Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4
Abstract
1. Introduction
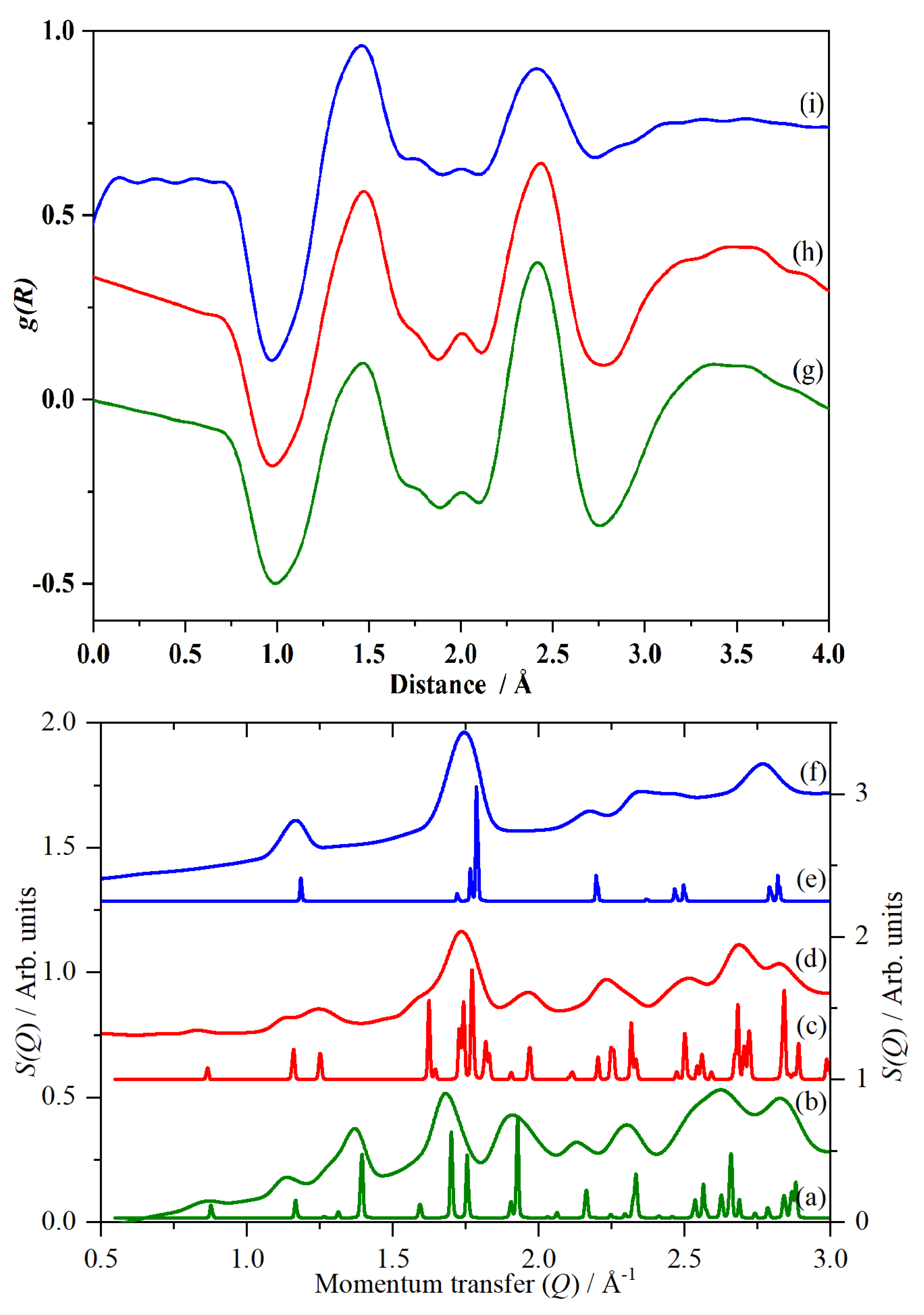
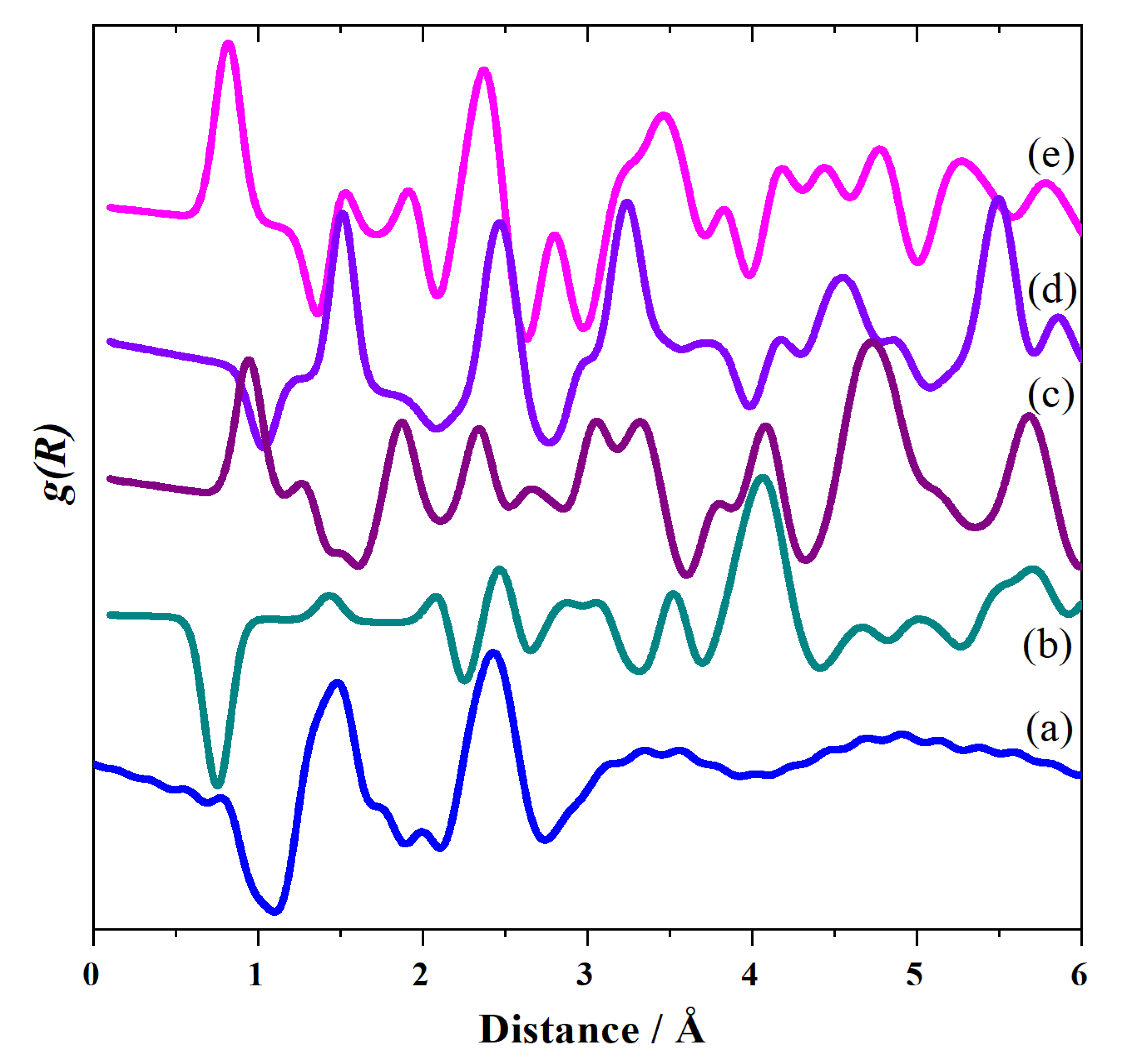
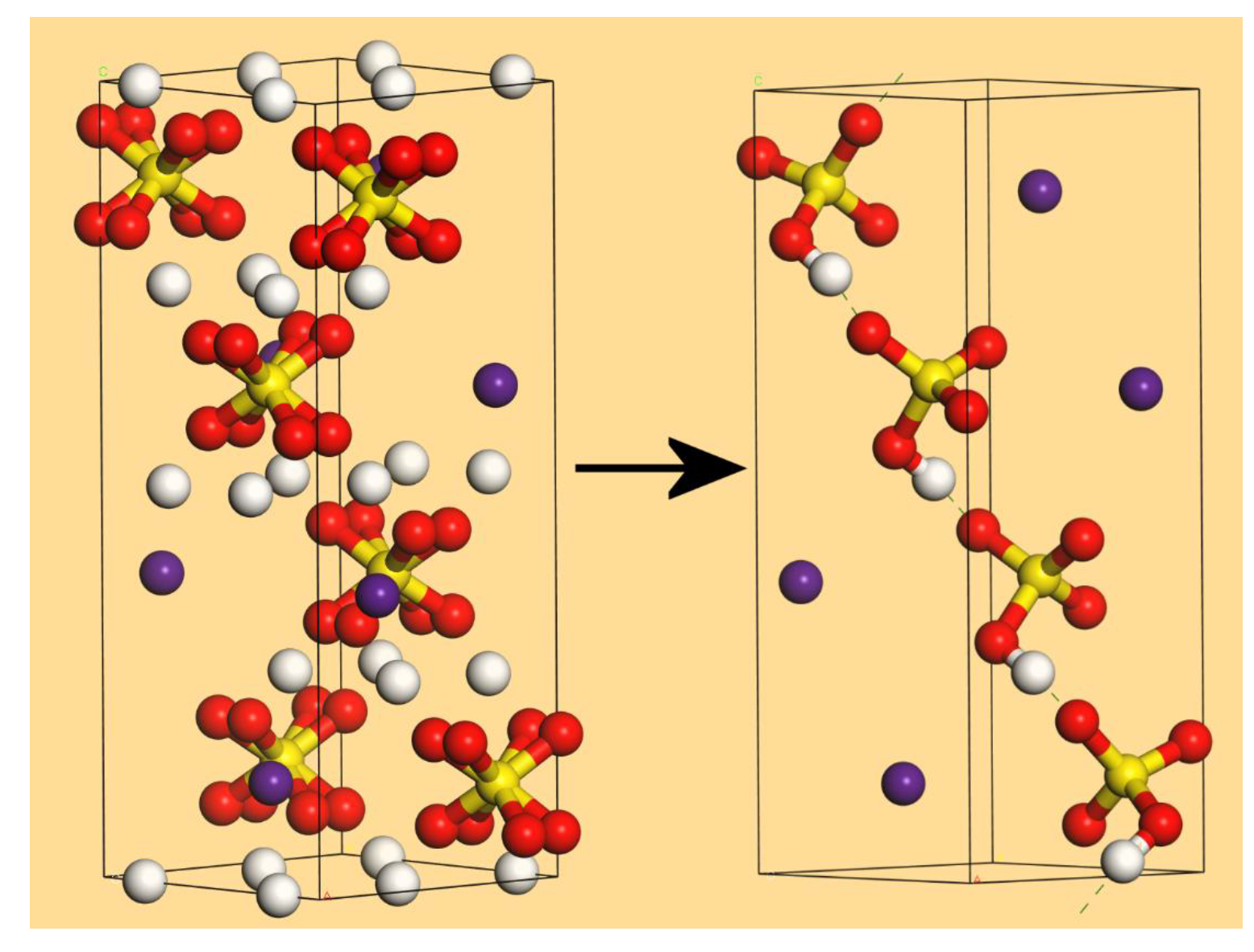
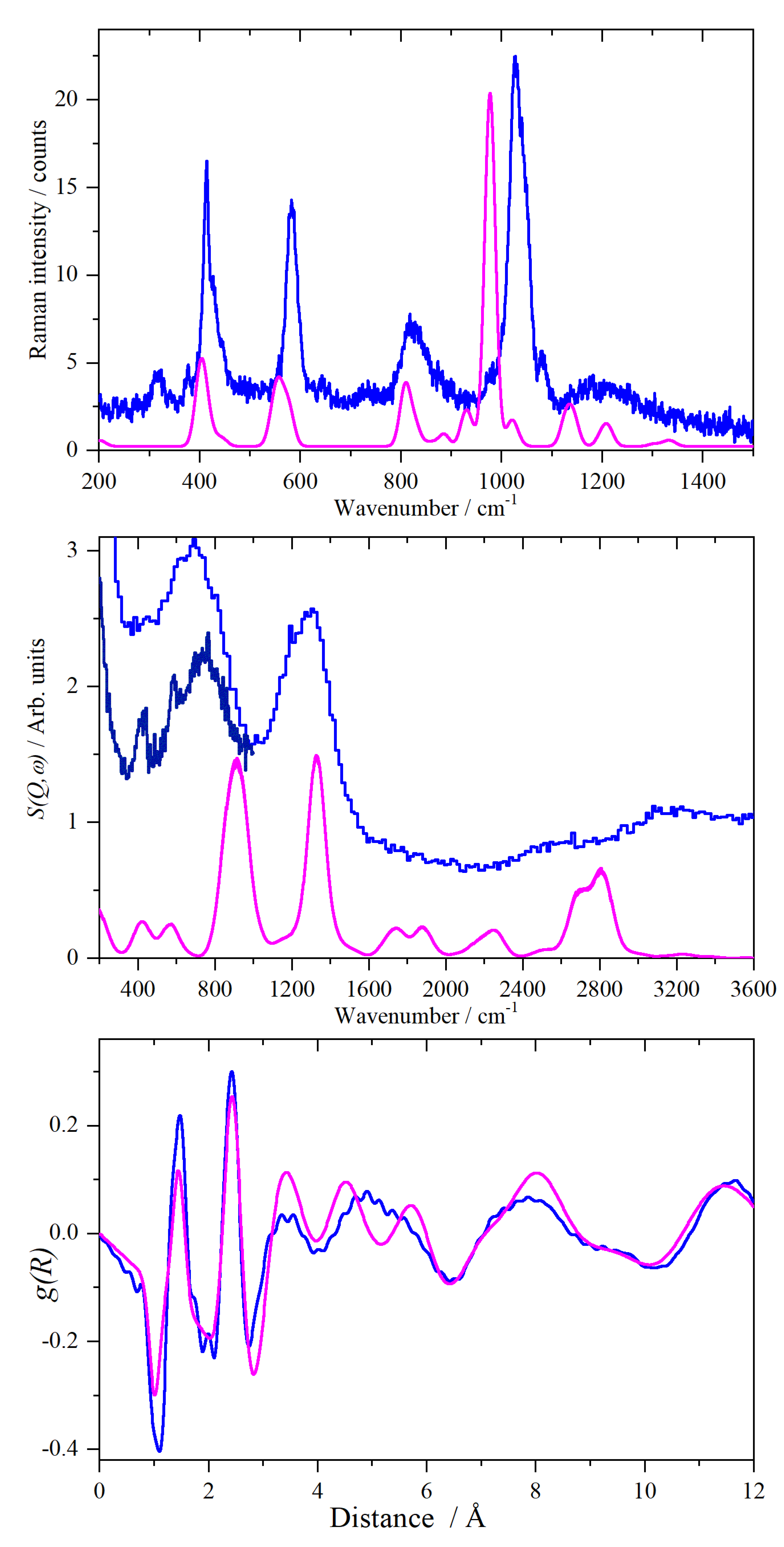
2. Results and Discussion
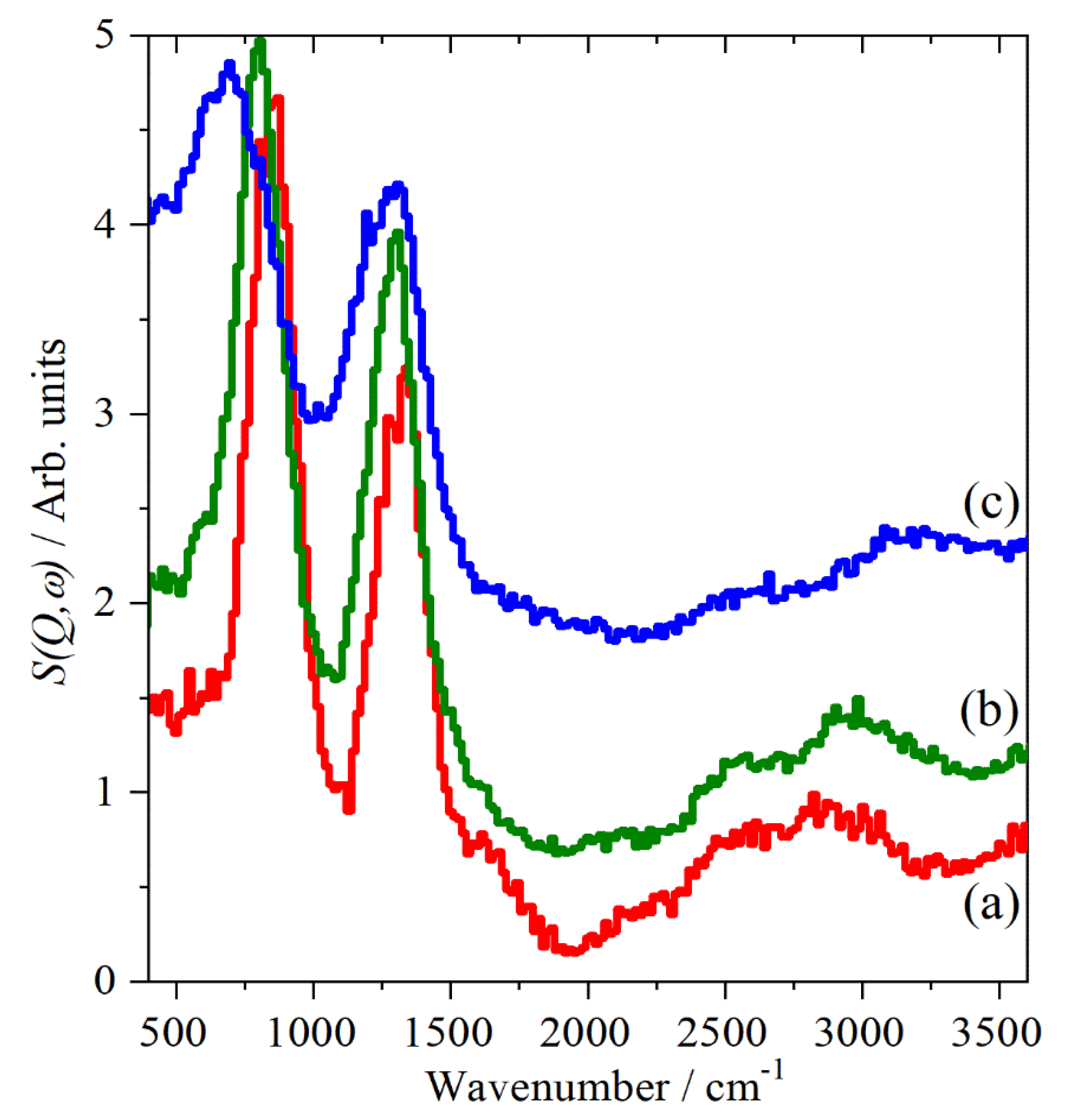
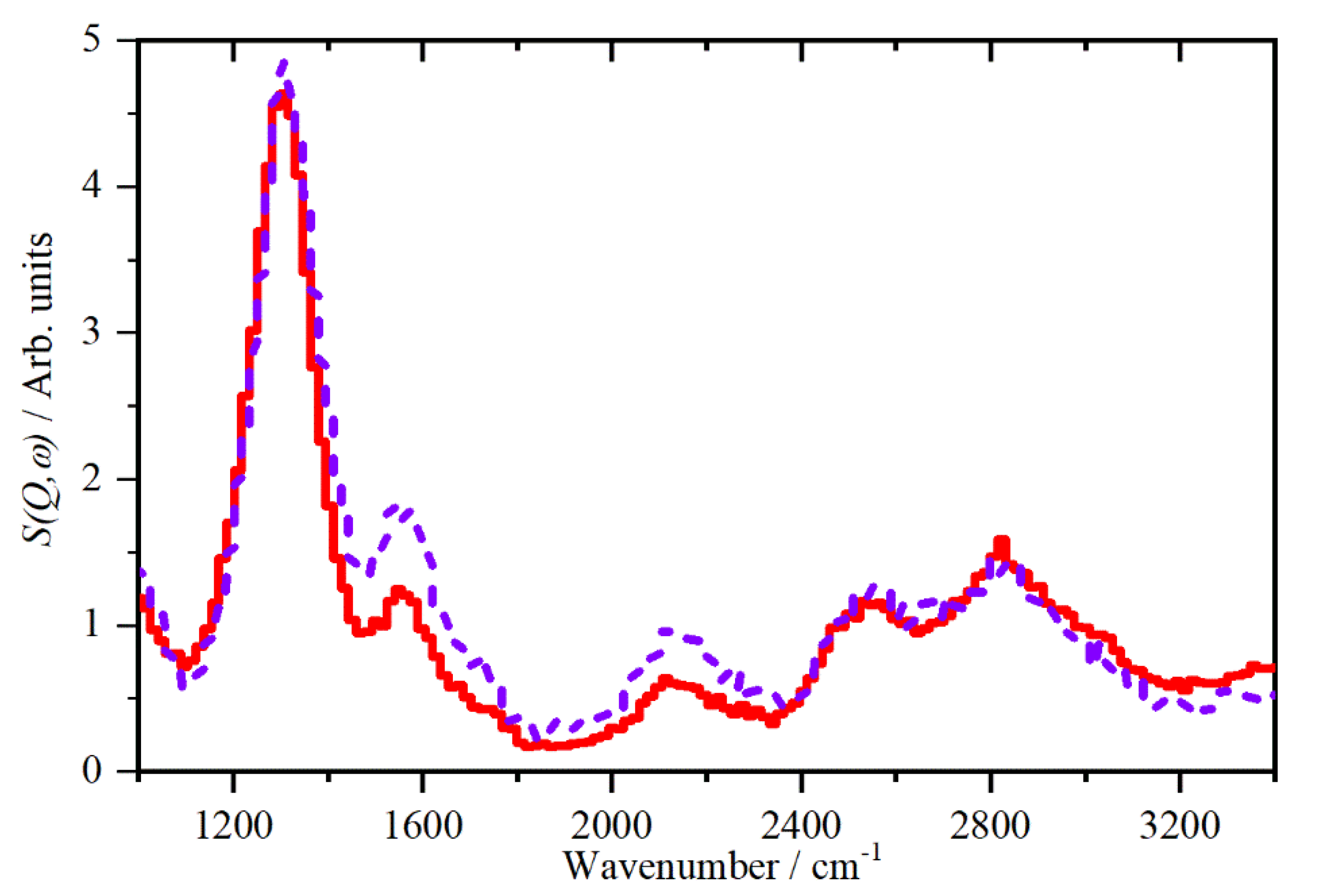
2.1. CsHSO4 Spectroscopy
2.2. CsDSO4 Spectroscopy
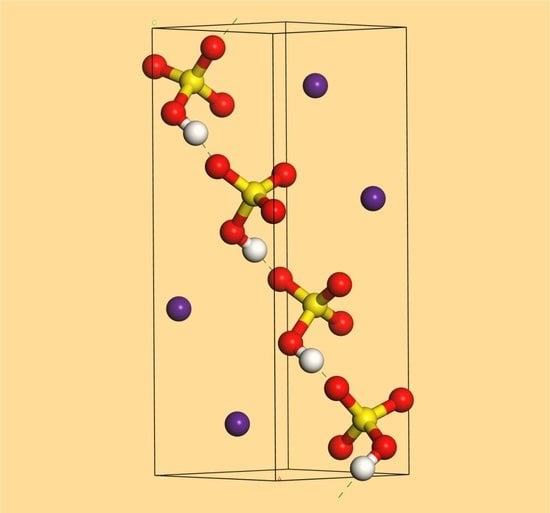
2.3. CsHSO4 Structure
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Norby, T. The promise of protonics. Nature 2001, 410, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.M.; Boysen, D.A.; Chisholm, C.R.I.; Merle, R.B. Solid acids as fuel cell electrolytes. Nature 2001, 410, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, B.; Lipkowski, J.; Lunden, A. On the phase transitions of cesium hydrogen sulfate (CsHSO4). J. Solid State Chem. 1995, 117, 412–413. [Google Scholar] [CrossRef]
- Nozik, Y.Z.; Lyakhovitskaya, L.I.; Shchagina, N.M.; Sarin, V.A. Neutron diffraction study of crystal structures of I, II, and III phases of cesium hydrogen sulfate using the full-profile analysis technique. Kristallografiya 1990, 35, 658–660. [Google Scholar]
- Balagurov, A.M.; Belushkin, A.V.; Beskrovnyi, A.I.; Vratislav, S.; Wasicki, J.; Dutt, I.D.; Dlouha, M.; Jirak, Z.; Natkaniec, I.; Savenko, B.N.; et al. Crystal data and symmetry of phases of cesium hydrogen sulfate and selenate. JINR Rapid Commun. (Dubna Russia) 1985, 13–85, 18–28. [Google Scholar]
