Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours
Abstract
1. Introduction
2. Results
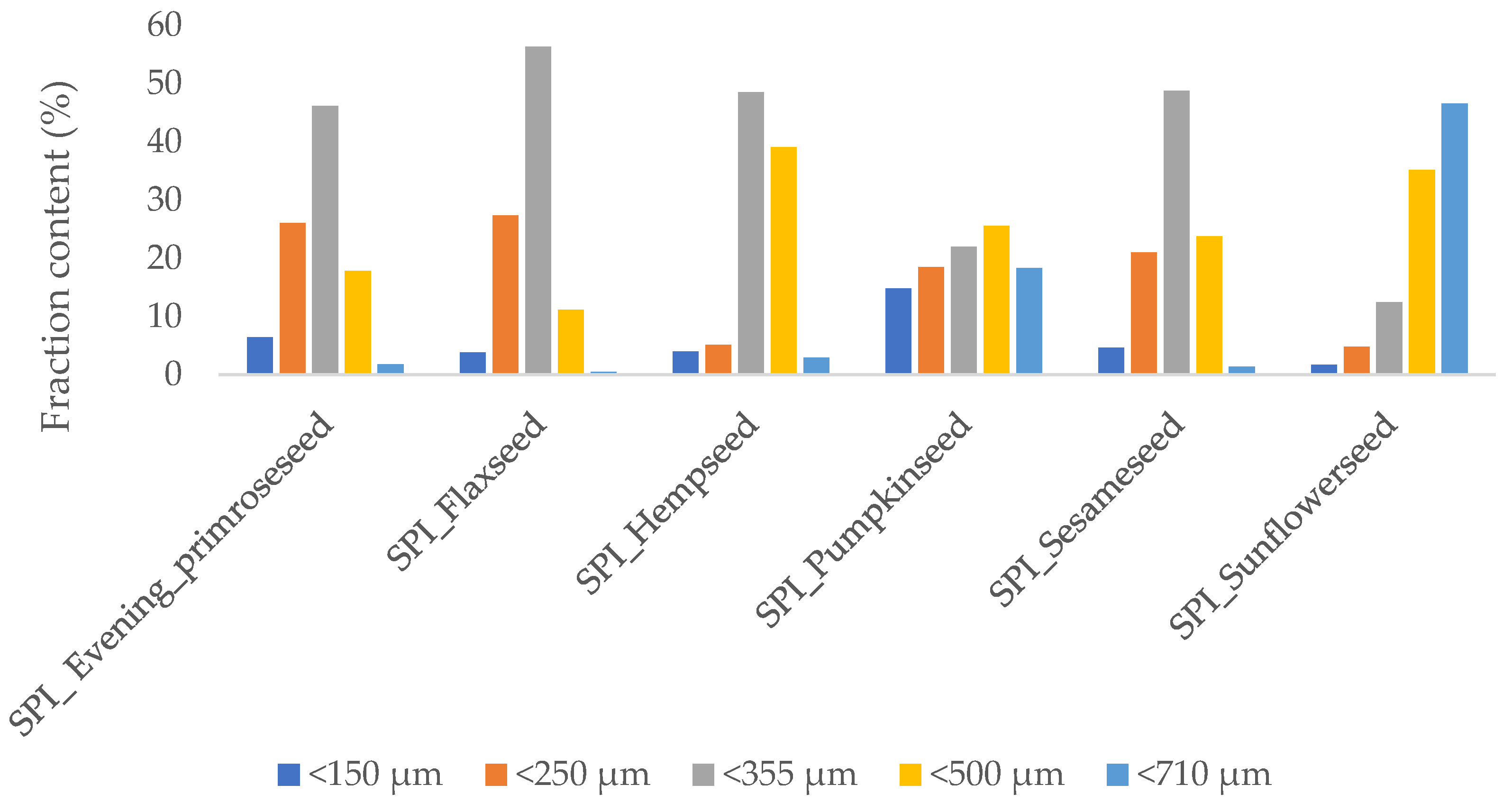
2.1. Oilseed Flour Particle Size Measurement
2.2. Physical Properties
2.2.1. Water Content
2.2.2. Thickness
2.2.3. Water Solubility
2.2.4. Swelling Index
2.2.5. Film Opacity
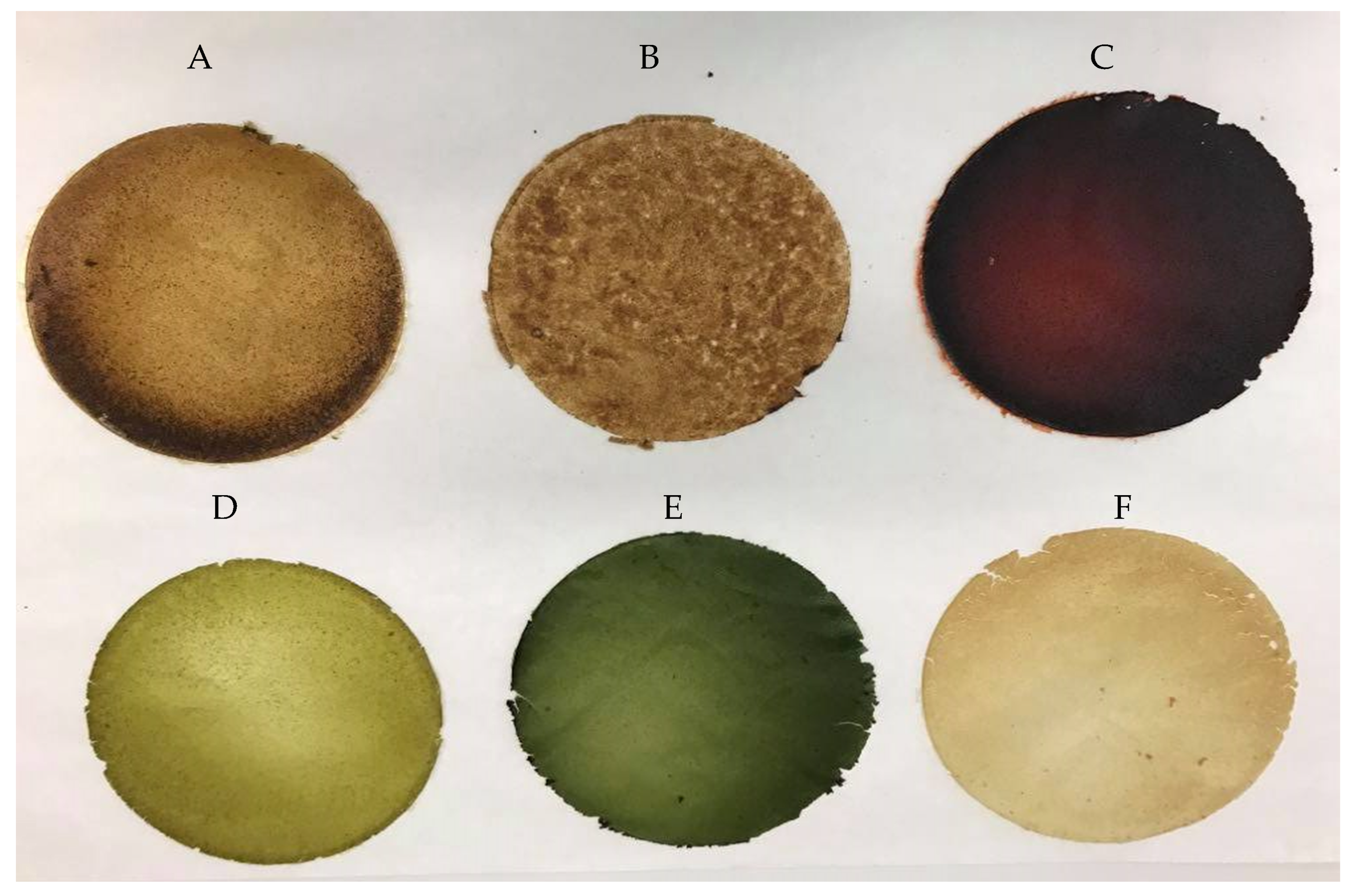
2.2.6. Color
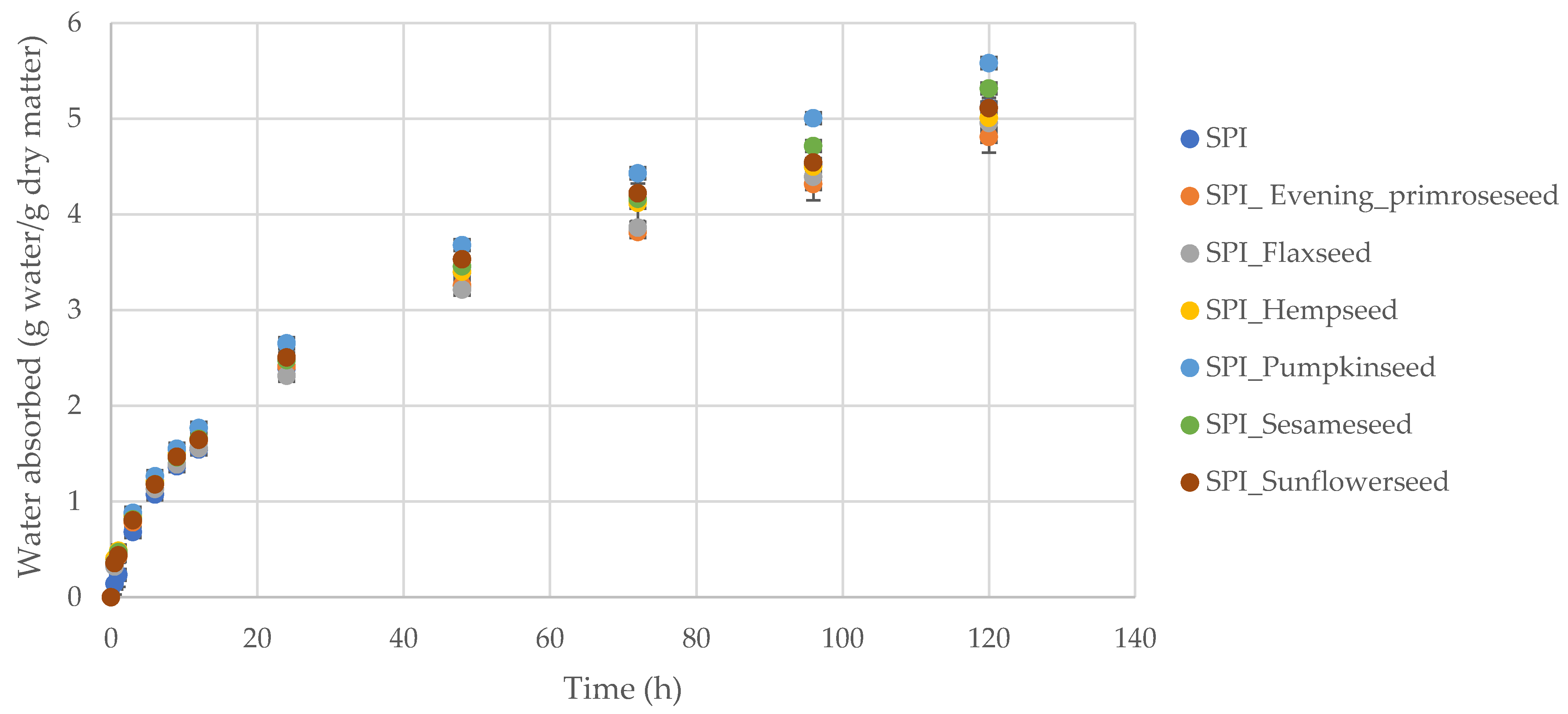
2.3. Water Vapor Permeability, Sorption and Diffusion
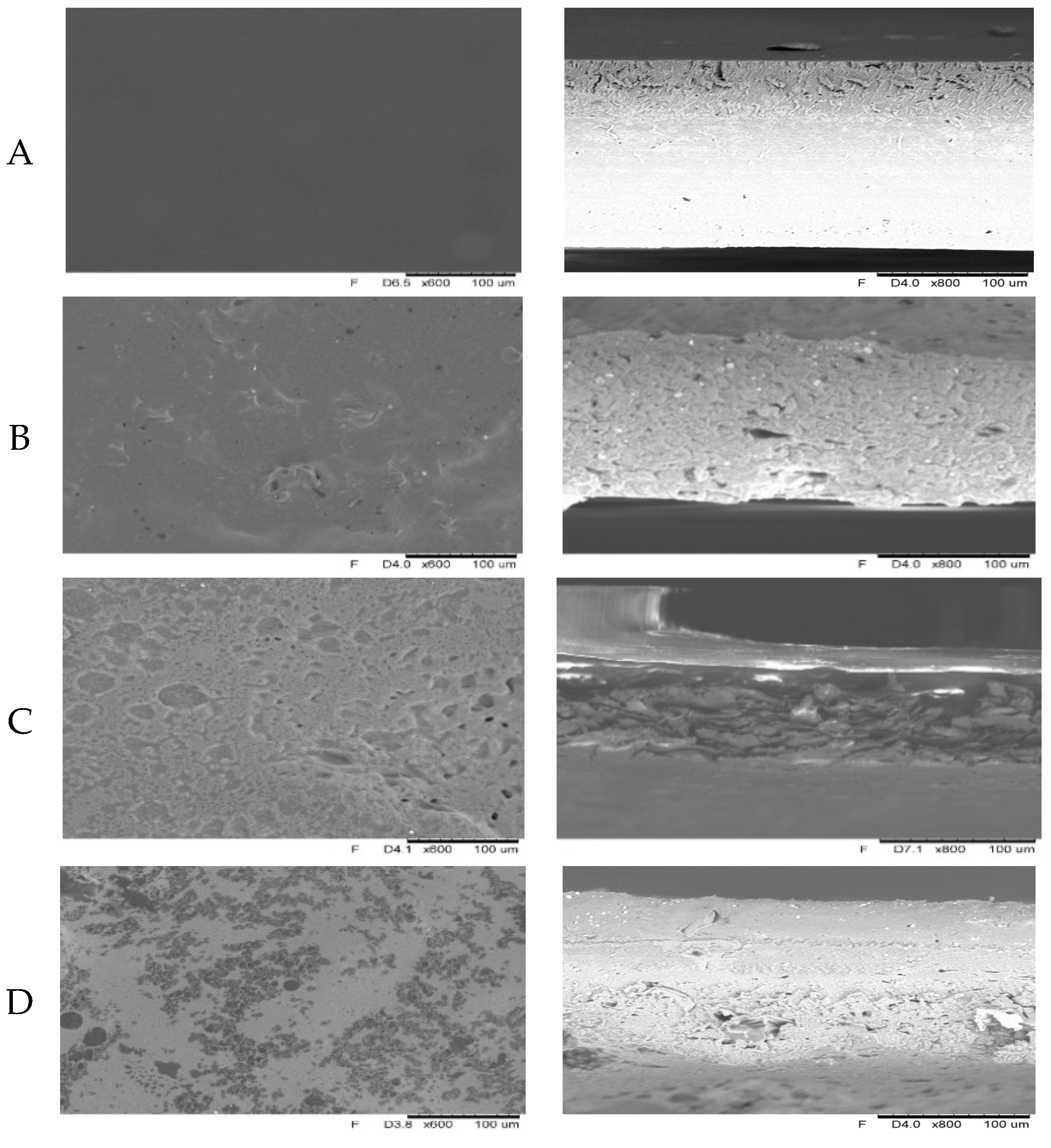
2.4. Microstructure
2.5. Mechanical Properties
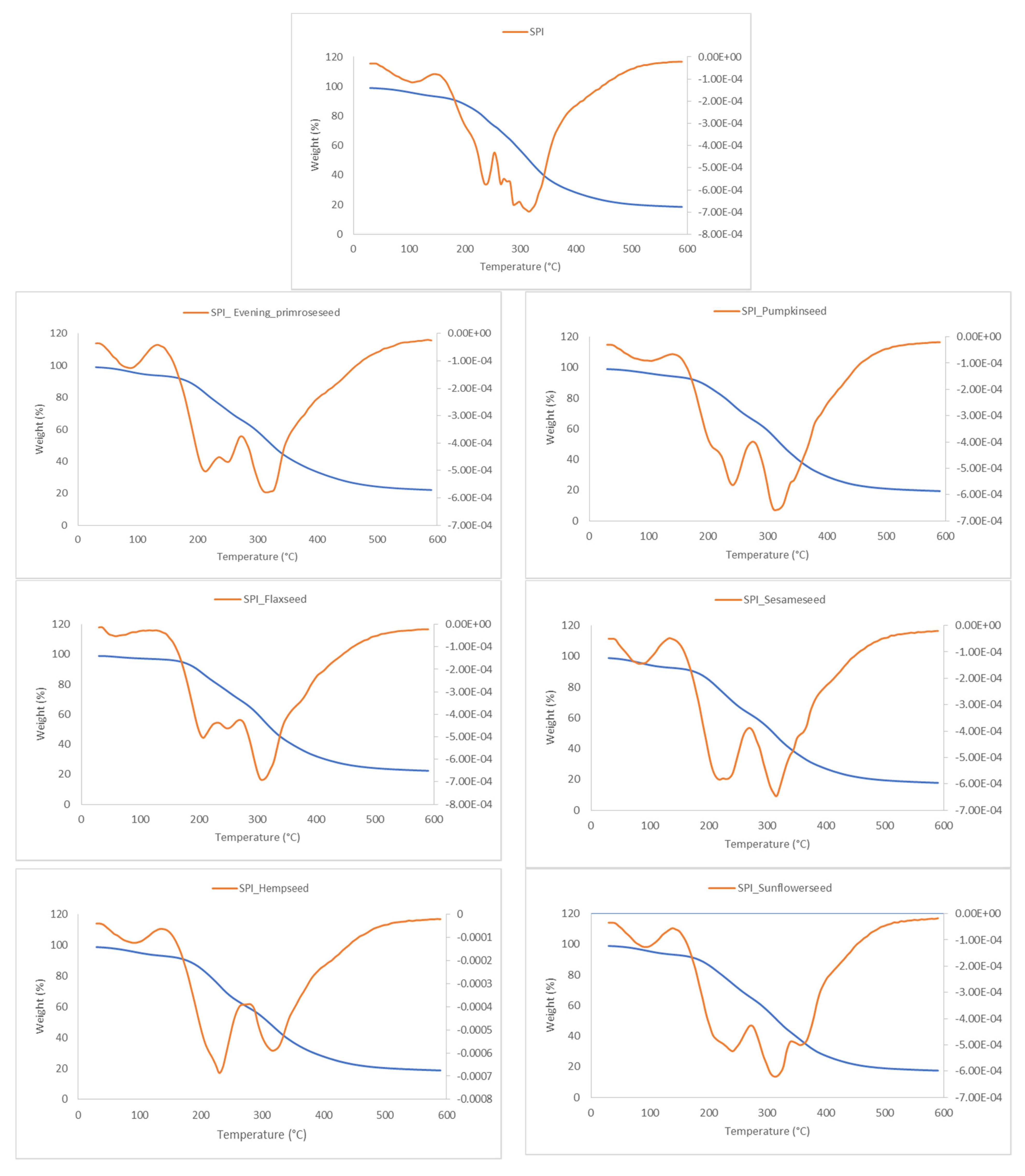
2.6. Thermal Properties
3. Materials and Methods
3.1. Materials
3.1.1. Characterization of Oilseed Flours
3.1.2. Film Preparation
3.2. Physical Properties
3.2.1. Water Content
3.2.2. Thickness
3.2.3. Water Solubility
3.2.4. Swelling Index
3.2.5. Film Opacity
3.2.6. Color
3.3. Water Vapor Permeability (WVP), Sorption and Diffusion
3.4. Microstructure
3.5. Mechanical Properties
3.6. Thermal Properties
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Díaz, O.; Ferreiro, T.; Rodríguez-Otero, J.L.; Cobos, A. Characterization of chickpea (Cicer arietinum L.) flour films: Effect of pH and plasticizer concentration. Int. J. Mol. Sci. 2019, 20, 1246. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and characterization of bioplastics from grass pea flour cast in the presence of microbial transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Galus, S. Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocoll. 2018, 85, 233–241. [Google Scholar] [CrossRef]
- Lamo, B.; Gómez, M. Bread enrichment with oilseeds. A review. Foods 2018, 7, 191. [Google Scholar] [CrossRef]
- Kaszuba, J.; Pycia, K.; Wiśniewski, R.; Jaworska, G.; Kuźniar, P. Effect of selected oil-bearing plant seeds contained in triticale bread on its quality. Żywność. Nauka. Technol. Jakość 2017, 24, 90–102. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, A.E.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Guimarães, R.M.; Ida, E.I.; Falcão, H.B.; de Rezende, T.A.M.; de Santana Silva, J.; Alves, C.C.F.; da Silva, M.A.P.; Egea, M.B. Evaluating technological quality of okara flours obtained by different drying processes. LWT 2020, 123, 109062. [Google Scholar] [CrossRef]
