Potential Application of Hippophae Rhamnoides in Wheat Bread Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Wheat Flour and Sea Buckthorn Berry Flour
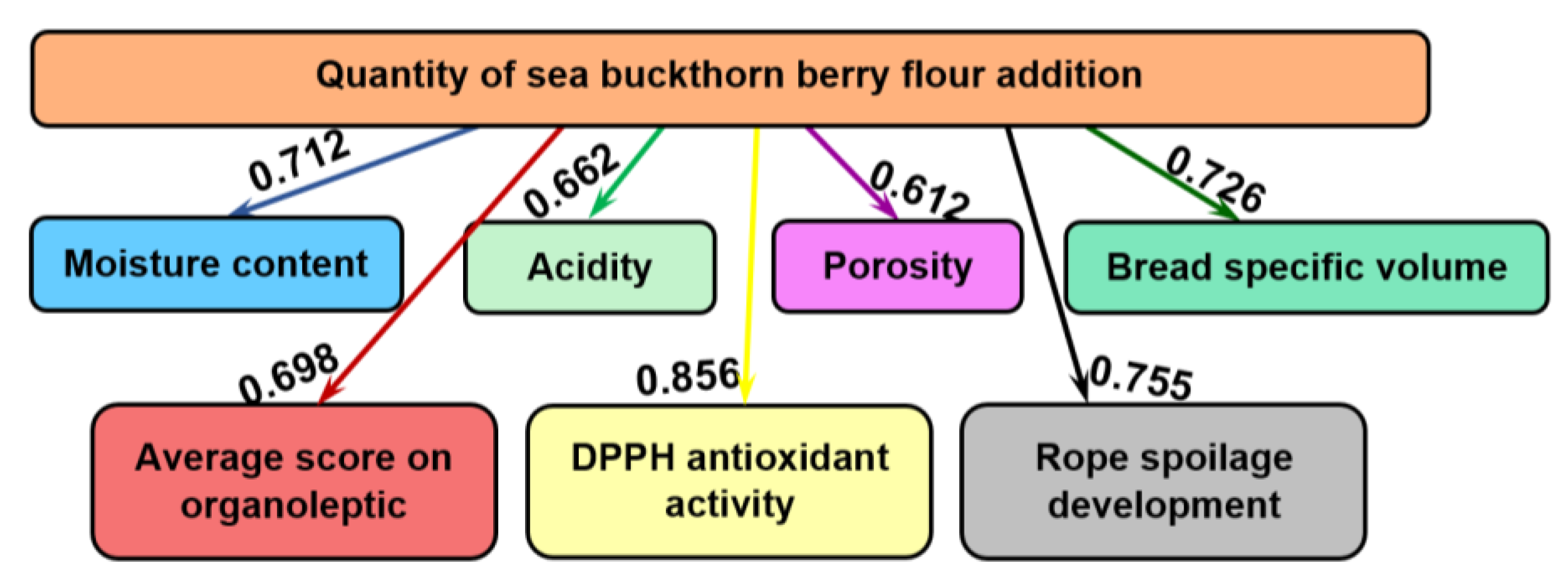
2.2. Effects of Sea Buckthorn Berry Flour Adition on Wheat Bread’s Properties
3. Materials and Methods
3.1. Extraction
3.2. Antioxidant Activity of the Sea Buckthorn Berry Flour by Reaction with ABTS (2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulphonic Acid)) Radical
3.3. Total Polyphenols and Flavonoids in Sea Buckthorn Berry Flour by Folin-Ciocalteu
3.4. Total Polyphenols in Sea Buckthorn Berry Flour by Absorbance at 280
3.5. Total Cinnamic Acids in Sea Buckthorn Berry Flour
3.6. Total Flavonols in Sea Buckthorn Berry Flour
3.7. Total Carotenoids in Sea Buckthorn Berry Flour
3.8. The Analysis of Polyphenols in Sea Buckthorn Berry Flour by HPLC
3.9. The Analysis of L-ascorbic Acid in Sea Buckthorn Berry Flour by HPLC
3.10. Bread Making
3.11. Antioxidant Activity of the Sea Buckthorn Berry Flour and Bread by DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Radical
3.12. Sensory Analysis of the Wheat Bread Samples
3.13. The Study of Rope Spoilage Development in Bread Samples
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Bureau of Statistics of the Republic of Moldova. National Bureau of Statistics of the Republic of Moldova. Anuarul statistic al Republicii Moldova (Statistical Yearbook of the Republic of Moldova) (In Romanian); National Bureau of Statistics of the Republic of Moldova: Chisinau, Republic of Moldova, 2018; p. 249. [Google Scholar]
- Vaičiulytė-Funk, L.; Žvirdauskienė, R.; Šalomskienė, J.; Šarkinas, A. The effect of wheat bread contamination by the Bacillus genus bacteria on the quality and safety of bread. Zemdirb. Agric. 2015, 102, 351–358. [Google Scholar] [CrossRef]
- Voysey, P.A. Rope: A problem for bakers. J. Appl. Bacteriol. 1989, 67, XXV–XXVI. [Google Scholar]
- Saranraj, P.; Geetha, M. Microbial Spoilage of Bakery Products and Its Control by Preservatives. Int. J. Pharm. Biol. Sci. Arch. 2012, 3, 38–48. [Google Scholar]
- Kaushal, M.; Sharma, P. Nutritional and antimicrobial property of sea buckthorn (Hippophae sp.) seed oil. J. Sci. Ind. Res. 2011, 70, 1033–1036. [Google Scholar]
- Ghendov-Moșanu, A.; Sturza, R.; Opriș, O.; Lung, I.; Popescu, L.; Popovici, V.; Soran, M.-L.; Patras, A. Effect of lipophilic sea buckthorn extract on cream cheese properties. J. Food Sci. Technol. 2020, 57, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Krejkarova, J.; Strakova, E.; Suchy, P.; Herzig, I.; Karaskova, K. Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceutics and its therapeutic possibilities-a review. Acta Vet. Brno 2015, 84, 257–268. [Google Scholar] [CrossRef]