- Itoh, K.; Ukeda, T.; Ozaki, T.; Nakamura, E. Redetermination of the structure of caesium hydrogensulfate. Acta Crystallogr. C 1990, 46, 358–361. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Adams, M.A.; Hull, S.; Shuvalov, L.A. P-T phase diagram of CsHSO4. Neutron scattering study of structure and dynamics. Solid State Ion. 1995, 77, 91–96. [Google Scholar] [CrossRef]
- Belushkin, A.V.; David, W.I.F.; Ibberson, R.M.; Shuvalov, L.A. High-resolution neutron powder diffraction studies of the structure of CsDSO4. Acta Crystallogr. B 1991, 47, 161–166. [Google Scholar] [CrossRef]
- Jirák, Z.; Dlouhá, M.; Vratislav, S.; Balagurov, A.M.; Beskrovnyi, A.I.; Gordelii, V.I.; Datt, I.D.; Shuvalov, L.A. A neutron diffraction study of the superioic phase of CsHSO4. Phys. Status Solidi A 1987, 100, K117–K122. [Google Scholar] [CrossRef]
- Merinov, B.V.; Baranov, A.I.; Shuvalov, L.A.; Maksimov, B.A. Crystal structure of superionic phase CsDSO4 and phase transitions in cesium hydro-and deuterosulfates (selenates). Kristallografiya 1987, 32, 86–92. [Google Scholar]
- Merinov, B.V. Localization of hydrogen atoms in protonic conductors with a dynamically disordered network of hydrogen bonds: Effect of anomalous manifestation of hydrogen atoms on electron-density maps. Crystallogr. Rep. 1997, 42, 906–917. [Google Scholar]
- Yamawaki, H.; Fujihisa, H.; Sakashita, M.; Honda, K. Vibrational spectra of CsHSO4 at high pressure and high temperature. Phys. Rev. B 2007, 75, 094111. [Google Scholar] [CrossRef]
- Egami, T.; Billinge, S.J.L. Underneath the Bragg Peaks, Structural Analysis of Complex Materials, 2nd ed.; Pergamon: Oxford, UK, 2012. [Google Scholar]
