Characterisation of Elastomers as Food Contact Materials–Part 1: Quantification of Extractable Compounds, Swelling of Elastomers in Food Simulants and Release of Elements
Abstract
1. Introduction
2. Results
2.1. Quantification of Extractable Compounds by SEC-ELSD
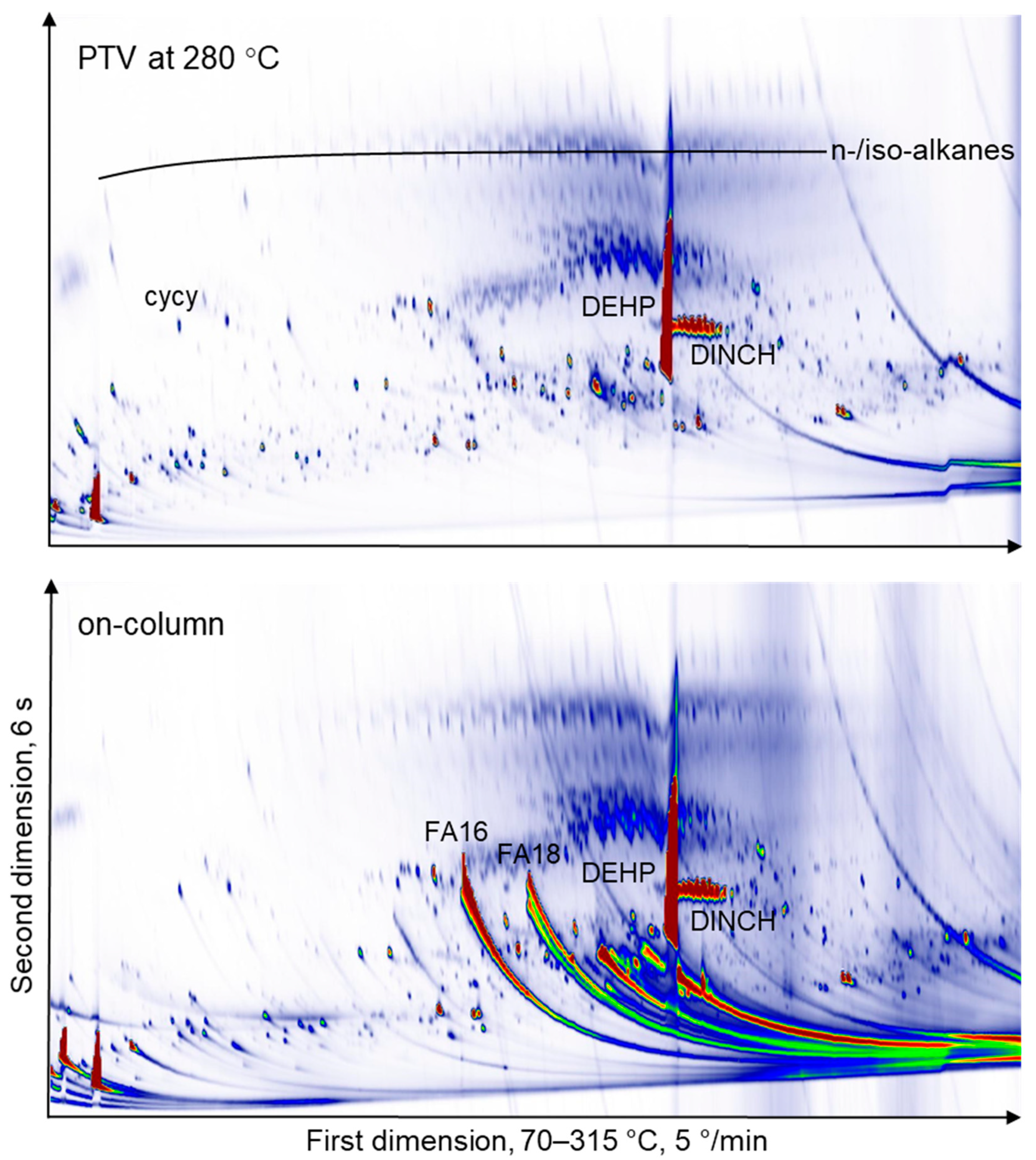
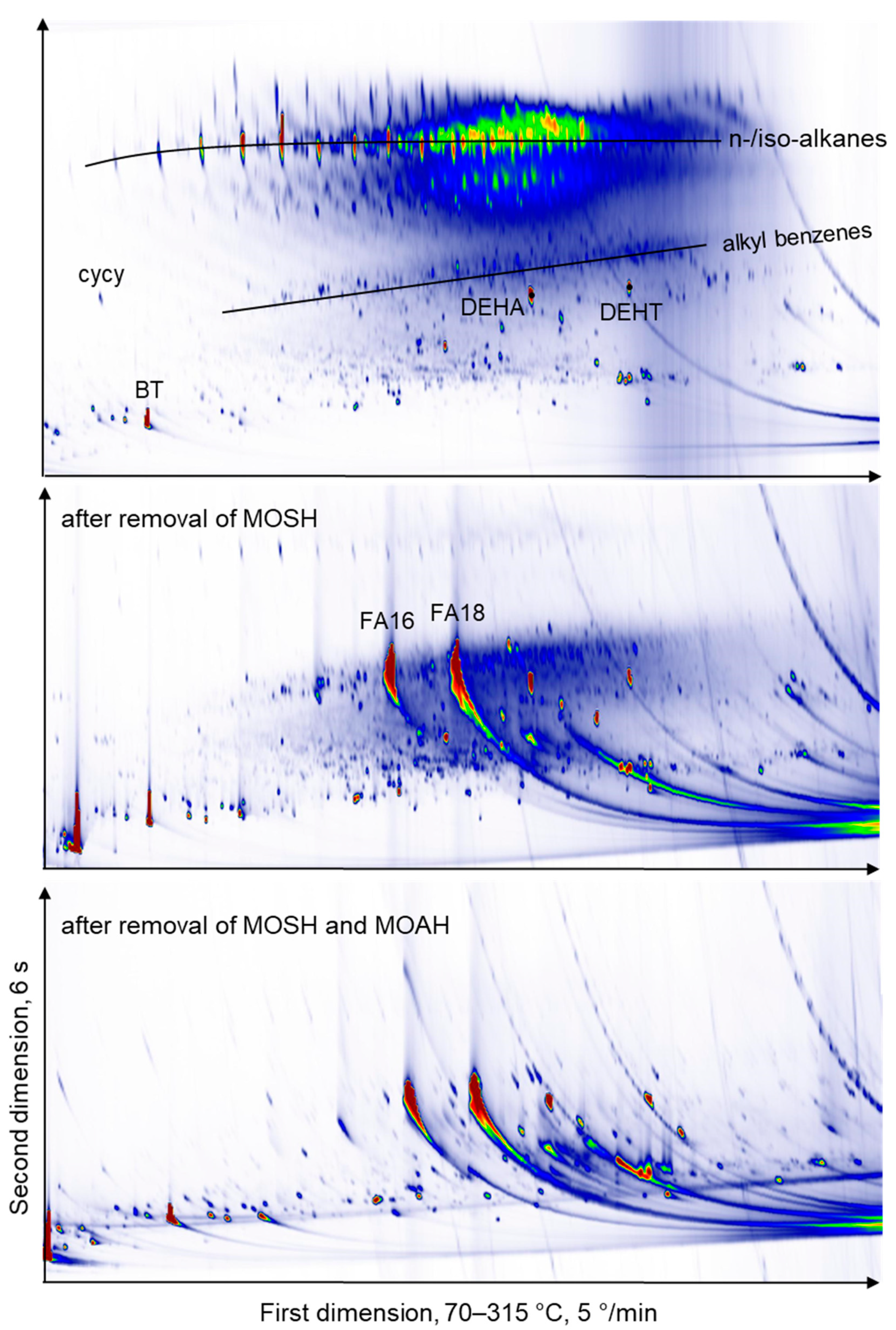
2.2. Comprehensive Two-dimensional GC × GC-FID or –MS Analysis of THF Extracts
2.3. Substances under 1000 Da in the Ethanol/Water Migrats
2.4. Swelling of Elastomer Samples
2.5. Release of Elements from Elastomers into Food Simulants
