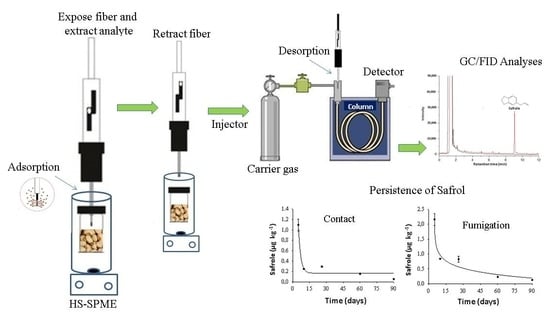
Method Validation and Evaluation of Safrole Persistence in Cowpea Beans Using Headspace Solid-Phase Microextraction and Gas Chromatography
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Cowpea Bean Characterization
2.3. Plant Material and Extraction of Essential Oil
2.4. P. hispidinervum Essential Oil Composition
2.5. Quantification of Absolute Safrole
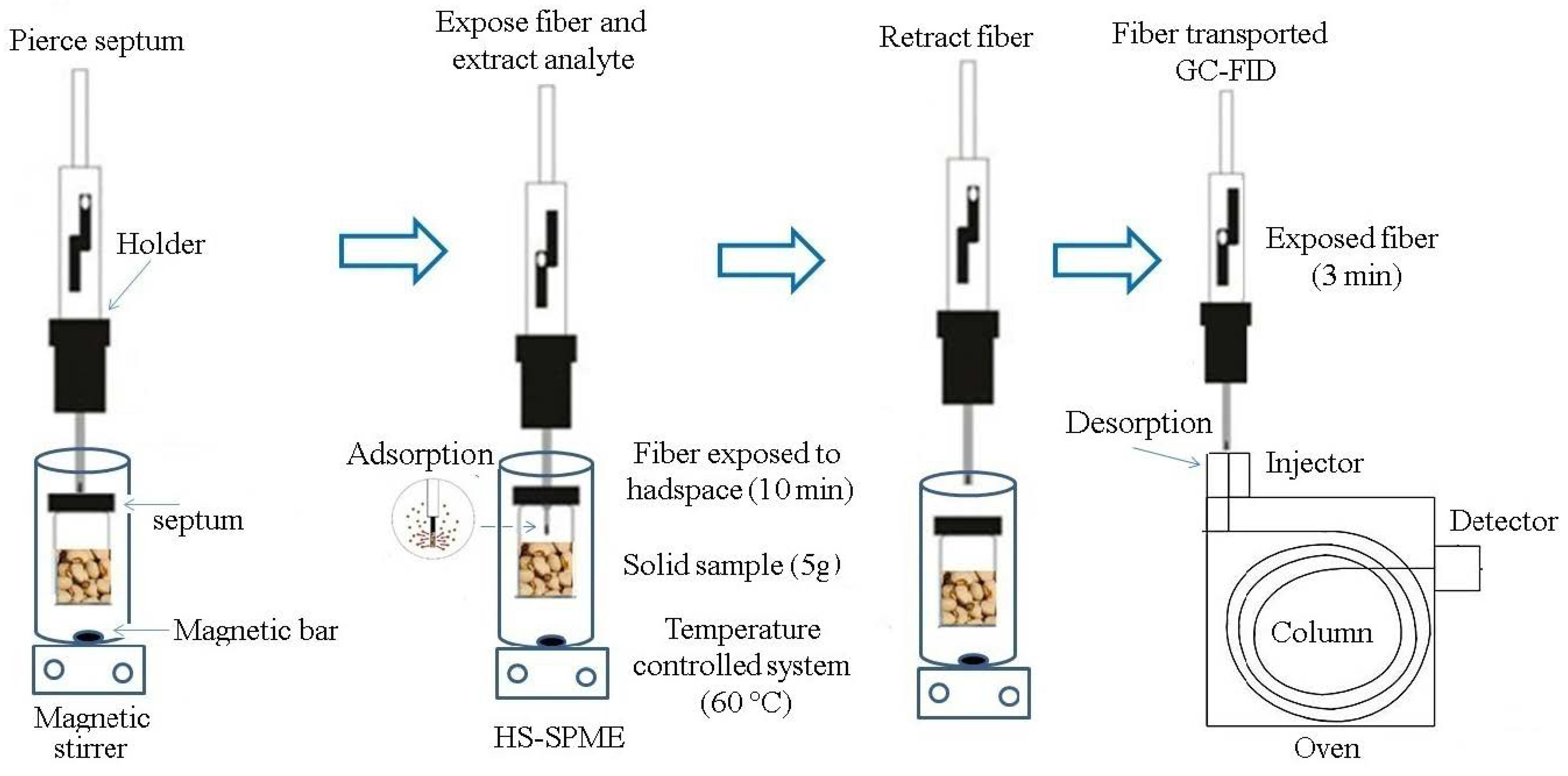
2.6. HS-SPME Procedure
2.7. Optimization of the Method of Safrole Extraction from Cowpea Beans
2.8. Validation of the Safrole Extraction and Analysis Method
2.9. Safrole Residue Persistence in Cowpea Beans
2.10. Statistical Analyses
3. Results and Discussion
3.1. Composition of the Essential Oil
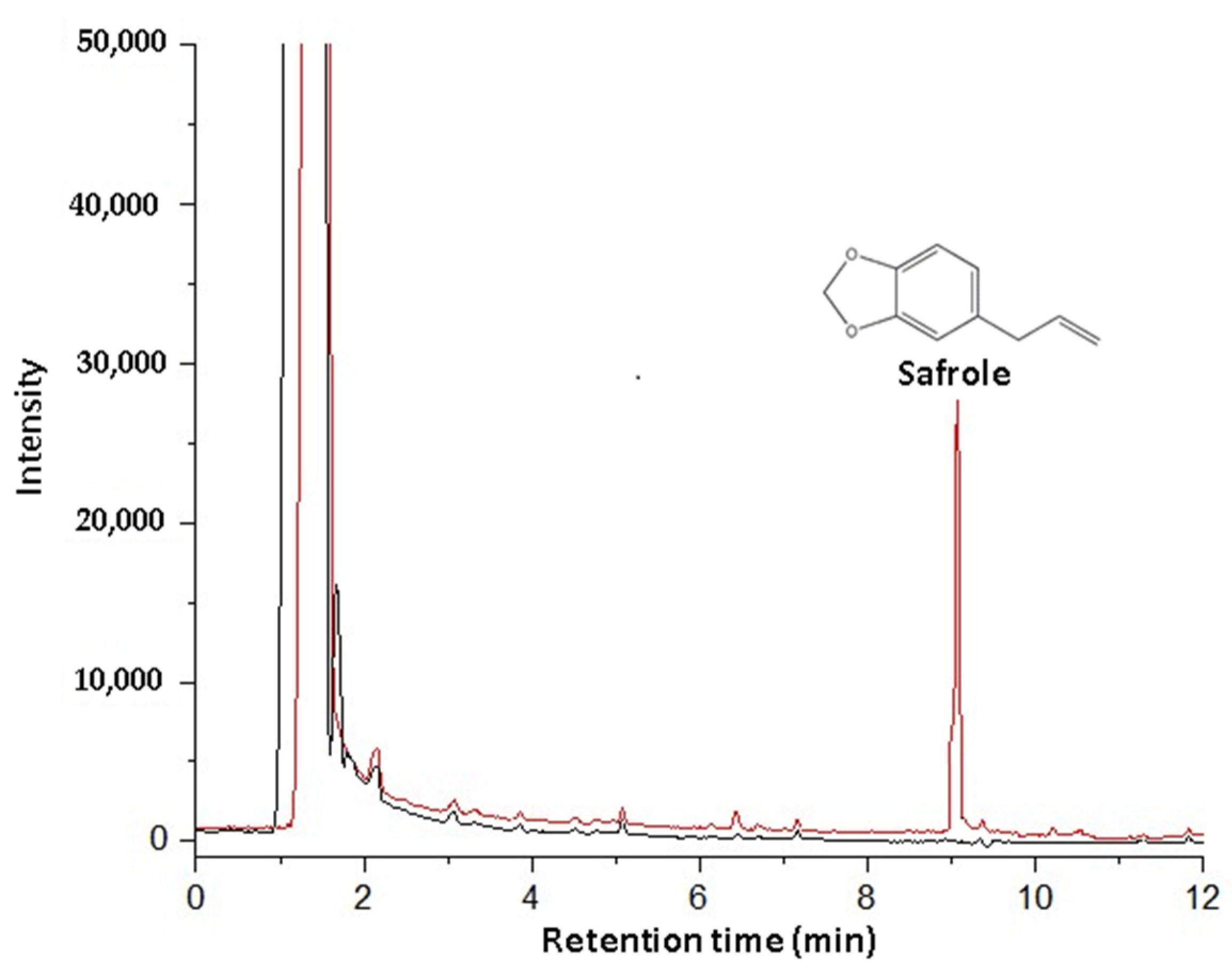
3.2. Safrole Quantification
3.3. Optimization of the Safrole Extraction Method
3.3.1. Choosing the Fiber
3.3.2. Optimal Conditions of Temperature and Extraction Time
