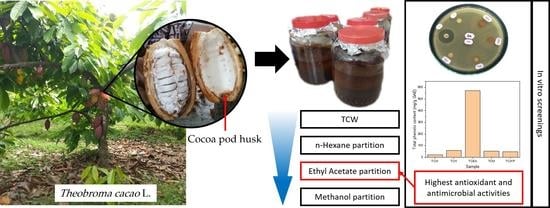
In-Vitro Screenings for Biological and Antioxidant Activities of Water Extract from Theobroma cacao L. Pod Husk: Potential Utilization in Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Specimen
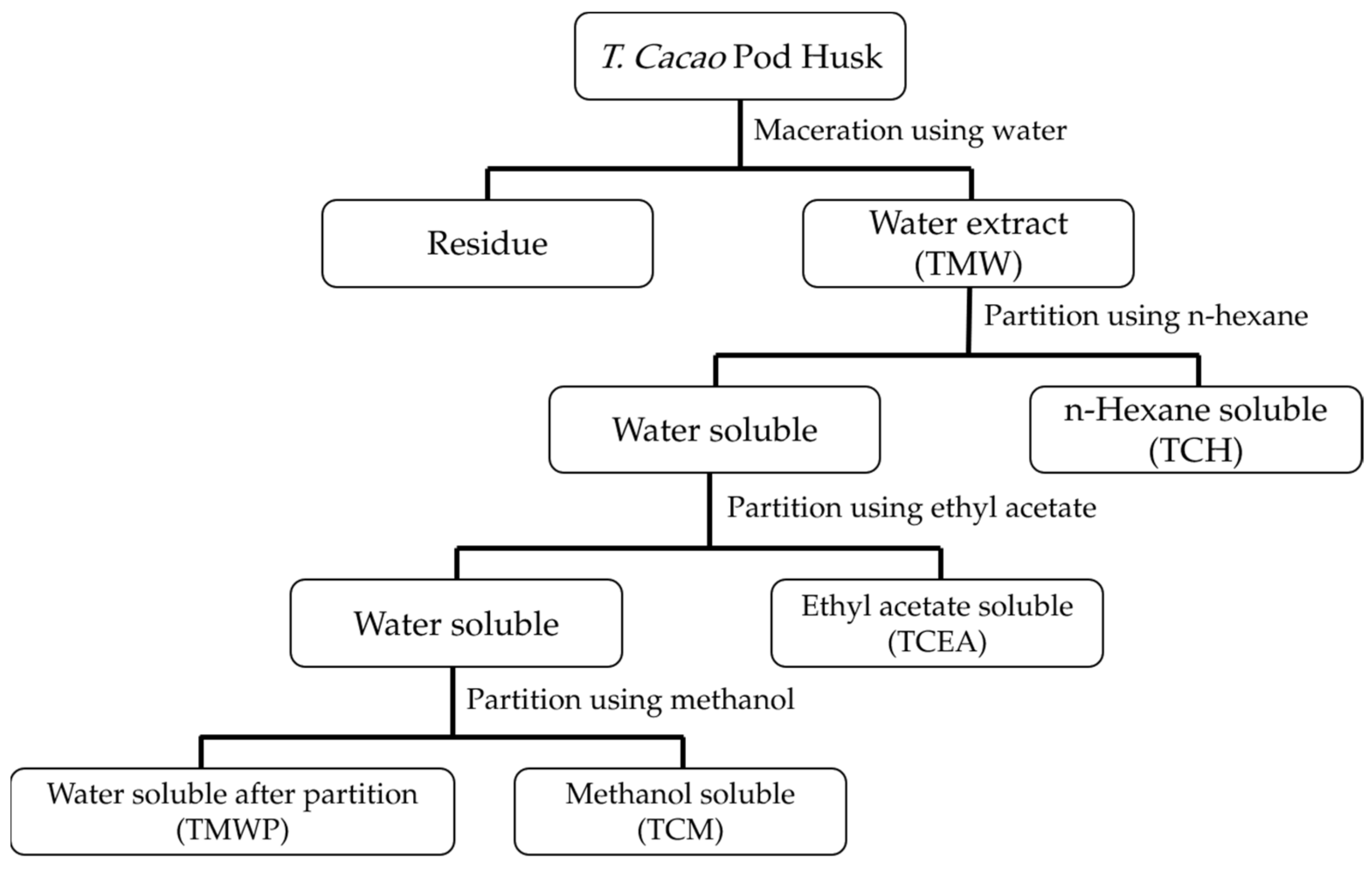
2.3. Extraction of T. cacao Pod Husks
2.4. Qualitative Phytochemical Analysis
2.5. Disc Diffusion Assay
2.6. Brine Shrimp Lethality Test (BSLT) Assay
2.7. Determination of Total Phenolic Content
2.8. Determination of Total Flavonoid Content
2.9. 2,2-Diphenyl-1-picrylhydrazyl Assay
2.10. Data Analysis
3. Results and Discussion
3.1. Yield and Phytochemical Contents
3.2. Antibacterial Activities
3.3. Antifungal Activities
3.4. Cytotoxicity
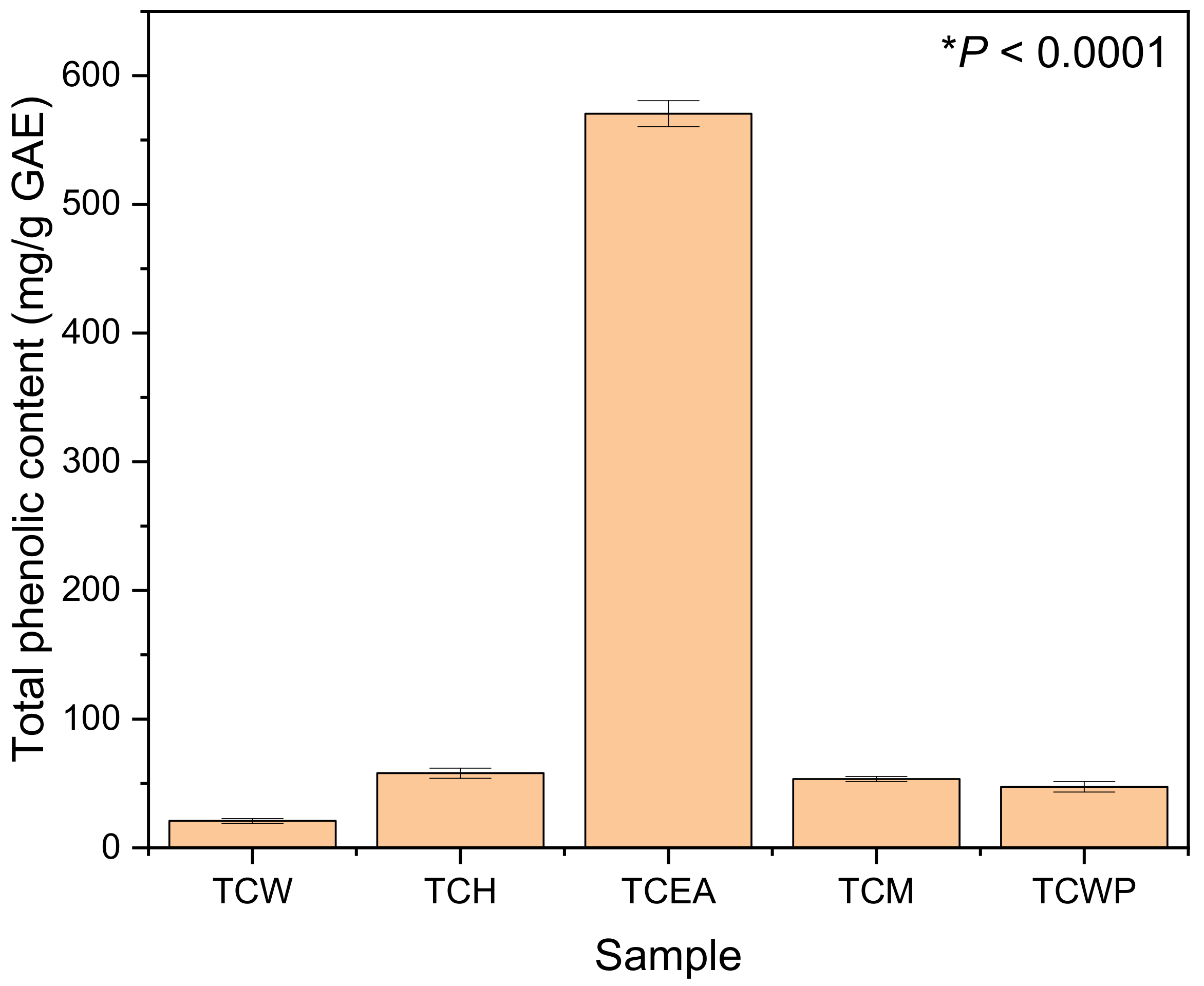
3.5. Total Phenolic Content (TPC)
3.6. Total Flavonoid Content (TFC)
3.7. Antioxidant Activities
3.8. Phytoconstituents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Voora, V.; Bermudez, S.; Larrea, C. Global Market Report; JSTOR: Cocoa, FL, USA, 2019; pp. 1–2. [Google Scholar]
- International Cocoa Organization November 2020 Quarterly Bulletin of Cocoa Statistics. Available online: https://www.icco.org/november-2020-quarterly-bulletin-of-cocoa-statistics/ (accessed on 5 October 2021).
- Vriesmann, L.C.; de Mello Castanho Amboni, R.D.; de Oliveira Petkowicz, C.L. Cacao pod husks (Theobroma cacao L.): Composition and hot-water-soluble pectins. Ind. Crop. Prod. 2011, 34, 1173–1181. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.L.; Lozano-Sanchez, J.; Contreras-Gámez, M.; Legeai-Mallet, L.; Fernández-Arroyo, S.; Segura-Carretero, A. Isolation, comprehensive characterization and antioxidant activities of Theobroma cacao extract. J. Funct. Foods 2014, 10, 485–498. [Google Scholar] [CrossRef]
- Gil, M.; Uribe, D.; Gallego, V.; Bedoya, C.; Arango-Varela, S. Traceability of polyphenols in cocoa during the postharvest and industrialization processes and their biological antioxidant potential. Heliyon 2021, 7, e07738. [Google Scholar] [CrossRef]
