Tyrosol Derivatives, Bearing 3,5-Disubstituted Isoxazole and 1,4-Disubstituted Triazole, as Potential Antileukemia Agents by Promoting Apoptosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemical Reagents
2.2. Cell Lines
2.3. Patient Leukemia Cells
- Group 1: Contained 10 CML patients at diagnosis;
- Group 2: Contained 5 CML patients resistant to imatinib or multiple TKIs with a bcr–abl/abl ratio > 10% at 3 months or >1% at 6 months.
2.4. Peripheral Blood Mononuclear Cells Isolation
2.5. Antitumor Assay
2.5.1. Cytotoxicity Assay
2.5.2. Anti-Proliferative Assay
2.6. Apoptosis-Induced Detection
2.7. Intracellular ROS Detection
2.8. Western Blot Analysis
2.9. Statistical Data Analysis
3. Results and Discussion
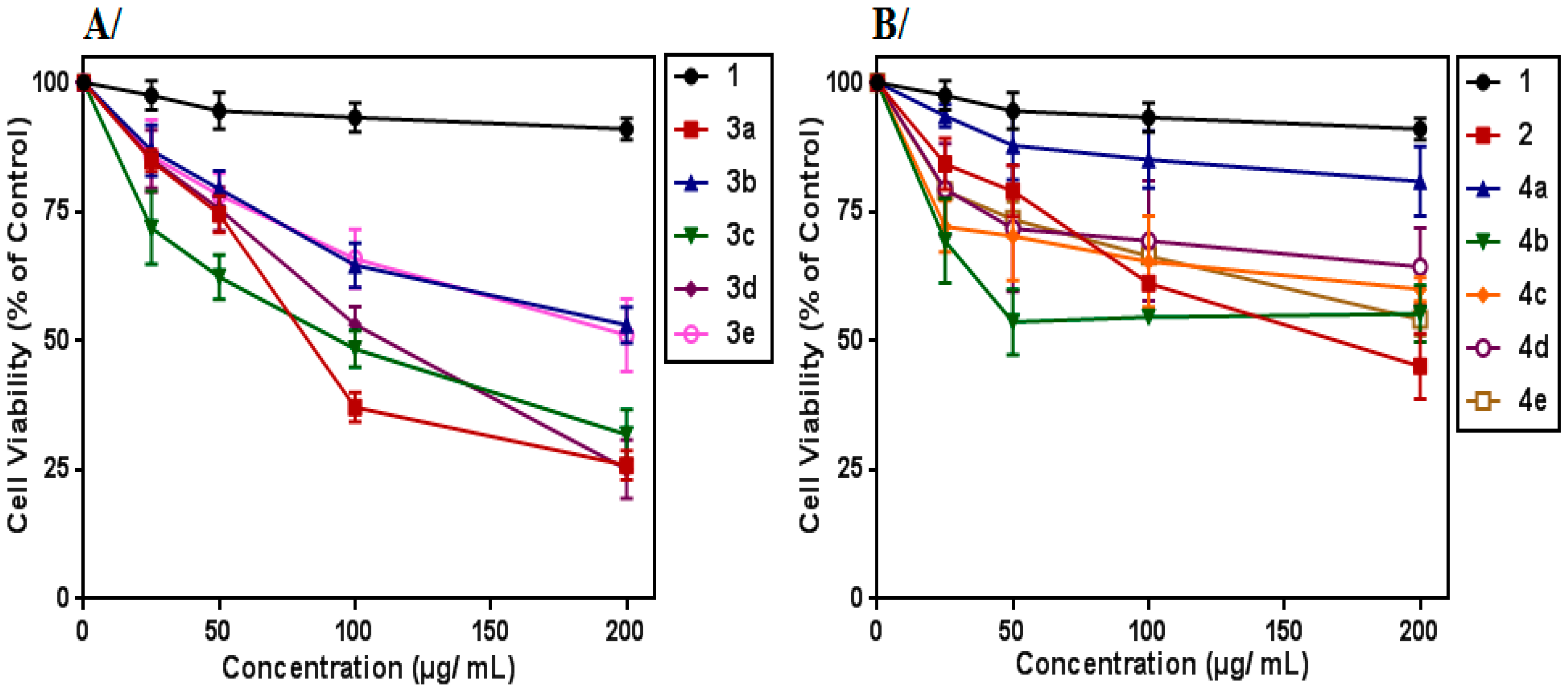
3.1. Cytotoxic Effect on K562 Cells
3.2. Anti-Proliferative Effect on K562 Cells
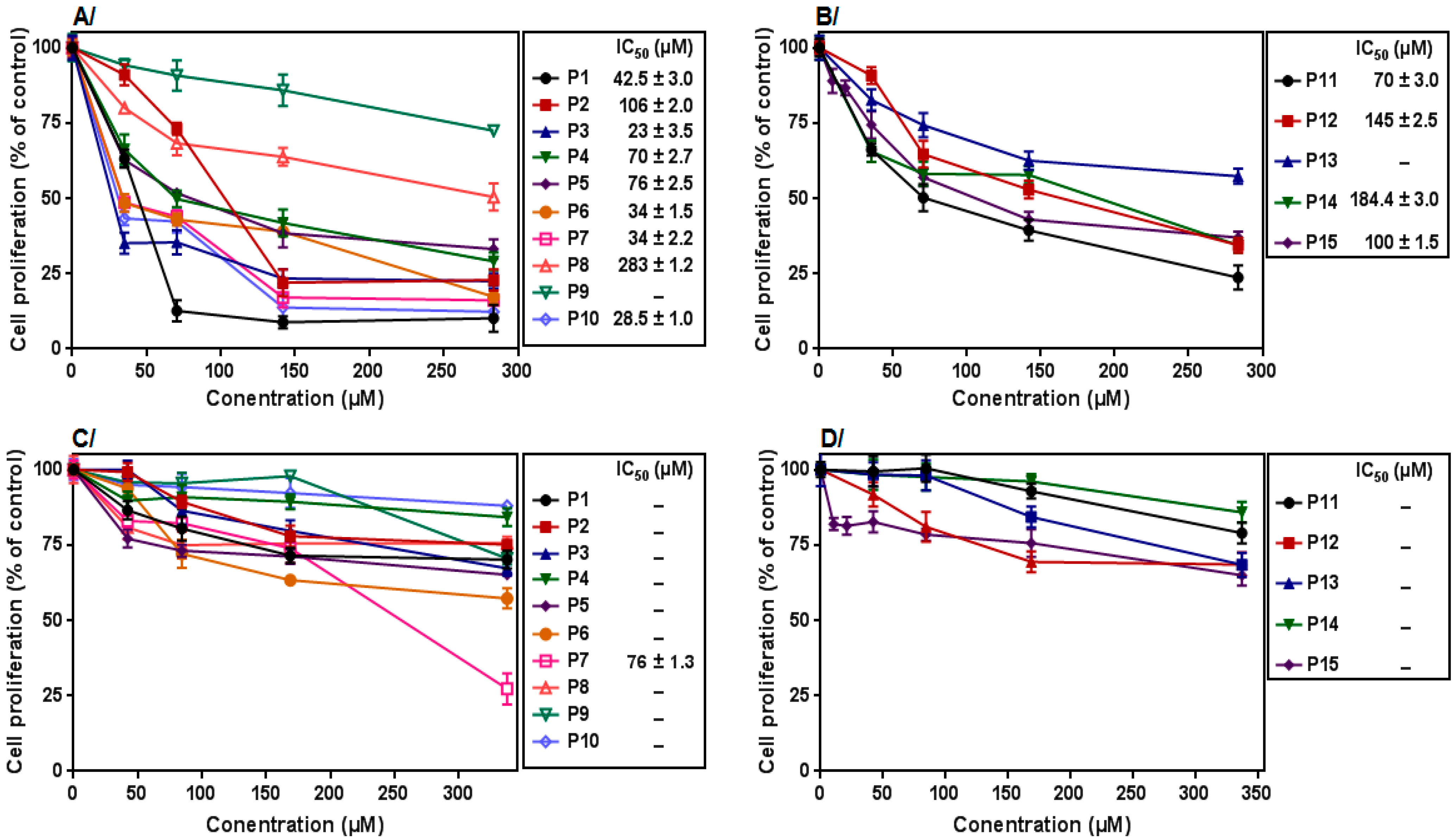
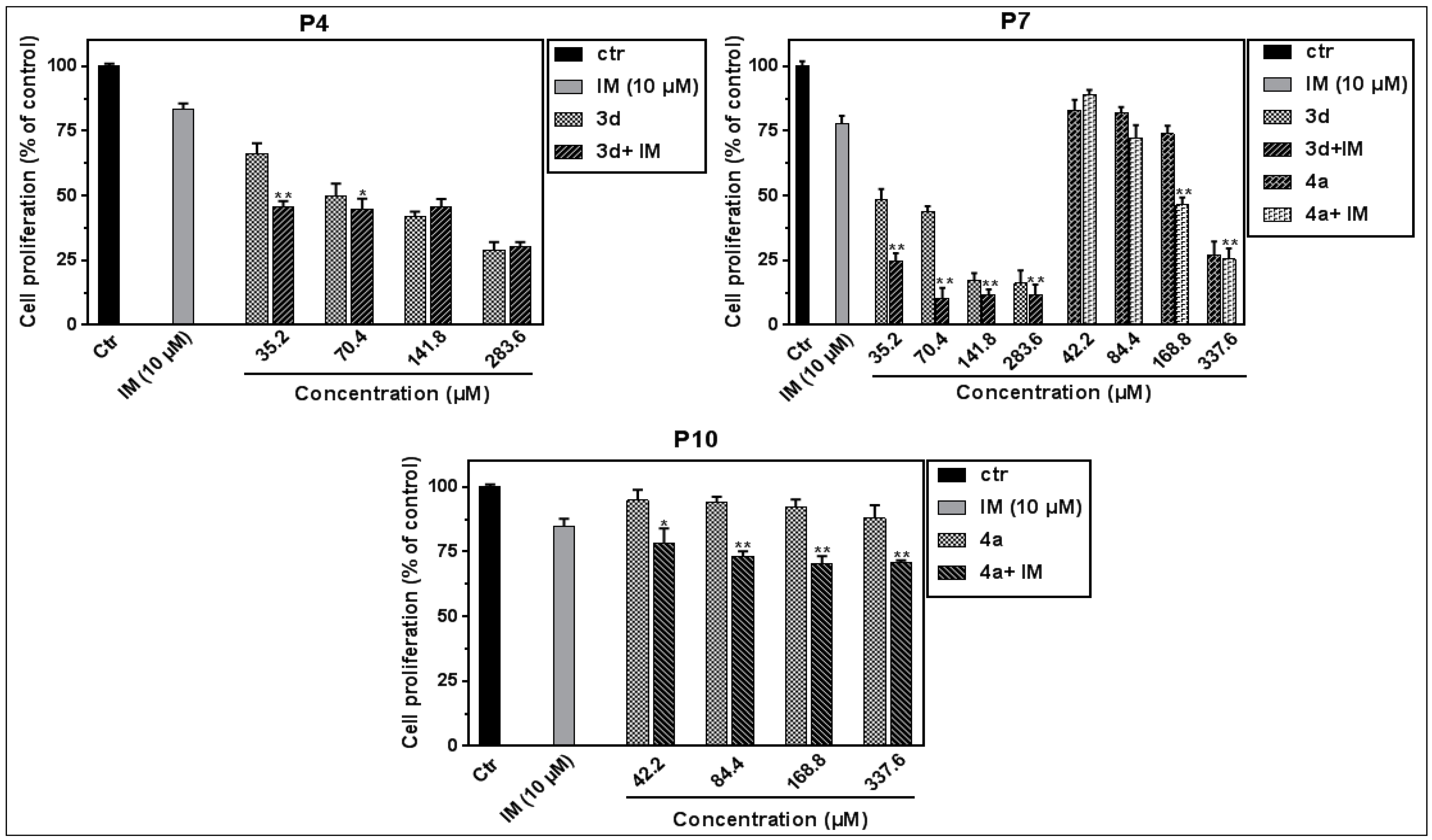
3.3. Effect on Leukemic PBMCs
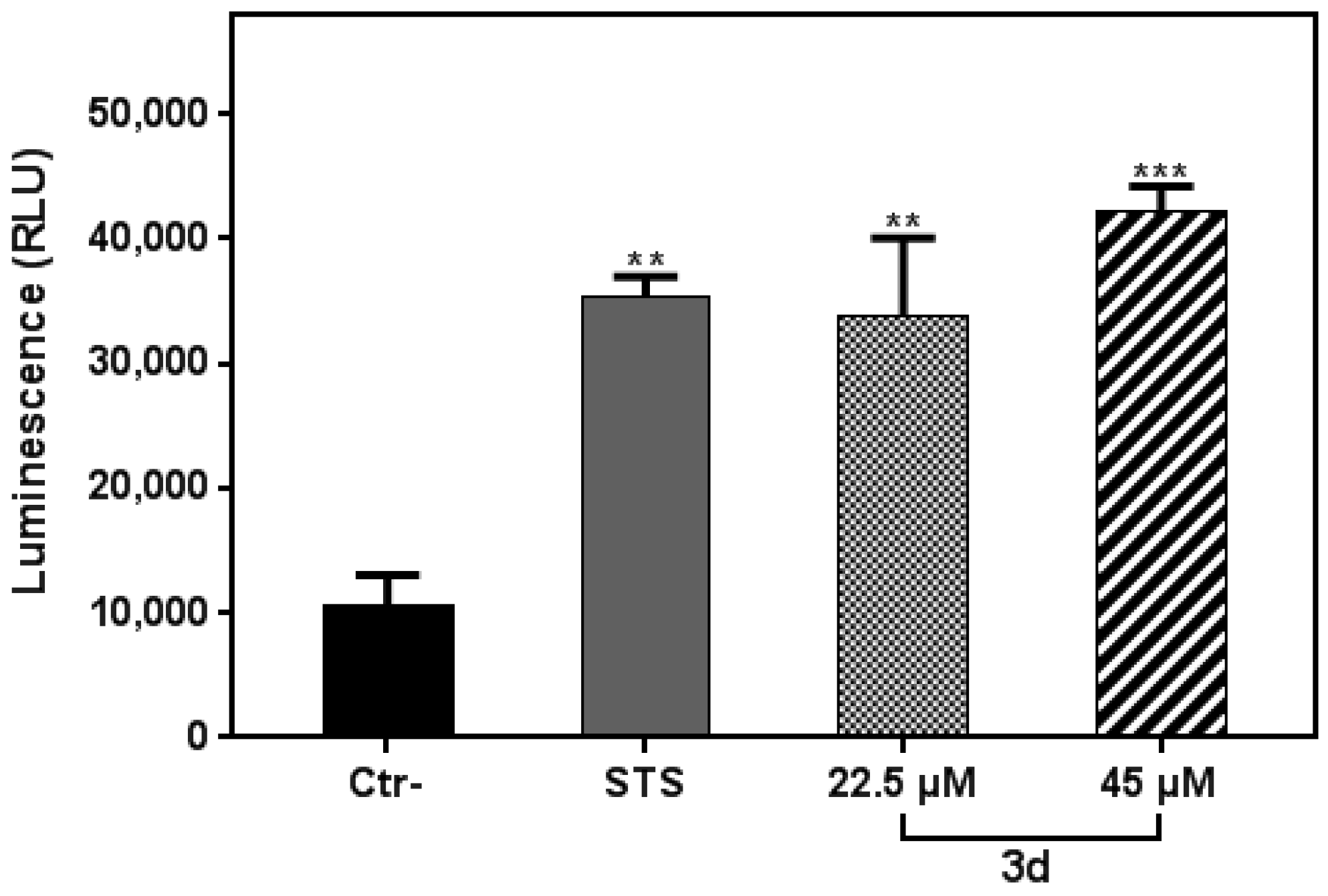
3.4. Compound 3d Induced Apoptosis in K562 Leukemia Cells
3.5. Compound 3d Triggered ROS Production in K562 Leukemia Cells
3.6. Effect of 3d Treatment on MAPK, Erk and Akt Activation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| CML | Chronic myeloid leukemia |
| TKI | Tyrosine kinase inhibitor |
| IM | Imatinib mesylate |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| FCS | Fetal calf serum |