3.4. Validation of the Method of Extraction and Analysis
3.5. Application of (HS-SPME) Method for Evaluating the Persistence of Safrole in Cowpea Beans
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tan, H.; Tie, M.; Luo, Q.; Zhu, Y.; Lai, J.; Li, H. A review of molecular makers applied in cowpea (Vigna unguiculata L. Walp.) breeding. J. Life Sci. 2012, 6, 1190–1199. [Google Scholar]
- Akami, M.; Chakira, H.; Andongma, A.A.; Khaeso, K.; Gbaye, A.O.; Nicolas, N.Y.; Nukenine, E.N.; Niu, C.Y. Essential oil optimizes the susceptibility of Callosobruchus maculatus and enhances the nutritional qualities of stored cowpea Vigna unguiculata. R. Soc. Open Sci. 2017, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop Prot. 2014, 56, 50–54. [Google Scholar] [CrossRef]
- Silva, T.T.; Costa, F.M. Survey of Insects that Attack Stored Bean Grains Vigna unguiculata (L.) and Phaseolus vulgaris L. in Porto Velho, Rondônia, Brazil. EntomoBrasilis 2016, 9, 124–128. [Google Scholar] [CrossRef]
- Gbaye, O.A.; Oyeniyi, E.A.; Ojo, O.B. Resistance of Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) populations in Nigeria to dichlorvos. Jordan J. Biol. Sci. 2016, 9, 41–46. [Google Scholar] [CrossRef]
- Oliveira, J.V.; França, S.M.; Barbosa, D.R.S.; Dutra, K.A.; Araújo, A.M.N.; Navarro, D.M.A.F. Fumigation and repellency of essential oils against Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae) in cowpea. Pesquisa Agropecuária Brasileira 2017, 52, 10–17. [Google Scholar] [CrossRef]
- Pimentel, M.; Oliveira, I.R.; Matrangolo, W.; Fernandes, D.; Ramos, G.; Eficiência de inseticidas alternativos para controle do caruncho-do-milho. Embrapa Milho e Sorgo-Boletim de Pesquisa e Desenvolvimento (INFOTECA-E) 2019. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1109817/1/bol186.pdf. (accessed on 31 September 2021).
