Influence of the Extraction Method on the Quality and Chemical Composition of Walnut (Juglans regia L.) Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Oil Extraction
2.3. Physicochemical Quality Parameters
2.4. Chlorophylls and Carotenoids Content
2.5. Fatty Acids’ Composition
2.6. Phytosterols Composition
2.7. Tocopherols Composition
2.8. Statistical Analysis
3. Results and Discussion
3.1. Oil Yields and Physicochemical Quality Parameters
3.2. Chlorophyll and Carotenoid Content
3.3. Fatty Acid Composition
3.4. Phytosterol Compositions
3.5. Tocopherol Compositions
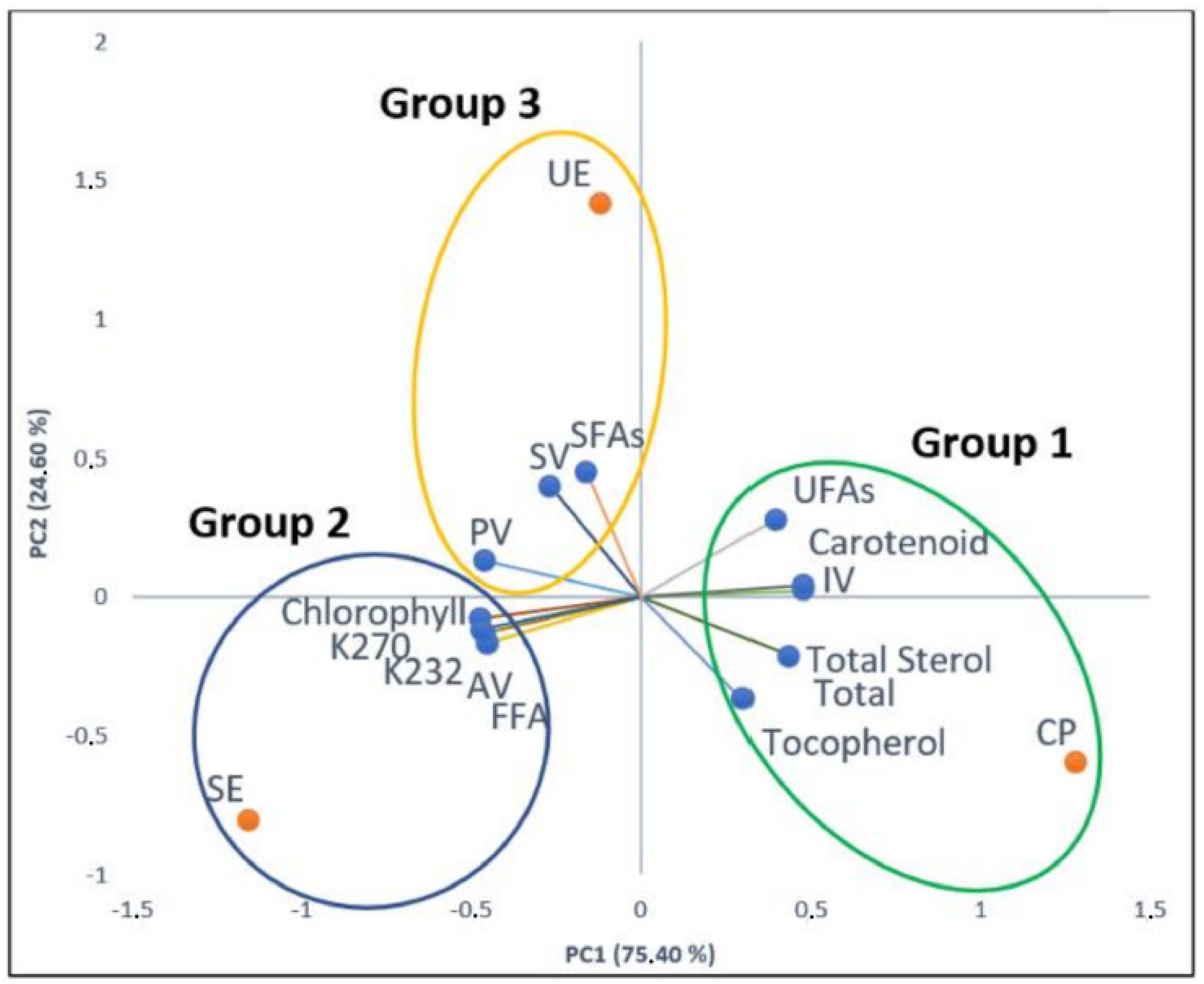
3.6. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, M.; Kumar, R. Microclimatic Buffering on Medicinal and Aromatic Plants: A Review. Ind. Crops Prod. 2021, 160, 113144. [Google Scholar] [CrossRef]
- Zouaoui, N.; Chenchouni, H.; Bouguerra, A.; Massouras, T.; Barkat, M. Characterization of Volatile Organic Compounds from Six Aromatic and Medicinal Plant Species Growing Wild in North African Drylands. NFS J. 2020, 18, 19–28. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Zidane, L. Ethnobotanical Study Aimed at Investigating the Use of Medicinal Plants to Treat Nervous System Diseases in the Rif of Morocco. J. Chiropr. Med. 2020, 19, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture Fisheries Rural Development Water and Forests. 2015. Available online: https://www.agriculture.gov.ma/en/node/13210 (accessed on 18 September 2022).
