Synthesis and Biological Activity of Unsymmetrical Monoterpenylhetaryl Disulfides
Abstract
1. Introduction
2. Results and Discussion
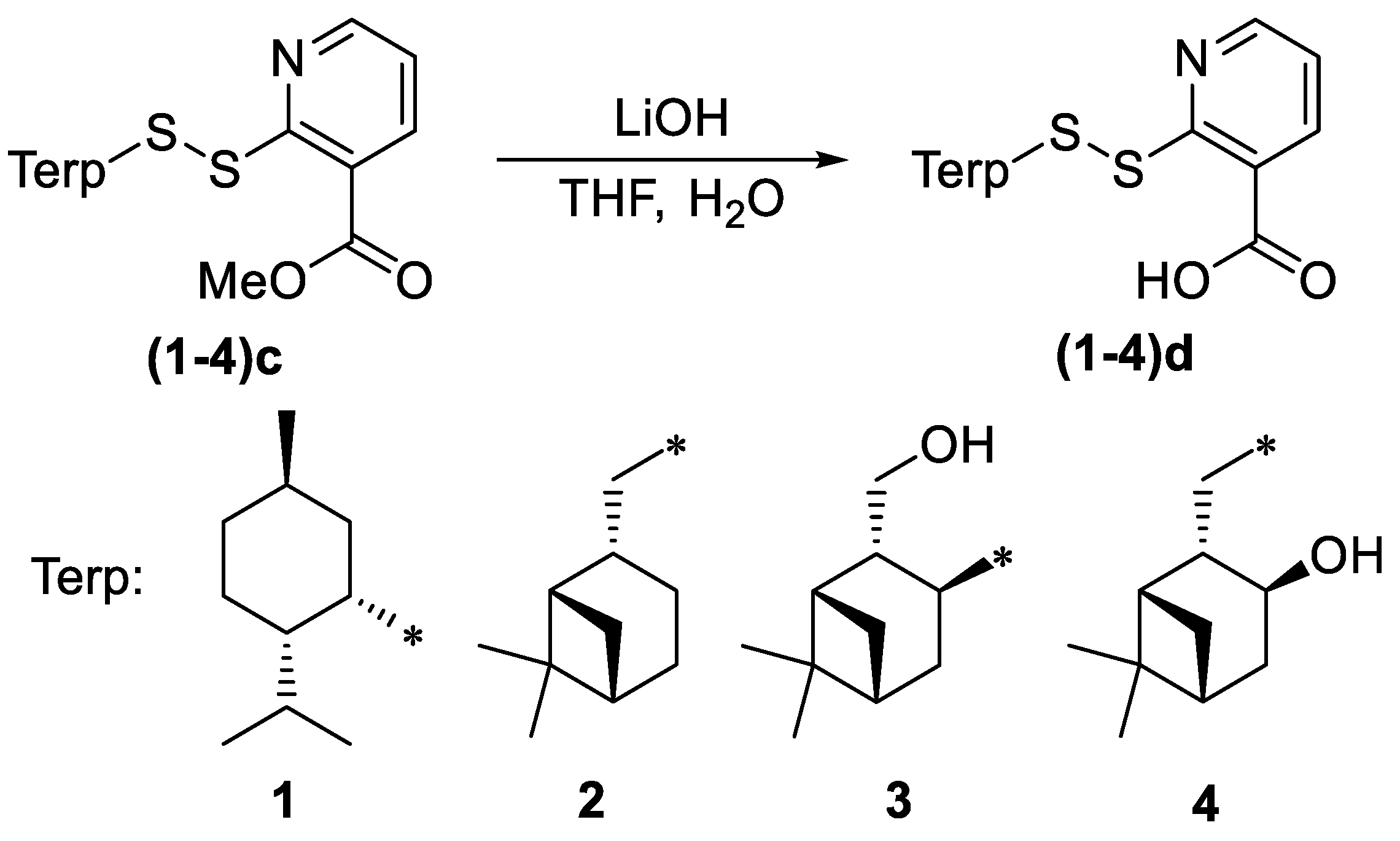
2.1. Synthesis of Unsymmetrical Monoterpenylhetaryl Disulfides
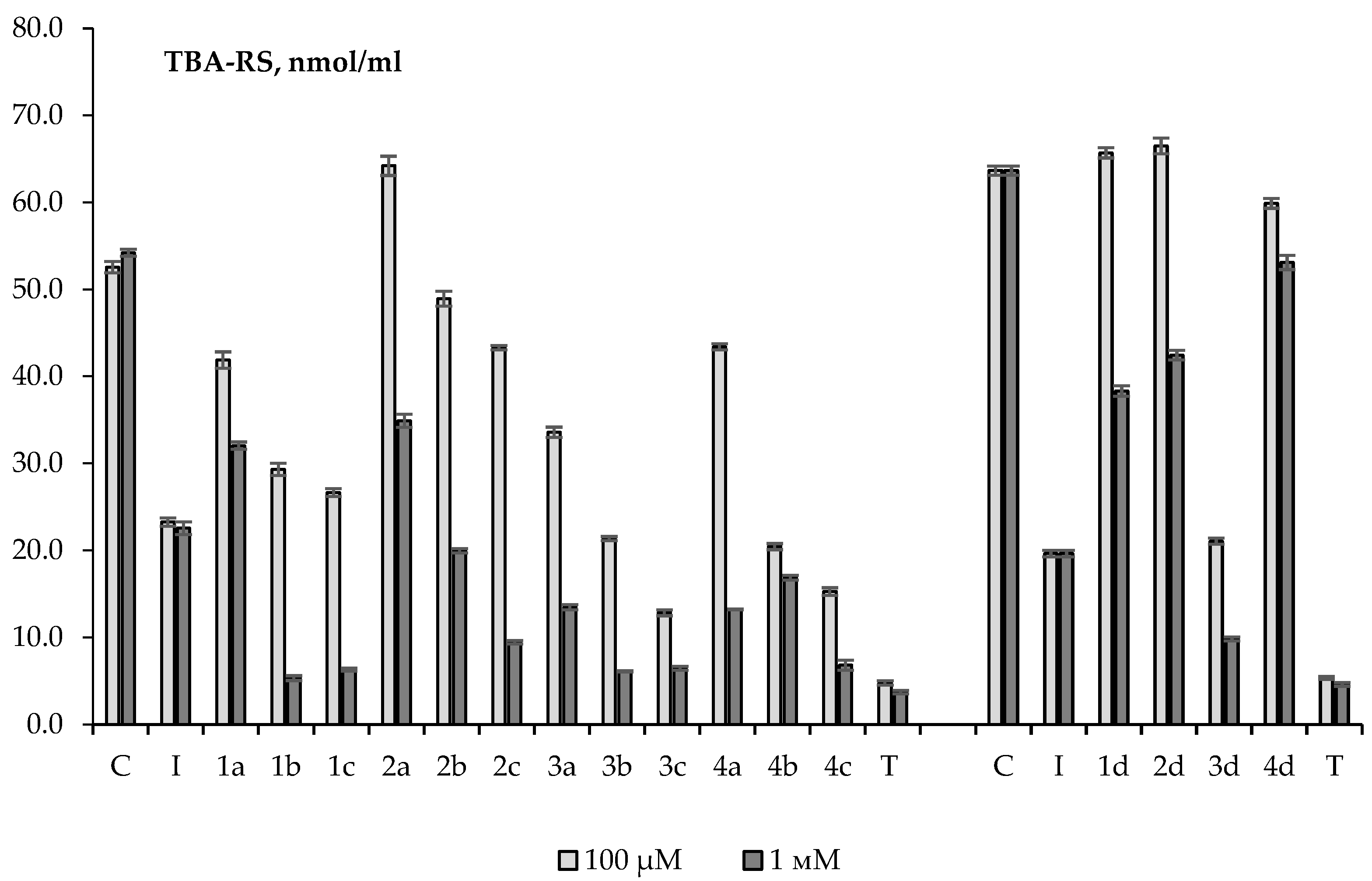
2.2. Antioxidant Activity in a Non-Cellular Model System (Brain Homogenates)
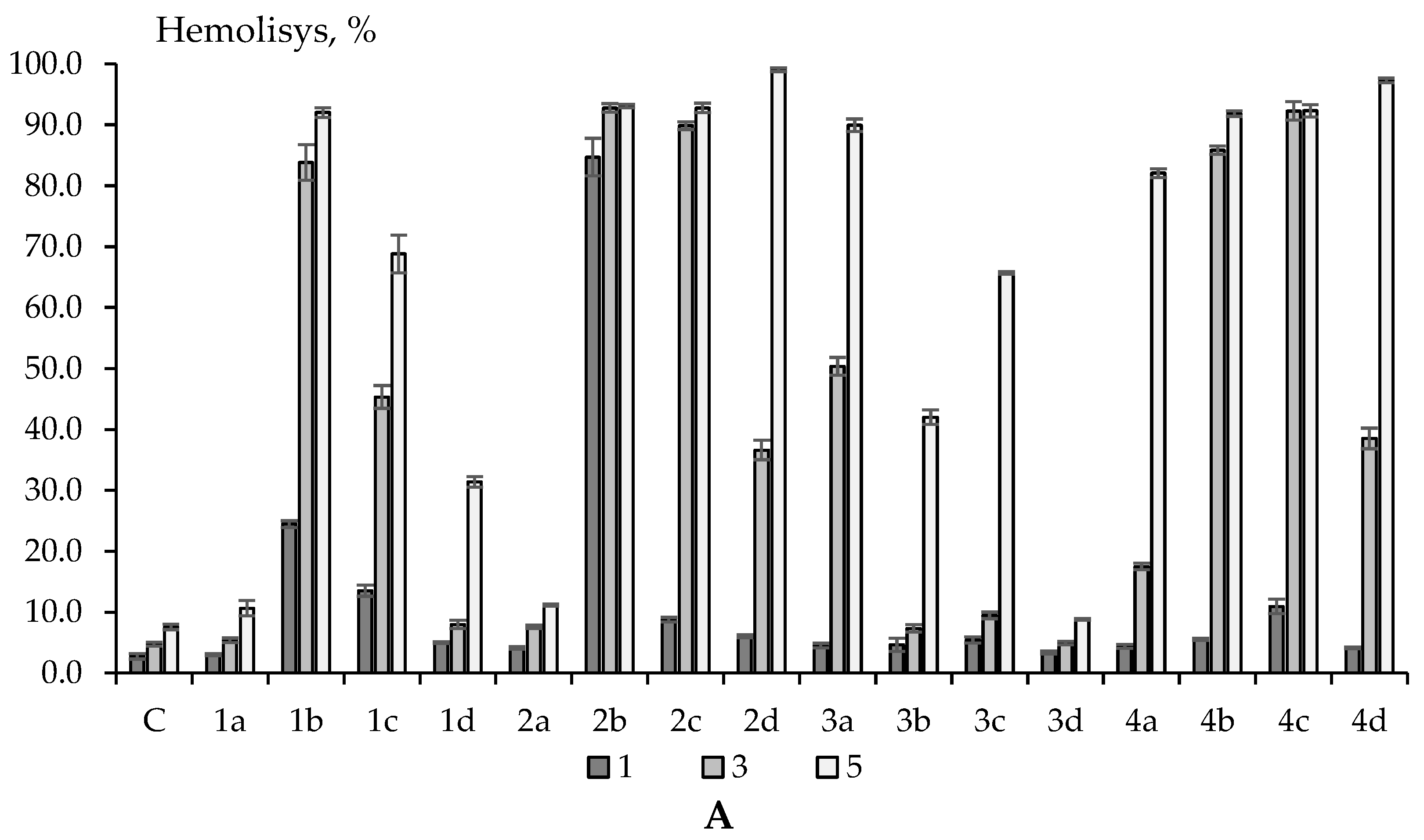
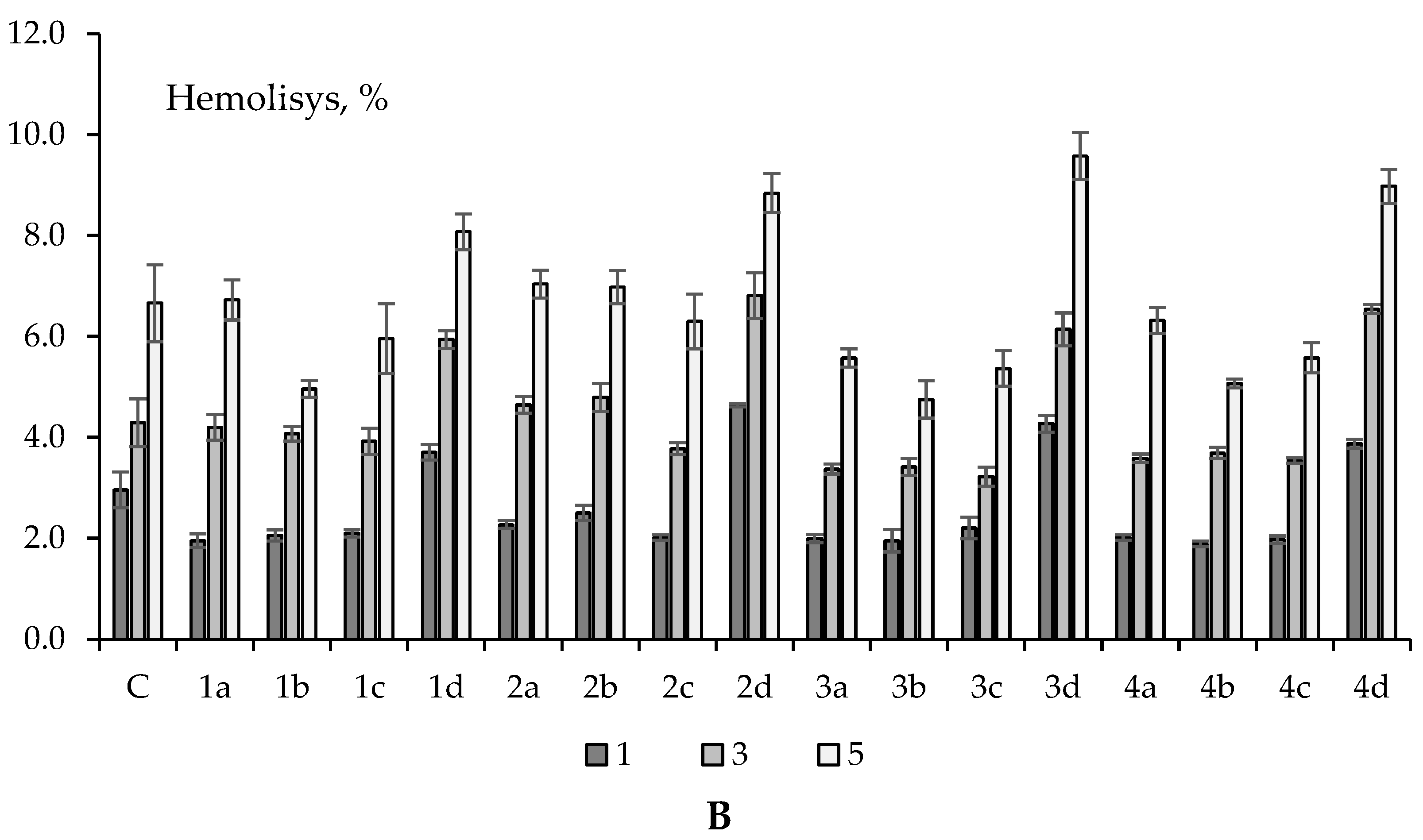
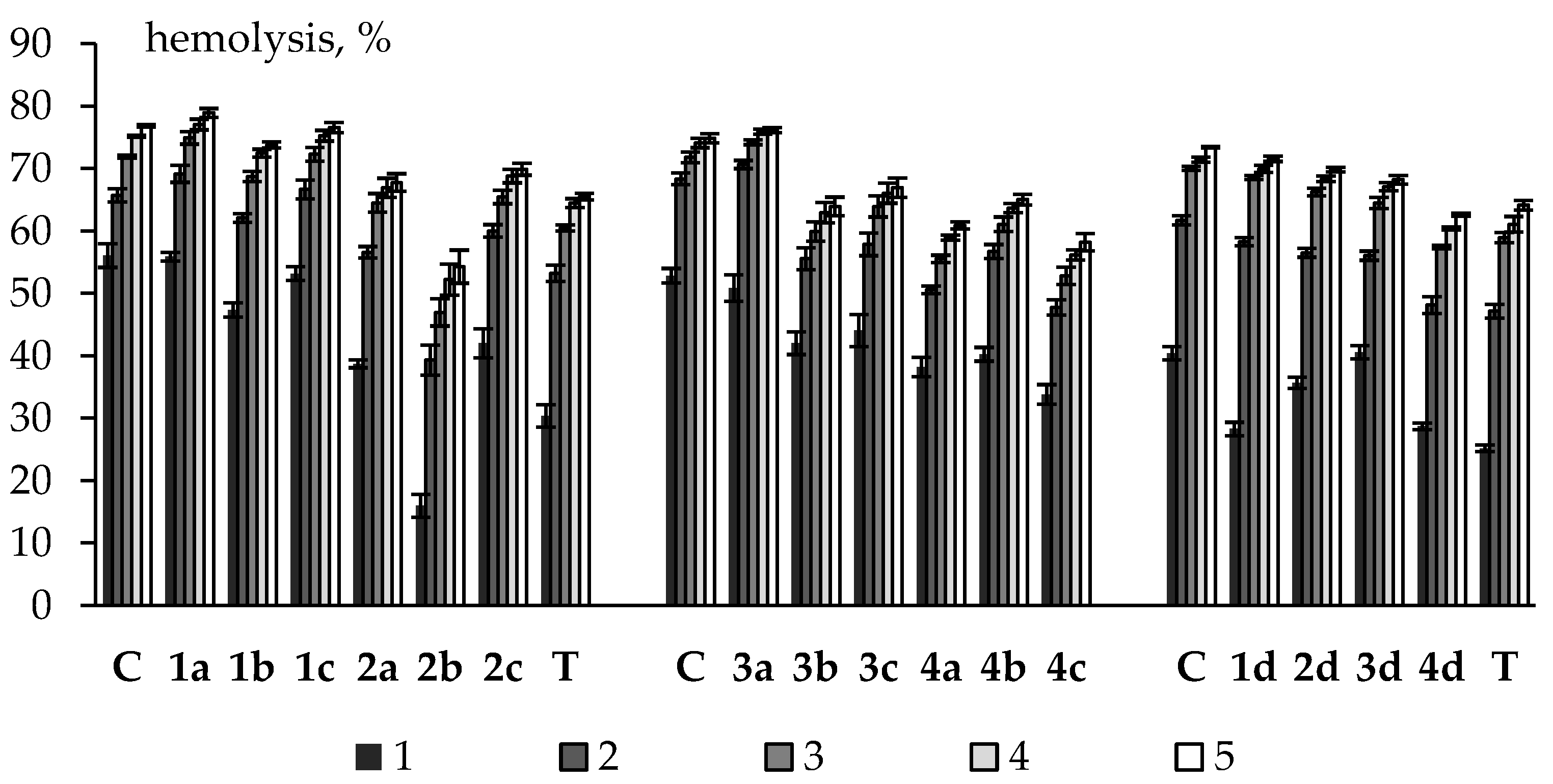
2.3. Erythrotoxicity, Membrane-Protective and Antioxidant Activity (on Erythrocytes)
2.4. Antimicrobial Activity
2.5. Antiviral Activity
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. General Procedure
4.2.1. General Procedure for the Synthesis of Unsymmetrical Disulfides
4.2.2. General Method for the Synthesis of Carboxylic Acids
4.3. Antioxidant Activity (Non-Cellular Model)
4.4. Erythrotoxicity, Antioxidant Activity, Membrane-Protective Activity (Cellular Model System)
4.5. Antibacterial Activity
4.6. Antifungal Activity
4.7. Mutagenicity and Cytotoxicity
4.8. Antiviral Activity Assessment
4.8.1. Toxicity Studies
4.8.2. Cell Protection Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Koval’, I.V. The Chemistry of Disulfides. Russ. Chem. Rev. 1994, 63, 735–750. [Google Scholar] [CrossRef]
- Heldreth, B.; Turos, E. Microbiological Properties and Modes of Action of Organosulfur-Based Anti-Infectives. Curr. Med. Chem. Anticancer Agents 2005, 4, 295–315. [Google Scholar] [CrossRef]
- Chung, J.; Chen, T.; Missiakas, D. Transfer of Electrons across the Cytoplasmic Membrane by DsbD, a Membrane Protein Involved in Thiol-Disulphide Exchange and Protein Folding in the Bacterial Periplasm. Mol. Microbiol. 2000, 35, 1099–1109. [Google Scholar] [CrossRef]
- Song, X.; Yue, Z.; Nie, L.; Zhao, P.; Zhu, K.; Wang, Q. Biological Functions of Diallyl Disulfide, a Garlic-Derived Natural Organic Sulfur Compound. J. Evid. Based Complement. Altern. Med. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Koh, S.-H.; Kwon, H.; Park, K.H.; Ko, J.K.; Kim, J.H.; Hwang, M.S.; Yum, Y.N.; Kim, O.-H.; Kim, J.; Kim, H.-T.; et al. Protective Effect of Diallyl Disulfide on Oxidative Stress-Injured Neuronally Differentiated PC12 Cells. Mol. Brain Res. 2005, 133, 176–186. [Google Scholar] [CrossRef]
