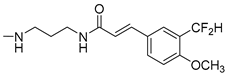
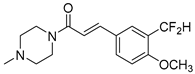
Abstract
Series of novel amides of isoferulic acid, where the phenolic hydroxyl was replaced by a difluoromethyl group, were synthesized and their in vitro antibacterial activities assayed against fourteen bacterial strains (six Gram-positive and eight Gram-negative). A one-pot methodology was developed to obtain the 3′-(difluoromethyl)-4′-methoxycinnamoyl amides using Deoxofluor® as a fluorinating agent. The N-isopropyl, N-isopentyl, and N-(2-phenylethyl) amides 11b, 11d and 11g were the most active and selective against Mycobacterium smegmatis (MIC = 8 µg/mL) with 11b and 11g displaying negligible or no cytotoxicity against HepG2 and A549 cells. Thirteen analogs of N-isopropylamide 11b were also synthesized and their antibacterial activity assayed. Results show that the difluoromethyl moiety enhanced antibacterial activity and selectivity towards M. smegmatis, changing the microorganism inhibition profile of the parent compound. The selectivity exhibited by some of the compounds towards M. smegmatis makes them potential leads in the search for new narrow spectrum antibiotics against M. tuberculosis.
1. Introduction
In the last 20 years, antimicrobial resistance has been recognized as a serious public health problem. The increased resistance of pathogenic microorganisms is related to the misuse/abuse of antibiotics and to their natural adaptation and evolution to marketed antimicrobial compounds (e.g., via biofilm formation, gene transfer from resistant counterparts, efflux pumps, cellular permeability, enzymes that confer resistance, and natural evolutionary mutations) [1,2,3]. This dangerous rise of pathogenic bacteria that are resistant to existing antibiotics constitutes a global human health threat and requires a continuous search for new chemical entities [4,5]. Broad spectrum antibiotics, effective with a wide range of pathogens, are important for first line treatment of bacterial infections as well as for prevention in risk situations (e.g., surgical procedures, organ transplant, etc.). Their use however damages the gut microbiota and favors the development of resistance mechanisms that may be readily transferred across species. Hence, once the pathogen has been identified, narrow spectrum therapy is recommended to minimize these adverse effects [6]. This requires the search for narrow spectrum antibiotics as well as uncovering the factors that influence selectivity towards specific bacteria.
Hydroxycinnamic acid derivatives (HCAs) have been shown to possess in vitro antibacterial features as well as health benefits [7,8,9]. HCAs possess a hydroxylated and/or methoxylated 3-phenylpropenoyl core (C6–C3) and are one of the most plentiful and ubiquitously distributed groups of plant secondary metabolites, commonly found in a variety of dietary products such as vegetables, fruits, chocolate, and beverages. The most common HCAs found in fruits and vegetables are p-coumaric (1), caffeic (2), ferulic (3), and chlorogenic (4) acids (Figure 1). Only a minor proportion of HCAs are present in their free form, and most are found as quinic or tartaric acid derivatives (e.g., chlorogenic acid 4 or hygromycin A 5). Daily intake of HCAs ranges from 25 to 1000 mg depending on the type of diet. Some of the most abundant sources of these compounds are coffee beans and yerba mate brews [9,10]. Surprisingly, the HCAs molecular target in terms of their antibacterial activity is still unknown, but in some cases experimental evidence has proven the interaction of these types of compounds with the pathogen cell membrane [11,12,13,14].
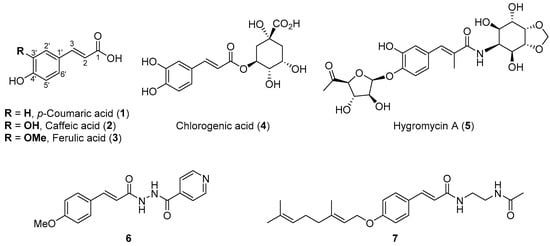
Figure 1.
Natural and synthetic HCAs showing antibacterial activity.