- Žilić, S. Phenolic compounds of wheat their content, antioxidant capacity and bioaccessibility. MOJ Food Process Technol. 2016, 2, 85–89. [Google Scholar] [CrossRef]
- Box, J.D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Hajazimi, E.; Landberg, R.; Zamaratskaia, G. Simultaneous determination of flavonols and phenolic acids by HPLC-CoulArray in berries common in the Nordic diet. LWT Food Sci. Technol. 2016, 74, 128–134. [Google Scholar] [CrossRef]
- Ma, X.; Laaksonen, O.; Zheng, J.; Yang, W.; Trepanier, M.; Kallio, H.; Yang, B. Flavonol glycosides in berries of two major subspecies of sea buckthorn (Hippophaë rhamnoides L.) and influence of growth sites. Food Chem. 2016, 200, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Arimboor, R.; Kumar, K.S.; Arumughan, C. Simultaneous estimation of phenolic acids in sea buckthorn (Hippophae rhamnoides) using RP-HPLC with DAD. J. Pharm. Biomed. Anal. 2008, 47, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sytařova, I.; Orsavová, J.; Snopek, L.; Mlček, J.; Byczyński, Ł.; Mišurcová, L. Impact of phenolic compounds and vitamins C and E on antioxidant activity of sea buckthorn (Hippophae rhamnoides L.) berries and leaves of diverse ripening times. Food Chem. 2020, 310, 125784. [Google Scholar] [CrossRef] [PubMed]
- Kuhkheil, A.; Naghdi Badi, H.; Mehrafarin, A.; Abdossi, V. Chemical constituents of sea buckthorn (Hippophae rhamnoides L.) fruit in populations of central Alborz Mountains in Iran. Res. J. Pharmacogn. 2017, 4, 1–12. [Google Scholar]
- Barl, B.; Akhov, L.; Dunlop, D.; Jana, S.; Schroeder, W.R. Flavonoid content and composition in leaves and berries of sea buckthorn (Hippophae rhamnoides) of different origin. Acta Hortic. 2003, 626, 397–405. [Google Scholar] [CrossRef]
- Burri, S.C.M.; Ekholm, A.; Hakansson, A.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef]
- Kant, V.; Mehta, M.; Varshneya, C. Antioxidant potential and total phenolic contents of sea buckthorn (Hippophae rhamnoides) pomace. Free Radic. Antiox. 2012, 2, 79–86. [Google Scholar] [CrossRef]
- Pop, R.M.; Weesepoel, Y.; Socaciu, C.; Pintea, A.; Vincken, J.P.; Gruppen, H. Carotenoid composition of berries and leaves from six Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014, 147, 1–9. [Google Scholar] [CrossRef]
- Andresson, S.C.; Olsson, M.E.; Johansson, E.; Rumpunen, K. Carotenoids in sea buckthorn (Hippophae rhamnoides L.) berries during ripening and use of pheophytin a as a maturity marker. J. Agric. Food Chem. 2009, 57, 250–258. [Google Scholar] [CrossRef]
- Yoo, A.Y.; Alnaeeli, M.; Park, J.K. Production control and characterization of antibacterial carotenoids from the yeast Rhodotorula mucilaginosa AY-01. Process Biochem. 2016, 51, 463–473. [Google Scholar] [CrossRef]
