Polysaccharide κ-Carrageenan as Doping Agent in Conductive Coatings for Electrochemical Controlled Release of Dexamethasone at Therapeutic Doses
Abstract
1. Introduction
2. Results and Discussion
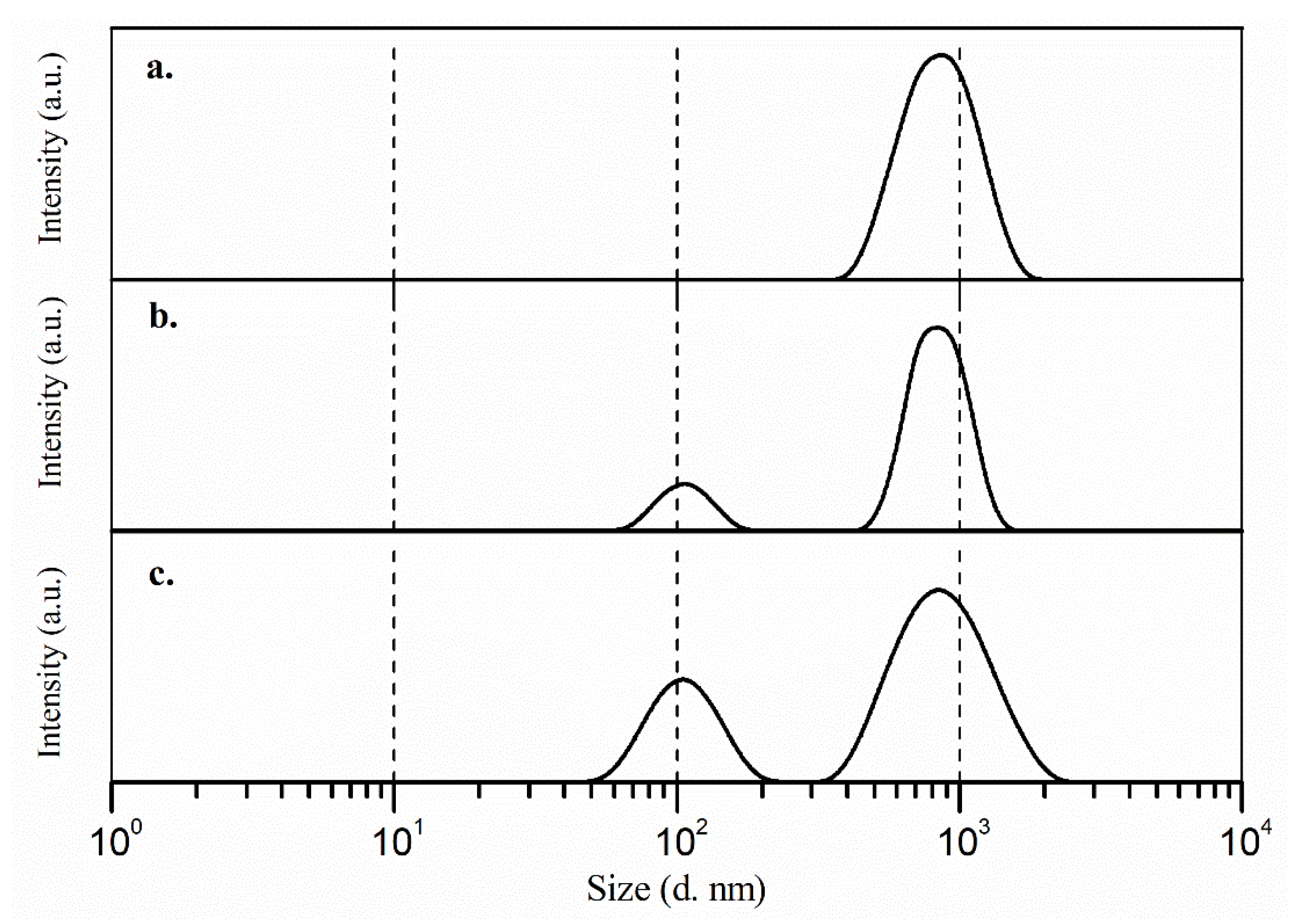
2.1. Evaluation of the Stability and Size of the Dispersion Systems
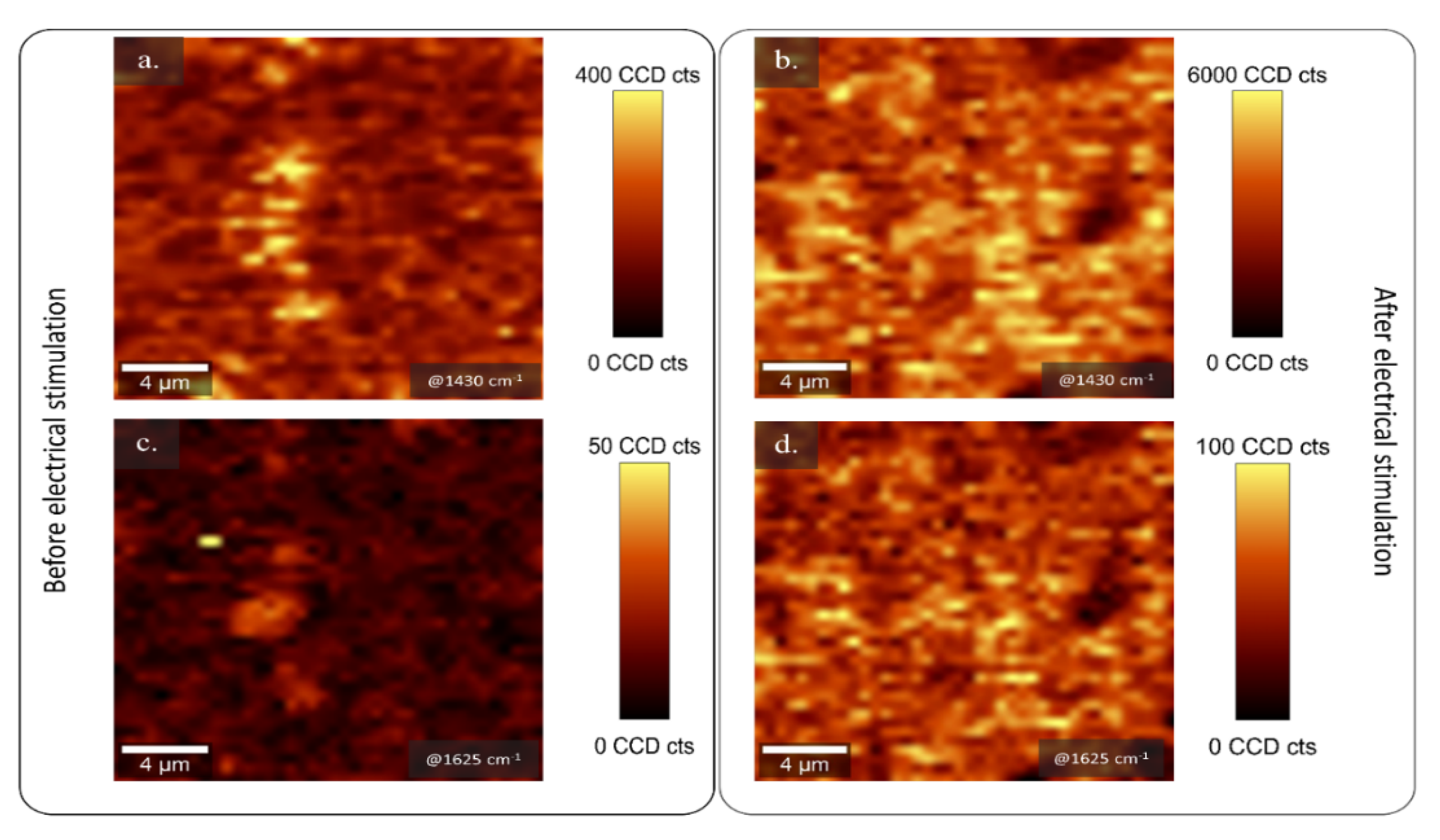
2.2. Analysis of the Topography and Composition of PEDOT:κC:Dx Coating by µ-Raman Spectroscopy and Profilometry Methods
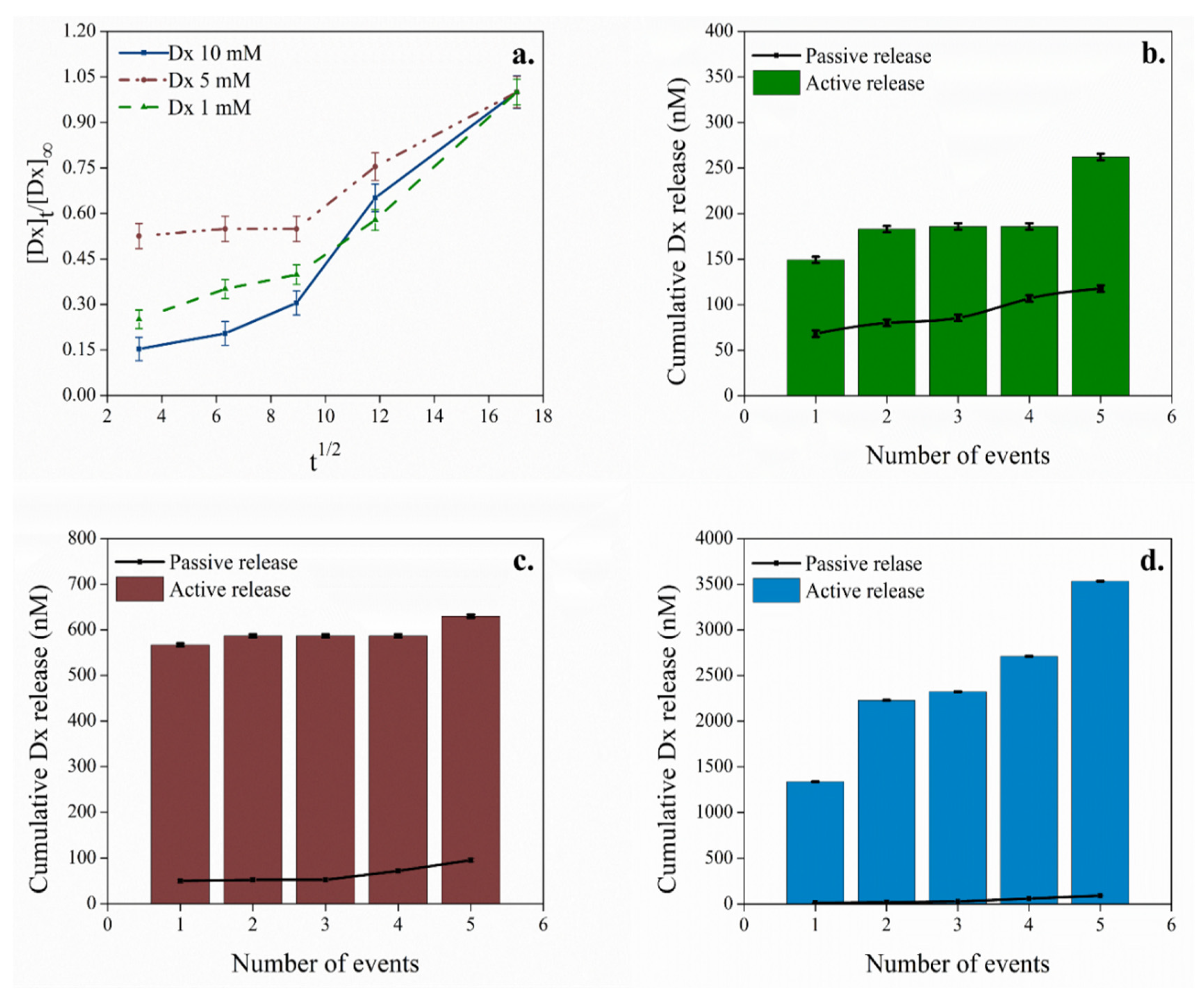
2.3. Dexamethasone Release Experiments from the PEDOT:κC:Dx Coating
3. Materials and Methods
3.1. Materials
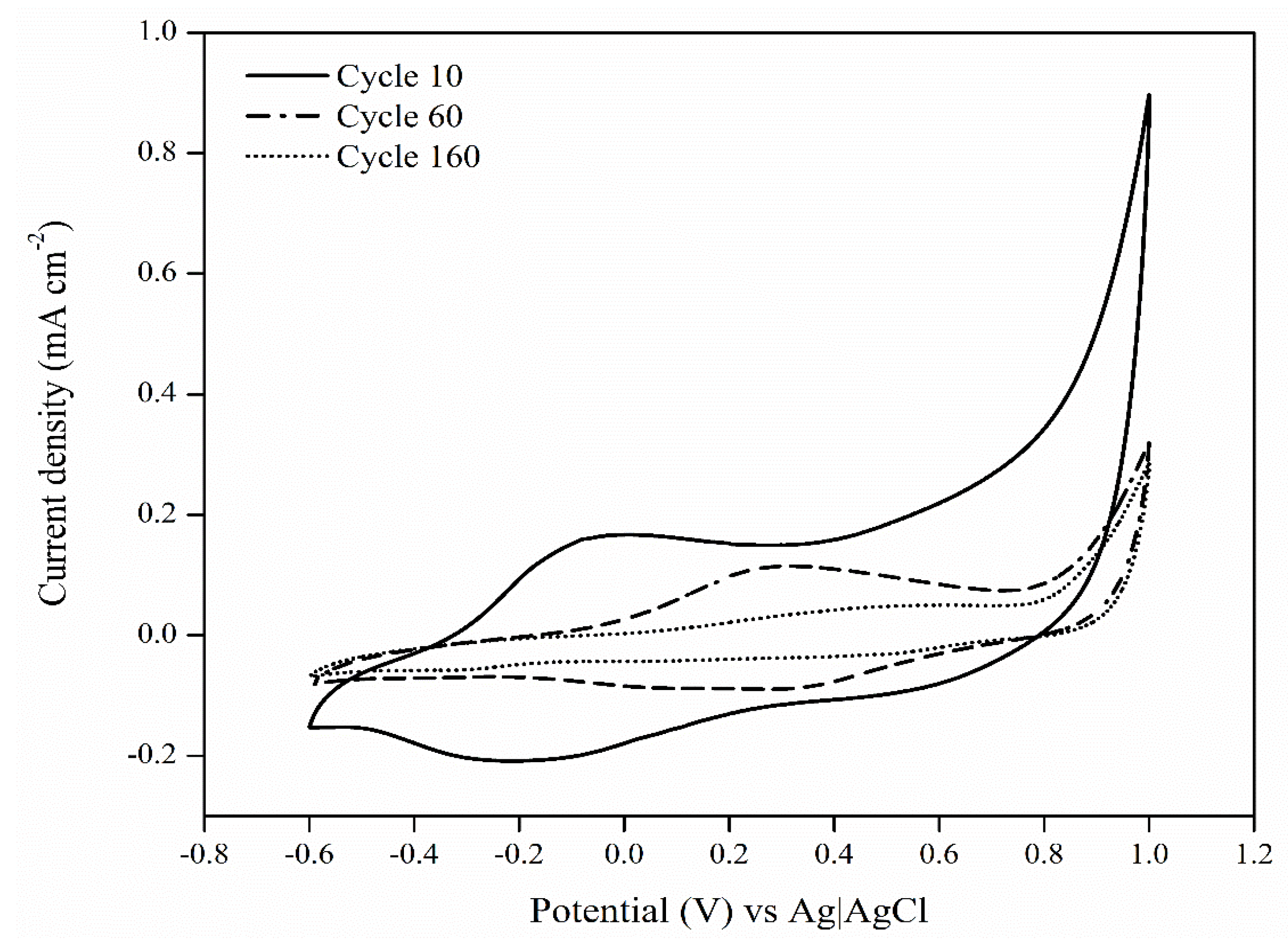
3.2. Synthesis and Preparation of the Modified PEDOT:κC:Dx Electrode
3.3. Evaluation of the Stability and Size of the Dispersion Systems
3.4. Analysis of the Topography and Composition of PEDOT:κC:Dx Coating by Profilometry and µ-Raman Spectroscopy Methods
3.5. Dexamethasone Release Experiments from the PEDOT:κC:Dx Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozkan, B.C.; Soganci, T.; Turhan, H.; Ak, M. Investigation of rGO and chitosan effects on optical and electrical properties of the conductive polymers for advanced applications. Electrochim. Acta 2019, 295, 1044–1051. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, K.; Alvarado-Hidalgo, F.; Zamora-Sequeira, R.; Sáenz-Arce, G.; Rojas-Carrillo, O.; Avedaño-Soto, E.; Ruepert, C.; Mena-Torres, F.; Starbird-Pérez, R. Biosensor based on the directly enzyme immobilization into a gold nanotriangles/conductive polymer biocompatible coat for electrochemical detection of Chlorpyrifos in water. Med. Devices Sens. 2019, 2, 1–18. [Google Scholar]
- Mantione, D.; del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) derivatives: Innovative conductive polymers for bioelectronics. Polymers (Basel) 2017, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani Zamani, F.; Moulahoum, H.; Ak, M.; Odaci Demirkol, D.; Timur, S. Current trends in the development of conducting polymers-based biosensors. TrAC Trends Anal. Chem. 2019, 118, 264–276. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Israr, M.; Jang, J.H.; Park, J.K. Nano-gold assisted highly conducting and biocompatible bacterial cellulose-PEDOT:PSS films for biology-device interface applications. Int. J. Biol. Macromol. 2018, 107, 865–873. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Kahoush, M.; Behary, N.; Cayla, A.; Mutel, B.; Guan, J.; Nierstrasz, V. Influence of remote plasma on PEDOT:PSS-coated carbon felt for improved activity of glucose oxidase. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Luo, S.C.; Ali, E.M.; Tansil, N.C.; Yu, H.H.; Gao, S.; Kantchev, E.A.B.; Ying, J.Y. Poly(3,4-ethylenedioxythiophene) (PEDOT) nanobiointerfaces: Thin, ultrasmooth, and functionalized PEDOT films with in vitro and in vivo biocompatibility. Langmuir 2008, 24, 8071–8077. [Google Scholar] [CrossRef]
- Starbird, R.; Bauhofer, W.; Meza-Cuevas, M.; Krautschneider, W.H. Effect of experimental factors on the properties of PEDOT-NaPSS galvanostatically deposited from an aqueous micellar media for invasive electrodes. In Proceedings of the 5th 2012 Biomedical Engineering International Conference, Ubon Ratchathani, Thailand, 5–7 December 2012; pp. 1–5. [Google Scholar]
- Li, Y.; Neoh, K.G.; Kang, E.T. Controlled release of heparin from polypyrrole-poly (vinyl alcohol) assembly by electrical stimulation. J. Biomed. Mater. Res. Part A 2005, 72, 171–180. [Google Scholar] [CrossRef]
- Boehler, C.; Oberueber, F.; Asplund, M. Tuning drug delivery from conducting polymer films for accurately controlled release of charged molecules. J. Control. Release 2019, 304, 173–180. [Google Scholar] [CrossRef]