- Filomeni, G.; Aquilano, K.; Rotilio, G.; Ciriolo, M.R. Reactive Oxygen Species-Dependent c-Jun NH2-Terminal Kinase/c-Jun Signaling Cascade Mediates Neuroblastoma Cell Death Induced by Diallyl Disulfide. Cancer Res. 2003, 63, 5940–5949. [Google Scholar]
- Agassi, S.F.T.; Yeh, T.-M.; Chang, C.-D.; Hsu, J.-L.; Shih, W.-L. Potentiation of Differentiation and Apoptosis in a Human Promyelocytic Leukemia Cell Line by Garlic Essential Oil and Its Organosulfur Compounds. Anticancer Res. 2020, 40, 6345–6354. [Google Scholar] [CrossRef]
- Li, W.-R.; Ma, Y.-K.; Xie, X.-B.; Shi, Q.-S.; Wen, X.; Sun, T.-L.; Peng, H. Diallyl Disulfide from Garlic Oil Inhibits Pseudomonas Aeruginosa Quorum Sensing Systems and Corresponding Virulence Factors. Front. Microbiol. 2019, 9, 3222. [Google Scholar] [CrossRef]
- Tsao, S.-M.; Liu, W.-H.; Yin, M.-C. Two Diallyl Sulphides Derived from Garlic Inhibit Meticillin-Resistant Staphylococcus Aureus Infection in Diabetic Mice. J. Med. Microbiol. 2007, 56, 803–808. [Google Scholar] [CrossRef]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The Role of Diallyl Sulfides and Dipropyl Sulfides in the In Vitro Antimicrobial Activity of the Essential Oil of Garlic, Allium Sativum L., and Leek, Allium Porrum L. Phytother. Res. 2013, 27, 380–383. [Google Scholar] [CrossRef]
- Maldonado, P.D.; Chánez-Cárdenas, M.E.; Pedraza-Chaverrí, J. Aged Garlic Extract, Garlic Powder Extract, S-Allylcysteine, Diallyl Sulfide and Diallyl Disulfide Do Not Interfere with the Antibiotic Activity of Gentamicin. Phytother. Res. 2005, 19, 252–254. [Google Scholar] [CrossRef]
- Liu, W.; Hsu, C.; Yin, M. In Vitro Anti-Helicobacter Pylori Activity of Diallyl Sulphides and Protocatechuic Acid. Phytother. Res. 2008, 22, 53–57. [Google Scholar] [CrossRef]
- Chung, J.G.; Chen, G.W.; Wu, L.T.; Chang, H.L.; Lin, J.G.; Yeh, C.C.; Wang, T.F. Effects of Garlic Compounds Diallyl Sulfide and Diallyl Disulfide on Arylamine N-Acetyltransferase Activity in Strains of Helicobacter Pylori from Peptic Ulcer Patients. Am. J. Chin. Med. 1998, 26, 353–364. [Google Scholar] [CrossRef]
