Abstract
The optimization of the production of thermoplastic starch (TPS) bionanocomposite films for their potential application in food packaging was carried out, according to the Box–Wilson Central Composite Design (CCD) with one center point, using Response Surface Methodology (RSM) and fillers based on lignin and nanofiber, which were derived from bamboo plant. The effects of the fillers on the moisture absorption (MAB), tensile strength (TS), percent elongation (PE) and Young’s modulus (YM) of the produced films were statistically examined. The obtained results showed that the nanocomposite films were best fitted by a quadratic regression model with a high coefficient of determination (R2) value. The film identified to be optimum has a desirability of 76.80%, which is close to the objective function, and contained 4.81 wt. % lignin and 5.00 wt. % nanofiber. The MAB, TS, YM and PE of the identified film were 17.80%, 21.51 MPa, 25.76 MPa and 48.81%, respectively. The addition of lignin and cellulose nanofiber to starch composite was found to have reduced the moisture-absorption tendency significantly and increased the mechanical properties of the films due to the good filler/matrix interfacial adhesion. Overall, the results suggested that the produced films would be suitable for application as packaging materials for food preservation.
1. Introduction
Synthetic plastics have dominated every field of human activity, particularly the packaging industries [1]. Despite their many merits, synthetic plastics have been a major environmental concern for some time. Since they are non-biodegradable and also dependent upon a non-renewable petroleum resource, the blooming usage of these plastics has caused grave energy crises, as well as environmental pollution associated with their disposal, including damage to the eco-system, water supplies and sewer systems, as well as rivers and streams. As a result, great attention is being drawn to natural polymers, e.g., starch, due to its potential as a resource for making environmentally friendly useable products to replace those derived from petroleum. The reason for starch being favored for this purpose is because it is inherently biodegradable, renewable, cost effective and available from many plants. In order to make it suitable for industrial application, such as packaging, starch is usually processed in the presence of a plasticizer to obtain a product called thermoplastic (TPS). However, TPSs alone cannot be employed for packaging because they have low mechanical strength and high water sensitivity [2], which falls below the requirements of a packaging material. Thus, in order to overcome these drawbacks, there is the need for the incorporation of reinforcing fillers, such as cellulosic fibers, whiskers, nanofibers, etc., to produce new and inexpensive starch biocomposites with improved properties [3,4,5,6,7,8]. Lignin and nanofibers are potential fillers which are currently in great demand due to their natural abundance and susceptibility to biodegradability, being derived from plants. The term “lignin”, from the Latin word lignum, meaning “wood”, was first used by Swiss botanist Candolle [9]. Lignin, a by-product that is mainly obtained from pulp and the paper industry, is the largest aromatic polymer in nature. Although its exact molecular structure is still subject to controversy, lignin is believed to result from the dehydrogenative polymerization of three monomer species, namely p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol. The amounts and proportions of the main functional reactive groups such as hydroxyl, methoxyl, carboxyl and carbonyl groups in lignin vary according to the plant species and extraction processes applied. Together with cellulose and hemicellulose, lignin forms the structural components of trees and various plants, and constitutes the most common natural polymers from plants. When lignin and cellulosic fibers are used as fillers in natural polymers, the resulting products are eco-friendly and have improved physical properties. Organic membranes prepared from cellulose derivatives usually have low mechanical strength as well as poor resistance to oxidation. In order to overcome these limitations, cellulose-based films make use of lignin [10]. The use of lignin and/or cellulosic fibers to improve the properties of TPS intended to be processed as packaging materials has been reported by a number of researchers. For example, in a study carried out by Kaushik et al. [11], cellulose nanofibrils were extracted from wheat straw using steam explosion, acidic treatment and high-shear mechanical treatment. These nanofibrils were dispersed in thermoplastic starch (TPS) using a Fluko high-shear mixer in varying proportions, and films were casted out of these nanocomposites. The results showed that the mechanical properties increased with the nanofiber concentration. The barrier properties also improved with the addition of nanofillers up to 10%, but further addition deteriorated the properties due to possible fiber agglomeration. In order to improve the mechanical properties and the resistance to water absorption of thermoplastic starch (TPS), Kaewtatip and Thongmee [12] employed kraft lignin (KL) and esterified lignin (EL) as fillers for the TPS matrix. EL was produced via esterification of the KL hydroxyl groups. The TPS/lignin composites were prepared using compression molding. The amount of each of the lignins used in the composites was 5 wt. % (dry starch basis). The TPS and composites were investigated using Fourier-transform infrared spectroscopy (FTIR), water absorption and tensile testing. The FTIR spectra showed that the interaction between the TPS and each lignin caused the peak of the OH stretching shift to lower the wavenumber. This result indicated that both the TPS/KL and the TPS/EL composites had improved mechanical properties over TPS. The tensile strength of the TPS/KL and TPS/EL composites was higher than for the TPS by about 17% and 32%, respectively. In addition, the presence of lignins in the TPS matrix significantly decreased the water-absorption properties. The combination of lignin and cellulosic fiber in the production of a new TPS composite was created by Narchamnan and Sakdaronnarong [13], using a laccasse-mediator system to enhance the binding efficiency of natural fibers and lignin compounds into a cassava starch composite matrix. In this work, violuric acid (VA) was tested for its effect as a mediator for laccase treatment. The influence of different fiber, lignin and water contents of the biocomposite was statistically investigated. The results showed that adding 15% (w/w) fibers into biocomposite at 44% (w/w) water content increased the flexural strength and modulus by four times compared with the control. A combination of fibers + VA gave the greatest enhancement of the modulus at 1140% and flexural strength at 375.8%, as much as neat starch biocomposite. The presence of fibers, lignin and VA as mediators for laccase treatment substantially enhanced the water resistance of starch biocomposite, which was detected by a change in the water drop contact angle on the biocomposite surface.