- Carrillo, L.C.; Londoño-Londoño, J.; Gil, A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Wulanjati, M.P.; Windarsih, A.; Bhattacharjya, D.K.; Suzuki, T.; Katayama, T. In vitro studies of antioxidant, antidiabetic, and antibacterial activities of Theobroma cacao, Anonna muricata and Clitoria ternatea. Biocatal. Agric. Biotechnol. 2021, 33, 101995. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Umri, R.J.; Maulana, I.; Ginting, B. Antioxidant and cytotoxic activity of ethyl acetate extracts of cocoa pod husk (Theobroma cacao L.). IOP Conf. Ser. Earth Environ. Sci. 2019, 364, 012026. [Google Scholar] [CrossRef]
- Ginting, B.; Purnama, A. Chemical composition and cytotoxic activities of n-Hexane extract from cacao pod husk (Theobroma cacao L.). Chem. Data Collect. 2020, 30, 100553. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Orlo, E.; Russo, C.; Nugnes, R.; Lavorgna, M.; Isidori, M. Natural Methoxyphenol Compounds: Antimicrobial Activity against Foodborne Pathogens and Food Spoilage Bacteria, and Role in Antioxidant Processes. Foods 2021, 10, 1807. [Google Scholar] [CrossRef]
- Zhu, L.; Li, W.; Deng, Z.; Li, H.; Zhang, B. The Composition and Antioxidant Activity of Bound Phenolics in Three Legumes, and Their Metabolism and Bioaccessibility of Gastrointestinal Tract. Foods 2020, 9, 1816. [Google Scholar] [CrossRef] [PubMed]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Ibrahim, N.H.; Mahmud, M.S.; Nurdin, S. Microwave-assisted extraction of β-sitosterol from cocoa shell waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012106. [Google Scholar] [CrossRef]
- Gonfa, T.; Teketle, S.; Kiros, T. Effect of extraction solvent on qualitative and quantitative analysis of major phyto-constituents and in-vitro antioxidant activity evaluation of Cadaba rotundifolia Forssk leaf extracts. Cogent. Food Agric. 2020, 6, 1853867. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Barreto de Deus, T.; Barros, L.S.S.; Mendes da Silva, R.; Karine da Silva Lima, W.; das Virgens Lima, D.; dos Santos Silva, A. Staphylococcus aureus and Escherichia coli in Curd Cheese Sold in the Northeastern Region of South America. Int. J. Microbiol. 2017, 2017, 8173741. [Google Scholar] [CrossRef]