- Alam, M.; Zubair, S.; Farazuddin, M.; Ahmad, E.; Khan, A.; Zia, Q.; Malik, A.; Mohammad, O. Development, Characterization and Efficacy of Niosomal Diallyl Disulfide in Treatment of Disseminated Murine Candidiasis. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 247–256. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.; Dimić, G.; Lević, J.; Tanackov, I.; Tepić, A.; Vujičić, B.; Gvozdanović-Varga, J. Effects of Onion (Allium cepa L.) and Garlic (Allium sativum L.) Essential Oils on the Aspergillus Versicolor Growth and Sterigmatocystin Production. J. Food Sci. 2012, 77, M278–M284. [Google Scholar] [CrossRef]
- Shoji, S.; Furuishi, K.; Yanase, R.; Miyazaka, T.; Kino, M. Allyl Compounds Selectively Killed Human Immunodeficiency Virus (Type 1)-Infected Cells. Biochem. Biophys. Res. Commun. 1993, 194, 610–621. [Google Scholar] [CrossRef]
- Hall, A.; Troupin, A.; Londono-Renteria, B.; Colpitts, T. Garlic Organosulfur Compounds Reduce Inflammation and Oxidative Stress during Dengue Virus Infection. Viruses 2017, 9, 159. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Hughes, R.; Pfefferkorn, J.A.; Barluenga, S.; Roecker, A.J. Combinatorial Synthesis through Disulfide Exchange: Discovery of Potent Psammaplin a Type Antibacterial Agents Active against Methicillin-ResistantStaphylococcus Aureus (MRSA). Chem. Eur. J. 2001, 7, 4280–4295. [Google Scholar] [CrossRef]
- Jing, Q.; Hu, X.; Ma, Y.; Mu, J.; Liu, W.; Xu, F.; Li, Z.; Bai, J.; Hua, H.; Li, D. Marine-Derived Natural Lead Compound Disulfide-Linked Dimer Psammaplin A: Biological Activity and Structural Modification. Mar. Drugs 2019, 17, 384. [Google Scholar] [CrossRef]
- Moriarty, R.M.; Roll, D.M.; Ku, Y.-Y.; Nelson, C.; Ireland, C.M. A Revised Structure for the Marine Bromoindole Derivative Citorellamine. Tetrahedron Lett. 1987, 28, 749–752. [Google Scholar] [CrossRef]
- Roll, D.M.; Ireland, C.M. Citorellamine, a New Bromoindole Derivative from Polycitorella-Mariae. Tetrahedron Lett. 1985, 26, 4303–4306. [Google Scholar] [CrossRef]
- Long, T.E. Repurposing Thiram and Disulfiram as Antibacterial Agents for Multidrug-Resistant Staphylococcus Aureus Infections. Antimicrob. Agents Chemother. 2017, 61, e00898-17. [Google Scholar] [CrossRef]
- Sheppard, J.G.; Frazier, K.R.; Saralkar, P.; Hossain, M.F.; Geldenhuys, W.J.; Long, T.E. Disulfiram-Based Disulfides as Narrow-Spectrum Antibacterial Agents. Bioorg. Med. Chem. Lett. 2018, 28, 1298–1302. [Google Scholar] [CrossRef]