- Ministry of Agriculture Fisheries Rural Development Water and Forests. Official Bulletin. In Official Bulletin; Ministry of Agriculture Fisheries Rural Development Water and Forests: Rabat, Morocco, 2015; pp. 959–960. [Google Scholar]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparison of Solvents for Extraction of Walnut Oils: Lipid Yield, Lipid Compositions, Minor-Component Content, and Antioxidant Capacity. LWT 2019, 110, 346–352. [Google Scholar] [CrossRef]
- Virtanen, J.K. Randomized Trials of Replacing Saturated Fatty Acids with N-6 Polyunsaturated Fatty Acids in Coronary Heart Disease Prevention: Not the Gold Standard? Prostaglandins Leukot. Essent. Fat. Acids 2018, 133, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Yang, H.; Zhang, P.; Wu, F.; Li, Y.; Zhou, Y.; Zhang, X.; Ma, H.; Zhang, W.; et al. α-Linolenic Acid but Not Linolenic Acid Protects against Hypertension: Critical Role of SIRT3 and Autophagic Flux. Cell Death Dis. 2020, 11, 83. [Google Scholar] [CrossRef]
- Yue, H.; Qiu, B.; Jia, M.; Liu, W.; Guo, X.-F.; Li, N.; Xu, Z.-X.; Du, F.-L.; Xu, T.; Li, D. Effects of α-Linolenic Acid Intake on Blood Lipid Profiles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2020, 61, 2894–2910. [Google Scholar] [CrossRef]
- Ullah, S.F.; Khan, N.M.; Ali, F.; Ahmad, S.; Khan, Z.U.; Rehman, N.; Jan, A.K.; Muhammad, N. Effects of Maillard Reaction on Physicochemical and Functional Properties of Walnut Protein Isolate. Food Sci. Biotechnol. 2019, 28, 1391–1399. [Google Scholar] [CrossRef]
- Uddin, Y.; Khan, N.M.; Ali, F.; Ahmad, S.; Ullah Khan, Z.; Asif Nawaz, M.; Wang, J. Estimation of Various Physicochemical Properties of Walnut Oil from Different Areas of Northern Kpk, Pakistan. J. Mex. Chem. Soc. 2021, 65, 572–581. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Kan, H.; Chen, S.X.; Thakur, K.; Wang, S.; Zhang, J.G.; Shang, Y.F.; Wei, Z.J. Comparison of Phenolic Compounds Extracted from Diaphragma Juglandis Fructus, Walnut Pellicle, and Flowers of Juglans Regia Using Methanol, Ultrasonic Wave, and Enzyme Assisted-Extraction. Food Chem. 2020, 321, 126672. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, G.; Liu, X.; Yu, Z.; Peng, S. Integrated Analysis of Seed MicroRNA and MRNA Transcriptome Reveals Important Functional Genes and MicroRNA-Targets in the Process of Walnut (Juglans Regia) Seed Oil Accumulation. Int. J. Mol. Sci. 2020, 21, 9093. [Google Scholar] [CrossRef]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Study of the Main Constituents of Some Authentic Walnut Oils. J. Agric. Food. Chem. 2005, 53, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from Two Species of Walnut: Juglans Regia and Juglans Sigillata. Food Chem. 2019, 279, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Jin, J.; Liu, R.; Jin, Q.; Wang, X. Chemical Compositions of Walnut (Juglans regia L.) Oils from Different Cultivated Regions in China. J. Am. Oil Chem. Soc. 2018, 95, 825–834. [Google Scholar] [CrossRef]