- Cahlíková, L.; Breiterová, K.; Opletal, L. Chemistry and Biological Activity of Alkaloids from the Genus Lycoris (Amaryllidaceae). Molecules 2020, 25, 4797. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Khan, M.A. Antibacterial, antioxidant and cytotoxic studies of total saponin, alkaloid and sterols contents of decoction of Joshanda: Identification of components through thin layer chromatography. Toxicol. Ind. Health 2015, 31, 202–208. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation—A Review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Kunicka-Styczyńska, A. Typing and virulence factors of food-borne Candida spp. isolates. Int. J. Food Microbiol. 2018, 279, 57–63. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.; Milenkovic, M.; Vidovic, B.; Todorovic, Z.; Sobajic, S. Correlation between Antimicrobial, Antioxidant Activity, and Polyphenols of Alkalized/Nonalkalized Cocoa Powders. J. Food Sci. 2017, 82, 1020–1027. [Google Scholar] [CrossRef]
- Ogidi, C.O.; Abioye, S.A.; Akinyemi, D.D.; Fadairo, F.B.; Bolaniran, T.; Akinyele, B.J. Bioactivity assessment of ethanolic extracts from Theobroma cacao and Cola spp. wastes after solid state fermentation by Pleurotus ostreatus and Calocybe indica. Adv. Tradit. Med. 2021. [Google Scholar] [CrossRef]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Hasballah, K.; Sarong, M.; Rusly, R.; Fitria, H.; Maida, D.R.; Iqhrammullah, M. Antiproliferative Activity of Triterpenoid and Steroid Compounds from Ethyl Acetate Extract of Calotropis gigantea Root Bark against P388 Murine Leukemia Cell Lines. Sci. Pharm. 2021, 89, 21. [Google Scholar] [CrossRef]
- Chan, W.; Shaughnessy, A.E.P.; van den Berg, C.P.; Garson, M.J.; Cheney, K.L. The Validity of Brine Shrimp (Artemia Sp.) Toxicity Assays to Assess the Ecological Function of Marine Natural Products. J. Chem. Ecol. 2021. [Google Scholar] [CrossRef]
- Kayaputri, I.L.; Sumanti, D.M.; Djali, M.; Indiarto, R.; Dewi, D.L. Kajian Fitokimia Ekstrak Kulit Biji Kakao (Theobroma cacao L.). Chim. Nat. Acta 2014, 2, 83–90. [Google Scholar] [CrossRef][Green Version]
- Martin, M.A.; Goya, L.; Ramos, S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food Chem. Toxicol. 2013, 56, 336–351. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Extraction of phenolic compounds from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: A comparative study of fifteen Indian cultivars. J. Food Sci. Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef]