- Nouraddini, M.; Esmaiili, M.; Mohtarami, F. Development and characterization of edible films based on eggplant flour and corn starch. Int. J. Biol. Macromol. 2018, 120, 1639–1645. [Google Scholar] [CrossRef]
- Vera, A.; Tapia, C.; Abugoch, L. Effect of high-intensity ultrasound trearment in combination with transglutaminase and nanoparticles on structural, mechanical, and physicochemical properties of quinoa proteins/chitosann edible films. Int. J. Biol. Macromol. 2020, 144, 536–543. [Google Scholar] [CrossRef]
- Dick, M.; Henrique Pagno, C.; Haas Costa, T.M.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Hickmann Flores, S. Edible films based on chia flour: Development and characterization. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Ochoa-Yepes, O.; Medina-Jaramillo, C.; Guz, L.; Famá, L. Biodegradable and edible starch composites with fiber-rich lentil flour to use as food packaging. Starch/Straerke 2018, 70, 1–34. [Google Scholar] [CrossRef]
- Andrade-Pizarro, R.D.; Skurtys, O.; Osorio-Lira, F. Effect of cellulose nanofibers concentration on mechanical, optical, and barrier properties of gelatin-based edible films. Dyna 2015, 82, 219–226. [Google Scholar] [CrossRef]
- Czerwińska, D. Charakterystyka i zastosowanie mąki z nasion roślin oleistych. Prz. Zboż. Młyn. 2010, 54, 16–17. [Google Scholar]
- Sharma, L.; Saini, C.S.; Sharma, H.K.; Sandhu, K.S. Biocomposite edible coatings based on cross linked-sesame protein and mango puree for the shelf life stability of fresh-cut mango fruit. J. Food Process Eng. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Patwa, A.; Malcolm, B.; Wilson, J.; Amrose, R.P.K. Particle size analysis of two distinct classes of wheat flour by sieving. ASABE 2016, 57, 151–159. [Google Scholar] [CrossRef]