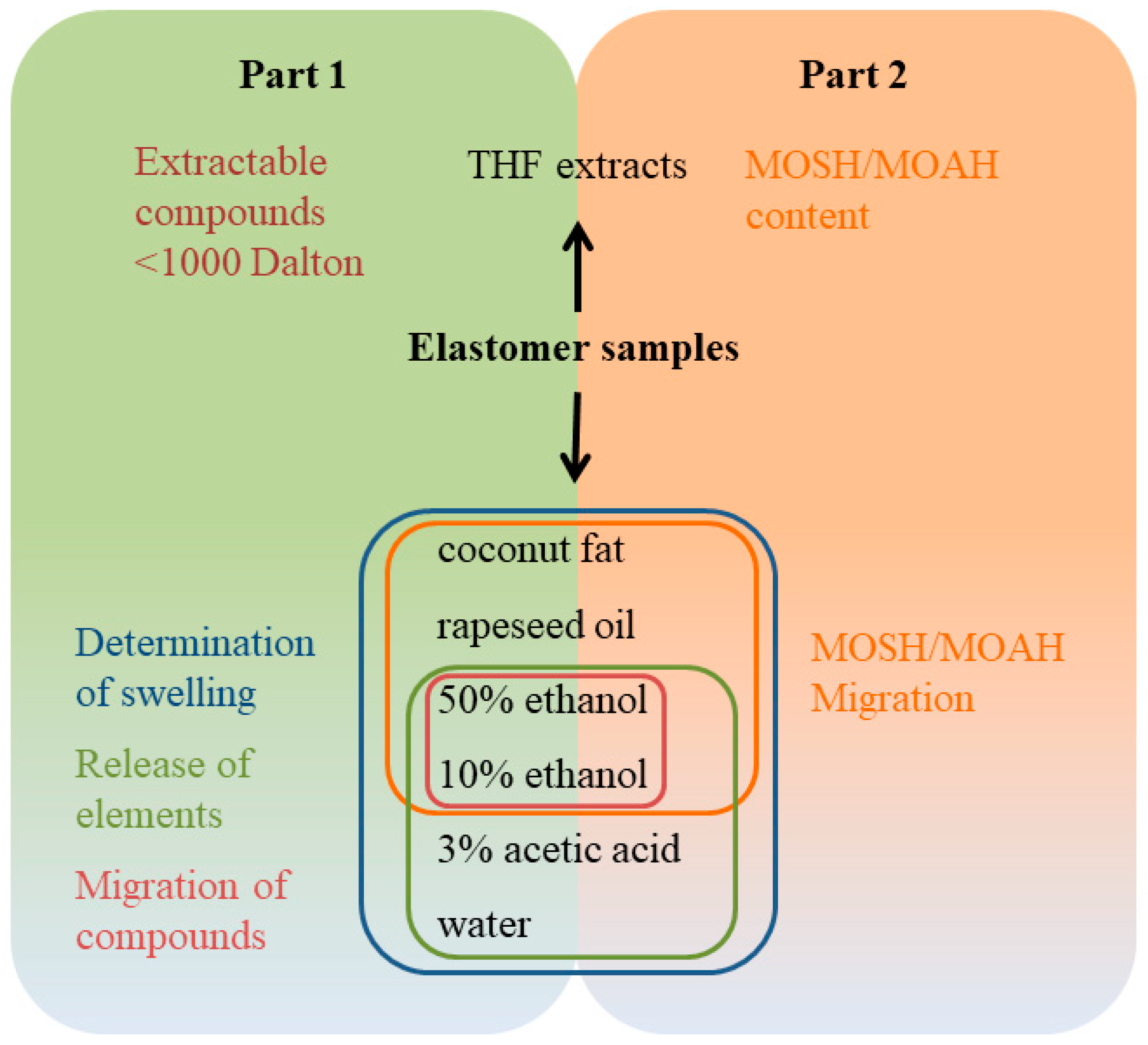
3. Materials and Methods
3.1. Materials
3.1.1. Solvents
3.1.2. Elastomer Samples
3.2. Methods
3.2.1. Extraction with THF
3.2.2. Migration Testing According to BfR Recommendation XXI
3.2.3. SEC-ELSD
3.2.4. Determination of Swelling Caused by Food Simulants
3.2.5. GC × GC-FID/GC × GC-MS
3.2.6. Determination of Elements by ICP-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alemán, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (iupac recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Kaiser, W. Elastomere. In Kunststoffchemie für ingenieure; Carl Hanser Verlag: Munich, Germany, 2007; pp. 471–490. [Google Scholar]
- European Commission (EC). Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come Into Contact With Food; European Commission (EC): Brussels, Belgium; Luxembourg, 2011. [Google Scholar]
- BfR. Recommendation XXI on Commodities Based on Natural and Synthetic Rubber; German Federal Institute for Risk Assessment (BfR): Berlin, Germany, 2016; Available online: https://bfr.ble.de/kse/faces/resources/pdf/210-english.pdf (accessed on 25 December 2020).