So far, studies based on the simultaneous incorporation of lignin and cellulose nanofibers into thermoplastic starch composite film, besides the one referenced above, have not been reported in the literature. Moreover, by changing the sources of the lignin and fibers used in TPS, new biocomposites with unique properties can result. In this work, the lignin and nanofibers were obtained from bamboo plant (Bambusa vulgaris). Further to this, work on the optimization of the experimental variables for the development of flexible films from starch for food packaging is limited, hence, the justification for the present study. Therefore, the objective of this research is to produce a lignin–cellulose nanofiber-filled thermoplastic starch composite film, with the optimization of key process variables, for potential application in food packaging.
2. Results and Discussion
2.1. Film Morphologies
The SEM micrographs of the pure starch (representing the negative control sample) and that of lignin–cellulose nanofiber-filled thermoplastic starch composite film (the target sample) are shown in Figure 1 and Figure 2, respectively. The SEM micrograph of the pure starch, as seen (Figure 1), showed the characteristic near-spherical morphology of the cassava starch granules with varying sizes [14]. The micrograph showed that the starch granules were generally in the order of sizes that ranged between 2 and 7 µm. This morphology is, however, destructured during the starch gelatinization process, subsequent to the incorporation of the fillers, in order to pave the way for good mixing in the composite formation stage. In Figure 2, the SEM micrograph shows that the fillers (lignin and cellulose nanofiber) were homogeneously distributed within the matrix of the starch nanocomposite film, a situation that usually results in improved mechanical properties. The white dots with varying sizes on the composite are considered as the fillers embedded in the starch matrix. The sizes of the cellulose nanofibers and lignin were evaluated using transmission electron microscopy (TEM), and their respective micrographs were as shown in Figure 3a,b. It was observed that the sizes of the cellulose nanofibers varied from 20 to 100 nm, while those of lignin fell between 20 and 200 nm. The determined characteristics of the used cellulose nanofiber are hereby shown in Table 1.
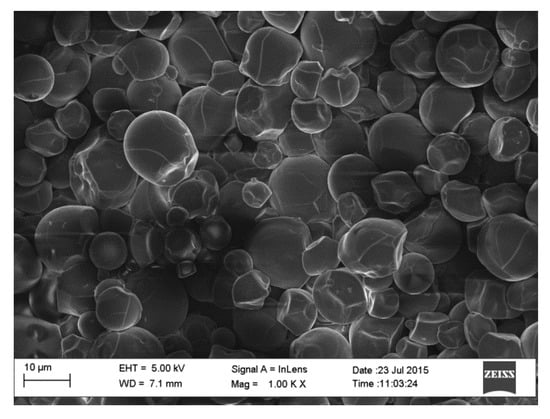
Figure 1.
SEM micrograph of pure cassava starch film.
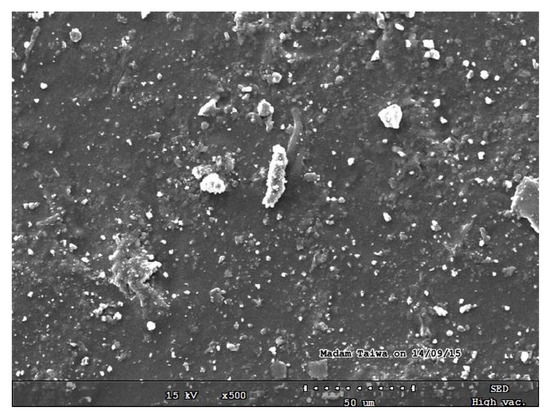
Figure 2.
SEM micrograph of lignin–cellulose nanofiber-filled thermoplastic starch composite film.
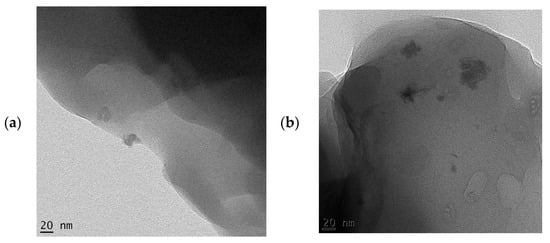
Figure 3.
TEM micrographs of (a) cellulose nanofiber; (b) lignin fillers.

Table 1.