- Bennett, E.L.; Murphy, P.J.; Imberti, S.; Parker, S.F. Characterisation of the hydrides in Stryker’s reagent: [HCu{P(C6H5)3}]6. Inorg. Chem. 2014, 53, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.F.; Bowron, D.T.; Imberti, S.; Soper, A.K.; Refson, K.; Lox, E.S.; Lopez, M.; Albers, P. Structure determination of adsorbed hydrogen on real catalysts. Chem. Commun. 2010, 46, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Zetterström, P.; Belushkin, A.V.; McGreevy, R.L.; Shuvalov, L.A. Structure and proton conduction in CsDSO4. Solid State Ion. 1999, 116, 321–329. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Carlile, C.J.; Shuvalov, L.A. The diffusion of protons in the superionic conductor CsHSO4 by quasielastic neutron scattering. J. Phys. Condens. Matter 1992, 4, 389–398. [Google Scholar] [CrossRef]
- Ortiz, E.; Vargas, R.A.; Mellander, B.-E. Phase behaviour of the solid proton conductor CsHSO4. J. Phys. Condens. Matter 2006, 18, 9561–9573. [Google Scholar] [CrossRef]
- Dimitriev, V.P.; Loshkarev, V.V.; Rabin, L.M.; Shuvalov, L.A.; Yuzyuk, T.I. Combinational light-scattering and transition mechanisms in cesium hydrosulfate. Kristallografiya 1986, 31, 673–677. [Google Scholar]
- Baran, J.J. Polarized infrared and Raman spectra of a CsHSO4 single crystal. J. Mol. Struct. 1987, 162, 211–228. [Google Scholar] [CrossRef]
- Baran, J.; Marchewka, M.K. Vibrational investigation of phase transitions in CsHSO4 crystal. J. Mol. Struct. 2002, 614, 133–149. [Google Scholar] [CrossRef]