- Boehler, C.; Kleber, C.; Martini, N.; Xie, Y.; Dryg, I.; Stieglitz, T.; Hofmann, U.G.; Asplund, M. Actively controlled release of dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials 2017, 129, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Lagenaur, C.F.; Cui, X.T. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J. Control. Release 2006, 110, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, T.; Xie, S.; Liu, X.; Li, H.; Linhardt, R.J.; Chi, L. Sequencing the oligosaccharide pool in the low molecular weight heparin dalteparin with offline HPLC and ESI–MS/MS. Carbohydr. Polym. 2018, 183, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Durney, A.R.; Anseth, K.S. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials 2007, 28, 66–77. [Google Scholar] [CrossRef]
- He, Q.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Bhise, N.S.; Evangelista, M.B.; Rouwkema, J.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Vrana, N.E.; Khademhosseini, A. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol. 2016, 34, 470–482. [Google Scholar] [CrossRef]
- Thevenot, P.T.; Nair, A.M.; Shen, J.; Lotfi, P.; Ko, C.Y.; Tang, L. The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 2010, 31, 3997–4008. [Google Scholar] [CrossRef]
- Goimil, L.; Jaeger, P.; Ardao, I.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A. Preparation and stability of dexamethasone-loaded polymeric scaffolds for bone regeneration processed by compressed CO2 foaming. J. CO2 Util. 2018, 24, 89–98. [Google Scholar] [CrossRef]
- Costa, P.F.; Puga, A.M.; Díaz-Gomez, L.; Concheiro, A.; Busch, D.H.; Alvarez-Lorenzo, C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int. J. Pharm. 2015, 496, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Handsche, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Helledie, T.; Dombrowski, C.; Rai, B.; Lim, Z.X.H.; Hin, I.L.H.; Rider, D.A.; Stein, G.S.; Hong, W.; van Wijnen, A.J.; Hui, J.H.; et al. Heparan sulfate enhances the self-renewal and therapeutic potential of mesenchymal stem cells from human adult bone marrow. Stem Cells Dev. 2012, 21, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Duarte, A.R.C.; Faria, S.; Marques, A.P.; Reis, R.L.; Neves, N.M. Osteogenic induction of hBMSCs by electrospun scaffolds with dexamethasone release functionality. Biomaterials 2010, 31, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Goimil, L.; Santos-Rosales, V.; Delgado, A.; Évora, C.; Reyes, R.