- Lane, K.; Derbyshire, E.; Li, W.; Brennan, C. Bioavailability and Potential Uses of Vegetarian Sources of Omega-3 Fatty Acids: A Review of the Literature. Crit. Rev. Food Sci. Nutr. 2014, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Lemos, B.S.; Birk, J.; Rosenberg, D.W. The influence of walnut consumption on microbial urolithin metabolism. Gastroenterology 2020, 158, S-1218–S-1219. [Google Scholar] [CrossRef]
- Esselun, C.; Dieter, F.; Sus, N.; Frank, J.; Eckert, G.P. Walnut Oil Reduces Aβ Levels and Increases Neurite Length in a Cellular Model of Early Alzheimer Disease. Nutrients 2022, 14, 1694. [Google Scholar] [CrossRef]
- Assunção, D.G.F.; da Silva, L.T.P.; Camargo, J.D.D.A.S.; Cobucci, R.N.; Ribeiro, K.D.D.S. Vitamin E Levels in Preterm and Full-Term Infants: A Systematic Review. Nutrients 2022, 14, 2257. [Google Scholar] [CrossRef]
- Khir, R.; Pan, Z. Walnuts. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 391–411. [Google Scholar]
- Nde, D.; Foncha, A. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Elhassan, S. Mechanical Expression of Oil from Sesame (Sesamum indicum L.); University of Khartoum: Khartoum, Sudan, 2009. [Google Scholar]
- Mohd Fuad, F.; Mat Don, M. Ultrasonic-Assisted Extraction of Oil from Calophyllum Inophyllum Seeds: Optimization of Process Parameters. J. Teknol. 2016, 78, 2180–3722. [Google Scholar] [CrossRef]
- Bimakr, M.; Ganjloo, A. Supercritical Carbon Dioxide Extraction of Bioactive Compounds. Food Nutr. J. 2016, 1, 21–27. [Google Scholar] [CrossRef]
- Olawale, O. Optimization and Predictive Capability of Rsm Using Controllable Variables in Azadiracha Indica Oilseeds Extraction Process. Int. J. Chem. Mater. Res. 2015, 3, 1–10. [Google Scholar] [CrossRef][Green Version]
- Mojtaba, A.; Fardin, K. Optimization of Enzymatic Extraction of Oil from Pistacia Khinjuk Seeds by Using Central Composite Design. Food Sci. Technol. 2013, 1, 37–43. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ghafoor, K.; Al Juhaimi, F.; Ahmed, I.A.M.; Babiker, E.E. Effect of Cold-Press and Soxhlet Extraction on Fatty Acids, Tocopherols and Sterol Contents of the Moringa Seed Oils. South Afr. J. Bot. 2019, 124, 333–337. [Google Scholar] [CrossRef]
- Rashed, M.M.A.; Tong, Q.; Abdelhai, M.H.; Gasmalla, M.A.A.; Ndayishimiye, J.B.; Chen, L.; Ren, F. Effect of Ultrasonic Treatment on Total Phenolic Extraction from Lavandula Pubescens and Its Application in Palm Olein Oil Industry. Ultrason. Sonochemistry 2016, 29, 39–47. [Google Scholar] [CrossRef]
- ISO 660; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2020.
- ISO 3960; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2017.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 7th ed.; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- El Moudden, H.; El Idrissi, Y.; El Guezzane, C.; Belmaghraoui, W.; El Yadini, A.; Harhar, H.; Tabyaoui, M. Tradition Mills’ Picholine Olive Oil Physicochemical Characterization and Chemical Profiling across Different Cities in Morocco. Sci. World J. 2020, 2020, 1804723. [Google Scholar] [CrossRef]
- ISO 9936; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. ISO: Geneva, Switzerland, 2016.