- European Commission (EC). Union Guidelines on Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come Into Contact With Food; European Commission (EC): Brussels, Belgium; Luxembourg, 2014. [Google Scholar]
- Kruger, R.H.; Boissiere, C.; Klein-Hartwig, K.; Kretzschmar, H.J. New phenylenediamine antiozonants for commodities based on natural and synthetic rubber. Food Addit. Contam. 2005, 22, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.A.; Castle, L.; Damant, A.P.; Read, W.A.; Speck, D.R. Development and application of an lc-ms method to determine possible migration of mercaptobenzothiazole, benzothiazole and related vulcanization residues from rubber used in contact with food and drink. Food Addit. Contam. 2003, 20, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Bouma, K.; Nab, F.M.; Schothorst, R.C. Migration of n-nitrosamines, n-nitrosatable substances and 2-mercaptobenzthiazol from baby bottle teats and soothers: A dutch retail survey. Food Addit. Contam. 2003, 20, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Liu, L.; Zhao, L.Y.; Zhou, Q.F.; Tan, T.W. Determination of volatile nitrosamines in latex products by hs-spme-gc-ms. Chromatographia 2011, 74, 817–825. [Google Scholar] [CrossRef]
- Sannino, A.; Bolzoni, L. Gc/ci-ms/ms method for the identification and quantification of volatile n-nitrosamines in meat products. Food Chem. 2013, 141, 3925–3930. [Google Scholar] [CrossRef]
- Sung, J.H.; Kwak, I.S.; Park, S.K.; Kim, H.I.; Lim, H.S.; Park, H.J.; Kim, S.H. Liquid chromatography-tandem mass spectrometry determination of n-nitrosamines released from rubber or elastomer teats and soothers. Food Addit. Contam. Part A 2010, 27, 1745–1754. [Google Scholar] [CrossRef]
- Kuhne, F.; Kappenstein, O.; Strabetagutl, S.; Weese, F.; Weyer, J.; Pfaff, K.; Luch, A. N-nitrosamines migrating from food contact materials into food simulants: Analysis and quantification by means of hplc-apci-ms/ms. Food Addit. Contam. Part A 2018, 35, 792–805. [Google Scholar] [CrossRef]
- Bouma, K.; Schothorst, R.C. Identification of extractable substances from rubber nettings used to package meat products. Food Addit. Contam. 2003, 20, 300–307. [Google Scholar] [CrossRef]
- Forrest, M.; Holding, S.; Howells, D. The use of two-dimensional gc-ms for the identification and quantification of low molecular weight compounds from high performance elastomers. Polym. Test. 2006, 25, 63–74. [Google Scholar] [CrossRef]
- Biedermann, M.; Grob, K. Advantages of comprehensive two-dimensional gas chromatography for comprehensive analysis of potential migrants from food contact materials. Anal. Chim. Acta 2019, 1057, 11–17. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on recent developments in the risk assessment of chemicals in food and their potential impact on the safety assessment of substances use in food contact materials. EFSA J. 2016, 14, 4357. [Google Scholar] [CrossRef]
- Grob, K. On-Column Injection in Capillary GS; Hüthing Verlag: Heidelberg, Germany, 1987. [Google Scholar]
- Grob, K. Split and Splitless Injection for Quantitative GC; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Abts, G. Spezifische eigenschaften der verschiedenen elastomere. In Einnführung in die Kautschuk Technologie; Carl Hanser Verlag: München, Germany, 2019; Volume 2. [Google Scholar]
- Bin Samsuri, A. Degradation of natural rubber and synthetic elastomers. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010; pp. 2407–2438. [Google Scholar]
- Noureddini, H.; Teoh, B.C.; Davis Clements, L. Viscosities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc. 1992, 69, 1189–1191. [Google Scholar] [CrossRef]