The characteristics of the used cellulose nanofiber prepared from bamboo (Bambusa vulgaris schrad).
2.2. Development of Regression Model Equations by Central Composite Design (CCD) for Lignin–Cellulose Nanofiber-Filled Thermoplastic Starch Composite Film
The quadratic models, in terms of the actual factors used in achieving the desired optimum or ideal films, were obtained from an analysis of variance (ANOVA) (Table 2, Table 3, Table 4 and Table 5), and are given in Equations (1)–(4). The positive signs in the models indicate synergetic effects, whereas the negative signs, antagonistic effects. The model for predicting the moisture-absorption capacity of the lignin–cellulose nanofiber-filled thermoplastic starch composite film in terms of actual factors is given in Equation (1).
where: MAB = moisture-absorption capacity, l = lignin (wt. %), n = nanofiber (wt. %).

Table 2.
ANOVA for response surface quadratic model for MAB of lignin–cellulose nanofiber-filled thermoplastic starch composite film.

Table 3.
ANOVA for response surface quadratic model for TS of lignin–cellulose nanofiber-filled thermoplastic starch composite film.

Table 4.
ANOVA for response surface quadratic model for YM lignin–cellulose nanofiber-filled thermoplastic starch composite film.

Table 5.
ANOVA for response surface quadratic model for PE of lignin–cellulose nanofiber-filled thermoplastic starch composite film.
The model was significant at a p-value of 0.0048, and the coefficient of determination (R2) of 0.9102 was obtained. This indicates that the statistical model could explain 91.02% of the variability, while the remaining 8.98 wt. % could not be accounted for by the independent variables [15]. The predicted of 64.30% reasonably agrees with the adjusted (85.63%), as should normally be the case for model adequacy due to the very small blocking effect of the data used. The precision of the model is high since the adequate precision value obtained was ~11.156 [16].
The model for predicting the percentage elongation of the lignin–cellulose nanofiber-filled thermoplastic starch composite film in terms of actual factors is given in Equation (2).
where: PE = elongation (wt. %); l and n retained their usual meaning.
The model was significant at a p-value of 0.1792, and the coefficient of determination (R2) of 0.8449 was obtained. This indicates that the model could describe 84.49 wt. % variability, while 15.51 wt. % was inexplicable by the independent variables [15]. In addition, the negative predicted value of −82.39 wt. % implied that the fitted model, though precise (with adequate precision value of 5.37), is not a suitable predictor of the percentage elongation of the starch composite film.
The model for predicting the tensile strength of the lignin–cellulose nanofiber-filled thermoplastic starch composite film with respect to the actual factors is given in Equation (3).
where: TS = tensile strength (MPa); l and n retained their usual meaning.
The model was significant at a p-value of 0.0045, and the coefficient of determination (R2) of 0.8346 was obtained. This indicates that the statistical model could explain the 83.46% value of the variability, while a 16.54% value was inexplicable by the independent variables [15]. In addition, the predicted of 61.72 wt. % agrees with the (adjusted) of 77.94%, as should normally be the case for adequate model. The model is precise in its capacity to predict the tensile strength of the starch mixture film, since an adequate precision value of 10.91 was recorded [16].
The model for predicting the Young’s modulus of the lignin–cellulose nanofiber-filled thermoplastic starch composite film in terms of actual factors is given in Equation (4).
where: YM = Young’s modulus (MPa); l and n retained their usual meaning.
The model was found to be significant at a 0.0001 p-value, and the coefficient of determination (R2) of 0.9501 was obtained. This indicates that the model could explain 95.01% of the variability, while 4.99% was inexplicable by the independent variables [15]. The predicted value of 91.81% reasonably agrees with the adjusted value of 93.34% for the adequate model, and this was caused by the small block effect of the data used in generating the model. In addition, the model is precise in its capability to forecast the lignin–cellulose nanofiber-filled thermoplastic starch composite film’s Young’s modulus, since the adequate precision recorded value was 20.51 [16].
2.3. Analyses of Response Surfaces
The 3D response surface plots of the joint effects of the independent variables (lignin and cellulose nanofiber) on the MAB, TS, YM and PE of lignin–cellulose nanofiber-filled thermoplastic starch composite film, as performed according to the working conditions stated in Table 6, are presented in Figure 4. The 3D response surfaces were plotted in order to investigate the probable interactions among the variables, and to determine the optimum conditions of each factor for the minimum moisture absorption and maximum strength of the starch nanocomposite films.

Table 6.
RSM factorial design matrix for lignin–cellulose nanofiber-filled thermoplastic starch composite film.
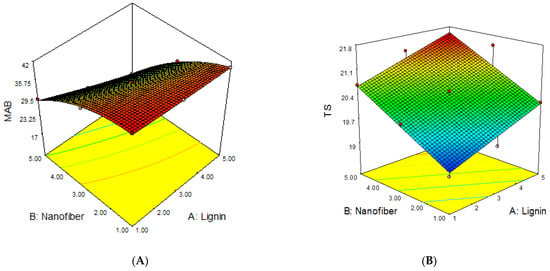
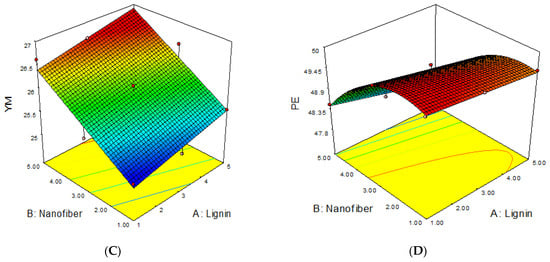
Figure 4.