- Nurcholis, W.; Sya’bani Putri, D.N.; Husnawati, H.; Aisyah, S.I.; Priosoeryanto, B.P. Total flavonoid content and antioxidant activity of ethanol and ethyl acetate extracts from accessions of Amomum compactum fruits. Ann. Agric. Sci. 2021, 66, 58–62. [Google Scholar] [CrossRef]
- Rosendal, E.; Ouédraogo, J.C.W.; Dicko, C.; Dey, E.S.; Bonzi-Coulibaly, Y.L. Geographical variation in total phenolics, flavonoids and antioxidant activities of Eucalyptus camaldulensis leaves in Burkina Faso. Afr. J. Pure Appl. Chem. 2020, 14, 51–59. [Google Scholar] [CrossRef]
- El-Chaghaby, G.A.; Ahmad, A.F.; Ramis, E.S. Evaluation of the antioxidant and antibacterial properties of various solvents extracts of Annona squamosa L. leaves. Arab. J. Chem. 2014, 7, 227–233. [Google Scholar] [CrossRef]
- Oresanya, I.O.; Sonibare, M.A.; Gueye, B.; Balogun, F.O.; Adebayo, S.; Ashafa, A.O.T.; Morlock, G. Isolation of flavonoids from Musa acuminata Colla (Simili radjah, ABB) and the in vitro inhibitory effects of its leaf and fruit fractions on free radicals, acetylcholinesterase, 15-lipoxygenase, and carbohydrate hydrolyzing enzymes. J. Food Biochem. 2020, 44, e13137. [Google Scholar] [CrossRef] [PubMed]
- Mwamatope, B.; Tembo, D.; Chikowe, I.; Kampira, E.; Nyirenda, C. Total phenolic contents and antioxidant activity of Senna singueana, Melia azedarach, Moringa oleifera and Lannea discolor herbal plants. Sci. Afr. 2020, 9, e00481. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Bubonja-Sonje, M.; Giacometti, J.; Abram, M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011, 127, 1821–1827. [Google Scholar] [CrossRef]
- Quiroz-Reyes, C.N.; Aguilar-Mendez, M.A.; Ramirez-Ortiz, M.E.; Ronquillo-De Jesús, E. Comparative study of ultrasound and maceration techniques for the extraction of polyphenols from cocoa beans (Theobroma cacao L.). Rev. Mex. Ing. Química 2013, 12, 11–18. [Google Scholar]