- Potula, H.-H.S.K.; Shahryari, J.; Inayathullah, M.; Malkovskiy, A.V.; Kim, K.-M.; Rajadas, J. Repurposing Disulfiram (Tetraethylthiuram Disulfide) as a Potential Drug Candidate against Borrelia Burgdorferi In Vitro and In Vivo. Antibiotics 2020, 9, 633. [Google Scholar] [CrossRef]
- Liegner, K.B. Disulfiram (Tetraethylthiuram Disulfide) in the Treatment of Lyme Disease and Babesiosis: Report of Experience in Three Cases. Antibiotics 2019, 8, 72. [Google Scholar] [CrossRef]
- Turos, E.; Revell, K.D.; Ramaraju, P.; Gergeres, D.A.; Greenhalgh, K.; Young, A.; Sathyanarayan, N.; Dickey, S.; Lim, D.; Alhamadsheh, M.M. Unsymmetric Aryl–Alkyl Disulfide Growth Inhibitors of Methicillin-Resistant Staphylococcus Aureus and Bacillus Anthracis. Bioorg. Med. Chem. 2008, 16, 6501–6508. [Google Scholar] [CrossRef][Green Version]
- Branowska, D.; Ławecka, J.; Sobiczewski, M.; Karczmarzyk, Z.; Wysocki, W.; Wolińska, E.; Olender, E.; Mirosław, B.; Perzyna, A.; Bielawska, A.; et al. Synthesis of Unsymmetrical Disulfanes Bearing 1,2,4-Triazine Scaffold and Their in Vitro Screening towards Anti-Breast Cancer Activity. Monatsh. Chem. 2018, 149, 1409–1420. [Google Scholar] [CrossRef]
- Wang, L.; Bao, B.-B.; Song, G.-Q.; Chen, C.; Zhang, X.-M.; Lu, W.; Wang, Z.; Cai, Y.; Li, S.; Fu, S.; et al. Discovery of Unsymmetrical Aromatic Disulfides as Novel Inhibitors of SARS-CoV Main Protease: Chemical Synthesis, Biological Evaluation, Molecular Docking and 3D-QSAR Study. Eur. J. Med. Chem. 2017, 137, 450–461. [Google Scholar] [CrossRef]
- Toropov, A.A.; Toropova, A.P.; Veselinović, A.M.; Leszczynska, D.; Leszczynski, J. SARS-CoV M pro Inhibitory Activity of Aromatic Disulfide Compounds: QSAR Model. J. Biomol. Struct. Dyn. 2022, 40, 780–786. [Google Scholar] [CrossRef]
- Pestova, S.V.; Izmest’ev, E.S.; Shevchenko, O.G.; Rubtsova, S.A.; Kuchin, A.V. Synthesis and Membranoprotective Properties of New Disulfides with Monoterpene and Carbohydrate Fragments. Russ. Chem. Bull. 2015, 64, 723–731. [Google Scholar] [CrossRef]
- Gyrdymova, Y.V.; Sudarikov, D.V.; Shevchenko, O.G.; Rubtsova, S.A.; Slepukhin, P.A.; Kutchin, A.V. Caryophyllane Thiols, Vinyl Thioethers, Di- and Bis-Sulfides: Antioxidant and Membrane Protective Activities. Chem. Biodivers. 2017, 14, e1700296. [Google Scholar] [CrossRef]