- Pham-Thi, M.; Colomban, P.; Novak, A.; Blinc, R. Phase transitions in superionic protonic conductors CsHSO4 and CsHSeO4. Solid State Commun. 1985, 55, 265–270. [Google Scholar] [CrossRef]
- Colomban, P.; Pham-Thi, M.; Novak, A. Thermal history and phase transitions in the superionic protonic conductors CsHSO4 and CsHSeO4. Solid State Ion. 1986, 20, 125–134. [Google Scholar] [CrossRef]
- Colomban, P.; Pham-Thi, M.; Novak, A. Influence of thermal and mechanical treatment and of water on structural phase transitions in CsHSO4. Solid State Ion. 1987, 24, 193–203. [Google Scholar] [CrossRef]
- Varma, V.; Rangavittal, N.; Rao, C.N.R. A study of superionic CsHSO4 and Cs1-xLixHSO4 by vibrational spectroscopy and X-ray diffraction. J. Solid State Chem. 1993, 106, 164–173. [Google Scholar] [CrossRef]
- Balagurov, A.M.; Belushkin, A.V.; Dutt, I.D.; Natkaniec, I.; Plakida, N.M.; Savenko, B.N.; Shuvalov, L.A.; Wasicki, J. Neutron scattering studies on structural phase transitions of superionic conductor CsHSO4. Ferroelectrics 1985, 63, 59–67. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Adams, M.A.; Kolesnikov, A.I.; Shuvalov, L.A. Lattice dynamics and effects of anharmonicity in different phases of caesium hydrogen sulphate. J. Phys. Condens. Matter 1994, 6, 5823–5832. [Google Scholar] [CrossRef]
- Krzystyniak, M.; Druzbicki, K.; Fernandez-Alonso, F. Nuclear dynamics in the metastable phase of the solid acid caesium hydrogen sulfate. Phys. Chem. Chem. Phys. 2015, 46, 31287–31296. [Google Scholar] [CrossRef]