| RPMI | Roswell Park Memorial Institute |
| PBS | Phosphate-buffered saline |
References
- Meenakshi Sundaram, D.N.; Jiang, X.; Brandwein, J.M.; Valencia-Serna, J.; Remant, K.C.; Uludağ, H. Current outlook on drug resistance in chronic myeloid leukemia (CML) and potential therapeutic options. Drug Discov. Today 2019, 24, 1355–1369. [Google Scholar] [CrossRef]
- The Biology of Chronic Myeloid Leukemia|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/nejm199907153410306 (accessed on 30 September 2021).
- Bavaro, L.; Martelli, M.; Cavo, M.; Soverini, S. Mechanisms of Disease Progression and Resistance to Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukemia: An Update. Int. J. Mol. Sci. 2019, 20, 6141. [Google Scholar] [CrossRef] [Green Version]
- Lussana, F.; Intermesoli, T.; Stefanoni, P.; Rambaldi, A. Mechanisms of Resistance to Targeted Therapies in Chronic Myeloid Leukemia. Handb. Exp. Pharmacol. 2018, 249, 231–250. [Google Scholar]
- Khan, T.; Gurav, P. PhytoNanotechnology: Enhancing Delivery of Plant Based Anti-cancer Drugs. Front. Pharmacol. 2017, 8, 1002. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Shahar Yar, M. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef]
- Shiri, P. Novel Hybrid Molecules Based on triazole-β-lactam as Potential Biological Agents. Mini. Rev. Med. Chem. 2021, 21, 536–553. [Google Scholar] [CrossRef]