The influence of filler contents on (A) MAB, (B) TS, (C) YM and (D) PE of the lignin–cellulose nanofiber-filled thermoplastic starch composite film.
The response surface plots for the influence of lignin and cellulose nanofiber contents on the MAB, TS, YM and PE of the lignin–cellulose nanofiber-filled thermoplastic starch composite film are presented in Figure 4.
The results revealed that the inclusion of cellulose nanofiber content had the most significant effect on the moisture absorption and mechanical properties of the lignin–cellulose nanofiber-filled thermoplastic starch composite film, followed by lignin inclusion content. The MAB reduced rapidly as the cellulose nanofiber content increased when compared to that of lignin content. The lignin and cellulose nanofiber contents interacted negatively. This shows that the moisture absorption reduced as the lignin and cellulose nanofiber contents increased. The decrease in MAB with the increase in the lignin and cellulose nanofiber contents was because of the strong filler–matrix interaction, which reduced the molecular mobility and diffusivity in the matrix material, thus, limiting the degree of moisture uptake [17]. This result is in accordance with the findings of Khan et al. [18], Mitchelle [19], and Taghizedeh and Sabouri [20], who reported low moisture-absorption rates for cocoyam, tapioca and modified corn starch films, respectively, with an increase in filler loading.
The results also showed that the TS and YM of the starch nanocomposite film increased as the lignin and cellulose nanofiber contents increased, while PE decreased with the increase in lignin and cellulose nanofiber contents. The significant increase in the TS and YM of the lignin–cellulose nanofiber-filled thermoplastic starch composite film with the increase in filler contents, particularly nanofiber, was due to the small sizes (nano-range), smooth surface and large surface area of these fibers, which produced a good fiber/matrix interaction, thus, improving the composite film’s strength [21]. It might also be that lignin improved the compatibility between the starch and cellulose nanofiber [22]. Moreover, Suarez et al. [23] also reported the fact that a good interfacial region increases stress transfer efficiency from the matrix to the fillers, thereby increasing the composite’s strength.
This result corroborated with that of Wang et al. [22], who confirmed a rise in the TS and YM, but a decrease in the elongation-at-break of a PLA nanocomposite reinforced with lignin–cellulose nanofiber (L-CNF), as the L-CNF content rose from 25 to 35 wt. %. Patpen et al. [24] also reported the effect of cellulose addition on the TS of polylactic acid biocomposite; they observed an increase in this parameter as cellulose loading increased. Tawakkal et al. [25] also reported how kenaf-derived cellulose (KDC)-loaded polylactic affected the material’s tensile properties and documented an improvement in both TS and tensile modulus, as KDC loading increased from 30 to 60 wt. %. On the contrary, Sawpan et al. [26] studied the mechanical properties of hemp fiber-reinforced PLA biocomposites and established a non-linear relationship between TS and fiber content, indicating that the strength of the biocomposite somewhat decreased as the fiber content increased from 0 to 40 wt. %.
2.4. Optimization of Lignin–Cellulose Nanofiber-Filled Thermoplastic Starch Composite Film Production
The result of the optimization of the experimental variables (lignin and cellulose nanofiber contents), showing the desirability function, with respect to the films prepared, is shown in Figure 5. The goal for both the water barrier and mechanical optimization was to minimize the moisture absorption and improve the mechanical property; thus, the target value of the responses, as obtained from the obtained experimental results, was at the lowest and uppermost values, respectively. The result from the optimization showed that increases in the lignin and cellulose nanofiber contents, from 1 to 5 wt. %, significantly affected the desirability of the films. Therefore, it is obvious from the optimization result that the selected film with a desirability closer to the goal is the one that was produced with the blend of 4.81 wt. % lignin and 5.00 wt. % nanofiber at 76.80% desirability. The corresponding MAB, TS, YM and PE of the selected film, apparently the optimum sample, are 17.80%, 21.51 MPa, 25.76 MPa and 48.81%, respectively. This finding is similar to that of Wang et al. [22], who documented optimum values of 21.6 MPa and 21.6% for the TS and elongation, respectively, for PLA composites reinforced with L-CNF. Akbar et al. [27], however, obtained optimum values of 50 MPa for the TS, 2.15 MPa for the YM and 141.07% for elongation, during the optimization of polyvinyl alcohol nanocomposite films. Patpen et al. [24] also reported an optimum value of 46.207 MPa for the optimization of PLA-based biocomposites that were reinforced with cellulose obtained from durian peel.