- Adjei-Fremah, S.; Worku, M.; Erive, M.O.D.; He, F.; Wang, T.; Chen, G. Effect of microfluidization on microstructure, protein profile and physicochemical properties of whole cowpea flours. Innov. Food Sci. Emerg. Technol 2019, 57, 1–10. [Google Scholar] [CrossRef]
- Pająk, P.; Przetaczek-Rożnowska, I.; Juszczak, L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M.M.; Tapia-Blácido, D.R.; Menegalli, F.C. Development and optimization of biodegradable films based on achira flour. Carbohydr. Polym. 2012, 88, 449–458. [Google Scholar] [CrossRef]
- Daudt, R.M.; Avena-Bustillos, R.J.; Williams, T.; Wood, D.F.; Külkamp-Guerreiro, I.C.; Marczak, L.D.F.; McHugh, T.H. Comparative study on properties of edible films based on pinhão (Araucaria angustifolia) starch and flour. Food Hydrocoll. 2016, 60, 279–287. [Google Scholar] [CrossRef]
- Chandla, N.K.; Khatkar, S.K.; Singh, S.; Saxena, D.C.; Jindal, N.; Bansal, V.; Wakchaure, N. Tensile strength and solubility studies of edible biodegradable films developed from pseudo-cereal starches: An inclusive comparison with commercial corn starch. Asian J. Dairy Food Res. 2020, 39, 139–146. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Aydogdu, A.; Kirtil, E.; Sumnu, G.; Oztop, M.H.; Aydogdu, Y. Utilization of lentil flour as a biopolymer source for the development of edible films. Inc. J. Appl. Polym. Sci. 2018, 135, 46356. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J. Are modified pumpkin flour/plum flour nanocomposite films biodegradable and compostable? Food Hydrocoll. 2018, 83, 397–410. [Google Scholar] [CrossRef]
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, properties and applications of soy-protein-based materials: A review. Int. J. Biol. Macromol. 2018, 48, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Debeaufort, F.; Lenart, A. Effect of oil lamination between plasticized starch layers on film properties. Food Chem. 2016, 195, 56–63. [Google Scholar] [CrossRef]