- Sharghi, H.; Aberi, M.; Shiri, P. Supported benzimidazole-salen Cu(II) complex: An efficient, versatile and highly reusable nanocatalyst for one-pot synthesis of hybrid molecules. Appl. Organomet. Chem. 2018, 32, e4446. [Google Scholar] [CrossRef]
- Tripathi, V.K.; Singh, J.; Ara, T.; Koul, S.; Farooq, S.; Kaul, A. Synthesis and biological evaluation of novel isoxazoles and triazoles linked 6-hydroxycoumarin as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2014, 24, 4243–4246. [Google Scholar]
- Naresh Kumar, R.; Jitender Dev, G.; Ravikumar, N.; Krishna Swaroop, D.; Debanjan, B.; Bharath, G.; Narsaiah, B.; Nishant Jain, S.; Gangagni Rao, A. Synthesis of novel triazole/isoxazole functionalized 7-(trifluoromethyl) pyrido[2,3-d]pyrimidine derivatives as promising anticancer and antibacterial agents. Bioorg. Med. Chem. Lett. 2016, 26, 2927–2930. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Etichetti CM, B.; Cicetti, S.; Girardini, J.E.; Spanevello, R.A.; Suárez, A.G.; Sarotti, A.M. Design, synthesis and evaluation of novel levoglucosenone derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2020, 30, 127247. [Google Scholar] [CrossRef]
- Dalidovich, T.S.; Hurski, A.L.; Morozevich, G.E.; Latysheva, A.S.; Sushko, T.A.; Strushkevich, N.V.; Gilep, A.A.; Misharin, A.Y.; Zhabinskii, V.N.; Khripach, V. A.New azole derivatives of [17(20)E]-21-norpregnene: Synthesis and inhibition of prostate carcinoma cell growth. Steroids 2019, 147, 10–18. [Google Scholar] [CrossRef]