The bioavailability of HCAs and their metabolites is crucial for their pharmacological properties; however, HCAs exhibit poor ADME (absorption, distribution, metabolism, and excretion) properties showing a rapid phase II metabolic transformation (e.g., methylation, glucuronidation, and sulfation) followed by urine and feces excretion. This leads to poor translation of in vitro features to in vivo therapeutic applications of HCAs [15]. Several attempts have been made to enhance the bioavailability and antibacterial selectivity of HCAs by means of lipophilization strategies such as amidation or esterification of the acyl moiety and functionalization of the phenolic hydroxyl with long alkyl or prenyl chains [13,16,17,18]. The latter approach has the disadvantage of poor atom economy and may also lead to self-aggregation and internalization to the lipidic core. Synthetic amides derived from HCAs have shown the most interesting antibacterial properties with several of the hydroxycinnamoyl amides and structurally related analogs being active against Mycobacterium tuberculosis including resistant strains (e.g., compounds 6 and 7, Figure 1) [19,20]. Some structural aspects relevant to the antibacterial potency and selectivity are the oxygen atom at C-4′ (free hydroxyl or alkoxy substituent) and the nitrogen atom at C-1 (amides, hydrazides, and nitrogen heterocycles) [7,8,21]. In this context, alternate approaches for increasing lipophilicity may provide access to new lead compounds with improved activities.
The difluoromethyl group has been shown to exhibit some distinctive properties that make it an interesting alternative to the widely used trifluoromethyl group when seeking to increase lipophilicity. In particular, due to its hydrogen bond donor properties, the CF2H group has been proposed as a lipophilic bioisostere of hydroxyl groups [22,23]. Recently, Zafrani and coworkers showed that, when bonded to an aryl scaffold, the difluoromethyl group effectively behaves as a lipophilic bioisoster of the phenolic OH, increasing lipophilicity while retaining significant hydrogen bonding acidity [24]. Hydrogen bonding acceptor properties of the difluoromethyl group have also been reported [25]. Previously, we also found an interesting bioisosteric relationship in the antioxidant behavior between difluoromethyl substituted arenes and phenols. Thus, replacement of a phenolic hydroxyl by a difluoromethyl moiety on hydroxycinnamic acid methyl ester scaffolds conferred radical scavenging ability only in lipophilic environments, even in the absence of free phenol groups, probably due to a hydrogen atom transfer mechanism [26]. We envisaged that the combination of this lipophilicity-booster fluorinated moiety together with the amidation strategy applied to HCAs could lead to analogs with enhanced ADME properties, with the added possibility of perturbing the bacterial redox homeostasis beyond the effects over the bacterial cell wall. Here, we report the synthesis of a series of difluoromethyl substituted methoxycinnamoyl amides and their biological evaluation against a panel of fourteen relevant microorganisms (Gram-positive and Gram-negative bacteria).
2. Results and Discussion
Our initial approach was to introduce a difluoromethyl moiety at the C-3′ position of a 4′-methoxycinnamoyl amide core and to evaluate the effect of the resulting analogs of isoferulic acid (8) on antibacterial activity (Figure 2). The difluoromethyl moiety was expected to enhance the lipophilicity while retaining, at least in part, the hydrogen bonding donor/acceptor capacity of the phenolic hydroxyl as well as its radical scavenging properties. The 4′-methoxy group was expected to provide the required oxygen atom with metabolic stability and to prevent formation of an o-quinomethane [27], while the acyl amide would confer antibacterial potency and selectivity, as well as allow fine tuning of drug-like properties by modifying the substituents on the nitrogen atom. This approach does not introduce major steric changes in the aromatic ring vicinity compared with caffeic acid and related HCAs.
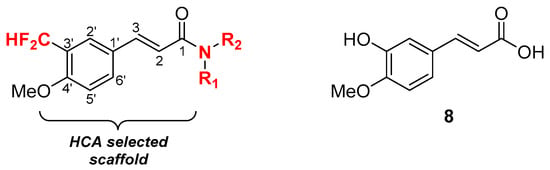
Figure 2.
Proposed modifications (in red) to the coumaric acid (1) scaffold and analogy with isoferulic acid (8).
2.1. -CF2H at C-3′ on the 4′-methoxycinnamyl Amide Scaffold
The synthetic strategy (Scheme 1) aimed to access, in a straightforward fashion, a variety of amides with the general structure 11. Commercially available 2-methoxybenzaldehyde was iodinated with iodine and silver nitrate in methanol and the resulting 5-iodo-2-methoxybenzaldehyde was then coupled with methyl acrylate under Heck reaction conditions using palladium acetate and tri(o-tolyl)phosphine as a ligand [28,29] to give the cinnamic acid derivative 9 in 73% isolated yield after alkaline hydrolysis. The structure of compound 9 was confirmed by 1H- and 13C-NMR spectra that showed resonances at δH/δC 12.35/167.6 for the carboxylic acid and at δH/δC 10.33/188.9 for the aldehyde moiety. The side chain double bond gave signals at δH/δC 7.59/142.6 and 6.47/118.3, with a 1H-1H coupling constant of 16 Hz that confirmed the E configuration.