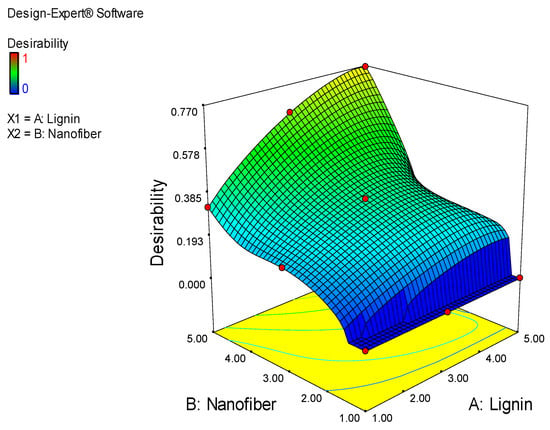
Figure 5.
Desirability function of selecting the ideal film prepared.
This study thus affirms that lignin and cellulose nanofiber addition to starch composite minimized MAB and improved the mechanical properties of the produced films because of the good filler/matrix interfacial adhesion, as evident by the SEM micrograph. Hence, the produced films can be applied to package food.
3. Materials and Methods
3.1. Plant Materials
Cassava (Manihot esculenta crantz) was purchased from a farm in Minna, Nigeria, whereas bamboo (Bambusa vulgaris schrad) was collected from the river banks of Gurara in Izom, Niger State. They were identified and authenticated by a botanist at the National Institute for Pharmaceutical Research and Development (NIPRD) Idu, Abuja. Specimen voucher numbers (NIPRD/H/6792 and NIPRD/H/6793), respectively, were placed at their herbarium for references in the future.
3.2. Chemicals
Analytical grade chemicals were employed for the study and included ethanol (BDH chemicals, London, UK); acetic anhydride (Sigma Aldrich, Burlington, MA); sodium hydroxide (Kermel, China); citric acid (BDH chemicals, UK); hydrochloric acid (Griffin and George, UK); sulfuric acid (BDH chemicals, UK); hydrogen peroxide (BDH chemicals, UK); sodium sulphate (BDH chemicals, UK); and ammonium hydroxide (Griffin and George, London, UK). Glycerol (BDH chemicals, UK) was used as the plasticizer for the study.
3.3. Preparation Lignin and Cellulose Nanofiber from Bamboo (Bambusa vulgaris Schrad)
Lignin was extracted from bamboo according to standard procedure, as reported by Yong et al. [28], Alemdar and Sain [29] and Ming-Fei et al. [30]. The bamboo stalks obtained were first sun-dried and then chipped into small pieces. The sun-dried pieces of bamboo were ground and screened to obtain a 40–60 µm mesh fraction. The ground bamboo stalk (20 g) was first soaked in NaOH (4% w/w) at room temperature for 24 h. It was then filtered and washed with distilled water (1 L) until it was free of alkali. The residue was re-dispersed in 1 L of distilled water, filtered again and treated with 10% (w/w) NaOH at 121 °C in an autoclave for 4 h. Furthermore, the residue was again washed in distilled water to free it of residual alkali (1 L) and filtered. Lignin was precipitated from the filtrate by acidifying it to pH 2 with H2SO4. The precipitates were separated from the mixture by filtration. The separated lignin was washed with water several times and then oven-dried at 40 °C. In order to obtain bamboo fiber, the supernatant liquid left after the alkali treatment was bleached in 8% (v/v) H2O2 at room temperature for 24 h. Finally, the material was again washed and filtered as before to obtain bamboo fiber. The bamboo fiber was then converted to nanofiber using acid hydrolysis [31]. The bamboo fibers obtained after lignin removal were steeped in HCl (10% w/w) with ultrasonic agitation at 60 °C for 2 h using an Ultrasonicator (SB25-12DT, Scientz, Ningbo, China). The material was given a final wash and then placed in a high shear homogenizer (Heidolph DIAX 900, Burladingen, Germany for 15 min to produce bamboo nanofibers.
3.4. Experimental Design and Optimization of Starch Nanocomposite Film Production
Design Expert software (version 7.0, Start-Ease Inc., Minneapolis, MN, USA) was employed for the experimental design, while Response Surface Methodology (RSM) was used for optimizing the conditions required for preparing the starch nanocomposite films. As a result, 1 center-point Box–Wilson Central Composite Design (CCD) was utilized. After the designed experiment was performed, linear regression was used to obtain the results. The design consisted of 9 experimental runs. The RSM considered the effect of two variables: lignin content (wt. %) and cellulose nanofiber content (wt. %) used as fillers in the nanocomposite preparation with 5 levels each. The response functions measured were moisture absorption (MAB), tensile strength (TS), percent elongation (PE) and Young’s modulus (YM). An analysis of variance (ANOVA) was employed to analyze the obtained data, in order to determine the interactions that exist between the process variables and the responses. Accurate and proper models were picked at p < 0.05 and had a significantcorrelation. The fitting model’s quality was expressed by the coefficient of determination R2 and adjusted R2. The factors’ level with their codings are shown in Table 7.

Table 7.
Experimental variables and their coded levels of variables levels for CCD.