- Chan, S.-H.; Leu, W.-J.; Swain, S.P.; Hsu, J.-L.; Hou, D.-R.; Guh, J.-H. Mechanistic Study of Triazole Based Aminodiol Derivatives in Leukemic Cells-Crosstalk between Mitochondrial Stress-Involved Apoptosis and Autophagy. Int. J. Mol. Sci. 2020, 21, 2470. [Google Scholar] [CrossRef] [Green Version]
- Ashwini, N.; Garg, M.; Mohan, C.D.; Fuchs, J.E.; Rangappa, S.; Anusha, S.; Swaroop, T.R.; Rakesh, K.S.; Kanojia, D.; Madan, V.; et al. Synthesis of 1,2-benzisoxazole tethered 1,2,3-triazoles that exhibit anticancer activity in acute myeloid leukemia cell lines by inhibiting histone deacetylases, and inducing p21 and tubulin acetylation. Bioorg. Med. Chem. 2015, 23, 6157–6165. [Google Scholar] [CrossRef]
- Pan, S.; Hu, J.; Zheng, T.; Liu, X.; Ju, Y.; Xu, C. Oleanolic acid derivatives induce apoptosis in human leukemia K562 cell involved in inhibition of both Akt1 translocation and pAkt1 expression. Cytotechnology 2015, 67, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Rong, J.; Feng, Z.-Z.; Shi, Y.-J.; Ren, J.; Xu, Y.; Wang, N.-Y.; Xue, Q.; Liu, K.L.; Zhou, S.Y.; Wei, W.; et al. Design, synthesis and biological evaluation of 3,5-dimethylisoxazole and pyridone derivatives as BRD4 inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 126577. [Google Scholar] [CrossRef]