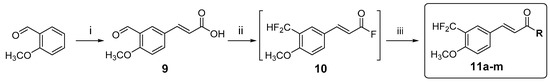
Scheme 1.
Synthesis of amides 11a–11m. Reagents and conditions: (i) (1) AgNO3, I2, MeOH, (2) Pd(AcO)2, (o-Tol)3P, methyl acrylate, Et3N, MeCN, and (3) K2CO3, MeOH-H2O; (ii) Deoxofluor®, PhCH3-CH2Cl2; and (iii) R1R2NH or R1R2NH·HX (see Table 1 for details).
The key step in this synthetic approach was the double deoxofluorination of the cinnamic acid derivative 9 to give 3′-difluoromethyl-4′-methoxycinnamoyl fluoride (10) and its subsequent conversion to amides 11a–11m in a one pot procedure (Table 1). At this stage, Deoxofluor® was chosen as the fluorinating reagent, as initial attempts to use XtalFluor-E® were unsuccessful due to long reaction times and undesired byproducts [30]. However, one drawback of using Deoxofluor® is the formation of N,N-bis(2-methoxyethyl)amine as a byproduct that can react with the acyl fluoride (Table 1, Method B), thus limiting the scope of the method to amines that are more nucleophilic than N,N-bis(2-methoxyethyl)amine [31,32,33,34]. We found that this undesired condensation could be avoided by filtering the reaction mixture through a short silica gel pad. The resulting solution containing the acyl fluoride 10 was concentrated and treated directly with the desired amine and triethylamine as a base (Table 1, Methods A and D). The intermediate acyl fluoride could also be isolated upon evaporation of the solvent and was characterized by 1H and 13C-NMR. This methodology allowed for the synthesis of primary and secondary amides and was also applicable to aromatic amines (e.g., aniline). Amines could be used either as free bases or as their salts (e.g., hydrochlorides and acetates), and even the highly unreactive amine 2,5-bis(trifluoromethyl)aniline gave the corresponding amide when the reaction was carried out in the presence of LDA as a base (Table 1, Method C). In the latter case, after filtration of the acyl fluoride solution, the solvent was completely removed under reduced pressure and replaced by THF. In contrast with previous reports [31,33], this procedure allows for the coupling of a wide variety of amines (either free or as their salts) with acyl fluorides, employing Deoxofluor® as the fluorinating agent. The procedure was easily scalable allowing the synthesis of several amides in parallel. Following this procedure, 13 amides of 3′-difluoromethyl-4′-methoxycinnamic acid (11a–11m) were synthesized in 46–84% isolated yields (Table 1). Structures were confirmed by 1H and 13C-NMR (1D and 2D) and mass spectrometry. Particularly diagnostic were the shifts of the -CF2H group at ca. δH 6.9 (triplet, JHF = 55.5 Hz) and δC 111.3 (triplet, JCF = 236 Hz).

Table 1.
Synthesis of 3′-difluoromethyl-4′-methoxycinnamoyl amides 11a–11m.
The series of amides 11a–11m was screened for antibacterial activity against a panel of 14 microorganisms: six Gram-positive and eight Gram-negative bacteria. The most susceptible microorganism was the Gram-positive bacillus Mycobacterium smegmatis (Table 2) with compounds 11b, 11d and 11g being the most promising candidates in terms of potency (MICs = 8 µg/mL) and selectivity. It has been shown that M. smegmatis can be used as a non-pathogenic model for M. tuberculosis in antibacterial screening, as activity against M. smegmatis usually results in activity against M. tuberculosis [35]. Marginal bacterial growth inhibition was detected for other compounds in the series against various microorganisms such as Streptococcus pyogenes, Streptococcus pneumoniae, Clostridum sporogenes, Pseudomona aeruginosa, and Acinetobacter guillouiae (MICs = 64 µg/mL). To carry out preliminary SAR studies, compound 11b was chosen as the better candidate due to its potency, selectivity towards M. smegmatis, and atom economy.

Table 2.
Antibacterial activity of compounds 11a–11m, 13a–13c, 15, 18a–18b, and 19–23 1.
2.2. Structure Activity Relationship Studies (SAR) on Compound 11b
To evaluate the influence of the 3′-CF2H moiety on antibacterial activity, we synthesized compounds 13a–13c by condensation of the corresponding acids 12a, 12b, or 8 and isopropylamine using (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP) as the activating agent (Scheme 2) [36]. While the bioisosteric replacement of a hydroxyl group by CF2H takes advantage of the hydrogen bonding donor (and possibly acceptor) ability of the CF2H to mimic the hydroxyl, replacement of a methyl group by CF2H makes use of the fluorine as a bioisostere of hydrogen due to its relatively small size. The CF2H has been shown to increase lipophilicity relative to the hydroxyl group (thus the lipophilic hydroxyl analogy) but to reduce lipophilicity when compared to the methyl analog [24].