- Gyrdymova, Y.V.; Sinegubova, E.O.; Muryleva, A.S.; Zarubaev, V.V.; Rubtsova, S.A. Correction to: Anti-Influenza Activity of Several Caryophyllane Thiosesquiterpenoids. Chem. Nat. Compd. 2020, 56, 191. [Google Scholar] [CrossRef]
- Startseva, V.A.; Kuznetsov, I.V.; Nikitina, L.E.; Osmanov, V.K.; Borisov, A.V.; Matsulevich, Z.V.; Klochkov, V.V. Hetarenesulfenyl(Selenyl) Chlorination of (+)-Camphene. Chem. Nat. Compd. 2015, 51, 671–674. [Google Scholar] [CrossRef]
- Pugh, K.C.; Gera, L.; Stewart, J.M. Synthesis and Stability of 3-Nitro-2-Pyridinesulfenyl Chloride (NpysCl). Int. J. Pept. Protein Res. 2009, 42, 159–164. [Google Scholar] [CrossRef]
- Sagara, T.; Okamura, M.; Shimohigashi, Y.; Ohno, M.; Kanematsu, K. Specific Affinity Labeling of μ Opioid Receptors in Rat Brain by S-Activated Sulfhydryldihydromorphine Analogs. Bioorg. Med. Chem. Lett. 1995, 5, 1609–1614. [Google Scholar] [CrossRef]
- Senter, P.D.; Pearce, W.E.; Greenfield, R.S. Development of a Drug-Release Strategy Based on the Reductive Fragmentation of Benzyl Carbamate Disulfides. J. Org. Chem. 1990, 55, 2975–2978. [Google Scholar] [CrossRef]
- Perlikowska, W.; Gouygou, M.; Mikolajczyk, M.; Daran, J.-C. Enantiomerically Pure Disulfides: Key Compounds in the Kinetic Resolution of Chiral PIII-Derivatives with Stereogenic Phosphorus. Tetrahedron Asymmetry 2004, 15, 3519–3529. [Google Scholar] [CrossRef]
- McCann, J.; Ames, B.N. A Simple Method for Detecting Environmental Carcinogens as Mutagens. Ann. N. Y. Acad. Sci. 1976, 271, 5–13. [Google Scholar] [CrossRef]
- Smee, D.F.; Hurst, B.L.; Evans, W.J.; Clyde, N.; Wright, S.; Peterson, C.; Jung, K.-H.; Day, C.W. Evaluation of Cell Viability Dyes in Antiviral Assays with RNA Viruses That Exhibit Different Cytopathogenic Properties. J. Virol. Methods 2017, 246, 51–57. [Google Scholar] [CrossRef]
- SAINT. Data Reduction and Correction Program; Bruker AXS: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Singh, W.M.; Baruah, J.B. Rosolic Acid–Catalyzed Conversion of Thiols to Disulfides. Synth. Commun. 2008, 39, 325–331. [Google Scholar] [CrossRef]