- Li, Y.-T.; Wang, J.-H.; Pan, C.-W.; Meng, F.-F.; Chu, X.-Q.; Ding, Y.; Qu, W.Z.; Li, H.Y.; Yang, C.; Zhang, Q. Syntheses and biological evaluation of 1,2,3-triazole and 1,3,4-oxadiazole derivatives of imatinib. Bioorg. Med. Chem. Lett. 2016, 26, 1419–1427. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Zhu, J.; Mo, J.; Lin, H.Z.; Chen, Y.; Sun, H.P. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065–3075. [Google Scholar] [CrossRef]
- Aissa, I.; Abdelkafi-Koubaa, Z.; Chouaïb, K.; Jalouli, M.; Assel, A.; Romdhane, A.; Harrath, A.H.; Marrakchi, N.; Ben Jannet, H. Glioblastoma-specific anticancer activity of newly synthetized 3,5-disubstituted isoxazole and 1,4-disubstituted triazole-linked tyrosol conjugates. Bioorg. Chem. 2021, 114, 105071. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Swoboda, D.M.; Grover, A.; Nodzon, L.; Zhang, L.; Pinilla-Ibarz, J. T315I-mutated myeloid sarcoma. Leuk. Res. Rep. 2019, 12, 100184. [Google Scholar] [CrossRef]
- Puxeddu, M.; Shen, H.; Bai, R.; Coluccia, A.; Bufano, M.; Nalli, M.; Sebastiani, J.; Brancaccio, D.; Da Pozzo, E.; Tremolanti, C.; et al. Discovery of pyrrole derivatives for the treatment of glioblastoma and chronic myeloid leukemia. Eur. J. Med. Chem. 2021, 221, 113532. [Google Scholar] [CrossRef]
- Liu, W.; Lin, L.C.; Wang, P.J.; Chen, Y.N.; Wang, S.C.; Chuang, Y.T.; Tsai, I.H.; Yu, S.Y.; Chang, F.R.; Cheng, Y.B. Nepenthes Ethyl Acetate Extract Provides Oxidative Stress-Dependent Anti-Leukemia Effects. Antioxidants 2021, 10, 1410. [Google Scholar] [CrossRef]