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; Gómez-Amoza, J.L.; Concheiro, A.; et al. ScCO2-foamed silk fibroin aerogel/poly(ϵ-caprolactone) scaffolds containing dexamethasone for bone regeneration. J. CO2 Util. 2019, 31, 51–64. [Google Scholar] [CrossRef]
- Chu, C.C.; Hsing, C.H.; Shieh, J.P.; Chien, C.C.; Ho, C.M.; Wang, J.J. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur. J. Pharmacol. 2014, 722, 48–54. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Ardao, I.; Starbird, R.; García-González, C.A. Conductive nanostructured materials based on poly-(3,4-ethylenedioxythiophene) (PEDOT) and starch/κ-carrageenan for biomedical applications. Carbohydr. Polym. 2018, 189, 304–312. [Google Scholar] [CrossRef]
- Hernández-Suarez, P.; Ramírez, K.; Alvarado, F.; Avendaño, E.; Starbird, R. Electrochemical characterization of poly(3,4-ethylenedioxythiophene)/κ-carrageenan as a biocompatible conductive coat for biologic applications. MRS Commun. 2018, 1–6. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Harzandi, A.M.; Hosseinzadeh, H. Modified carrageenan 3. Synthesis of a novel polysaccharide-based superabsorbent hydrogel via graft copolymerization of acrylic acid onto kappa-carrageenan in air. Eur. Polym. J. 2004, 40, 1363–1370. [Google Scholar] [CrossRef]
- Hunter, R. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2013; pp. 6–7. [Google Scholar]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Ali, H.; Kalashnikova, I.; White, M.A.; Sherman, M.; Rytting, E. Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int. J. Pharm. 2013, 454, 149–157. [Google Scholar] [CrossRef]
- Pargaonkar, N.; Lvov, Y.M.; Li, N.; Steenekamp, J.H.; De Villiers, M.M. Controlled release of dexamethasone from microcapsules produced by polyelectrolyte layer-by-layer nanoassembly. Pharm. Res. 2005, 22, 826–835. [Google Scholar] [CrossRef]