- Xu, L.; Liu, T.; Cao, H.; Zheng, L.; Zhu, C.; Karrar, E.; Ouyang, Y.; Shen, X. Influence of Different Extraction Methods on the Chemical Composition, Antioxidant Activity, and Overall Quality Attributes of Oils from Trichosanthes Kirilowii Maxim Seed. Food Control 2022, 142, 109201. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparison of Different Processing Methods of Iron Walnut Oils (Juglans Sigillata): Lipid Yield, Lipid Compositions, Minor Components, and Antioxidant Capacity. Eur. J. Lipid Sci. Technol. 2018, 120, 1800151. [Google Scholar] [CrossRef]
- Gaber, M.A.F.M.; Tujillo, F.J.; Mansour, M.P.; Juliano, P. Improving Oil Extraction from Canola Seeds by Conventional and Advanced Methods. Food Eng. Rev. 2018, 10, 198–210. [Google Scholar] [CrossRef]
- Mat Yusoff, M.; Gordon, M.H.; Niranjan, K. Aqueous Enzyme Assisted Oil Extraction from Oilseeds and Emulsion De-Emulsifying Methods: A Review. Trends Food Sci. Technol. 2015, 41, 60–82. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and Enzymatic Processes for Edible Oil Extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Sarmento, C.M.P.; Ferreira, S.R.S.; Hense, H. Supercritical Fluid Extraction (SFE) of Rice Bran Oil to Obtain Fractions Enriched with Tocopherols and Tocotrienols. Braz. J. Chem. Eng. 2006, 23, 243–249. [Google Scholar] [CrossRef]
- Boujemaa, I.; el Bernoussi, S.; Harhar, H.; Tabyaoui, M. The Influence of the Species on the Quality, Chemical Composition and Antioxidant Activity of Pumpkin Seed Oil. OCL-Oilseeds Fats Crops Lipids 2020, 27, 40. [Google Scholar] [CrossRef]
- Prescha, A. Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to ImproveOil Stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef]
- CAC. FAO/WHO Food Standards Programme Codex Alimentarius Commission. In Codex Alimentarius, 42nd ed.; CAC: Rome, Italy, 2019; pp. 2–7. [Google Scholar]
- Grilo, F.S.; Wang, S.C. Walnut (Juglans regia L.) Volatile Compounds Indicate Kernel and Oil Oxidation. Foods 2021, 10, 329. [Google Scholar] [CrossRef]
- el Bernoussi, S.; Boujemaa, I.; Harhar, H.; Belmaghraoui, W.; Matthäus, B.; Tabyaoui, M. Evaluation of Oxidative Stability of Sweet and Bitter Almond Oils under Accelerated Storage Conditions. J. Stored Prod. Res. 2020, 88, 101662. [Google Scholar] [CrossRef]
- Moodley, R.; Kindness, A.; Jonnalagadda, S.B. Elemental Composition and Chemical Characteristics of Five Edible Nuts (Almond, Brazil, Pecan, Macadamia and Walnut) Consumed in Southern Africa. J. Environ. Sci. Health B 2007, 42, 585–591. [Google Scholar] [CrossRef]
- Spitz, L. Glossary. In Soap Manufacturing Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 267–280. [Google Scholar] [CrossRef]
- Amin, M.Z.; Islam, T.; Mostofa, F.; Uddin, M.J.; Rahman, M.M.; Satter, M.A. Comparative Assessment of the Physicochemical and Biochemical Properties of Native and Hybrid Varieties of Pumpkin Seed and Seed Oil (Cucurbita maxima Linn.). Heliyon 2019, 5, e02994. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Markakis, P. Olive Oil: A Review. Adv. Food Res. 1988, 31, 453–482. [Google Scholar] [CrossRef]