- Muniandy, K.S.E.; Thomas, A.G. Diffusion of liquids and solids in rubber. In Natural Rubber Science and Technology; Roberts, A.D., Ed.; Oxford University Press: New York, NY, USA, 1988; pp. 820–852. [Google Scholar]
- Pham, B.; Phalen, R.; Sasanuma, N.; Dorsainvil, Y.; Tol, J.; Zhang, R. Mechanism on the permeation of ethanol in nitrile gloves studied using positron annihilation lifetime spectroscopy. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- EDQM. Metals and Alloys Used in Food Contact Materials and Articles—A Practical Guide for Manufacturers and Regulators. Council of Europe—European Directorate for Quality of Medicines & Healthcare; EDQM: Strasbourg, France, 2013. [Google Scholar]
- SCCS. Opinion on the Safety of Aluminium in Cosmetic Products; European Commission—Scientific Committee on Consumer Safety: Brussels, Belgium; Luxembourg, 2014. [Google Scholar]
- European Food Safety Authority (EFSA). Safety of Aluminium from Dietary Intake—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 6, 754. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Update of the Risk Assessment of Nickel in Food and Drinking Water. EFSA J. 2020, 18, 6268. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion of the Efsa Panel on Contaminants in the Food Chain (Contam) on the Risks to Public Health Related to the Presence of Nickel in Food and Drinking Water. EFSA J. 2015, 13, 4002. [Google Scholar] [CrossRef]
- European Comission (EC). Commission Regulation (EU) 2017/752 of 28 April 2017 Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come Into Contact With Food; European Comission (EC): Brussels, Belgium; Luxembourg, 2011. [Google Scholar]
- Ferradino, A.G. Antioxidant selection for peroxide cure elastomer applications. Rubber Chem. Technol. 2003, 76, 694–718. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion on dietary reference values for manganese. EFSA J. 2013, 11, 3419. [Google Scholar] [CrossRef]
- Sachse, B.; Kolbaum, A.E.; Ziegenhagen, R.; Andres, S.; Berg, K.; Dusemund, B.; Hirsch-Ernst, K.I.; Kappenstein, O.; Müller, F.; Röhl, C.; et al. Dietary manganese exposure in the adult population in germany—What does it mean in relation to health risks? Mol. Nutr. Food Res. 2019, 63. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Abts, G. Kautschukchemikalien. In Einnführung in die Kautschuk Technologie; Carl Hanser Verlag: München, Germany, 2019; Volume 2. [Google Scholar]
- King, J.C.; Cousins, R. Zinc. In Modern Nutrition in Health and Disease; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Alphen aan den Rijn, The Netherlands, 2014. [Google Scholar]
- Hedera, P.; Peltier, A.; Fink, J.K.; Wilcock, S.; London, Z.; Brewer, G.J. Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin ii. The denture cream is a primary source of excessive zinc. Neurotoxicology 2009, 30, 996–999. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on dietary reference values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- European Comission (EC). Commission Regulation (EU) 2016/1416 of 24 August 2016 Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come Into Contact With Food; European Comission (EC): Brussels, Belgium; Luxembourg, 2016. [Google Scholar]
- IARC. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans—Arsenic, Metals, Fibres, and Dusts; IARC Press: Lyon, France, 2012; pp. 41–94. [Google Scholar]