- Sagbas, S.; Butun, S.; Sahiner, N. Modifiable chemically crosslinked poli(κ-carrageenan) particles. Carbohydr. Polym. 2012, 87, 2718–2724. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Zhuravleva, I.L.; Cardinaels, R.; Moldenaers, P. Macromolecular complexes of lysozyme with kappa carrageenan. Food Hydrocoll. 2018, 74, 227–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Grijpma, D.W.; Feijen, J. Poly(trimethylene carbonate) and monomethoxy poly(ethylene glycol)-block-poly(trimethylene carbonate) nanoparticles for the controlled release of dexamethasone. J. Control. Release 2006, 111, 263–270. [Google Scholar] [CrossRef]
- Walsh, F.C.; Ponce De Leon, C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. Inst. Met. Finish. 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Kokkinos, P.A.; Koutsoukos, P.G.; Deligianni, D.D. Detachment strength of human osteoblasts cultured on hydroxyapatite with various surface roughness. Contribution of integrin subunits. J. Mater. Sci. Mater. Med. 2012, 23, 1489–1498. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Tran-Van, F.; Garreau, S.; Louarn, G.; Froyer, G.; Chevrot, C. Fully undoped and soluble oligo(3,4-ethylenedioxythiophene)s: Spectroscopic study and electrochemical characterization. J. Mater. Chem. 2001, 11, 1378–1382. [Google Scholar] [CrossRef]
- Stevenson, G.; Moulton, S.E.; Innis, P.C.; Wallace, G.G. Polyterthiophene as an electrostimulated controlled drug release material of therapeutic levels of dexamethasone. Synth. Met. 2010, 160, 1107–1114. [Google Scholar] [CrossRef]
- Leprince, L.; Dogimont, A.; Magnin, D.; Demoustier-Champagne, S. Dexamethasone electrically controlled release from polypyrrole-coated nanostructured electrodes. J. Mater. Sci. Mater. Med. 2010, 21, 925–930. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Bredas, J.; Street, B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Thomas, D.; Nair, V.V.; Latha, M.S.; Thomas, K.K. Theoretical and experimental studies on theophylline release from hydrophilic alginate nanoparticles. Futur. J. Pharm. Sci. 2019, 5, 2. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Kleber, C.; Lienkamp, K.; Rühe, J.; Asplund, M. Electrochemically controlled drug release from a conducting polymer hydrogel (PDMAAp/PEDOT) for local therapy and bioelectronics. Adv. Healthc. Mater. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Hong, D.; Chen, H.X.; Xue, Y.; Li, D.M.; Wan, X.C.; Ge, R.; Li, J.C. Osteoblastogenic effects of dexamethasone through upregulation of TAZ expression in rat mesenchymal stem cells. J. Steroid Biochem. Mol. Biol. 2009, 116, 86–92. [Google Scholar] [CrossRef]
- Simann, M.; Schneider, V.; Le Blanc, S.; Dotterweich, J.; Zehe, V.; Krug, M.; Jakob, F.; Schilling, T.; Schütze, N. Heparin affects human bone marrow stromal cell fate: Promoting osteogenic and reducing adipogenic differentiation and conversion. Bone 2015, 78, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Spataro, L.; Dilgen, J.; Retterer, S.; Spence, A.J.; Isaacson, M.; Turner, J.N.; Shain, W. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp. Neurol. 2005, 194, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Montero-Rodríguez, J.J.; Ramirez-Sanchez, K.; Valladares-Castrillo, G.; Avendano-Soto, E.D.; Starbird-Perez, R. Design and simulation of flexible thin-film electrodes for cell culture stimulation. In Proceedings of the 2020 Latin American Electron Devices Conference (LAEDC), San José, Costa Rica, 25–28 Febuary 2020. [Google Scholar]
- Wakkad, E.; Shams, D. The Anodic oxidation of metals at very low current density. Part V.