- Boulfane, S.; Maata, N.; Anouar, A.; Hilali, S. Caractérisation Physicochimique Des Huiles d’olive Produites Dans Les Huileries Traditionnelles de La Région de La Chaouia-Maroc. J. Appl. Biosci. 2015, 87, 8022. [Google Scholar] [CrossRef]
- Wiesner, M.; Hanschen, F.S.; Maul, R.; Neugart, S.; Schreiner, M.; Baldermann, S. Nutritional Quality of Plants for Food and Fodder. In Encyclopedia of Applied Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 285–291. [Google Scholar]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.L.; Wang, T.; Inglett, G.E. Oil and Tocopherol Content and Composition of Pumpkin Seed Oil in 12 Cultivars. J. Agric. Food Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.L.; Mattea, M.A.; Maestri, D.M. Pressing and Supercritical Carbon Dioxide Extraction of Walnut Oil. J. Food Eng. 2008, 88, 399–404. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Hamedi, M.; Rezaei, K.; Khodaiyan, F. Evaluation of Physicochemical Properties and Antioxidant Activities of Persian Walnut Oil Obtained by Several Extraction Methods. Ind. Crops Prod. 2013, 45, 133–140. [Google Scholar] [CrossRef]
- Mariod, A.A.; Elkheir, S.; Ahmed, Y.M.; Matthäus, B. Annona Squamosa and Catunaregam Nilotica Seeds, the Effect of the Extraction Method on the Oil Composition. J. Am. Oil Chem. Soc. 2010, 87, 763–769. [Google Scholar] [CrossRef]
- Oliveira, R.; Rodrigues, M.F.; Bernardo-Gil, M.G. Characterization and Supercritical Carbon Dioxide Extraction of Walnut Oil. J. Am. Oil Chem. Soc. 2002, 79, 225–230. [Google Scholar] [CrossRef]
- Gao, P.; Cao, Y.; Liu, R.; Jin, Q.; Wang, X. Phytochemical Content, Minor-Constituent Compositions, and Antioxidant Capacity of Screw-Pressed Walnut Oil Obtained from Roasted Kernels. Eur. J. Lipid Sci. Technol. 2019, 121, 1800292. [Google Scholar] [CrossRef]
- Kafkas, E.; Burgut, A.; Ozcan, H.; Ozcan, A.; Sutyemez, M.; Kafkas, S.; Türemis, N. Fatty Acid, Total Phenol and Tocopherol Profiles of Some Walnut Cultivars: A Comparative Study. Food Nutr. Sci. 2017, 8, 1074–1084. [Google Scholar] [CrossRef]
- Ada, M.; Paizila, A.; Bİlgİn, Ö.F.; Attar, Ş.H.; Türemİş, N.F.; Kafkas, S.; Kafkas, N.E. Determination of Fat, Fatty Acids and Tocopherol Content of Several Turkish Walnut Cultivars. Int. J. Agric. For. Life Sci. 2021, 5, 94–100. [Google Scholar]
- Nasri, C.; Halabi, Y.; Harhar, H.; Mohammed, F.; Bellaouchou, A.; Guenbour, A.; Tabyaoui, M. Chemical Characterization of Oil from Four Avocado Varieties Cultivated in Morocco. OCL 2021, 28, 19. [Google Scholar] [CrossRef]
- de Oliveira, C.; de Mattos, A.B.M.; Silva, C.B.R.; Mota, J.F.; Zemdegs, J.C.S. Nutritional and Hormonal Modulation of Adiponectin and Its Receptors AdipoR1 and AdipoR2. Vitam. Horm. 2012, 90, 57–94. [Google Scholar] [CrossRef]
- Manchanda, S.; Chandra, A.; Bandopadhyay, S.; Deb, P.K.; Tekade, R.K. Formulation Additives Used in Pharmaceutical Products. In Dosage Form Design Considerations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 773–831. [Google Scholar]