- Acquah, C.; Zhang, Y.; Dube, M.A.; Udenigwe, C. Formation and characterization of protein-based films from yellow pea (Pisum sativum) protein isolate and concentrate for edible applications. Curr. Res. Nutr. Food Sci. 2020, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and moisture sensitivity of fully bio-based dialdehyde carboxymethyl cellulose cross-linked soy protein isolate films. Carbohydr. Polym. 2017, 10, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.O.; Cortez-Vega, W.R.; Prentice, C.; Fonseca, G.G. Development and characterization of biopolimer films based on bocaiuva (Acromonia aculeata) flour. Int. J. Biol. Macromol. 2019, 155, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N. Relationship between protein characteristics and film-forming properties of kidney bean, field pea and amaranth protein isolates. Int. J. Food Sci. Technol. 2015, 50, 1033–1043. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Oliveira de Moraes, J.; Vicente, A.R.; Laurindo, J.B.; Mauri, A.N. Scale-up of the production of soy (Glycine max L.) protein films using tape casting: Formulation of film-forming suspension and drying conditions. Food Hydrocoll. 2017, 66, 110–117. [Google Scholar] [CrossRef]
- De Faria Arquelau, P.B.; Silva, V.D.M.; Garcia, M.A.V.T.; de Araujo, R.L.B.; Fante, C.A. Characterization of edible coatings based on ripe ‘Prata’ banana peel flour. Food Hydrocoll. 2019, 89, 570–578. [Google Scholar] [CrossRef]
- Anonymous. Barwa i Jakość; Heidelberg Druckmaschinen AG: Kurfursten-Anlage, Germany, 1999; pp. 52–60. [Google Scholar]
- Maniglia, B.C.; Tessaro, L.; Lucas, A.A.; Tapia-Blácido, D.R. Bioactive films based on babassu mesocarp flour and starch. Food Hydrocoll. 2017, 70, 383–391. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; do Amaral Sobral, P.J.; Menegalli, F.C. Effect of drying conditions and plasticizer type on some physical and mechanicalproperties of amaranth flour films. LWT 2013, 50, 392–400. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Zhang, H.; Li, X.; Fan, W. Reaction-diffusion approach to modeling water diffusion in glutinous rice flour particles during dynamic vapor adsorption. J. Food Sci. Technol. 2019, 56, 4605–4615. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; González, G. Effect of cross-linking with aloe vera gel on surface and physicochemical properties of edible films made from plantain flour. Food Biophys. 2017, 12, 11–22. [Google Scholar] [CrossRef]