- Liu, X.; Zhang, Q.; Li, S.; Mi, P.; Chen, D.; Zhao, X.; Feng, X. Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): A comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 2018, 199, 16–25. [Google Scholar] [CrossRef]
- Huang, Y.; Li, F.; Liu, M.; Wang, Y.; Shen, F.; Tang, P. Susceptibility of Tribolium castaneum to phosphine in China and functions of cytochrome P450s in phosphine resistance. J. Pest. Sci. 2019, 92, 1239–1248. [Google Scholar] [CrossRef]
- Starner, K.; Goh, K.S. Detections of the Neonicotinoid Insecticide Imidacloprid in Surface Waters of Three Agricultural Regions of California, USA, 2010–2011. Bull. Environ. Contam. Toxicol. 2012, 88, 316–321. [Google Scholar] [CrossRef]
- Freitas, R.S.; Faroni, L.R.A.; Sousa, A.H. Hermetic storage for control of common bean weevil, Acanthoscelides obtectus (Say). J. Stored Prod. Res. 2016, 66, 1–5. [Google Scholar] [CrossRef]
- Correa, Y.D.C.G.; Faroni, L.R.A.; Haddi, K.; Oliveira, E.E.; Pereira, E.J.G. Locomotory and physiological responses induced by clove and cinnamon essential oils in the maize weevil Sitophilus zeamais. Pestic. Biochem. Physiol. 2015, 125, 31–37. [Google Scholar] [CrossRef]
- Smith, G.H.; Roberts, J.M.; Pope, T.W. Terpene based biopesticides as potential alternatives to synthetic insecticides for control of aphid pests on protected ornamentals. Crop Prot. 2018, 110, 125–130. [Google Scholar] [CrossRef]
- Moura, E.S.; Faroni, L.R.A.; Rodrigues, A.A.Z.; Heleno, F.F.; Queiroz, M.E.L.R.; Vilela, A.O. Evaluation of the Persistence of Linalool and Estragole in Maize Grains via Headspace Solid-Phase Microextraction and Gas Chromatography. Food Anal. Methods. 2021, 14, 217–229. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappal, L. Essential oils in stored product insect pest control. J. Food Qual. 2018, 6, 1–18. [Google Scholar] [CrossRef]
- Moura, E.S.; Faroni, L.R.A.; Heleno, F.F.; Rodrigues, A.A.Z.; Prates, L.H.F.; Queiroz, M.E.L.R. Optimal extraction of Ocimum basilicum essential oil by association of ultrasound and hydrodistillation and its potential as a biopesticide against a major stored grains pest. Molecules 2020, 25, 2781. [Google Scholar] [CrossRef]
- Tak, J.; Isman, M.B. Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crops Prod. 2017, 108, 786–792. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dubey, N.K. Antifungal and antiaflatoxigenic properties of Cuminum cyminum (L.) seed essential oil and its efficacy as a preservative in stored commodities. Int. J. Food Microbiol. 2014, 168–169, 1–7. [Google Scholar] [CrossRef]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014, 116, 1149–1163. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control. 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Andrés, M.F.; Rossa, G.E.; Cassel, E.; Vargas, R.M.F.; Santana, O.; Díaz, C.E.; Gonzalez-Coloma, A. Biocidal effects of Piper hispidinervum (Piperaceae) essential oil and synergism among its main components. Food Chem. Tox. 2017, 109, 1086–1092. [Google Scholar] [CrossRef]
- Sauter, I.P.; Rossa, G.E.; Lucas, A.M.; Cibulski, S.P.; Roehec, P.M.; Silva, L.A.A.; Rott, M.B.; Vargas, M.F.; Cassel, E.; Poser, G.L. Chemical composition and amoebicidal activity of Piper hispidinervum (Piperaceae) essential oil. Ind. Crop. Prod. 2012, 40, 292–295. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the lariasis fi mosquito Culex quinquefasciatus and the housefly Musca Domestica. Ind. Crop. Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Baher, Z.F.; Mirza, M.; Ghorbanli, M.; Rezaii, M.B. The influence of water stress on plant height, herbal and essential oil yield and composition in Satureja hortensis L. Flavour Fragr. J. 2002, 17, 275–277. [Google Scholar] [CrossRef]
- Coitinho, R.L.B.C.; Oliveira, J.V.; Gondim Junior, M.G.C.; Câmara, C.A.G. Persistência de óleos essenciais em milho armazenado, submetido à infestação de gorgulho do milho. Ciência Rural 2010, 40, 1492–1496. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Silva, M.L.; Luz, A.I.R.; Zoghbi, M.G.B.; Ramos, L.S. Espécies de Piper da Amazônia rica em safrol. Química Nova 1987, 10, 200–204. [Google Scholar]