- Moreira, M.D.; Magalhaes, M.M.; Coimbra, J.M.; dos Reis, K.C.; Freitas Schwan, R.; Ferreira Silva, C. Solid coffee waste as alternative to produce carotenoids with antioxidant and antimicrobial activities. Waste Manag. 2018, 82, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food. Principles and Practices; Springer: Berlin, Germany, 2010. [Google Scholar]
- Ameh, M.O.; Gernah, D.I.; Igbabul, B.D. Physicochemical and sensory evaluation of wheat bread supplemented with stabilized undefatted rice bran. Food Nutr. Sci. 2013, 4, 43–48. [Google Scholar] [CrossRef]
- Greene, J.L.; Bovell-Benjamin, A.C. Macroscopic and Sensory Evaluation of Bread Supplemented with Sweet Potato Flour. J. Food Sci. 2004, 69, 167–173. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Liepiņa, I.; Nikolajeva, V.; Jākobsone, I. Antimicrobial activity of extracts from fruits of Aronia melanocarpa and Sorbus aucuparia. Environ. Exp. Biol. 2013, 11, 195–199. [Google Scholar]
- Anastasiadi, M.; Chorianopoulos, N.; Nychas, G.; Haroutounian, S. Antilisterial activities of polyphenol-rich extracts of grapes and vinification by-products. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.; Mustapha, A. Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef]
- Karrar, E.M.A. A review on: Antioxidant and its impact during the bread making process. Int. J. Nutr. Food Sci. 2014, 3, 592–593. [Google Scholar] [CrossRef]
- Quadir, M.I.; Abbas, K.; Younus, A.; Shaikh, R.S. Antibacterial activity of sea buckthorn (Hippophae rhamnoides L.) against methicillin resistant Staphylococus aureus (MRSA). Pak. J. Pharm. Sci. 2016, 29, 1711–1713. [Google Scholar]
- Gill, N.S.; Sharma, R.; Arora, R.; Bali, M. Antioxidant and antibacterial activity of Hippophae rhamnoides methanolic leaf extracts from dry temperate agro-climatic region of Himachal-Pradesh. J. Plant Sci. 2012, 7, 194–200. [Google Scholar] [CrossRef][Green Version]
- Pilar de Torre, M.; Cavero, R.Y.; Calvo, M.I.; Vizmanos, J.L. A Simple and a Reliable Method to Quantify Antioxidant Activity In Vivo. Antioxidants 2019, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, U. Determination of Moisture in Dehydrated Vegetables. In ISI Handbook of Food Analysis (Part VIII); Indian Standards Institution: New Delhi, India, 1984; p. 12. [Google Scholar]
- ISO 2173:2003 Fruit and vegetable products. Determination of soluble solids. In Refractometric Method; International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 750:1998 Fruit and vegetable products. In Determination of Titratable Acidity; International Organization for Standardization: Geneva, Switzerland, 1998.