- Drakos, A.; Kyriakakis, G.; Evageliou, V.; Protonotariou, S.; Mandala, I.; Ritzoulis, C. Influence of jet milling and particle sizeon the composition, physicochemical and mechanical properties of barley and rye flours. Food Chem. 2017, 215, 326–332. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Mechanical and barrier properties of chitosan combined with other components as food packaging film. Environ. Chem. Lett. 2019, 18, 257–267. [Google Scholar] [CrossRef]
- Ramadhani, F.S.; Rostini, I.; Anna, Z.; Rochima, E. Characterization of edible film from seaweed flour (Eucheuma cottonii Weber-van Bosse, 1993) with different types of plasticizer. WSN 2019, 133, 23–33. [Google Scholar]
- Gutiérrez, T.J.; Herniou-Julien, C.; Álvarez, K.; Álvarez, V.A. Structural properties and in vitro digestability of edible and pH-sensitive films made from guinea arrowroot starch and wastes from wine manufacture. Carbohydr. Polym. 2018, 184, 135–143. [Google Scholar] [CrossRef]
- González, A.; Barrera, G.N.; Galimberti, P.I.; Ribotta, P.D.; Igarzabal, C.I.A. Developmet of edible films prepared by soy protein and the galactomannan fraction extracted grom Gleditsia triacanthos (Fabaceae) seed. Food Hydrocoll. 2019, 97, 105227. [Google Scholar] [CrossRef]
- Andrade Martins, Y.A.; Ferreira, S.V.; Silva, N.M.; Sandre, M.F.B.; Filho, J.G.O.; Leão, P.V.T.; Leão, K.M.; Nicolau, E.S.; Plácido, G.R.; Egea, M.B.; et al. Edible Films of Whey and Cassava Starch: Physical, Thermal, and Microstructural Characterization. Coatings 2020, 10, 1059. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Emulsion film based on fish skin gelatin and palm oil: Physical, structural and thermal properties. Food Hydrocoll. 2015, 48, 248–259. [Google Scholar] [CrossRef]