- Mitchell, P.C.H.; Parker, S.F.; Ramirez-Cuesta, A.J.; Tomkinson, J. Vibrational Spectroscopy with Neutrons, with Applications in Chemistry, Biology, Materials Science and Catalysis; World Scientific: Singapore, 2005. [Google Scholar]
- Parker, S.F.; Lennon, D.; Albers, P.W. Vibrational spectroscopy with neutrons: A review of new directions. Appl. Spectrosc. 2011, 65, 1325–1341. [Google Scholar] [CrossRef]
- Parker, S.F.; Lennon, D. Applications of neutron scattering to heterogeneous catalysis. J. Phys. Conf. Ser. 2016, 746, 012066. [Google Scholar] [CrossRef]
- Tomkinson, J.; Parker, S.F.; Lennon, D. No evidence for Evans’ holes in the A, B, C vibrational structure of potassium dihydrogen arsenate. J. Chem. Phys. 2010, 133, 034508. [Google Scholar] [CrossRef]
- Parker, S.F. The role of hydroxyl groups in low temperature carbon monoxide oxidation. Chem. Commun. 2011, 47, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Fernandez-Alonso, F.; Parker, S.F.; Cutler, D.J.; King, A. Simultaneous neutron scattering and Raman scattering. Appl. Spectrosc. 2009, 63, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Colognesi, D.; Celli, M.; Cilloco, F.; Newport, R.J.; Parker, S.F.; Rossi-Albertini, V.; Sacchetti, F.; Tomkinson, J.; Zoppi, M. TOSCA neutron spectrometer; the final configuration. Appl. Phys. A 2002, 74, S64–S66. [Google Scholar] [CrossRef]
- Albers, P.W.; Glenneberg, J.; Refson, K.; Parker, S.F. IINS study of the molecular properties of pure hydrogen peroxide and its water mixtures of different concentration. J. Chem. Phys. 2014, 140, 16450. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.K.; Callison, J.; Winfield, J.M.; Carr, R.H.; Eaglesham, A.; Sutherland, A.; Parker, S.F.; Lennon, D. Spectroscopic characterisation of model compounds, reactants and byproducts connected with an isocyanate production chain. Ind. Eng. Chem. Res. 2018, 57, 7355–7362. [Google Scholar] [CrossRef]
- Mazzei, L.; Perrichon, A.; Mancini, A.; Wahnström, G.; Malavasi, L.; Parker, S.F.; Börjesson, L.; Karlsson, M. Local structure and vibrational dynamics in indium-doped barium zirconate. J. Mater. Chem. A 2019, 7, 7360–7372. [Google Scholar] [CrossRef]
- ISIS Neutron and Muon Source. Available online: http://www.isis.stfc.ac.uk/Pages/sandals.aspx (accessed on 23 January 2020).
- ISIS Neutron and Muon Source. Available online: http://www.isis.stfc.ac.uk/ (accessed on 23 January 2020).
- Chisholm, C.R.I.; Haile, S.M. Entropy evaluation of the superprotonic phase of CsHSO4: Pauling’s ice rules adjusted for systems containing disordered hydrogen-bonded tetrahedra. Chem. Mater. 2007, 19, 270–279. [Google Scholar]
- Hannon, A.C.; Howells, W.S.; Soper, A.K. ATLAS: A suite of programs for the analysis of time-of-flight neutron diffraction data from liquid and amorphous samples. Inst. Phys. Conf. Ser. 1990, 107, 193–211. [Google Scholar]
- Farrow, C.L.; Juhás, P.; Liu, J.W.; Bryndin, D.; Božin, E.S.; Bloch, J.; Proffen, T.; Billinge, S.J.L. PDFfit2 and PDFgui: Computer programs for studying nanostructure in crystals. J. Phys. Condens. Matter 2007, 19, 335219. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist.-Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational density-functional perturbation theory for dielectrics and lattice dynamics. Phys. Rev. B 2006, 73, 155114. [Google Scholar] [CrossRef]