| CP | SE | UE | |
|---|---|---|---|
| AV (mg KOH/g) | 1.52 ± 0.09 a | 3.90 ± 0.02 b | 2.13 ± 0.06 c |
| FFA (%) | 0.76 ± 0.11 a | 1.95 ± 0.02 b | 1.07 ± 0.03 a |
| PV (meq O2/kg) | 4.14 ± 0.09 a | 5.12 ± 0.34 b | 4.95 ± 0.27 b |
| IV (mg I2/100 g) | 152.27 ± 0.13 a | 151.45 ± 0.17 a | 151.83 ± 0.11 a |
| SV (mg KOH/1 g) | 189.0 ± 0.12 a | 190.18 ± 0.23 a | 191.42 ± 0.33 a |
| Chlorophyll | 0.13 ± 0.01 a | 0.20 ± 0.02 b | 0.16 ± 0.01 b |
| Carotenoid | 14.83 ± 0.61 a | 10.11 ± 0.43 b | 12.46 ± 0.56 c |
| K232 | 1.30 ± 0.05 a | 2.73 ± 0.07 b | 1.77 ± 0.05 c |
| K270 | 0.12 ± 0.02 a | 2.49 ± 0.08 b | 0.96 ± 0.02 c |
| CP (%) | SE (%) | UE (%) | Std (%) | |
|---|---|---|---|---|
| Myristic acid (C14:0) | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | ND |
| Palmitic acid (C16:0) | 7.76 ± 0.11 a | 7.89 ±0.13 a | 7.89 ±0.08 a | 6.0–8.0 |
| Palmitoleic acid (C16:1) | 0.18 ±0.09 a | 0.19 ±0.03 a | 0.21 ±0.07 a | ND–0.4 |
| Heptadecanoic acid (C17:0) | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | ND–0.1 |
| Heptadecenoic acid (cis-10) (C17:1) | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | ND–0.1 |
| Stearic Acid (C18:0) | 2.52 ± 0.06 a | 2.52 ± 0.07 a | 2.88 ±0.09 a | 1.0–3.0 |
| Oleic Acid (C18:1) | 16.83 ±0.21 a | 16.38 ± 0.18 a | 16.80 ±0.15 a | 14.0–23.0 |
| Linoleic Acid (C18:2) | 57.84 ±0.18 a | 57.58 ±0.10 a | 57.08 ± 0.09 a | 54.0–65.0 |
| Linolenic Acid (C18:3) | 15.11 ± 0.10 a | 14.51 ±0.12 b | 14.84 ±0.07 a,b | 9.0–15.4 |
| Arachidic acid (C20:0) | 0.09 ± 0.04 a | 0.09 ±0.07 a | 0.09 ± 0.03 a | ND–0.3 |
| Gadoleic Acid (C20:1) | 0.13 ± 0.02 a | 0.13 ± 0.03 a | 0.13 ± 0.03 a | ND–0.3 |
| (SFAs) | 10.44 | 10.57 | 10.93 | |
| (MUFAs) | 17.16 | 16.72 | 17.61 | |
| (PUFAs) | 72.35 | 69.37 | 71.92 | |
| (M/S) | 1.64 | 1.58 | 1.57 | |
| (P/S) | 6.93 | 6.88 | 6.58 |
| CP | SE | UE | ||||
|---|---|---|---|---|---|---|
| mg/kg | % | mg/kg | % | mg/kg | % | |
| Cholesterol | 5.35 ± 0.17 a | 0.41 ± 0.12 a | 4.33 ± 0.14 b | 0.37 ± 0.10 a | 4.44 ± 0.13 b | 0.38 ± 0.09 a |
| Campesterol | 68.31 ± 0.13 a | 5.23 ± 0.09 a | 58.10 ± 0.11 b | 4.96 ± 0.08 a | 58.31 ± 0.10 b | 4.99 ± 0.07 a |
| Stigmasterol | 3.27 ± 0.06 a | 0.25 ± 0.04 a | 1.99 ± 0.04 b | 0.17 ± 0.03 a | 3.86 ± 0.03 c | 0.33 ± 0.02 a |
| Clerosterol | 8.36 ± 0.14 a | 0.64 ± 0.10 a | 7.84 ± 0.10 a | 0.67 ± 0.07 a | 6.78 ± 0.08 b | 0.58 ± 0.06 a |
| β-Sitosterol | 1113.52 ± 0.17 a | 85.26 ± 0.12 a | 1005.79 ± 0.16 b | 85.87 ± 0.11 a | 989.18 ± 0.11 c | 84.65 ± 0.08 a,b |
| Δ5-Avenasterol | 83,72 ± 0.07 a | 6.41 ± 0.05 a | 74.61 ± 0.08 b | 6.37 ± 0.06 a | 73.62 ± 0.06 c | 6.30 ± 0.04 a |
| Δ7-Stigmasterol | 0.91 ± 0.04 a | 0.07 ± 0.03 a | 1.05 ± 0.06 a | 0.09 ± 0.04 a | 16.83 ± 0.06 b | 1.44 ± 0.04 b |
| Δ7-Avenasterol | 1,57 ±0.07 a | 0.12 ± 0.05 a | 0.82 ± 0.04 b | 0.07 ± 0.03 a | 1.64 ± 0.03 a | 0.14 ± 0.02 a |
| Total (mg/Kg) | 1306.03 ± 0.16 a | 1171.29 ± 0.30 b | 1168.55 ± 0.20 c | |||
| α-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total | ||||
|---|---|---|---|---|---|---|---|