- Çalışkan, B.; Sinoplu, E.; İbiş, K.; Akhan Güzelcan, E.; Çetin Atalay, R.; Banoglu, E. Synthesis and cellular bioactivities of novel isoxazole derivatives incorporating an arylpiperazine moiety as anticancer agents. J. Enzyme. Inhib. Med. Chem. 2018, 33, 1352. [Google Scholar] [CrossRef] [Green Version]



| N | Age | Sex | Diagnosis | Phase | Variant BCR–ABL | BCR–ABL/ABL Ratio | White Cell Count/mm3 | Platelets/mm3 |
|---|---|---|---|---|---|---|---|---|
| Group 1 | ||||||||
| 1 | 60 | M | CML | At diagnosis | b2a2 | 154,620 | _ | |
| 2 | 24 | M | CML | At diagnosis | b2a2 | 157,000 | 98,000 | |
| 3 | 51 | M | CML | At diagnosis | b3a2 | 95,590 | 629,000,000 | |
| 4 | 49 | M | CML | At diagnosis | b2a2 | 647,380 | 407,000 | |
| 5 | 61 | F | CML | At diagnosis | b2a2/b3a2 | 40,000 | 281,000 | |
| 6 | _ | F | CML | At diagnosis | e1a2 (P190) | 50,000 | 219,000 | |
| 7 | 42 | F | CML | At diagnosis | b2a2 | 127,000 | 436,000 | |
| 8 | 47 | F | CML | At diagnosis | b3a2 | 60,240 | 454,000 | |
| 9 | 54 | M | CML | At diagnosis | b2a2 (T315I?) | 132,300 | 384,000 | |
| 10 | 60 | F | CML | At diagnosis | b2a2 | 100,000 | 120,000 | |
| Group 2 | ||||||||
| 11 | 48 | F | CML | Resistant | b2a2 | 3 months 41% 6 months 32% | 4630 | 196,000 |
| 12 | 61 | F | CML | Resistant | b2a2 | 64% | 71,520 | 82,000 |
| 13 | 55 | F | CML | Resistant | b3a2 | |||