- Milman, V.; Perlov, A.; Refson, K.; Clark, S.J.; Gavartin, J.; Winkler, B. Structural, electronic and vibrational properties of tetragonal zirconia under pressure: A density functional theory study. J. Phys. Condens. Matter 2009, 21, 485404. [Google Scholar] [CrossRef] [PubMed]
- Refson, K. Phonons and Related Calculations in CASTEP. Available online: http://www.castep.org/ (accessed on 23 January 2020).
- Dassault Systèmes BIOVIA. Available online: https://www.3dsbiovia.com/products/collaborative-science/biovia-materials-studio/ (accessed on 23 January 2020).
- Ramirez-Cuesta, A.J. aCLIMAX 4.0.1, The new version of the software for analyzing and interpreting INS spectra. Comput. Phys. Commun. 2004, 157, 226–238. [Google Scholar] [CrossRef]
- Dymkowski, K.; Parker, S.F.; Fernandez-Alonso, F.; Mukhopadhyay, S. AbINS: The modern software for INS interpretation. Phys. B 2018, 551, 443–448. [Google Scholar] [CrossRef]


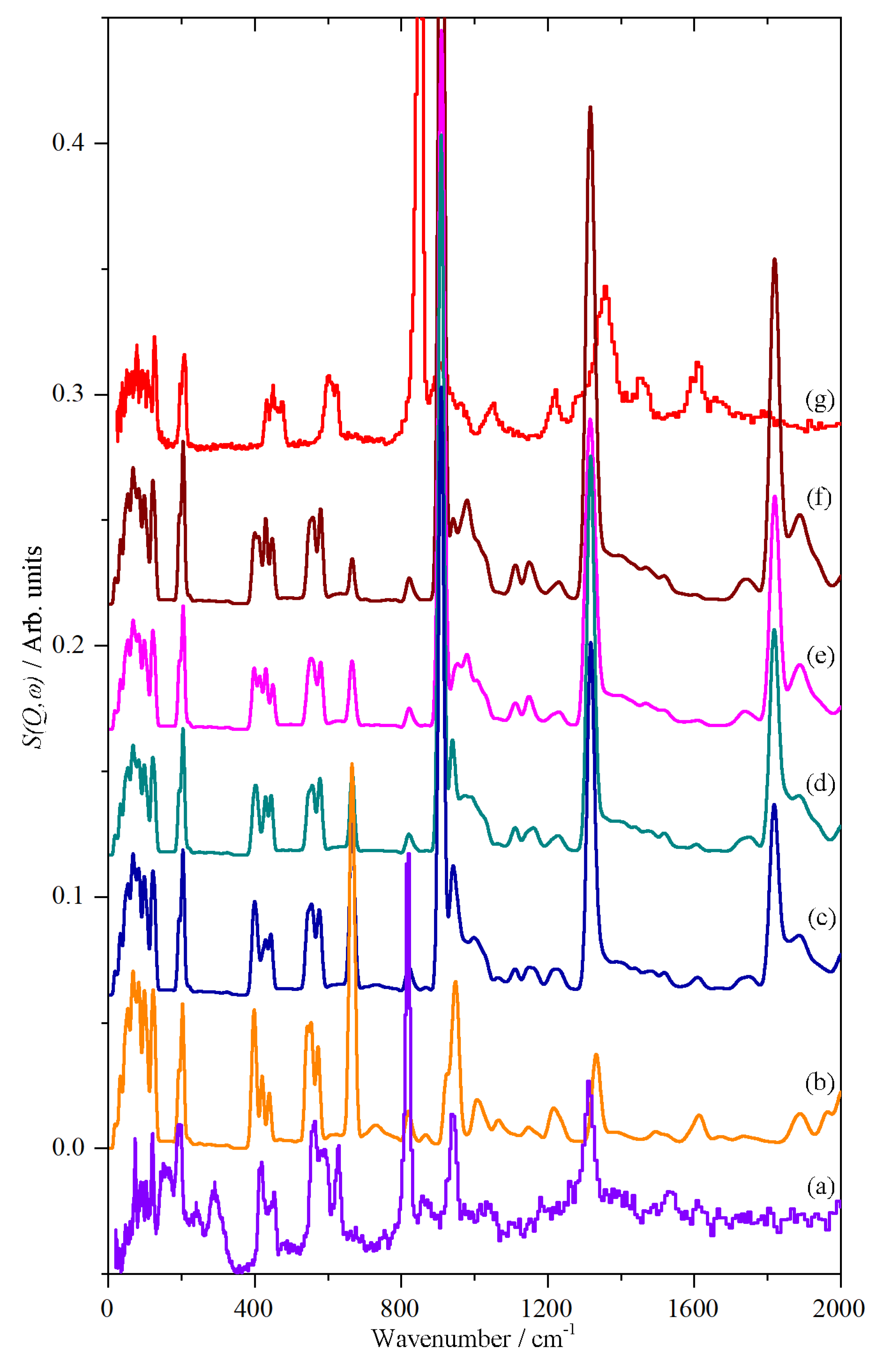
| Phase III | Phase II | Phase I | Assign 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cal 1 | INS 2 | Raman | Cal | INS | Raman | Cal | INS | Raman | |
| 10 K | 10 K | 10 K | 10 K | 430 K | 430 K | ||||
| 0–96 | 32–88 w | 0–96 | 25–1010 w | 0–93 | Translations | ||||
| 102 | 107 m | 103 | 105 w | 101 | HSO4 Libration | ||||
| 116 | 120 w | 123 | 123 w | 117 | HSO4 Libration | ||||
| 219 | 202,212,218 w,w,w | 221 w | 199 | 192,203 sh,w | 194 | HSO4 Libration | |||
| 399 | 398 | 404 | 414 s | ν2 O–S–O bend | |||||