In the optimization selection, two factors (lignin and cellulose nanofiber contents) were considered in order to build desirability indices. The objective was to reduce the MAB while improving TS, PE and YM; therefore, the target value of the responses was lowest for MAB and highest for TS, PE and YM from the experimental results obtained.
3.5. Preparation of Lignin–Cellulose Nanofiber-Filled Thermoplastic Starch Composite Film
Cassava starch (2 g) granules were dispersed in 50 mL of distilled water and heat was applied at 70 °C for 20 min under constant stirring over a magnetic stirrer. Glycerol (50 wt. % based on dry cassava starch content) was added to the dispersion while the heating at 70 °C was continued under constant stirring speed for the next 2 min. Next, the lignin and cellulose nanofiber (varied wt. %, with respect to the dry cassava starch content, and based on the statistical formulation of the Central Composite Design adopted) were added to the dispersion under the same conditions for another 2 min. Before being introduced into the plasticized starch mixture, the cellulose nanofibers were sonicated for 10 min by using a 60 W rated Sonicator. The mixture of dispersion was then cast into a mold and oven-dried at 50 °C using a still-air oven for 18 h, in order to obtain dry lignin–cellulose nanofiber-filled thermoplastic starch composite films [32,33], whose average thickness was found to be 0.12 mm. The control (TPS) sample was also prepared using the same process mentioned above, except that there were no fillers added to it. All films were conditioned at 55 ± 5% RH and 25 ± 2 °C before testing their permeability and mechanical properties, as described by the ASTM standard D882-09 [34] and Detduangchan et al. [35]. The conditioning was performed by inserting the films into desiccators containing a saturated solution of Mg (NO3)2•6H2O for 72 h. Part of the films were tested for water absorption, TS, PE and YM, and the rest, which were for other tests, were kept in plastic bags and inserted into desiccators.
3.6. Characterization of Lignin–Cellulose Nanofiber-Filled Thermoplastic Starch Composite Film Water-Absorption Test
The film pieces (20 mm × 20 mm) were pre-conditioned by drying in the oven at 50 °C for 24 h and then weighed to determine the dry weight. They were then immersed in a bath containing distilled water at room temperature. The film samples were removed from distilled water after intervals of 1, 3, 5, 7, 9 and 11 h and, after wiping off the excess water on their surfaces with tissue, their weights were determined. The water-absorption capability (WAC) was, thus, calculated using Equation (5) [32,36]:
where: Wwet = Wet specimen weight and Wdry represents the dry specimen weight.
3.7. Mechanical Properties
The TS, PE and the YM values were determined with a universal testing machine (DBBMTCL-2500kg Testometric, Rochdale, UK), according to the ASTM D882 Standard [34]. Prior to testing, each sample was conditioned at a temperature of 25 °C and a relative humidity (RH) of 55% for 24 h. The average thickness of the samples was about 0.12 mm. The tensile test was carried out using 1.3 mm/min crosshead speed. Each of the determinations was obtained from triplicate specimens.
3.8. Scanning Electron Microscopy (SEM)
The morphological structures of the lignin–cellulose nanofiber-filled thermoplastic starch composite films were determined using SEM (Zeiss Auriga HRSEM) at an accelerating voltage of 15 kV. The as-prepared samples were, respectively, placed on a stub with a double-sided adhesive tape, after which they were coated with a thin layer of gold. The micrographs were captured using a magnification of 350 times the original specimen size [33,37].
3.9. Statistical Analysis
Design Expert Software (version 7.0) was employed to analyze the obtained data. The determination of interaction effects between the factors and a quadratic surface plot was generated using an analysis of variance (ANOVA). The model adequacy was examined using the ANOVA, a normal probability plot and a residual plot, according to the method described in the literature [38]. An F-Test was also employed to determine the model’s statistical significance and the regression coefficients’ significance.
4. Conclusions
In conclusion, the film with a desirability of ~76.80%—which is closest to the objective function—and containing 4.81 wt. % lignin and 5.00 wt. % cellulose nanofiber, was selected as the optimum sample. The MAB, TS, YM and PE of the selected film were 17.80%, 21.51 MPa, 25.76 MPa and 48.81%, respectively. This film presented the maximum mechanical potency and minimal moisture-absorption capacity. The addition of lignin and cellulose nanofiber concurrent to the TPS matrix evidently caused a decrease in moisture absorption, while at the same time an improvement in the mechanical properties of the films. Consequently, the prepared films have the potential to be employed as films for packaging foods.