- Araújo, A.M.N.; Faroni, L.R.A.; Oliveira, J.V.; Barbosa, D.R.S.; Breda, M.O.; França, S.M. Lethal and sublethal responses of Sitophilus zeamais populations to essential oils. J. Pest. Sci. 2017, 90, 589–600. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Guerrero-Beltrán, J.A. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 2014, 142, 455–460. [Google Scholar] [CrossRef]
- Clemes, S.M.; Santos, T.G.; Rebelo, R.A.; Laps, R.R.; Pescador, R. Seasonality and hydrodistillation time effects on the yield and chemical composition of leaves essential oil of Piper mikanianum (Kunth) Steudel. Eclética Química 2015, 40, 117–125. [Google Scholar] [CrossRef]
- Rivera, P.N.; Mosquera, T.; Baldisserotto, A.; Abad, J.; Aillon, C.; Cabezas, D.; Piedra, J.; Coronel, I.; Manfredini, S. Chemical composition and in-vitro biological activities of the essential oil from leaves of Peperomia inaequalifolia Ruiz & Pav. Am. J. Essent. Oils Nat. Prod. 2015, 2, 29–31. [Google Scholar]
- Mossi, A.J.; Zanella, C.A.; Kubiak, G.; Lerin, L.A.; Cansian, R.L.; Frandoloso, F.S.; Prá, V.D.; Mazutti, M.A.; Costa, J.A.V.; Treichel, H. Essential oil of Ocotea odorifera: An alternative against Sitophilus zeamais. Renew. Agric. Food Syst. 2013, 29, 161–166. [Google Scholar] [CrossRef]
- Pandey, R.; Mahar, R.; Hasanain, M.; Shukla, S.K.; Sarkar, J.; Rameshkumar, K.B.; Brijesh Kumar, B. Rapid screening and quantitative determination of bioactive compounds from fruit extracts of Myristica species and their in vitro antiproliferative activity. Food Chem. 2016, 211, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.S.; Barbosa, Q.P.S.; Vanin, S.A. Metabolism of safrole by Heraclides thoas brasiliensis (Papilionidae). J. Lepid. Soc. 2014, 68, 283–285. [Google Scholar] [CrossRef]
- Sohilait, H.J.; Kainama, H. Synthesis of 1-(3,4-methylenedioxyphenyl)-1-butene-3- one from safrole. EJPAC 2016, 3, 66–70. [Google Scholar] [CrossRef]
- Siano, F.; Ghizzoni, C.; Gionfriddo, F.; Colombo, E.; Servillo, L.; Castaldo, D. Determination of estragole, safrole and eugenol methyl ether in food products. Food Chem. 2003, 81, 469–475. [Google Scholar] [CrossRef]
- Hung, T.; Chou, C.; Sun, T.; Liang, W.; Cheng, J.; Fang, Y.; Li, Y.; Shieh, P.; Ho, C.; Kuo, C.; et al. The Mechanism of safrole-induced [Ca2+]i rises and non-Ca2+-triggered cell death in SCM1 human gastric cancer cells. CJP 2015, 58, 302–311. [Google Scholar] [CrossRef]
- Yang, A.; Zhang, L.; Zhi, D.; Liu, W.; Gao, X.; He, X. Identification and analysis of the reactive metabolites related to the hepatotoxicity of safrole. Xenobiotica 2017, 48, 1164–1172. [Google Scholar] [CrossRef]
- IARC. List of classifications. International Agency for Research on Cancer. 2015. Available online: http://monographs.iarc.fr/ENG/Classification/index.php (accessed on 10 August 2018).
- Jeong, J.M.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Kim, E.M.; Lee, J. One-step sample preparation for convenient examination of volatile monoterpenes and phenolic compounds in peppermint leaves using deep eutectic solventes. Food Chem. 2018, 251, 69–76. [Google Scholar] [CrossRef]
- CODEX—Codex Alimentarius Commission. Codex Committee on Food Additives and Contaminants (2006). Available online: http://www.fao.org/fao-who-codexalimentarius/shproxy/ru/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FShared%2BDocuments%252FArchive%252FMeetings%252FCCFAC%252Fccfac38%252Ffa38_12e.pdf (accessed on 10 July 2021).
- ANVISA. Resolution RDC nº 2, de 15 de January de 2007. Ministério da Saúde - MS. Agência Nacional de Vigilância Sanitária - ANVISA. Available online: http://portal.anvisa.gov.br/documents/10181/2718376/RDC_02_2007_COMP.pdf/c966caff-1c19-4a2f-87a6-05f7a09e940b (accessed on 10 August 2018).