- Stoyanov, S.; Petkov, I.; Antonov, L.; Stoyanova, T.; Karagiannidis, P.; Aslanidis, P. Thione–Thiol Tautomerism and Stability of 2- and 4-Mercaptopyridines and 2-Mercaptopyrimidines. Can. J. Chem. 1990, 68, 1482–1489. [Google Scholar] [CrossRef]
- Izmest’ev, E.S.; Sudarikov, D.V.; Rubtsova, S.A.; Slepukhin, P.A.; Kuchin, A.V. Asymmetric Synthesis of New Optically Active Sulfinamides of Menthane Series and Their Derivatives. Russ. J. Org. Chem. 2012, 48, 184–192. [Google Scholar] [CrossRef]
- Banach, A.; Ścianowski, J.; Ozimek, P. The Use of Sulfides Derived from Carane, P-Menthane, Pinane, and Bornane in the Synthesis of Optically Active Epoxides. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 274–284. [Google Scholar] [CrossRef]
- Martıínez-Ramos, F.; Vargas-Dıíaz, M.E.; Chacón-Garcıía, L.; Tamariz, J.; Joseph-Nathan, P.; Zepeda, L.G. Highly Diastereoselective Nucleophilic Additions Using a Novel Myrtenal-Derived Oxathiane as a Chiral Auxiliary. Tetrahedron Asymmetry 2001, 12, 3095–3103. [Google Scholar] [CrossRef]
- Banina, O.A.; Sudarikov, D.V.; Krymskaya, Y.V.; Frolova, L.L.; Kuchin, A.V. Synthesis of Chiral Hydroxythiols Based on Oxygen-Containing α- and β-Pinene Derivatives. Chem. Nat. Compd. 2015, 51, 261–265. [Google Scholar] [CrossRef]
- Hayashi, K.; Ichimaru, Y.; Sugiura, K.; Maeda, A.; Harada, Y.; Kojima, Y.; Nakayama, K.; Imai, M. Efficiency of Lithium Cations in Hydrolysis Reactions of Esters in Aqueous Tetrahydrofuran. Chem. Pharm. Bull. 2021, 69, 581–584. [Google Scholar] [CrossRef]
- Acker, C.I.; Brandão, R.; Rosário, A.R.; Nogueira, C.W. Antioxidant Effect of Alkynylselenoalcohol Compounds on Liver and Brain of Rats in Vitro. Environ. Toxicol. Pharmacol. 2009, 28, 280–287. [Google Scholar] [CrossRef]
- Stefanello, S.T.; Prestes, A.S.; Ogunmoyole, T.; Salman, S.M.; Schwab, R.S.; Brender, C.R.; Dornelles, L.; Rocha, J.B.T.; Soares, F.A.A. Evaluation of in Vitro Antioxidant Effect of New Mono and Diselenides. Toxicol. In Vitro 2013, 27, 1433–1439. [Google Scholar] [CrossRef]
- Bellé, N.A.V.; Dalmolin, G.D.; Fonini, G.; Rubin, M.A.; Rocha, J.B.T. Polyamines Reduces Lipid Peroxidation Induced by Different Pro-Oxidant Agents. Brain Res. 2004, 1008, 245–251. [Google Scholar] [CrossRef]