- Muñoz, L.; Aguilera, J.; Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Characterization and microstructure of films made from mucilage of Salvia hispanica and whey protein concentrate. J. Food Eng. 2012, 111, 511–518. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; De Oliveira, R.A. Extraction and characterization of arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Borges, S.V.; Marconcini, J.M.; Yoshida, M.I.; Neto, A.R.S.; Pereira, T.C.; Pereira, C.F.G. Effect of replacement of corn starch by whey protein isolate in biodegradable film blends obtained by extrusion. Carbohydr. Polym. 2017, 157, 971–980. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Lee, J.H.; Ng, P.K.W. Mechanical and barrier properties of biodegradable soy protein isolate-based films coated with polylactic acid. LWT 2007, 40, 232–238. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheet 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Sobral, P.J.; dos Santos, J.S.; Garcia, F.T. Effect of protein and plasticizer concentration in film forming solutions on physical properties of edible films based on muscle proteins of a Thai Tilapia. J. Food Eng. 2005, 70, 93–100. [Google Scholar] [CrossRef]
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity and structure affect water vapor permeability of model edible films. J. Food Sci. 1993, 58, 428–434. [Google Scholar] [CrossRef]
- Galus, S.; Mathieu, H.; Lenart, A.; Debeaufort, F. Effect of modified starch or maltodextrines incorporation on the barrier and mechanical properties, moisture sensitivity and appearance of soy protein isolate-based edible films. Innov. Food Sci. Emerg. Technol. 2012, 16, 148–154. [Google Scholar] [CrossRef]
- Crank, J. Mathematics of Diffusion, 2nd ed.; Oxford Science Publications: Oxford, UK, 1975. [Google Scholar]


| Film | Water Content (%) | Thickness (μm) | Water Solubility (%) | Swelling in Water (%) |
|---|---|---|---|---|
| SPI | 6.72 ± 0.07 ab | 120.50 ± 12.50 b | 100% | n.d. |
| SPI_ Evening_primroseseed | 6.00 ± 0.55 a | 173.50 ± 15.21 a | 14.39 ± 0.63 abc | 191.78 ± 4.93 bc |