Author Contributions
Conceptualization and methodology, E.C.E. and S.S.O.; software and validation, E.C.E. and T.A.-A.; formal analysis, investigation, resources, data curation writing—original draft preparation, T.A.-A.; writing—review and editing, All; visualization, supervision and project administration, E.C.E. and S.S.O.; funding acquisition, T.A.-A. E.R.S. proofread the manuscript for technical compliance before submission of original manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Notwithstanding, E.C.E, by virtue of being part of ACE for Mycotoxin and Food Safety, Federal University of Technology, Minna, Nigeria, qualified for financial support to help defray, in part, the publication fee of the manuscript. Such support is usually extended to ACE member(s) whose manuscript(s) is/are accepted for publication in Q1 or Q2 journals, such as this one.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The corresponding author, S.S. Ochigbo, hereby acknowledges financial support received from the Raw Materials Research and Development Council (RMRDC), Abuja, Nigeria, during his postdoctoral research in the University of the Free State (Qwaqwa Campus), South Africa, from June, 2008 to December, 2009. This opportunity was instrumental to the acquisition of both the knowledge and research skills for production of biodegradable starch films. That experience was a key factor that positively impacted upon the supervisory role that he brought to bear on the work leading to this publication. Furthermore, the authors heartily appreciate the support of all the technical staff in the Step-B Centre, Federal University of Technology, Minna, Nigeria, for providing the necessary facilities used in carrying out the laboratory work.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds may still be available from the first author who carried out the laboratory work.
References
- Vert, M.; Santos, I.D.; Ponsart, S.; Alauzet, N.; Morgat, J.-L.; Coudance, J.; Garreau, H. Degradable polymers in a living environment: Where do you end up? Polym. Int. 2002, 51, 840–844. [Google Scholar] [CrossRef]
- Bhattacharya, M. Stress relaxation of starch/synthetic polymer blends. J. Mat. Sci. 1998, 33, 4131–4139. [Google Scholar] [CrossRef]
- Garcia, N.L.; Famá, L.; Dufresne, A.; Aranguren, A.; Goyanes, S. A comparison between the physico-chemical properties of tuber and cereal starches. Food Res. Int. 2009, 42, 976–982. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation and properties of nanocomposites. Biomacromolecules 2009, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Famá, L.; Bittante, A.M.B.Q.; Sobral, P.J.A.; Goyanes, S.; Gerschenson, L.N. Garlic powder and wheat bran as fillers: Their effect on the physicochemical properties of edible biocomposites. Mat. Sci. Eng. C 2010, 30, 853–859. [Google Scholar] [CrossRef]
- Famá, L.M.; Pettarin, V.; Goyanes, S.; Bernal, C.R. Starch based nanocomposites with improved mechanical properties. Carbohydr. Polym. 2011, 83, 1226–1231. [Google Scholar] [CrossRef]
- Famá, L.M.; Gañan, P.; Bernal, C.R.; Goyanes, S. Biodegradable starch nanocomposites with low water vapor permeability and high storage modulus. Carbohydr. Polym. 2012, 87, 1989–1993. [Google Scholar] [CrossRef]
- Pérez-Pacheco, E.; Canto-Pinto, J.C.; Moo-Huchin, V.M.; Estrada-Mota, I.A.; Estrada-León, R.J.; Chel-Guerrero, L. Thermoplastic Starch (TPS)-Cellulosic Fibers Composites: Mechanical Properties and Water Vapor Barrier: A Review. In Composites from Renewable and Sustainable Materials; BoD–Books on Demand: Norderstedt, Germany, 2016; Chapter 5. [Google Scholar] [CrossRef]
- Garcia Calvo-Flores, F.; Dobado, J.A. Lignin as renewable raw material. Chemsuschem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Wang, X.; Han, G.; Shen, Z.; Sun, R. Fabrication, Property, and Application of Lignin-Based Nanocomposites. In Eco-Friendly Polymer Nanocomposites, Advanced Structured Materials; Thakur, V.K., Thakur, M.K., Eds.; Springer: New Delhi, India, 2015; Volume 74. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, M.; Verma, G. Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohydr. Polym. 2010, 82, 337–345. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Thongmee, J. Effect of kraft lignin and esterified lignin on the properties of thermoplastic starch. Mater. Des. 2013, 49, 701–704. [Google Scholar] [CrossRef]
- Narkchamnan, S.; Sakdaronnarong, C. Thermo-molded biocomposite from cassava starch, natural fibers and lignin associated by laccase-mediator system. Carbohydr. Polym. 2013, 96, 109–117. [Google Scholar] [CrossRef]
- Szymonska, J.; Targosz-Korecka, M.; Krok, F. Characterization of starch nanoparticles. J. Phys. Conf. Ser. 2009, 146, 1–6. [Google Scholar] [CrossRef]
- Amenaghawon, N.A.; Okieimen, C.O.; Ogbeide, S.E. Modelling and Statistical Optimization of dilute acid hydrolysis of eucalyptus wood chips using Response Surface Methodology. Pac. J. Sci. Technol. 2014, 15, 245–256. [Google Scholar]