- Sirikhansaeng, P.; Tanee, T.; Sudmoon, R.; Chaveerach, A. Major Phytochemical as γ -Sitosterol Disclosing and Toxicity Testing in Lagerstroemia Species. Evidence-Based Complement. Altern. Med. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Cerri, M.; Reale, L.; Zadra, C. Metabolite Storage in Theobroma cacao L. Seed: Cyto-Histological and Phytochemical Analyses. Front. Plant Sci. 2019, 10, 1599. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Effect of roasting conditions on the fat, tocopherol, and phytosterol content and antioxidant capacity of the lipid fraction from cocoa beans of different Theobroma cacao L. cultivars. Eur. J. Lipid Sci. Technol. 2014, 116, 1002–1014. [Google Scholar] [CrossRef]
- Labib, R.M.; Ayoub, N.A.; Singab, A.B.; Al-Azizi, M.M.; Sleem, A. Chemical constituents and pharmacological studies of Lagerstroemia indica. Phytopharmacology 2013, 4, 373–389. [Google Scholar]
- Endrini, S.; Rahmat, A.; Ismail, P.; Taufiq-Yap, Y.H. Cytotoxic effect of γ-sitosterol from Kejibeling (Strobilanthes crispus) and its mechanism of action towards c-myc gene expression and apoptotic pathway. Med. J. Indones. 2015, 23, 203–208. [Google Scholar] [CrossRef]
- Balamurugan, R.; Duraipandiyan, V.; Ignacimuthu, S. Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2011, 667, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.-H.; Quan, Y.-C.; Jiang, H.-Y.; Wen, Z.-S.; Qu, Y.-L.; Guan, L.-P. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 2015, 768, 131–138. [Google Scholar] [CrossRef]
- Carpenter, D.R.; Hammerstone, J.F.; Romanczyk, L.J.; Aitken, W.M. Lipid composition of Herrania and Theobroma seeds. J. Am. Oil Chem. Soc. 1994, 71, 845–851. [Google Scholar] [CrossRef]
- Wang, T.; Hicks, K.B.; Moreau, R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J. Am. Oil Chem. Soc. 2002, 79, 1201–1206. [Google Scholar] [CrossRef]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Batta, A.K.; Xu, G.; Honda, A.; Miyazaki, T.; Salen, G. Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism 2006, 55, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. Biomed. Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef] [PubMed]

| Sample | Yield (%) | Phytochemical Contents | |||||
|---|---|---|---|---|---|---|---|
| Alkaloids | Flavonoids | Terpenoids | Steroids | Saponins | Phenolics | ||
| TCW | 33.712 | + | + | − | − | + | + |
| TCH | 0.047 | − | − | + | + | − | − |
| TCEA | 0.134 | + | + | + | − | − | + |