- European Food Safety Authority (EFSA). Dietary exposure to inorganic arsenic in the european population. EFSA J. 2014, 12, 3597. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific pinion of the efsa panel on contaminants in the food chain (contam) on arsenic in food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- Bundesinstitut für Risikobewertung (BfR). BfR. Stellungnahme nr. 024/2018 des bfr vom 26. Jul 2018: Freisetzung von Metallen aus Emaillierten Grillrosten: Einige Geben zu viel ab; Bundesinstitut für Risikobewertung (BfR): Berlin, Germany, 2018. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific opinion of the EFSA panel on contaminants in the food chain on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans—Inorganic and Organic Lead Compounds; IARC Press: Lyon, France, 2006; pp. 1–432. [Google Scholar]
- European Chemicals Agency (ECHA). Opinion of the Committee for Risk Assessment (Rac) on a Dossier Proposing Harmonised Classification and Labeling at EU Level of Lead; European Chemicals Agency (ECHA): Helsinki, Finland, 2013. [Google Scholar]
- Bundesinstitut für Risikobewertung. BfR. Stellungnahme nr. 043/2020 des bfr vom 21. September 2020: Geschirr aus Keramik: Bfr Empfiehlt Niedrigere Freisetzungsmengen für blei und Cadmium; Bundesinstitut für Risikobewertung (BfR): Berlin, Germany, 2020. [Google Scholar]
- Simoneau, C.; Beldi, G.; Jakubowska, N.; Peltzer, M. Towards Suitable Tests for the Migration of Metals From Ceramic and Crystal Tableware: Work in Support of the Revision of the Ceramic Directive 84/500/eec; JRC Technical Reports; European Commission: Brussels, Belgium; Luxembourg, 2017. [Google Scholar]
- Li, X.H.; Qian, P.P. Identification of an exposure risk to heavy metals from pharmaceutical-grade rubber stoppers. J. Food Drug Anal. 2017, 25, 723–730. [Google Scholar] [CrossRef]
- Biedermann, M.; Grob, K. Polyadipates used as plasticizers in food contact: Fraction below 1000 da determined by size exclusion chromatography with evaporative light scattering detection and segmental response linearization or UV detection. J. Sep. Sci. 2006, 29, 114–122. [Google Scholar] [CrossRef]
- Biedermann, M.; Grob, K. Comprehensive two-dimensional gas chromatography for characterizing mineral oils in foods and distinguishing them from synthetic hydrocarbons. J. Chromatogr. A 2015, 1375, 146–153. [Google Scholar] [CrossRef]


| Sample Type | Elastomer (Sample #) | Labelled as FCM | Extractable Content with THF [%] | |||||
|---|---|---|---|---|---|---|---|---|
| <1000 Da | >1000 Da | |||||||
| Extraction # | Extraction # | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | |||
| pre-product | IIR (1) | no | 10 | 1.5 | 0.4 | 2.4 | 1.1 | 1.4 |
| pre-product | EPDM (2) | yes | 28 | 3.3 | 0.2 | 3.0 | 0.4 | 0.3 |
| pre-product | NR/SBR (3) | yes | 6 | 1.8 | 0.4 | 2.5 | 3.0 | 4.5 |
| pre-product | NBR (4) | yes | 6.5 | 1.2 | 0.3 | 2.6 | 5.2 | 7.3 |
| pre-product | TPE (5) | yes | 35 | 2.5 | 0.1 | 30 | 2.6 | 0.2 |
| gasket, bottle swing stopper | TPE (6) | yes | 45 | 4.3 | 0.3 | 28 | 2.0 | 0.3 |
| pre-product | NBR (7) | yes | 5.5 | 1.2 | 0.2 | 2.5 | 0.5 | 0.2 |
| pre-product | PUR (8) | yes | 1.0 | 0.2 | 0.0 | 1.6 | 0.7 | 0.2 |
| gasket, preserving jar | NR/SBR (9) | yes | 7.5 | 2.5 | 0.9 | 8.0 | 13 | 7.4 |
| gasket, espresso maker | SBS/NR (10) | yes | 5.0 | 0.7 | 0.2 | 0.8 | 1.8 | 2.2 |
| Sample (#) | Number of Substances (excl. MOSH/MOAH) | % of Elastomer Weight (signals incl. MOSH/MOAH) | Number of Mass Spectra | % Tentatively Assigned Spectra | |
|---|---|---|---|---|---|
| 1–10 ppm | >10 ppm | [%] | [%] | ||
| IIR (1) | 280 | 53 | 6.4 | 80 | 61 |
| EPDM (2) | 147 | 7 | 12.0 | 56 | 59 |
| NR/SBR (3) | 327 | 78 | 3.1 | 97 | 44 |
| NBR (4) | 471 | 194 | 2.1 | 186 | 29 |
| TPE (5) | 57 | 4 | 19.3 | 40 | 45 |
| TPE (SEBS) (6) | 103 | 22 | 14.0 | 48 | 48 |
| NBR (7) | 484 | 68 | 2.6 | 158 | 47 |
| PUR (8) | 54 | 17 | 0.8 | 42 | 64 |
| NR/SBR (9) | 569 | 121 | 4.2 | 101 | 28 |