| 14 | 52 | M | CML | Resistant | b2a2 | 3 months 22% 6 months 7% | 218,000 | _ |
| 15 | 60 | F | CML | Resistant | b2a2 | 3 months 20% 6 months 7% | 4600 | 80,000 |
| Compound | Chemical Formula |
|---|---|
| 1 | 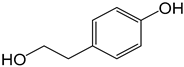 |
| 2 | 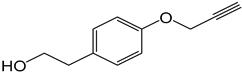 |
| 3a | 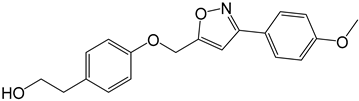 |
| 3b | 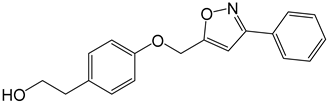 |
| 3c | 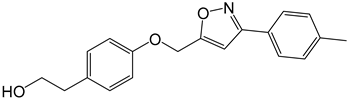 |
| 3d | 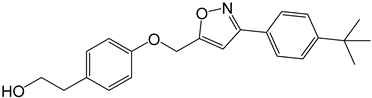 |
| 3e | 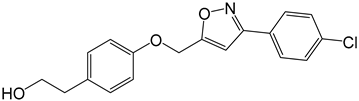 |
| 4a |  |
| 4b | 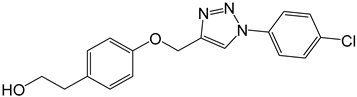 |
| 4c | 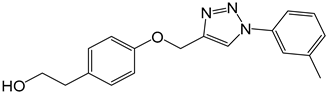 |
| 4d | 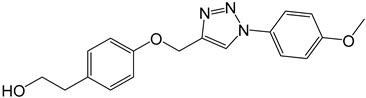 |
| 4e | 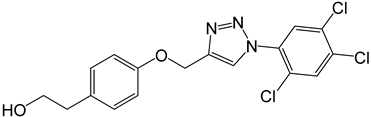 |
| Compound | 1 | 2 | 3a | 3b | 3c | 3d | 3e | 4a | 4b | 4c | 4d | 4e |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | >100 | 18 ± 1.5 | 18 ± 2.0 | 24 ± 0.9 | 18 ± 3.0 | 16 ± 1.0 | 18 ± 2.5 | 18 ± 0.8 | 24 ± 2.0 | 50 ± 1.5 | 25 ± 1.2 | 25 ± 0.5 |
| IC50 (µM) | >400 | 101 ± 8.4 | 55 ± 6.1 | 81 ± 3 | 58 ± 9.6 | 45 ± 2.8 | 54.5 ± 7.5 | 61 ± 2.7 | 72.5 ± 6 | 161 ± 4.8 | 76.5 ± 3.6 | 63 ± 1.2 |
| Patient | % Inhibition |
|---|---|
| Group 1 | |
| 1 | 34.8 ± 0.84 |
| 2 | 27.3 ± 2.2 |
| 3 | 23.8 ± 1.0 |
| 4 | 16.4 ± 0.8 |
| 5 | 29.9 ± 0.5 |
| 6 | 50.9 ± 1.3 |
| 7 | 22.1 ± 0.9 |
| 8 | 23.4 ± 1.2 |
| 9 | 21 ± 2.4 |
| 10 | 15.3 ± 3.3 |
| Group 2 | |
| 11 | 35.9 ± 1.5 |