- Kreutz, T.; Lucca, L.G.; Loureiro-Paes, O.A.R.; Teixeira, H.F.; Veiga, V.F., Jr.; Limberger, R.P.; Ortega, G.G.; Koester, L.S. Optimization, validation and application of headspace solid-phase microextraction gas chromatography for the determination of 1-nitro-2-phenylethane and methyleugenol from Aniba canelilla (H.B.K.) Mez essential oil in skin permeation samples. J. Chromatogr. A 2018, 1564, 163–175. [Google Scholar] [CrossRef]
- Moreira, N.; Araújo, A.M.; Rogerson, F.; Vasconcelos, I.; Freitas, V.; Pinho, P.G. Development and optimization of a HS-SPME-GC-MS methodology to quantify volatile carbonyl compounds in Port wines. Food Chem. 2019, 270, 518–526. [Google Scholar] [CrossRef]
- Behzadi, M.; Noroozian, E.; Mirzaei, M. A novel coating based on carbon nanotubes/poly-ortho-phenylenediamine composite for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. Talanta 2013, 108, 66–73. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, Q.; Niu, Y.; Liu, Q.; Chen, F.; Ma, N.; Zhou, X.; Zhu, J. Optimization of headspace solid-phase micro-extraction and its application in analysis of volatile compounds in cherry tomato by gas chromatography. Food Anal. Methods 2017, 10, 596–609. [Google Scholar] [CrossRef]
- Prates, L.H.F.; Faroni, L.R.A.; Heleno, F.F.; Queiroz, M.E.L.R.; Sousa, A.H.; Assis Silva, M.V. Eugenol diffusion coefficient and its potential to control Sitophilus zeamais in rice. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas. Cromatography/Mass Spectroscopy, 4th ed.; Allured publishing corporation: Carol Stream, IL, USA, 2007; p. 47. [Google Scholar]
- Vilela, A.D.O.; Faroni, L.R.A.; Rodrigues, A.A.Z.; Heleno, F.F.; de Queiroz, M.E.L.R.; Moura, E.D.S.; Gomes, J.L. Headspace Solid-Phase Microextraction: Validation of the Method and Determination of Allyl Isothiocyanate Persistence in Cowpea Beans. ACS omega. 2020, 5, 21364–21373. [Google Scholar] [CrossRef]
- SANTE. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; nº. SANTE/11813/2017; European Commission, Directorate General for Health and Food Safety, Safety of the Food Chain Pesticides and Biocides: Brussels, Belgium, 2017; p. 42. [Google Scholar]
- Ducki, S.; Miralles-Garcia, J.; Zumbe, A.; Tornero, A.; Storey, D.M. Evaluation of solid-phase micro-extraction coupled to gas chromatography–mass spectrometry for the headspace analysis of volatile compounds in cocoa products. Talanta 2008, 74, 1166–1174. [Google Scholar] [CrossRef]