- (AACC) International. Approved Methods of American Association of Cereal Chemists, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Spranger, I.; Sun, B.; Mateus, A.M.; de Freitas, V.; Ricardo-da-Silva, J. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology—Volume 2, The Chemistry of Wine Stabilization and Treatments; John Wiley and Sons Ltd.: Chichester, UK, 2006; pp. 171–174. [Google Scholar]
- Demir, N.; Yioldiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Pop, E.A.; Diaconeasa, Z.M.; Fetea, F.; Bunea, A. Carotenoids, Tocopherols and Antioxidant Activity of Lipophilic Extracts from Sea Buckthorn Berries (Hippophae rhamnoides), Apricot Pulp and Apricot Kernel (Prunus armeniaca). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2015, 72, 169–176. [Google Scholar] [CrossRef]
- Cristea, E.; Sturza, R.; Jauragi, P.; Niculaua, M.; Ghendov-Moșanu, A.; Patras, A. Influence of pH and ionic strength on the color parameters and antioxidant properties of an ethanolic red grape marc extract. J. Food Biochem. 2019, 43, e12788. [Google Scholar] [CrossRef]
- Popovici, V.; Sturza, R.; Ghendov-Moșanu, A.; Soran, L.; Lung, I.; Patras, A. Influența condițiilor de extracție asupra compoziției și activității antioxidante a extractelor liposolubile de măceșe (In Romanian) (The influence of extraction conditions on the composition and antioxidant properties of rose hip liposoluble extracts). Meridian Ing. 2018, 1, 23–27. [Google Scholar]
- Wheat Bread Flour; GOST 27669-88; Method for Experimental Laboratory Breadmaking: Euro-Asian Council for Standardization, Metrology and Certification (EASC): Moscow, Russia, 1988.
- SR 91:2007 Bread and bakery products. Methods of analysis. In Pâine și Produse de Patiserie. Metode de Analiză; Romanian Standards Association ASRO: Bucharest, Romania, 2007.
- Monsen, E.R. Iron nutrition and absorption: Dietary factors which impact iron bioavailability. J. Am. Diet. Assoc. 1988, 88, 786–790. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thompson, J.M.; Waites, W.M.; Dodd, C.E.R. Detection of rope spoilage in bread caused by Bacillus species. J. Appl. Microbiol. 1998, 85, 481–486. [Google Scholar] [CrossRef]
- Fellin, W. Analyzying Uncertainty in Engineering; Springer: Berlin, Germany, 2005. [Google Scholar]
- Scafetta, N. An Entropic Approach to the Analysis of Time Series. Ph.D. Thesis, University of North Texas, Denton, TX, USA, 2001. [Google Scholar]
Sample Availability: Samples are available from the first author. |

| Indicator | Value |
|---|---|
| Moisture content, % | 13.3 ± 0.1 |
| Ash content, % | 0.520 ± 0.001 |
| Wet gluten content, % | 25.56 ± 0.2 |
| Acidity, degrees | 2.1 ± 0.1 |
| Sea Buckthorn Berry Flour Characteristics | Value |
|---|---|
| Moisture content, % | 7.80 ± 0.20 |
| Ascorbic acid, mg/100 g | 352.5 ± 23.4 |
| Total polyphenols (Folin-Ciocalteu), mg GAE/100 g | 1467 ± 471 |
