyl-1-butanal | 904 | 924 |
| −0.703 | 2(E)-Octenal | 1458 | 1466 |
| −0.663 | Pentanal | 963 | 985 |
| Ester and Lactone (1) | |||
| −0.747 | Hexanoic acid, ethenyl ester | 1667 | N/A |
| Furan (1) | |||
| −0.718 | 2-Ethyl-furan | 937 | 945 |
| Hydrocarbon (3) | |||
| −0.815 | Naphthalene | 1749 | 1765 |
| −0.682 | Toluene | 1031 | 1022 |
| Ketone (3) | |||
| −0.803 | 2,3-Pentanedione | 1046 | 1050 |
| −0.699 | 3(E),5(E)-octadien-2-one | 1540 | 1531 |
| −0.678 | 3-Hydroxy-2-butanone (Acetoin) | 1309 | 1295 |
| Terpene (1) | |||
| −0.664 | Linalool | 1552 | 1551 |
| VID Coefficient | Tentative Identity | RIobserved | RIliterature |
|---|---|---|---|
| Cowpea | |||
| Positive VID | |||
| Sulphur-containing (1) | |||
| 0.948 | Dimethyl sulfide | 802 | 777 |
| Furan (2) | |||
| 0.869 | 2-Ethyl-furan | 929 | 945 |
| 0.603 | 2-Methyl-furan | 861 | 888 |
| Ketone (1) | |||
| 0.628 | 6-Methyl-5-hepten-2-one | 1355 | 1319 |
| Terpene (1) | |||
| 0.600 | γ-Terpinene | 1260 | 1255 |
| Negative VID | |||
| Acid (1) | |||
| −0.611 | Hexanoic acid | 1811 | 1865 |
| Alcohol (5) | |||
| −0.742 | 1-Hexanol | 1364 | 1357 |
| −0.738 | 3(Z)-Hexen-1-ol | 1398 | 1381 |
| −0.677 | 2-Penten-1-ol | 1328 | 1321 |
| −0.652 | 1-Nonanal | 1415 | 1412 |
| −0.618 | 1-Penten-3-ol | 1149 | 1174 |
| Hydrocarbon (5) | |||
| −0.720 | Decane | 972 | N/A |
| −0.675 | Branched hydrocarbon | 850 | N/A |
| −0.662 | Branched hydrocarbon | 1116 | N/A |
| −0.628 | Toluene | 1023 | 1014 |
| −0.604 | Branched hydrocarbon | 1303 | N/A |
| Aldehyde (9) | |||
| −0.779 | 2(E)-Hexenal | 1229 | 1238 |
| −0.771 | 2(E)-Nonenal | 1553 | 1550 |
| −0.748 | 2(E),4(E)-Nonadienal | 1700 | 1664 |
| −0.748 | Hexanal | 1069 | 1078 |
| −0.745 | 2(E)-Octenal | 1453 | 1400 |
| −0.736 | 2(E),4(E)-Heptadienal | 1485 | 1471 |
| −0.691 | 5-Ethylcyclopent-1-enecarboxaldehyde | 1445 | 1416 |
| −0.657 | Heptanal | 1189 | 1176 |
| −0.632 | Pentadecanal | 1946 | 2024 |
| Ester and Lactone (3) | |||
| −0.742 | Hexyl acetate | 1283 | 1259 |
| −0.737 | α-Terpinyl acetate | 1695 | 1704 |
| −0.658 | Ethyl 3-methylbutanoate | 1049 | 1053 |
| Terpene (3) | |||
| −0.720 | D-Limonene | 1208 | 1190 |
| −0.719 | p-Cymene | 1288 | 1280 |
| −0.631 | Eucalyptol | 1219 | 1179 |
| Furan (1) | |||
| −0.490 | 2-Pentyl-furan | 1240 | 1224 |
| Ketone (1) | |||
| −0.729 | 3,5-Dimethyl-2-octanone | 1053 | N/A |
| Sulphur−containing (1) | |||
| −0.674 | Hydroxyethyl methyl sulfide | 1545 | 1537 |
| Chickpea | |||
| Positive VID | |||
| Aldehyde (1) | |||
| 0.844 | Benzaldehyde | 1547 | 1506 |
| Furan (1) | |||
| 0.832 | 2-Ethyl-furan | 929 | 945 |
| Ketone (2) | |||
| 0.739 | 6-Methyl-2-heptanone | 1248 | 1319 |
| 0.725 | 2-Propanone | 829 | 832 |
| Pyridine (1) | |||
| 0.763 | Pyridine | 1185 | 1213 |
| Sulphur−containing (2) | |||
| 0.774 | Dimethyl sulfide | 803 | 777 |
| 0.766 | 2-Acetylthiazole | 1654 | 1650 |
| Negative VID | |||
| Acid (1) | |||
| −0.646 | Hexanoic acid | 1811 | 1865 |
| Alcohol (8) | |||
| −0.754 | 1-Heptanol | 1466 | 1462 |
| −0.717 | 1-Octen-3-ol | 1458 | 1461 |
| −0.699 | 1-Hexanol | 1364 | 1357 |
| −0.662 | 1-Octanol | 1562 | 1549 |
| −0.656 | 2(E)-Hepten-1-ol | 1518 | 1517 |
| −0.639 | 2(E)-Octen-1-ol | 1610 | 1580 |
| −0.635 | 1-Nonanol | 1652 | 1658 |
| −0.625 | 1-Penten-3-ol | 1149 | 1174 |
| Ketone (1) | |||
| −0.648 | 2-Octanone | 1300 | 1323 |