- Gharari, H.; Farjaminezhad, M.; Marefat, A.; Fakhari, A.R. All-in-one solid-phase microextraction: Development of a selective solid-phase microextraction fiber assembly for the simultaneous and efficient extraction of analytes with different polarities. J. Sep. Sci. 2016, 39, 1709–1716. [Google Scholar] [CrossRef]
- Liu, W.; Hu, Y.; Zhao, J.; Xu, Y.; Guan, Y. Physically incorporated extraction phase of solid-phase microextraction by sol–gel technology. J. Chromatogr. A 2006, 1102, 37–43. [Google Scholar] [CrossRef]
- Abad, F.C.; Winck, P.R.; Silva, J.M.; Caramão, E.B.; Zini, C.A. Multiresidue determination of pesticides in carrots using pressurized liquid extraction and gas chromatography with mass spectrometry detector. J. Braz. Chem. Soc. 2010, 21, 461–468. [Google Scholar] [CrossRef][Green Version]
- Ermer, J.; Miller, J.H.M. Method validation in pharmaceutical analysis: A guide to best practice; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; p. 403. [Google Scholar] [CrossRef]
- Pereira, A.C.R.L.; Oliveira, J.V.; Gondim Junior, M.G.C.; Câmara, C.A.G. Influência do período de armazenamento do caupi [Vigna unguiculata (L.) Walp.], tratado com óleos essenciais e fixos, no controle de Callosobruchus maculatus (Fabricius, 1775) (Coleoptera, Chrysomelidae, Bruchinae). Ciência Agrotecnologia 2009, 33, 319–325. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, J.; Wang, Z.; Liu, F.; Wan, Z. Fumigation toxicity of allicin against three stored product pests. J. Stored Prod. Res. 2013, 55, 48–54. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]

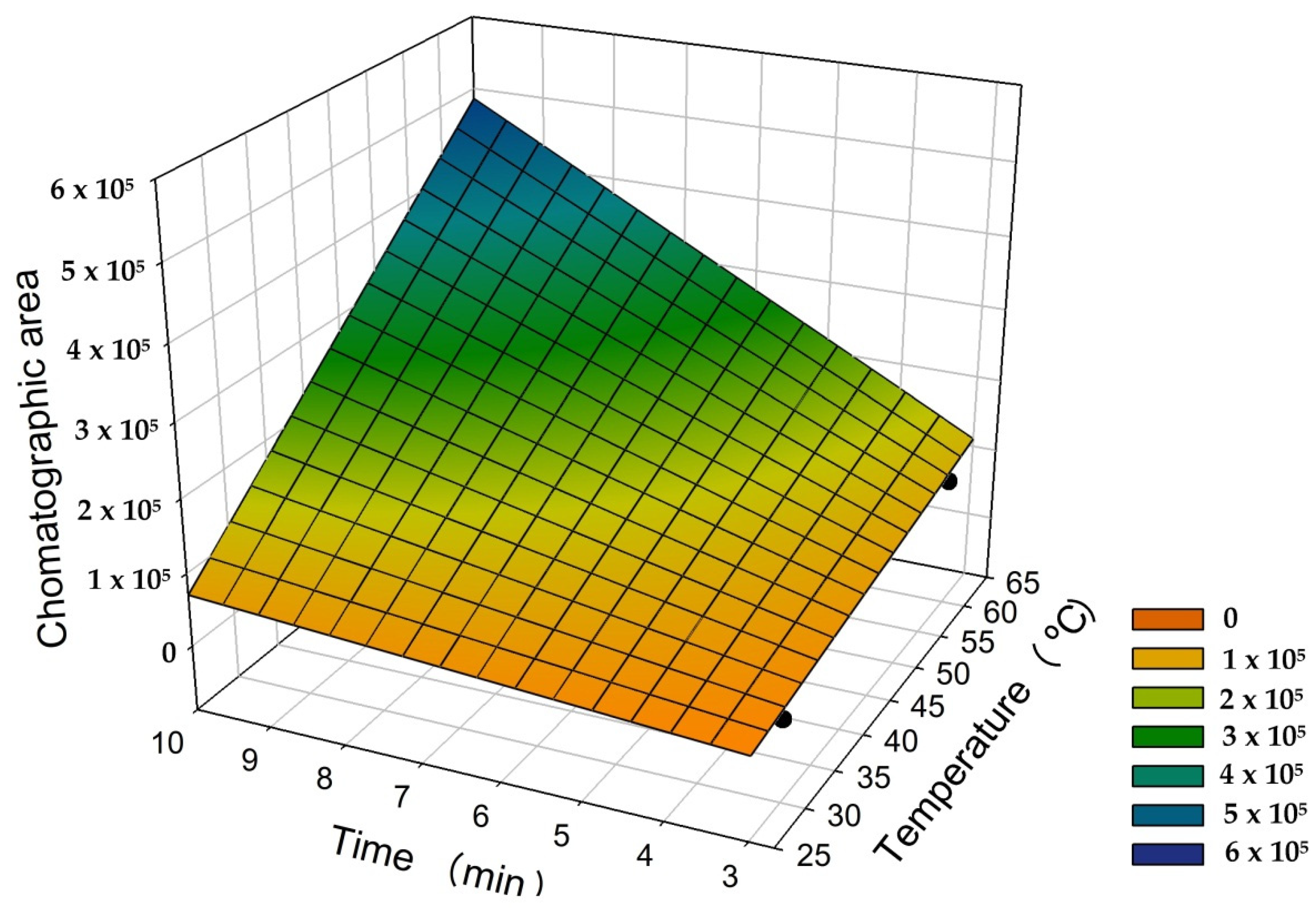

| Experiments | Time (min) a | Temperature (°C) b | ||
|---|---|---|---|---|
| Real | Codified | Real | Codified | |
| 1 | 3 | − | 30 | − |
| 2 | 3 | − | 60 | + |
| 3 | 10 | + | 30 | − |
| 4 | 10 | + | 60 | + |
| 5 | 3 | − | 30 | − |
| 6 | 3 | − | 60 | + |
| 7 | 10 | + | 30 | − |
| 8 | 10 | + | 60 | + |
| Component | RIa Literature | RIb Calculated | Relative % |
|---|---|---|---|
| p-Cymen-8-ol | 1179 | 1184 | 1.20 |
| Safrole | 1285 | 1292 | 93.00 |
| (E)-Caryophyllene | 1417 | 1415 | 0.69 |
| Bicyclogermacrene | 1500 | 1493 | 2.05 |
| Pentadecan | 1500 | 1498 | 1.60 |
| Spatulenol | 1577 | 1573 | 1.46 |
| Theoretical Concentration | Nominal Concentration ± 1SE | Accuracy (n = 6) | Precision |