| TCM | 0.878 | + | + | − | − | + | + |
| TCWP | 89.390 | + | + | − | − | + | + |
| Sample | Inhibition Diameter (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Candida albicans | ||||||||||
| 1% | 5% | 10% | 20% | 1% | 5% | 10% | 20% | 1% | 5% | 10% | 20% | |
| TCW | − | − | − | − | − | − | − | − | − | − | − | − |
| TCH | − | − | 6.10 ± 0.04 a | 6.55 ± 0.04 a | − | − | − | − | − | 6.70 ± 0.10 | 7.73 ± 0.08 | 9.68 ± 0.02 a |
| TCEA | − | − | 6.14 ± 0.04 a | 6.62 ± 0.10 a | − | − | − | 6.52 ± 0.02 | − | − | − | 11.72 ± 0.36 b |
| TCM | − | − | − | 6.38 ± 0.08 b | − | − | − | − | − | − | − | − |
| Control * | 19.41 ± 1 | 14.61 ± 1.1 | 13.12 ± 1.08 ** | |||||||||
| Sample | Mortality (%) | Linear Equation | LC50 (μg/mL) | ||||
|---|---|---|---|---|---|---|---|
| 1 μg/mL | 10 μg/mL | 100 μg/mL | 500 μg/mL | 1000 μg/mL | |||
| TCW | 33 | 37 | 43 | 43 | 47 | y = 0.116x + 4.567 | 5495.4 |
| TCH | 3 | 10 | 20 | 23 | 27 | y =0.458x + 3.038 | 19,054.6 |
| TCEA | 23 | 27 | 33 | 60 | 80 | y = 0.472x + 4.046 | 104.7 |
| TCM | 20 | 30 | 37 | 43 | 93 | y = 0.562x + 3.948 | 74.1 |
| Sample | Inhibition (%) | EC50 (μg/mL) | ||||
|---|---|---|---|---|---|---|
| 6.25 μg/mL | 12.5 μg/mL | 25 μg/mL | 50 μg/mL | 100 μg/mL | ||
| TCW | 12.74 ± 0.88 a | 25.60 ± 0.77 a | 28.86 ± 0.23 a | 42.16 ± 0.68 a | 46.71 ± 0.86 a | 97.69 ± 0.46 a |
| TCH | 35.04 ± 0.45 b | 35.30 ± 0.39 b | 38.44 ± 0.79 b | 44.11 ± 0.73 a | 46.56 ± 0.70 a | 116.70 ± 0.86 b |
| TCEA | 11.84 ± 0.85 a | 23.07 ± 0.92 c | 33.99 ± 0.68 c | 48.30 ± 0.65 b | 61.04 ± 0.77 b | 9.61 ± 0.64 c |
| TCM | 19.19 ± 0.91 c | 23.11 ± 0.86 c | 24.56 ± 0.63 d | 35.89 ± 0.57 c | 46.49 ± 0.95 a | 108.33 ± 0.77 d |
| TCWP | 30.04 ± 0.83 d | 31.56 ± 0.66 d | 37.97 ± 0.99 b | 42.49 ± 0.89 a | 45.60 ± 0.92 a | 115.52 ± 0.78 b |
| Compound (Formula) | Retention (Min) | Molecular Weight (g/mol) | Area (%) |
|---|---|---|---|
| Dodecanoic acid (C12H24O2) | 16.58 | 200 | 0.22 |
| Tetradecanoic acid (C14H28O2) | 20.27 | 228 | 0.20 |
| Heptadecane C17H36 | 20.76 | 240 | 0.23 |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester (C16H22O4) | 21.64 | 278 | 0.33 |
| n-Hexadecanoic acid (C16H32O2) | 22.43 | 256 | 1.43 |
| Dibutyl phthalate (C16H22O4) | 22.56 | 278 | 0.24 |