| 12 | 21.3 ± 2.1 |
| 13 | 22.1 ± 0.8 |
| 14 | 21.8 ± 2.9 |
| 15 | 30.6 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkafi-Koubaa, Z.; Aissa, I.; Ben Jannet, H.; Srairi-Abid, N.; Marrakchi, N.; Menif, S. Tyrosol Derivatives, Bearing 3,5-Disubstituted Isoxazole and 1,4-Disubstituted Triazole, as Potential Antileukemia Agents by Promoting Apoptosis. Molecules 2022, 27, 5086. https://doi.org/10.3390/molecules27165086
Abdelkafi-Koubaa Z, Aissa I, Ben Jannet H, Srairi-Abid N, Marrakchi N, Menif S. Tyrosol Derivatives, Bearing 3,5-Disubstituted Isoxazole and 1,4-Disubstituted Triazole, as Potential Antileukemia Agents by Promoting Apoptosis. Molecules. 2022; 27(16):5086. https://doi.org/10.3390/molecules27165086
Chicago/Turabian StyleAbdelkafi-Koubaa, Zaineb, Imen Aissa, Hichem Ben Jannet, Najet Srairi-Abid, Naziha Marrakchi, and Samia Menif. 2022. "Tyrosol Derivatives, Bearing 3,5-Disubstituted Isoxazole and 1,4-Disubstituted Triazole, as Potential Antileukemia Agents by Promoting Apoptosis" Molecules 27, no. 16: 5086. https://doi.org/10.3390/molecules27165086
APA StyleAbdelkafi-Koubaa, Z., Aissa, I., Ben Jannet, H., Srairi-Abid, N., Marrakchi, N., & Menif, S. (2022). Tyrosol Derivatives, Bearing 3,5-Disubstituted Isoxazole and 1,4-Disubstituted Triazole, as Potential Antileukemia Agents by Promoting Apoptosis. Molecules, 27(16), 5086. https://doi.org/10.3390/molecules27165086