| 441 | 421 s | 432 | 425,441,462 m,m,m | 415,430 s,s | 426 | 430 m | 432 sh | ν2 O–S–O bend | |
| 547 | 549 | 552 | ν4 O–S–O bend | ||||||
| 560 | 572,589 m,m | 570,589 m,s | 561 | 590 m | 579,587 m,s | 561 | ν4 O–S–O bend | ||
| 593 | 606,614 m,m | 605,619 m,m | 580 | 600,612 m,m | 601 s | 577 | 582 s | ν4 O–S–O bend | |
| 822 | 866 s | 821 | 863 s | 818 | 823 m,br | S–OH stretch | |||
| 942 | 865,902 vs,s | 913 | 830 vs | 904 | 746 s | S–O–H oop bend | |||
| 984 | 995 vs | 1006 | 1032 w | 1016 vs | 1011 | 1027 vs | ν1 S–O sym stretch | ||
| 1133 | 1178 s | 1146 | 1173 m | 1140 | 1177 w,br | ν3 S–O asym stretch | |||
| 1252 | 1255 m | 1215 | 1256 m | 1210 | 1210 w,br | ν3 S–O asym stretch | |||
| 1372 | 1368 s | 1320 | 1332 s | 1324 | 1295 s | S–OH ip bend | |||
| 2589 | 2733 | 2757 | 3185 | O–H stretch | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, S.F.; Cavaye, H.; Callear, S.K. Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4. Molecules 2020, 25, 1271. https://doi.org/10.3390/molecules25061271
Parker SF, Cavaye H, Callear SK. Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4. Molecules. 2020; 25(6):1271. https://doi.org/10.3390/molecules25061271
Chicago/Turabian StyleParker, Stewart F., Hamish Cavaye, and Samantha K. Callear. 2020. "Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4" Molecules 25, no. 6: 1271. https://doi.org/10.3390/molecules25061271
APA StyleParker, S. F., Cavaye, H., & Callear, S. K. (2020). Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4. Molecules, 25(6), 1271. https://doi.org/10.3390/molecules25061271