- Cao, G.; Ren, N.; Wang, A.; Lee, D.J.; Guo, W.; Liu, B.; Feng, Y.; Zhao, Q. Acid Hydrolysis of Corn Stover for Biohydrogen Production using Thermoanaerobacterium thermosaccharolyticum W16. Int. J. Hydrog. Energy 2009, 34, 7182–7188. [Google Scholar] [CrossRef]
- Bendahou, A.; Kaddami, H.; Dufresne, A. Investigation on the effect of cellulosic nanoparticles morphology on the properties of natural rubber based nanocomposites. Eur. Polym. J. 2010, 46, 609–620. [Google Scholar] [CrossRef]
- Khan, K.H.; Ali, T.M.; Hasnain, A. Effect of chemical modifications on the functional and rheological properties of potato (Solanum tuberosum) starches. J. Anim. Plant Sci. 2014, 24, 550–555. [Google Scholar]
- Michelle, L.H.T. Development of Citric Acid Cross-Linked Starch for Controlled-Release Fertilizer (CRF); B.Eng Project Report; Department of Chemical Engineering, Universiti Teknologi Petronas: Tronoh, Perak, 2013. [Google Scholar]
- Taghizadeh, M.T.; Sabouri, N. Biodegradation behaviors and water adsorption of poly (vinyl alcohol)/starch/carboxymethyl cellulose/clay nanocomposites. Taghizadeh Sabouri Int. Nano Lett. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Jaszkiewicz, A. Mechanical performance of biocomposites based on PLA and PHBV reinforced with natural fibres—a comparative study to PP. Compos. Sci. Technol. 2010, 70, 1687–1696. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Bai, H.; Zhang, L. Thermal, Mechanical, and Degradation Properties of Nanocomposites Prepared using Lignin-Cellulose Nanofibers and Poly (Lactic Acid). Bioresources 2014, 9, 3211–3224. [Google Scholar] [CrossRef]
- Suarez, J.C.M.; Continho, F.M.B.; Sydenstricker, T.H. SEM studies of tensile fracture surfaces of polypropylene-sawdust composites. Polym. Test. 2003, 22, 819–824. [Google Scholar] [CrossRef]
- Patpen, P.; Russly, A.; Rosnita, A.T.; Khalina, A. Response Surface Methodology for the Optimization of Preparation of Biocomposites Based on Poly(lactic acid) and Durian Peel Cellulose. Sci. World J. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Talib, R.A.; Abdan, K.; Chin, N.L. Optimisation of processing variables of kenaf derived cellulose reinforced polylactic acid. Asian J. Chem. 2010, 22, 6652–6662. [Google Scholar]
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Improvement of mechanical performance of industrial hemp fibre reinforced polylactide biocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 310–319. [Google Scholar] [CrossRef]
- Akbar, J.; Mohamad, H.A.; Zohre, H.E. Effects of ultrasound time on the properties of polyvinyl alcohol-based nanocomposite films. Nutr. Food Sci. Res. 2015, 2, 29–38. [Google Scholar]
- Zhang, Y.; Lu, X.B.; Gao, C.; Lv, W.J.; Yao, J.M. Preparation and characterization of crystalline cellulose from bamboo fibers by controlled cellulose hydrolysis. J. Fiber Bioeng. Inform. 2012, 5, 263–271. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues, wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Li, M.F.; Fan, Y.M.; Xu, F.; Sun, R.C. Characterization of extracted lignin of bamboo (Neosinocalamus affinis) pretreated with sodium hydroxide/urea solution at low temperature. Bioresources 2010, 5, 1762–1778. [Google Scholar]
- Saniwan, S.; Lalita, V.; Chularat, K. Starch/cellulose biocomposites prepared by high-shear homogenization/compression molding. J. Mater. Sci. Eng. B 2012, 2, 213–222. [Google Scholar]
- AbdulRasheed-Adeleke, T.; Egwim, E.C.; Ochigbo, S.S.; Ossai, P.C. Effect of acetic anhydride and citric acid modification on biodegradability of cassava starch nanocomposite films. J. Mater. Sci. Eng. B 2015, 5, 372–379. [Google Scholar] [CrossRef]
- Ochigbo, S.S.; Luyt, A.S.; Mofokeng, J.P.; Antic, Z.; Dramicanin, M.D.; Djokovic, V. Dynamic Mechanical and Thermal Properties of the Composites of Thermoplastic Starch and Lanthanum Hydroxide Nanoparticles. J. Appl. Polym. Sci. 2012, 127, 699–709. [Google Scholar] [CrossRef]
- ASTM Standard D882-09; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Standards for Testing and Materials International: West Conshohocken, PA, USA, 2009.
- Detduangchan, N.; Sridach, W.; Wittaya, T. Enhancement of the properties of biodegradable rice starch films by using chemical crosslinking agents. Int. Food Res. J. 2014, 21, 1189–1199. [Google Scholar]
- Lei, Y.; Wu, Q.; Yao, F.; Xu, Y. Preparation and properties of recycled HDPE/natural fiber composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1664–1674. [Google Scholar] [CrossRef]
- Piyaporn, K.; Kawee, S.; Duanghathai, P. Preparation of Cassava starch/Montnorillonite Nanocomposite Film. J. Sci. Res. 2004, 29, 2. [Google Scholar]
- Chieng, B.W.; Ibrahim, N.A.; Wan Yunus, W.M.Z. Optimization of tensile strength of poly (lactic acid)/grapheme nanocomposites using response surface methodology. Polym.-Plast. Technol. 2012, 51, 791–799. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).