| Aldehyde (14) | |||
| −0.745 | 2(E)-Nonenal | 1554 | 1550 |
| −0.741 | 5-Ethylcyclopent-1-enecarboxaldehyde | 1445 | 1416 |
| −0.735 | 2(E)-Decenal | 1650 | 1655 |
| −0.727 | 4-Oxononanal | 1802 | N/A |
| −0.715 | Hexanal | 1069 | 1078 |
| −0.701 | 2(E),4(E)-Decadienal | 1788 | 1770 |
| −0.668 | 2(E)-Octenal | 1453 | 1400 |
| −0.666 | Heptanal | 1189 | 1176 |
| −0.664 | 3-Methyl-butanal | 898 | 934 |
| −0.654 | 2(E),4(E)-Nonadienal | 1700 | 1664 |
| −0.653 | 2(Z)-Heptenal | 1346 | 1349 |
| −0.646 | 2-Undecenal | 1740 | 1740 |
| −0.629 | Nonanal | 1415 | 1412 |
| −0.602 | Octanal | 1305 | 1307 |
| Ester and Lactone (2) | |||
| −0.740 | Dihydro-5-pentyl-2(3h)-furanone | 1956 | 2005 |
| −0.678 | Hexanoic acid, ethenyl ester | 1663 | N/A |
| Furan (1) | |||
| −0.701 | 2-Pentyl-furan | 1240 | 1224 |
| Hydrocarbon (3) | |||
| −0.657 | 1-Ethyl-1-methyl-cyclopentane | 1425 | N/A |
| −0.656 | 2-Methyl-6-propylphenol | 1820 | N/A |
| −0.644 | Branched hydrocarbon | 983 | N/A |
| Pyran (1) | |||
| −0.732 | Hexahydro-2,5-methano-2H-furo [3,2-b]pyran | 1257 | N/A |
| Terpene (1) | |||
| −0.648 | p-Xylene | 1141 | 1194 |
| Kidney bean | |||
| Positive VID | |||
| Ketone (1) | |||
| 0.606 | 3-Methyl-2-butanone | 954 | 949 |
| Pyran (1) | |||
| 0.789 | 2H-1-Benzopyran | 1549 | N/A |
| Sulphur−containing (1) | |||
| 0.612 | Dimethyl sulfide | 802 | 777 |
| Negative VID | |||
| Alcohol (5) | |||
| −0.746 | 3(Z)-Hexen-1-ol | 1398 | 1381 |
| −0.718 | 1-Hexanol | 1364 | 1357 |
| −0.713 | 1-Octen-3-ol | 1458 | 1461 |
| −0.705 | 1-Nonanol | 1652 | 1658 |
| −0.623 | 1-Octanol | 1562 | 1549 |
| Aldehyde (7) | |||
| −0.686 | 2(E),4(E)-Heptadienal | 1486 | 1471 |
| −0.685 | 2(E)-Pentenal | 1127 | 1146 |
| −0.684 | 2(E)-Hexenal | 1230 | 1238 |
| −0.661 | 2(E)-Octenal | 1453 | 1400 |
| −0.655 | Heptanal | 1190 | 1176 |
| −0.653 | 2(E)-Nonenal | 1553 | 1550 |
| −0.618 | Hexanal | 1069 | 1078 |
| Hydrocarbon (1) | |||
| −0.741 | Branched hydrocarbon | 1445 | N/A |
| Ketone (2) | |||
| −0.776 | 3,5-Octadien-2-one | 1536 | 1531 |
| −0.668 | 2-Octanone | 1300 | 1323 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khrisanapant, P.; Kebede, B.; Leong, S.Y.; Oey, I. Effects of Hydrothermal Processing on Volatile and Fatty Acids Profile of Cowpeas (Vigna unguiculata), Chickpeas (Cicer arietinum) and Kidney Beans (Phaseolus vulgaris). Molecules 2022, 27, 8204. https://doi.org/10.3390/molecules27238204
Khrisanapant P, Kebede B, Leong SY, Oey I. Effects of Hydrothermal Processing on Volatile and Fatty Acids Profile of Cowpeas (Vigna unguiculata), Chickpeas (Cicer arietinum) and Kidney Beans (Phaseolus vulgaris). Molecules. 2022; 27(23):8204. https://doi.org/10.3390/molecules27238204
Chicago/Turabian StyleKhrisanapant, Prit, Biniam Kebede, Sze Ying Leong, and Indrawati Oey. 2022. "Effects of Hydrothermal Processing on Volatile and Fatty Acids Profile of Cowpeas (Vigna unguiculata), Chickpeas (Cicer arietinum) and Kidney Beans (Phaseolus vulgaris)" Molecules 27, no. 23: 8204. https://doi.org/10.3390/molecules27238204
APA StyleKhrisanapant, P., Kebede, B., Leong, S. Y., & Oey, I. (2022). Effects of Hydrothermal Processing on Volatile and Fatty Acids Profile of Cowpeas (Vigna unguiculata), Chickpeas (Cicer arietinum) and Kidney Beans (Phaseolus vulgaris). Molecules, 27(23), 8204. https://doi.org/10.3390/molecules27238204