*. J. Chem. Soc. 1946, 1, 3098–3102. [Google Scholar]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Konermann, L. Addressing a common misconception: Ammonium acetate as neutral ph “buffer” for native electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sequeira, R.; Alvarado-Hidalgo, F.; Robles-Chaves, D.; Saénz-Arce, G.; Avendaño-Soto, E.; Sànchez-Kooper, A.; Starbird-Pérez, R. Degradation for wastewater treatment using a sensor based on poly (3, 4-ethylenedioxythiophene)(PEDOT) modified with carbon nanotubes and gold nanoparticles. Polymers (Basel) 2019, 11, 1449. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| System | ζ-potential (mV) | SD (mV) |
|---|---|---|
| Dx | −69.40 | 1.14 |
| κC | −43.30 | 3.31 |
| κC:Dx | −42.63 | 1.67 |
| EDOT:Dx | −70.83 | 1.09 |
| κC:EDOT | −48.46 | 1.70 |
| EDOT:κC:Dx | −48.70 | 1.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez Sánchez, K.; Ledezma-Espinoza, A.; Sánchez-Kopper, A.; Avendaño-Soto, E.; Prado, M.; Starbird Perez, R. Polysaccharide κ-Carrageenan as Doping Agent in Conductive Coatings for Electrochemical Controlled Release of Dexamethasone at Therapeutic Doses. Molecules 2020, 25, 2139. https://doi.org/10.3390/molecules25092139
Ramírez Sánchez K, Ledezma-Espinoza A, Sánchez-Kopper A, Avendaño-Soto E, Prado M, Starbird Perez R. Polysaccharide κ-Carrageenan as Doping Agent in Conductive Coatings for Electrochemical Controlled Release of Dexamethasone at Therapeutic Doses. Molecules. 2020; 25(9):2139. https://doi.org/10.3390/molecules25092139
Chicago/Turabian StyleRamírez Sánchez, Karla, Aura Ledezma-Espinoza, Andrés Sánchez-Kopper, Esteban Avendaño-Soto, Mónica Prado, and Ricardo Starbird Perez. 2020. "Polysaccharide κ-Carrageenan as Doping Agent in Conductive Coatings for Electrochemical Controlled Release of Dexamethasone at Therapeutic Doses" Molecules 25, no. 9: 2139. https://doi.org/10.3390/molecules25092139
APA StyleRamírez Sánchez, K., Ledezma-Espinoza, A., Sánchez-Kopper, A., Avendaño-Soto, E., Prado, M., & Starbird Perez, R. (2020). Polysaccharide κ-Carrageenan as Doping Agent in Conductive Coatings for Electrochemical Controlled Release of Dexamethasone at Therapeutic Doses. Molecules, 25(9), 2139. https://doi.org/10.3390/molecules25092139