|---|---|---|---|
| (µg kg−1) | (µg kg−1) | Recovery (%) | 2CV (%) |
| 0.019 | 0.018 ± 0.0010 | 99.26 | 12.76 |
| 0.095 | 0.094 ± 0.0057 | 100.48 | 14.82 |
| 0.190 | 0.196 ± 0.012 | 104.85 | 14.61 |
| Treatment | Model | Equation | R2 a | RMSE b | P |
|---|---|---|---|---|---|
| Contact | Linear | y = − 0.0079 x + 0.67 | 0.46 | 0.27 | 0.21 |
| Quadratic | y = 0.0002x2 − 0.025x + 0.86 | 0.58 | 0.25 | 0.42 | |
| Exponential | y = 10.54еxp−0.4861x + 0.17 | 0.98 | 0.08 | 0.043 | |
| Potential | y = 18.06(1 + x) −1.5842 | 0.88 | 0.13 | 0.018 | |
| Logarithmic | y = − 0.065ln (x − 5.0000) + 0.40 | 0.98 | 0.49 | 0.021 | |
| Fumigation | Linear | y = − 0.018x + 1.5 | 0.64 | 0.43 | 0.10 |
| Quadratic | y = 0.0004x2 − 0.052x + 1.9 | 0.76 | 0.38 | 0.24 | |
| Exponential | y = 6.53еxp−0.2606x + 0.38 | 0.89 | 0.24 | 0.11 | |
| Potential | y = 11.16(1 + x) −0.95 | 0.92 | 0.21 | 0.011 | |
| Logarithmic | y = − 0.27ln (x − 4.94) + 1.4 | 0.97 | 0.13 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, M.S.S.; Faroni, L.R.D.; Heleno, F.F.; de Sousa, A.H.; Prates, L.H.F.; Rodrigues, A.A.Z. Method Validation and Evaluation of Safrole Persistence in Cowpea Beans Using Headspace Solid-Phase Microextraction and Gas Chromatography. Molecules 2021, 26, 6914. https://doi.org/10.3390/molecules26226914
Ferraz MSS, Faroni LRD, Heleno FF, de Sousa AH, Prates LHF, Rodrigues AAZ. Method Validation and Evaluation of Safrole Persistence in Cowpea Beans Using Headspace Solid-Phase Microextraction and Gas Chromatography. Molecules. 2021; 26(22):6914. https://doi.org/10.3390/molecules26226914
Chicago/Turabian StyleFerraz, Maria Suely Siqueira, Lêda Rita D’Antonino Faroni, Fernanda Fernandes Heleno, Adalberto Hipólito de Sousa, Lucas Henrique Figueiredo Prates, and Alessandra Aparecida Zinato Rodrigues. 2021. "Method Validation and Evaluation of Safrole Persistence in Cowpea Beans Using Headspace Solid-Phase Microextraction and Gas Chromatography" Molecules 26, no. 22: 6914. https://doi.org/10.3390/molecules26226914
APA StyleFerraz, M. S. S., Faroni, L. R. D., Heleno, F. F., de Sousa, A. H., Prates, L. H. F., & Rodrigues, A. A. Z. (2021). Method Validation and Evaluation of Safrole Persistence in Cowpea Beans Using Headspace Solid-Phase Microextraction and Gas Chromatography. Molecules, 26(22), 6914. https://doi.org/10.3390/molecules26226914