| mg/Kg | % | mg/Kg | % | mg/Kg | % | mg/Kg | |
| CP | 15.75 ± 0.20 a | 1.85 ± 0.09 a | 754.83 ± 0.14 a | 88.63 ± 0.08 a | 81.06 ± 0.06 a | 9.52 ± 0.02 a | 851.64 ± 0.28 a |
| SE | 7.40 ± 0.13 b | 1.63 ± 0.02 a | 408.90 ± 0.11 b | 89.87 ± 0.05 b | 38.66 ± 0.14 b | 8.50 ± 0.06 b | 454.96 ± 0.38 b |
| UE | ND | ND | 128.32 ± 0.08 c | 87.71 ± 0.04 c | 17.99 ± 0.20 c | 12.30 ± 0.09 c | 146.31 ± 0.12 c |
| F1 | F2 | |
|---|---|---|
| Eigenvalue | 9.802 | 3.198 |
| Variability (%) | 75.402 | 24.598 |
| % Cumulative | 75.402 | 100.000 |
| Variables | AV | FFA | PV | IV | SV | Chlorophyll | Carotenoid | K232 | K270 | T. Sterol | T. Tocopherol | SFA | UFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV | 1 | ||||||||||||
| FFA | 1.000 | 1 | |||||||||||
| PV | 0.807 | 0.806 | 1 | ||||||||||
| IV | −0.951 | −0.951 | −0.950 | 1 | |||||||||
| SV | 0.235 | 0.234 | 0.764 | −0.524 | 1 | ||||||||
| Chlorophyll | 0.982 | 0.982 | 0.904 | −0.992 | 0.414 | 1 | |||||||
| Carotenoid | −0.963 | −0.962 | −0.937 | 0.999 | −0.490 | −0.996 | 1 | ||||||
| K232 | 0.997 | 0.997 | 0.848 | −0.971 | 0.306 | 0.993 | −0.980 | 1 | |||||
| K270 | 0.994 | 0.994 | 0.864 | −0978 | 0.336 | 0.996 | −0.986 | 1.000 | 1 | ||||
| T. Sterol | −0.687 | −0.686 | −0.984 | 0.878 | −0.868 | −0.812 | 0.858 | −0.739 | −0.760 | 1 | |||
| T. Tocopherol | −0.318 | −0.317 | −0.817 | 0.595 | −0.996 | −0.491 | 0.563 | −0.387 | −0.416 | 0.907 | 1 | ||
| SFA | −0.013 | −0.014 | 0.581 | −0.297 | 0.969 | 0.176 | −0.258 | 0.061 | 0.092 | −0.717 | −0.944 | 1 | |
| UFA | −0.967 | −0.968 | −0.630 | 0.841 | 0.019 | −0.902 | 0.862 | −0.946 | −0.935 | 0.480 | 0.067 | 0.266 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elouafy, Y.; El Yadini, A.; El Moudden, H.; Harhar, H.; Alshahrani, M.M.; Awadh, A.A.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A.; Tabyaoui, M. Influence of the Extraction Method on the Quality and Chemical Composition of Walnut (Juglans regia L.) Oil. Molecules 2022, 27, 7681. https://doi.org/10.3390/molecules27227681
Elouafy Y, El Yadini A, El Moudden H, Harhar H, Alshahrani MM, Awadh AAA, Goh KW, Ming LC, Bouyahya A, Tabyaoui M. Influence of the Extraction Method on the Quality and Chemical Composition of Walnut (Juglans regia L.) Oil. Molecules. 2022; 27(22):7681. https://doi.org/10.3390/molecules27227681
Chicago/Turabian StyleElouafy, Youssef, Adil El Yadini, Hamza El Moudden, Hicham Harhar, Mohammed Merae Alshahrani, Ahmed Abdullah Al Awadh, Khang Wen Goh, Long Chiau Ming, Abdelhakim Bouyahya, and Mohamed Tabyaoui. 2022. "Influence of the Extraction Method on the Quality and Chemical Composition of Walnut (Juglans regia L.) Oil" Molecules 27, no. 22: 7681. https://doi.org/10.3390/molecules27227681
APA StyleElouafy, Y., El Yadini, A., El Moudden, H., Harhar, H., Alshahrani, M. M., Awadh, A. A. A., Goh, K. W., Ming, L. C., Bouyahya, A., & Tabyaoui, M. (2022). Influence of the Extraction Method on the Quality and Chemical Composition of Walnut (Juglans regia L.) Oil. Molecules, 27(22), 7681. https://doi.org/10.3390/molecules27227681