| SPI_Flaxseed | 8.50 ± 0.78 c | 164.89 ± 12.97 a | 12.18 ± 2.04 ab | 362.16 ± 4.01 d |
| SPI_Hempseed | 6.88 ± 0.31 ab | 164.78 ± 16.69 a | 15.76 ± 1.41 bc | 170.43 ± 16.23 b |
| SPI_Pumpkinseed | 6.50 ± 0.53 ab | 155.88 ± 18.04 a | 11.38 ± 2.33 a | 131.10 ± 1.86 a |
| SPI_Sesameseed | 7.92 ± 0.48 bc | 167.57 ± 16.20 a | 17.25 ± 1.41 c | 139.78 ± 5.52 a |
| SPI_Sunflowerseed | 8.45 ± 0.56 c | 137.60 ± 14.25 b | 13.80 ± 1.65 abc | 206.58 ± 8.68 c |
| Film | L* | a* | b* | ΔE | Film Opacity (A mm−1) |
|---|---|---|---|---|---|
| SPI | 91.84 ± 1.05 f | −3.18 ± 0.21 b | 25.72 ± 2.39 b | 92.04 ± 1.51 f | 1.27 ± 0.28 c |
| SPI_ Evening_primroseseed | 34.91 ± 1.13 b | 6.99 ± 0.71 d | 1.35 ± 0.66 c | 64.82 ± 0.99 a | 9.57 ± 0.74 e |
| SPI_Flaxseed | 54.28 ± 3.03 a | 13.62 ± 0.96 e | 21.06 ± 2.11 a | 65.42 ± 2.47 a | 7.15 ± 0.96 b |
| SPI_Hempseed | 63.16 ± 3.18 c | 8.66 ± 0.90 d | 30.24 ± 1.33 d | 47.17 ± 2.04 c | 3.85 ± 0.64 d |
| SPI_Pumpkinseed | 71.46 ± 1.56 d | −1.80 ± 0.73 b | 34.40 ± 0.39 e | 71.69 ± 1.36 e | 5.59 ± 1.22 a |
| SPI_Sesameseed | 83.40 ± 1.08 e | 1.15 ± 0.37 c | 25.21 ± 1.05 b | 28.74 ± 1.34 b | 6.12 ± 0.56 a |
| SPI_Sunflowerseed | 51.97 ± 2.85 a | −5.13 ± 0.99 a | 19.88 ± 2.23 a | 51.18 ± 1.83 d | 7.57 ± 0.67 b |
| Film | WVP (10−9 g m−1 s−1 Pa−1) | Water Diffusion (10−14 m2 s−1) |
|---|---|---|
| SPI | 1.49 ± 0.13 a | 0.80 ± 0.00 e |
| SPI_ Evening_primroseseed | 2.28 ± 0.33 e | 1.65 ± 0.07 c |
| SPI_Flaxseed | 1.82 ± 0.28 c | 1.40 ± 0.06 ab |
| SPI_Hempseed | 1.94 ± 0.17 d | 1.55 ± 0.07 bc |
| SPI_Pumpkinseed | 2.41 ± 0.27 f | 1.35 ± 0.07 a |
| SPI_Sesameseed | 2.70 ± 0.38 g | 1.50 ± 0.06 ab |
| SPI_Sunflowerseed | 1.64 ± 0.21 b | 1.15 ± 0.07 d |
| Film | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| SPI | 1.74 ± 0.23 c | 1.19 ± 0.37 a | 3.95 ± 0.15 c |
| SPI_ Evening_primroseseed | 3.09 ± 0.34 d | 18.75 ± 4.40 d | 24.54 ± 4.75 b |
| SPI_Flaxseed | 2.04 ± 0.36 c | 10.20 ± 2.33 c | 20.03 ± 3.37 ab |
| SPI_Hempseed | 1.21 ± 0.19 b | 5.12 ± 1.72 b | 16.57 ± 3.87 a |