- Chawla, R.; Arora, R.; Kumar, R.; Sharma, A.; Prasad, J.; Singh, S.; Sagar, R.; Chaudhary, P.; Shukla, S.; Kaur, G.; et al. Antioxidant Activity of Fractionated Extracts of Rhizomes of High-Altitude Podophyllum Hexandrum: Role in Radiation Protection. Mol. Cell. Biochem. 2005, 273, 193–208. [Google Scholar] [CrossRef]
- Asakawa, T.; Matsushita, S. Coloring Conditions of Thiobarbituric Acid Test for Detecting Lipid Hydroperoxides. Lipids 1980, 15, 137–140. [Google Scholar] [CrossRef]
- Takebayashi, J.; Chen, J.; Tai, A. A Method for Evaluation of Antioxidant Activity Based on Inhibition of Free Radical-Induced Erythrocyte Hemolysis. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 594, pp. 287–296. ISBN 978-1-60761-410-4. [Google Scholar]
- Leclercq, R.; Cantón, R.; Brown, D.F.J.; Giske, C.G.; Heisig, P.; MacGowan, A.P.; Mouton, J.W.; Nordmann, P.; Rodloff, A.C.; Rossolini, G.M.; et al. EUCAST Expert Rules in Antimicrobial Susceptibility Testing. Clin. Microbiol. Infect. 2013, 19, 141–160. [Google Scholar] [CrossRef]
- Rex, J.H. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeats: Approved Standard, 3rd ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008; pp. 1–25. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

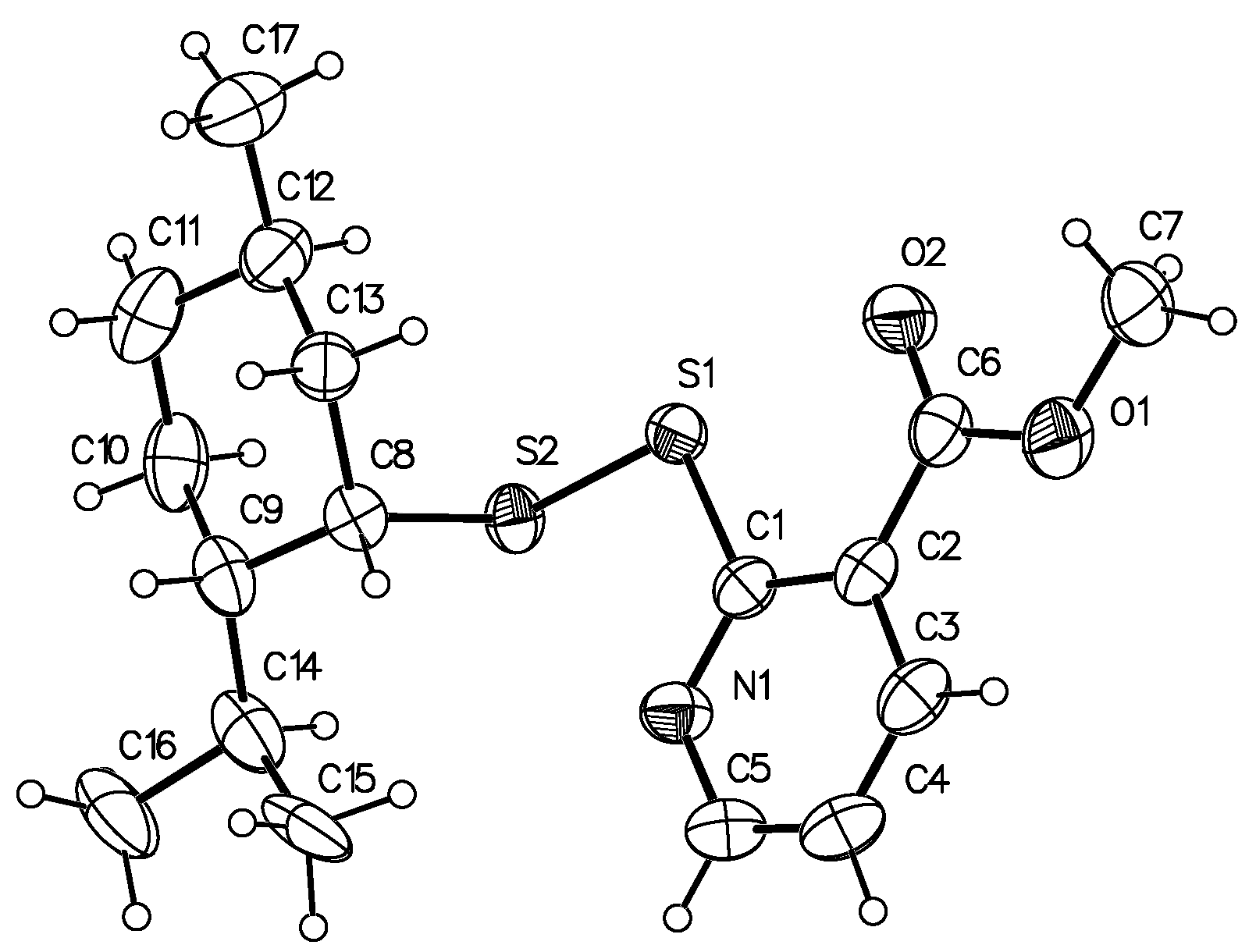
| Compound | MIC, µg/mL | CC50 EBL, μg/mL | Mutagenicity in the Ames Test | ||||
|---|---|---|---|---|---|---|---|
| S. aureus ATCC 29213 (MSSA) | S. aureus MRSA Clinical Isolate | P. aeruginosa ATCC 27853 | C. albicans 703 Clinical Isolate | ||||
| 1. | 1a | 16 | 16 | 16 | 512 | 11.7 | TA 100 |
| 2. | 1b | 16 | 32 | 32 | 512 | 14.0 | TA 102 |
| 3. | 1c | 32 | 32 | 32 | 512 | 18.3 | NF 2 |
| 4. | 1d | 32 | 64 | 1024 | 64 | 47.8 | NF |
| 5. | 2a | 128 | 128 | 64 | 1024 | 104.3 | NF |
| 6. | 2b | 16 | 64 | 32 | >1024 | 13.6 | NF |
| 7. | 2c | >1024 | 64 | 1024 | 1024 | 122.6 | NF |
| 8. | 2d | 32 | 512 | 1024 | 128 | 18.1 | NF |
| 9. | 3a | 32 | 32 | 64 | 1024 | 7.1 | NF |
| 10. | 3b | 32 | 32 | 1024 | 512 | 8.2 | NF |
| 11. | 3c | 64 | 32 | 1024 | 1024 | 8.1 | NF |
| 12. | 3d | 512 | 1024 | 1024 | 32 | 117.7 | NF |
| 13. | 4a | 256 | >1024 | 1024 | >1024 | ND 1 | ND |
| 14. | 4b | >1024 | >1024 | >1024 | >1024 | ND | ND |
| 15. | 4c | 1024 | >1024 | >1024 | 1024 | ND | ND |
| 16. | 4d | 512 | 64 | 1024 | 16 | 11.14 | NF |