| Total polyphenols (Abs280), mg GAE/100 g | 1311 ± 105 |
| Total flavonoids, mg GAE/100 g | 555 ± 61 |
| Cinnamic acids, mg CAE/100 g | 425 ± 34 |
| Flavonols, mg QE/100 g | 668 ± 33 |
| Total carotenoids, mg/100 g | 34.93 ± 1.30 |
| ABTS Antioxidant activity, mmol TE/100 g | 7.64 ± 0.41 |
| DPPH Antioxidant activity, % | 67.99 ± 1.20 |
| Catechin, mg/100 g | 35.3 ± 5.1 |
| Hyperoside, mg/100 g | 23.6 ± 12.1 |
| Chlorogenic acid, mg/100 g | 11.1 ± 6.3 |
| Cis-resveratrol, mg/100 g | 10.8 ± 7.5 |
| Trans-resveratrol, mg/100 g | 10.4 ± 0.4 |
| Ferulic acid, mg/100 g | 10.3 ± 1.6 |
| Protocatechuic acid, mg/100 g | 7.0 ± 0.9 |
| Procyanidin B2, mg/100 g | 4.3 ± 1.7 |
| Epicatechin, mg/100 g | 2.5 ± 1.8 |
| Gallic acid, mg/100 g | 2.2 ± 0.5 |
| Procyanidin B1, mg/100 g | 1.6 ± 0.2 |
| Quercetin, mg/100 g | 0.9 ± 0.8 |
| p-hydroxybenzoic acid, mg/100 g | 0.8 ± 0.2 |
| Syringic acid, mg/100 g | 0.7 ± 0.3 |
| m-hydroxybenzoic acid, mg/100 g | 0.5 ± 0.1 |
| Vanillic acid, mg/100 g | 0.5 ± 0.2 |
| p-coumaric acid, mg/100 g | 0.3 ± 0.2 |
| Caffeic acid, mg/100 g | 0.2 ± 0.0 |
| Sinapic acid, mg/100 g | nd |
| Polydatine, mg/100 g | nd |
| Salicylic acid, mg/100 g | nd |
| Ferulic acid methyl ester, mg/100 g | nd |
| Gentisic acid, mg/100 g | nd |
| Control | 1% SBBF | 3% SBBF | 5% SBBF | |
|---|---|---|---|---|
| Product shape and volume | 4.00 ± 0.00 c | 4.00 ± 0.00 c | 3.34 ± 0.01 b | 3.20 ± 0.05 a |
| Crust appearance and color | 4.00 ± 0.00 c | 4.00 ± 0.00 c | 3.72 ± 0.02 b | 3.58 ± 0.03 a |
| Baking degree, state and appearance of bread core | 5.80 ± 0.02 b | 6.00 ± 0.00 c | 6.00 ± 0.00 c | 5.60 ± 0.04 a |
| Bread core porosity and pore structure | 6.00 ± 0.00 c | 6.00 ± 0.00 c | 5.30 ± 0.06 b | 5.10 ± 0.07 a |
| Aroma | 4.00 ± 0.00 c | 4.00 ± 0.00 c | 3.60 ± 0.04 b | 3.20 ± 0.07 a |
| Taste | 6.00 ± 0.00 c | 6.00 ± 0.00 c | 5.40 ± 0.03 b | 5.20 ± 0.05 a |
| Total score on organoleptic | 29.80 ± 0.20 c | 30.00 ± 0.00 c | 27.36 ± 0.24 b | 25.88 ± 0.31 a |
| Bread Quality Parameters | Control | 1% SBBF | 3% SBBF | 5% SBBF |
|---|---|---|---|---|
| Moisture content, % | 42.0 ± 0.32 a | 42.5 ± 0.28 a | 43.2 ± 0.30 b | 43.7 ± 0.25 b |
| Acidity, degrees. | 1.2 ± 0.1 a | 2.4 ± 0.2 b | 3.2 ± 0.2 c | 4.7 ± 0.2d |
| Porosity, % | 72.3 ± 1.4 a | 72.7 ± 1.3 a | 68.2 ± 1.2 b | 59.7 ± 1.5 c |
| Bread specific volume, cm3/100 g | 237 ± 18 a | 247 ± 14 a | 195 ± 12 b | 181 ± 10 b |
| Storage Time of Wheat Bread Samples Before Appearance of Rope Spoilage, Hours | Control | 1.0% SBBF | 3.0% SBBF | 5.0% SBBF |
|---|---|---|---|---|
| 24 | − | − | − | − |
| 48 | − | − | − | − |
| 72 | + | − | − | − |
| 96 | ++ | + | − | − |
| 120 | +++ | +++ | + | − |
| 144 | +++ | +++ | ++ | + |
| Sensory Characteristic | Scale | Product Description | Points |
|---|---|---|---|
| Shape and volume | 0...4 | Correct shape, symmetrical, aesthetic. Volume well developed. Non-flattened or bulged. | 4 |