| SBS/NR (10) | 487 | 154 | 4.1 | 83 | 42 |
| Elastomer (sample #) | SEC * < 1000 Da | 10% Ethanol | 50% Ethanol | 10% Ethanol | 50% Ethanol | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 day | 10 days | 1 day | 10 days | 1 day | 10 days | 1 day | 10 days | ||
| [mg/dm2] | [mg/dm2] | mg migrated [%] ** | |||||||
| IIR (1) | 900 | - | - | - | 12 | - | - | - | 1.3 |
| EPDM (2) | 2581 | - | - | - | 14 | - | - | - | 0.5 |
| NR/SBR (3) | 627 | - | 6.5 | 18 | 62 | - | 1.0 | 2.9 | 9.9 |
| NBR (4) | 718 | - | 7.5 | 27 | 95 | - | 1.0 | 3.8 | 13.3 |
| TPE-S (5) | 3169 | - | - | - | <6 | - | - | - | <0.2 |
| TPE-S (6) | 3938 | - | - | - | <6 | - | - | - | <0.2 |
| NBR (7) | 713 | - | 7.0 | 20 | 76 | - | 1.0 | 2.8 | 10.7 |
| PUR (8) | 92 | 11 | 24 | 33 | 75 | 12 | 26 | 36 | 81 |
| NR/SBR (9) | 982 | - | - | - | 20 | - | - | - | 2.0 |
| SBS/NR (10) | 651 | - | - | - | 22 | - | - | - | 3.3 |
| Elastomer Type (Sample #) | H2O | 3% Acetic Acid | 10% Ethanol | 50% Ethanol | Coconut Oil | Rapeseed Oil | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 10 d | 24 h | 10 d | 24 h | 10 d | 24 h | 10 d | 24 h | 10 d | 24 h | 10 d | |
| IIR (1) | 0.5 | 1.9 | 1.2 | 5.4 | 0.5 | 2.1 | 0.8 | 2.4 | 5.1 | 16.4 | 2.1 | 6.7 |
| EPDM (2) | 0.2 | 0.6 | 1.9 | 5.8 | 0.2 | 0.7 | 0.2 | 0.5 | 2.6 | 10.7 | −2.4 | 0.9 |
| NR/SBR (3) | 0.5 | 1.4 | 64.4 | 31.0 | 0.6 | 1.5 | 0.7 | −1.3 | 20.0 | 64.2 | 17.2 | 54.2 |
| NBR (4) | 1.2 | 3.7 | 3.8 | 3.8 | 1.8 | 4.8 | 3.3 | 4.8 | 2.8 | 9.1 | 2.0 | 6.8 |
| TPE-S (5) | - | - | - | - | - | - | - | - | −0.9 | −0.9 | −5.9 | −8.9 |
| TPE-S (6) | 0.1 | 0.2 | 2.0 | 17.4 | 0.1 | 0.3 | 0.3 | 0.4 | −2.7 | −2.4 | −5.9 | −4.5 |
| NBR (7) | 2.3 | 9.3 | 6.6 | 11.5 | 3.7 | 11.4 | 6.8 | 15.3 | 1.5 | 4.4 | 1.0 | 3.2 |
| PUR (8) | 1.6 | 2.7 | 2.2 | 2.0 | 2.5 | 2.8 | 15.8 | 15.3 | 0.6 | −0.6 | −0.3 | −0.8 |
| NR/SBR (9) | 0.5 | 1.4 | 40.9 | 16.8 | 0.6 | 1.5 | 3.9 | 1.9 | 11.7 | 55.4 | 16.8 | 49.3 |
| SBS/NR (10) | 0.4 | 1.5 | 1.9 | 4.2 | 0.8 | 1.8 | 1.2 | 2.9 | 5.2 | 24.1 | 4.6 | 19.2 |
| Element | Maximal Permitted Migration | Source/Comments |
|---|---|---|
| Al | 1000 μg/kg food (simulant) | Specific migration limit taking into account other sources of intake (EU Regulation No 10/2011) |
| Ni | 20 μg/kg food (simulant) | Specific migration limit taking into account other sources of intake (EU Regulation No 10/2011); (Regulation EU 2017/752) |
| Zn | 5000 μg/kg mg/kg food (simulant) | Specific migration limit taking into account other sources of intake (EU Regulation No 10/2011) |
| Mn | 600 μg/kg food (simulant) | Specific migration limit taking into account other sources of intake (EU Regulation No 10/2011) |
| As | 2 μg/kg food (simulant) | Release limit for As from metals and alloys |
| Pb | 10 μg/kg food (simulant) | Release limit for Pb from metals and alloys |
| Sample Type (sample #) | Food Simulant | Migration Time | Unit | Al | Mn | Ni | Zn | As | Pb |
|---|---|---|---|---|---|---|---|---|---|
| IIR (1) | 3% ac. acid | 24 h | [μg/L] | 50.27 | <LOQ | 2.94 | 1351 | 0.01 | 0.61 |
| 10 d | [μg/L] | 20.96 | 0.70 | 2.06 | 3115 | <LOQ | 1.30 | ||
| 50% ethanol | 24 h | [μg/L] | 7.00 | 0.25 | 0.71 | 217 | <LOQ | 2.56 | |
| 10 d | [μg/L] | 19.85 | 0.98 | 3.37 | 553 | <LOQ | 3.61 | ||
| EPDM (2) | 3% ac. acid | 24 h | [μg/L] | 63.62 | <LOQ | 1.05 | 2401 | 0.01 | 0.38 |
| 10 d | [μg/L] | 137.93 | 0.97 | 11.86 | 2837 | <LOQ | 0.34 | ||
| 50% ethanol | 24 h | [μg/L] | 3.74 | <LOQ | 0.93 | 410 | <LOQ | <LOQ | |
| 10 d | [μg/L] | 7.75 | 1.15 | 0.46 | 919 | <LOQ | 3.03 | ||
| NR/SBR (3) | 3% ac. acid | 24 h | [μg/L] | 47.59 | 0.29 | 2.89 | 4882 | 0.02 | 149.27 |