| SPI_Pumpkinseed | 0.82 ± 0.23 a | 1.85 ± 0.94 a | 8.49 ± 2.46 d |
| SPI_Sesameseed | 0.60 ± 0.24 a | 1.01 ± 0.72 a | 6.04 ± 2.60 cd |
| SPI_Sunflowerseed | 1.28 ± 0.15 b | 6.36 ± 1.14 b | 19.77 ± 1.97 ab |
| Film | First Stage | Second Stage | Third Stage | |||
|---|---|---|---|---|---|---|
| Temperature (°C) | Weight Loss (%) | Temperature (°C) | Weight Loss (%) | Temperature (°C) | Weight Loss (%) | |
| SPI | 99.98 | 7.06 | 266.2 | 7.41 | 320.7 | 20.96 |
| SPI_ Evening_primroseseed | 82.09 | 6.25 | 210.6 | 17.88 | 318.4 | 13.93 |
| SPI_Flaxseed | 53.20 | 3.47 | 205.1 | 15.84 | 310.7 | 13.07 |
| SPI_Hempseed | 85.87 | 6.85 | 230.6 | 19.75 | 320.33 | 12.83 |
| SPI_Pumpkinseed | 86.79 | 5.88 | 242.4 | 18.13 | 316.8 | 14.53 |
| SPI_Sesameseed | 80.12 | 7.65 | 218.6 | 29.81 | 316.9 | 35.41 |
| SPI_Sunflowerseed | 86.38 | 7.04 | 242.2 | 28.49 | 318.3 | 37.44 |
| Evening Primroseed | Flaxseed | Hempseed | Pumpkinseed | Sesameseed | Sunflowerseed | |
|---|---|---|---|---|---|---|
| Energy (kcal) | 363.8 | 316 | 356 | 425.2 | 397.5 | 346 |
| Fat (g) | 8 | 8.9 | 6 | 16 | 10 | 9.5 |
| of which saturates (g) | 1 | 0.8 | 1 | 3 | 2 | 1.2 |
| Carbohydrate (g) | 45 | 4.8 | 41 | 13 | 21 | 8 |
| of which sugars (g) | 1 | 4.1 | 3 | 1 | 1 | 6.5 |
| Protein (g) | 29 | 38 | 38 | 58 | 55 | 48 |
| Salt (g) | 0 | 0.16 | 0 | 0 | 0 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. https://doi.org/10.3390/molecules26123738
Mikus M, Galus S, Ciurzyńska A, Janowicz M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules. 2021; 26(12):3738. https://doi.org/10.3390/molecules26123738
Chicago/Turabian StyleMikus, Magdalena, Sabina Galus, Agnieszka Ciurzyńska, and Monika Janowicz. 2021. "Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours" Molecules 26, no. 12: 3738. https://doi.org/10.3390/molecules26123738
APA StyleMikus, M., Galus, S., Ciurzyńska, A., & Janowicz, M. (2021). Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules, 26(12), 3738. https://doi.org/10.3390/molecules26123738