| Amikacin | 4 | 4 | 4 | ND | ND | ND | |
| Fluconazole | ND | ND | ND | 16 | ND | ND | |
| Compound | CC50, µM | IC50, µM | SI | |
|---|---|---|---|---|
| 1. | 1a | 36 ± 3 | 5 ± 1 | 8 |
| 2. | 1b | 29 ± 2 | 13 ± 2 | 2 |
| 3. | 1c | 31 ± 2 | 12 ± 2 | 3 |
| 4. | 1d | 38 ± 3 | >34 | 1 |
| 5. | 2a | 99 ± 5 | 13 ± 3 | 8 |
| 6. | 2b | 35 ± 3 | 13 ± 2 | 3 |
| 7. | 2c | 104 ± 8 | 19 ± 3 | 6 |
| 8. | 2d | 60 ± 4 | 5 ± 1 | 12 |
| 9. | 3a | 30 ± 3 | 9 ± 3 | 4 |
| 10. | 3b | 83 ± 4 | 16 ± 3 | 5 |
| 11. | 3c | >283 | 255 ± 31 | 1 |
| 12. | 3d | 285 ± 14 | 132 ± 16 | 2 |
| 13. | 4a | 68 ± 6 | >31 | 2 |
| 14. | 4b | 34 ± 2 | 12 ± 3 | 3 |
| 15. | 4c | 40 ± 2 | 34 ± 4 | 1 |
| 16. | 4d | 178 ± 11 | >97 | 2 |
| Rimantadine | 367 ± 28 | 57 ± 8 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudarikov, D.V.; Gyrdymova, Y.V.; Borisov, A.V.; Lukiyanova, J.M.; Rumyantcev, R.V.; Shevchenko, O.G.; Baidamshina, D.R.; Zakarova, N.D.; Kayumov, A.R.; Sinegubova, E.O.; et al. Synthesis and Biological Activity of Unsymmetrical Monoterpenylhetaryl Disulfides. Molecules 2022, 27, 5101. https://doi.org/10.3390/molecules27165101
Sudarikov DV, Gyrdymova YV, Borisov AV, Lukiyanova JM, Rumyantcev RV, Shevchenko OG, Baidamshina DR, Zakarova ND, Kayumov AR, Sinegubova EO, et al. Synthesis and Biological Activity of Unsymmetrical Monoterpenylhetaryl Disulfides. Molecules. 2022; 27(16):5101. https://doi.org/10.3390/molecules27165101
Chicago/Turabian StyleSudarikov, Denis V., Yulia V. Gyrdymova, Alexander V. Borisov, Julia M. Lukiyanova, Roman V. Rumyantcev, Oksana G. Shevchenko, Diana R. Baidamshina, Nargiza D. Zakarova, Airat R. Kayumov, Ekaterina O. Sinegubova, and et al. 2022. "Synthesis and Biological Activity of Unsymmetrical Monoterpenylhetaryl Disulfides" Molecules 27, no. 16: 5101. https://doi.org/10.3390/molecules27165101
APA StyleSudarikov, D. V., Gyrdymova, Y. V., Borisov, A. V., Lukiyanova, J. M., Rumyantcev, R. V., Shevchenko, O. G., Baidamshina, D. R., Zakarova, N. D., Kayumov, A. R., Sinegubova, E. O., Volobueva, A. S., Zarubaev, V. V., & Rubtsova, S. A. (2022). Synthesis and Biological Activity of Unsymmetrical Monoterpenylhetaryl Disulfides. Molecules, 27(16), 5101. https://doi.org/10.3390/molecules27165101