| Heneicosane (C21H44) | 22.75 | 296 | 0.22 |
| 1-Nonadecene (C19H38) | 23.46 | 266 | 0.12 |
| 4a-But-3-enyl-2-t-butyl-tetrahydrocyclopenta[1,3]dioxin-4-one (C15H24O3) | 23.62 | 252 | 0.14 |
| Octanoic acid, 4,6-dimethyl-, methyl ester, (4S,6S)-(+)- (C11H22O2) | 23.76 | 186 | 0.14 |
| Octadecane (C18H38) | 24.27 | 254 | 0.11 |
| Methyl octadec-17-ynoate (C19H34O2) | 24.86 | 294 | 2.28 |
| 9-t-Butyltricyclo[4.2.1.1(2,5)]decane-9,10-diol (C14H24O2) | 24.95 | 224 | 0.39 |
| Campesterol (C28H48O) | 25.10 | 400 | 5.61 |
| Ethyl stearate, 9,12-diepoxy (C20H36O4) | 25.82 | 340 | 0.18 |
| Oxiraneoctanoic acid, 3-octyl-, cis- (C18H34O3) | 25.94 | 298 | 0.18 |
| Stigmasterol (C29H48O) | 26.25 | 412 | 20.46 |
| Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (C19H38O4) | 26.60 | 330 | 0.43 |
| Bis(2-ethylhexyl) phthalate (C24H38O4) | 26.96 | 390 | 1.82 |
| Cyclononasiloxane, octadecamethyl- (C18H54O9Si9) | 27.99 | 666 | 0.12 |
| gamma-Sitosterol (C29H50O) | 28.42 | 414 | 64.74 |
| Stigmastanol C29H52O | 28.80 | 416 | 0.27 |
| Decanoic acid, 2-hydroxy-3-[(1-oxooctyl)oxy]propyl ester C21H40O5 | 29.13 | 372 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, M.; Ginting, B.; Saidi, N. In-Vitro Screenings for Biological and Antioxidant Activities of Water Extract from Theobroma cacao L. Pod Husk: Potential Utilization in Foods. Molecules 2021, 26, 6915. https://doi.org/10.3390/molecules26226915
Yahya M, Ginting B, Saidi N. In-Vitro Screenings for Biological and Antioxidant Activities of Water Extract from Theobroma cacao L. Pod Husk: Potential Utilization in Foods. Molecules. 2021; 26(22):6915. https://doi.org/10.3390/molecules26226915
Chicago/Turabian StyleYahya, Mustanir, Binawati Ginting, and Nurdin Saidi. 2021. "In-Vitro Screenings for Biological and Antioxidant Activities of Water Extract from Theobroma cacao L. Pod Husk: Potential Utilization in Foods" Molecules 26, no. 22: 6915. https://doi.org/10.3390/molecules26226915
APA StyleYahya, M., Ginting, B., & Saidi, N. (2021). In-Vitro Screenings for Biological and Antioxidant Activities of Water Extract from Theobroma cacao L. Pod Husk: Potential Utilization in Foods. Molecules, 26(22), 6915. https://doi.org/10.3390/molecules26226915