| 10 d | [μg/L] | 386.99 | 5.16 | 10.86 | 22,304 | 0.12 | 558.99 | ||
| 50% ethanol | 24 h | [μg/L] | <LOQ | 1.86 | 3.47 | 2779 | <LOQ | 108.70 | |
| 10 d | [μg/L] | <LOQ | 3.58 | 6.10 | 4693 | <LOQ | 89.47 | ||
| NBR (4) | 3% ac. acid | 24 h | [μg/L] | 288.75 | 20.35 | 2.46 | 137,029 | 0.12 | 38.84 |
| 10 d | [μg/L] | 1265.49 | 58.68 | 8.61 | 333,017 | 0.36 | 54.73 | ||
| 50% ethanol | 24 h | [μg/L] | <LOQ | 0.40 | 0.69 | 2563 | <LOQ | 30.07 | |
| 10 d | [μg/L] | <LOQ | 1.88 | 7.75 | 9885 | <LOQ | 57.89 | ||
| TPE-S (5) | No sample available | ||||||||
| TPE-S (6) | 3% ac. acid | 24 h | [μg/L] | 13.67 | 0.48 | 4.07 | 95 | 0.01 | 0.33 |
| 10 d | [μg/L] | 15.09 | 2.26 | 2.78 | 342 | <LOQ | 0.23 | ||
| 50% ethanol | 24 h | [μg/L] | <LOQ | <LOQ | 2.35 | 133 | <LOQ | <LOQ | |
| 10 d | [μg/L] | 4.17 | 0.92 | 2.04 | 1433 | <LOQ | 40.11 | ||
| NBR (7) | 3% ac. acid | 24 h | [μg/L] | 224.70 | 264.11 | 18.41 | 143,810 | 0.52 | 8.26 |
| 10 d | [μg/L] | 848.44 | 1068.35 | 114.42 | 291,942 | 1.70 | 29.09 | ||
| 50% ethanol | 24 h | [μg/L] | <LOQ | <LOQ | 20.95 | 1062 | <LOQ | 8.62 | |
| 10 d | [μg/L] | <LOQ | 1.03 | 47.21 | 2469 | <LOQ | 14.21 | ||
| PUR (8) | 3% ac. acid | 24 h | [μg/L] | 6.85 | <LOQ | 0.41 | <LOQ | 0.004 | <LOQ |
| 10 d | [μg/L] | 5.35 | <LOQ | <LOQ | 106 | <LOQ | 0.03 | ||
| 50% ethanol | 24 h | [μg/L] | <LOQ | <LOQ | <LOQ | 129 | <LOQ | <LOQ | |
| 10 d | [μg/L] | 10.76 | 0.70 | 12.53 | 412 | <LOQ | 11.38 | ||
| NR/SBR (9) | 3% ac. acid | 24 h | [μg/L] | 221.09 | 1.30 | 0.47 | 44,568 | 0.09 | <LOQ |
| 10 d | [μg/L] | 290.37 | 2.97 | <LOQ | 108,417 | 0.28 | 0.08 | ||
| 50% ethanol | 24 h | [μg/L] | <LOQ | 1.60 | <LOQ | 3626 | <LOQ | 0.17 | |
| 10 d | [μg/L] | 39.07 | 7.87 | 30.68 | 9621 | <LOQ | 3.82 | ||
| SBS/NR (10) | 3% ac. acid | 24 h | [μg/L] | 288.65 | 46.37 | 5.32 | 91,447 | 0.79 | 40.02 |
| 10 d | [μg/L] | 1234.87 | 219.53 | 18.86 | 242,867 | 3.62 | 204.29 | ||
| 50% ethanol | 24 h | [μg/L] | <LOQ | <LOQ | 11.92 | 3651 | 0.22 | 232.86 | |
| 10 d | [μg/L] | 15.51 | 5.32 | 19.71 | 5575 | 0.47 | 540.52 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kühne, F.; Biedermann, M.; Eicher, A.; Felder, F.; Sander, S.; Schmidt, R.; Lehmann, S.; McCombie, G.; Merkel, S.; Kappenstein, O.; et al. Characterisation of Elastomers as Food Contact Materials–Part 1: Quantification of Extractable Compounds, Swelling of Elastomers in Food Simulants and Release of Elements. Molecules 2021, 26, 509. https://doi.org/10.3390/molecules26020509
Kühne F, Biedermann M, Eicher A, Felder F, Sander S, Schmidt R, Lehmann S, McCombie G, Merkel S, Kappenstein O, et al. Characterisation of Elastomers as Food Contact Materials–Part 1: Quantification of Extractable Compounds, Swelling of Elastomers in Food Simulants and Release of Elements. Molecules. 2021; 26(2):509. https://doi.org/10.3390/molecules26020509
Chicago/Turabian StyleKühne, Friederike, Maurus Biedermann, Angela Eicher, Florian Felder, Stefan Sander, Roman Schmidt, Saskia Lehmann, Gregor McCombie, Stefan Merkel, Oliver Kappenstein, and et al. 2021. "Characterisation of Elastomers as Food Contact Materials–Part 1: Quantification of Extractable Compounds, Swelling of Elastomers in Food Simulants and Release of Elements" Molecules 26, no. 2: 509. https://doi.org/10.3390/molecules26020509
APA StyleKühne, F., Biedermann, M., Eicher, A., Felder, F., Sander, S., Schmidt, R., Lehmann, S., McCombie, G., Merkel, S., Kappenstein, O., & Luch, A. (2021). Characterisation of Elastomers as Food Contact Materials–Part 1: Quantification of Extractable Compounds, Swelling of Elastomers in Food Simulants and Release of Elements. Molecules, 26(2), 509. https://doi.org/10.3390/molecules26020509