| Correct shape, but asymmetrical. Volume well developed. | 2 | ||
| Deformed shape. | 0 | ||
| Crust appearance and color | 0...4 | Beautiful yellowish crust for white bread. Uniform color; smooth, glossy crust surface, without defects. Crispy crust. | 4 |
| Uniformly browned crust, too dark or too pale parts, rough surface. Cracks less than 1 cm wide and less than 5 cm long. Not crispy and slightly soft crust. | 2 | ||
| Whitish crust or too browned parts larger than ¼ of the wrinkled surface or dirty crust. Cracks 1 cm wide and 5 cm long. | 0 | ||
| Baking degree, state and appearance of bread core | 0...6 | Well baked. Very elastic core, uniform color, not crumbly. | 6 |
| Sufficiently baked. Soft crust. Medium elastic core, not crumbly. | 3 | ||
| Unbaked dough residues. Not elastic core, crumbly. | 0 | ||
| Bread core porosity and pore structure | 0...6 | Uniform core porosity, fine (fluffy) pore structure, maximum two holes of maximum 1 cm2 are present in section. | 6 |
| Uniform core porosity, fine (fluffy) pore structure, pores of maximum size 1 cm2 are present in section. | 4 | ||
| Uneven core porosity, maximum four gaps of approx. 2 cm2 are present in section. | 2 | ||
| Large holes in the section and very low porosity. | 0 | ||
| Aroma | 0...4 | Pleasant pronounced aroma of well fermented and well baked bread. | 4 |
| Mild aroma, without any foreign notes. | 2 | ||
| No aroma. Foreign odors are present. | 0 | ||
| Taste | 0...6 | Pleasant, mildly sour-sweet, characteristic for every assortment | 6 |
| Relatively good, satisfactory taste. | 4 | ||
| Slightly unpleasant, sour, flat or salty taste | 2 | ||
| Pronounced sour, flat or salty taste. | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Padureanu, S.; Deseatnicova, O.; Turculet, N.; Boestean, O.; Niculaua, M. Potential Application of Hippophae Rhamnoides in Wheat Bread Production. Molecules 2020, 25, 1272. https://doi.org/10.3390/molecules25061272
Ghendov-Mosanu A, Cristea E, Patras A, Sturza R, Padureanu S, Deseatnicova O, Turculet N, Boestean O, Niculaua M. Potential Application of Hippophae Rhamnoides in Wheat Bread Production. Molecules. 2020; 25(6):1272. https://doi.org/10.3390/molecules25061272
Chicago/Turabian StyleGhendov-Mosanu, Aliona, Elena Cristea, Antoanela Patras, Rodica Sturza, Silvica Padureanu, Olga Deseatnicova, Nadejda Turculet, Olga Boestean, and Marius Niculaua. 2020. "Potential Application of Hippophae Rhamnoides in Wheat Bread Production" Molecules 25, no. 6: 1272. https://doi.org/10.3390/molecules25061272
APA StyleGhendov-Mosanu, A., Cristea, E., Patras, A., Sturza, R., Padureanu, S., Deseatnicova, O., Turculet, N., Boestean, O., & Niculaua, M. (2020). Potential Application of Hippophae Rhamnoides in Wheat Bread Production. Molecules, 25(6), 1272. https://doi.org/10.3390/molecules25061272