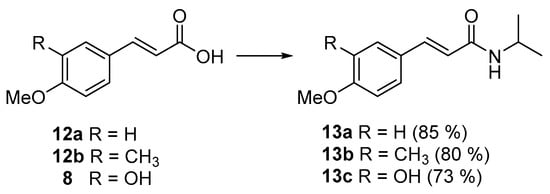
Scheme 2.
Synthesis of amides 13a–c. Reagents and conditions: BOP, Et3N, CH2Cl2, i-PrNH2, 0–30 °C.
Both the non-functionalized counterpart 13a and the 3′-methyl analog 13b were only moderately active against M. smegmatis, with a four-fold activity decrease compared to the difluoromethylated analog 11b (see Table 2). On the other hand, the isoferulic acid amide 13c with a phenolic hydroxyl at position 3′ showed a different activity profile, namely that it was inactive against M. smegmatis but moderately active against the Gram-negative bacteria Shigella boydii and Shigella flexneri. These results suggest that the balance between lipophilicity (see Table S1) and hydrogen bond donor/acceptor capacities of the CF2H moiety is a significant contributor to the observed activity and selectivity towards M. smegmatis of the N-isopropylamide derivative. Furthermore, to analyze the aromatic ring substitution pattern, the regioisomer 15 was obtained from the methyl cinnamate 14 (Scheme 3) [26]. Compound 15 presented a different antibacterial activity profile (MIC = 32 µg/mL against Clostridium sporogenes, see Table 2) compared to compound 11b, proving the importance of the substitution pattern in the selective activity against Mycobacterium smegmatis.
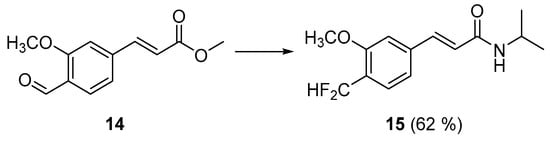
Scheme 3.
Synthesis of amide 15. Reagents and conditions: (1) K2CO3, MeOH-H2O; (2) Deoxofluor®, PhCH3-CH2Cl2; and (3) i-PrNH2, Et3N.
To evaluate the role of the methoxy group at the 4′ position of the aromatic ring, acetate 18a and the free phenol 18b were synthesized (Scheme 4). Compounds 18a and 18b showed a significant loss of potency (Table 2) and, in the case of 18a, an increased cytotoxicity (see below).
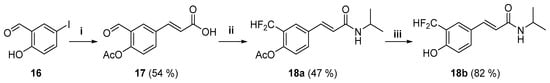
Scheme 4.
Synthesis of amides 18a and 18b. Reagents and conditions: (i) (1) Ac2O, K2CO3, acetone and (2) Pd(AcO)2, (o-Tol)3P, acrylic acid, Et3N, MeCN; (ii) (1) Deoxofluor®, PhCH3-CH2Cl2 and (2) i-PrNH2, Et3N-CH2Cl2; and (iii) H2SO4, MeOH.
We then focused our attention on the importance of the alkenoyl chain at position C1′ (i.e., hybridization, planarity, and length). Chain modified analogs 19–25 (Figure 3) were obtained with minor modifications to the synthetic strategy used for compound 11b. Briefly, the cyclopropyl analog 19 was obtained by a sequence involving a Corey–Chaykovsky reaction over the Weinreb amide 11h, followed by a Gassman’s “anhydrous hydroxide” saponification and a BOP condensation with isopropylamine [37,38]. Compound 20 was synthesized by catalytic hydrogenation of 11b. Compound 21 was obtained by replacing acrylic acid with methacrylic acid in the Heck reaction and ester 22 was obtained by treating the acyl fluoride 10 with isopropanol instead of isopropylamine. The vinylogue 23 was obtained by a Rieche formylation of commercial p-methoxybenzoic acid followed by the deoxofluorination/condensation protocol used for the synthesis of 11b [39,40]. Naphthalene derivatives 24 and 25 were obtained in a similar way as 23. Compounds 19–25 showed loss of activity and selectivity, proving the importance of the structural features of the side chain and validating the hydroxycinnamic acid scaffold as a privileged structure.
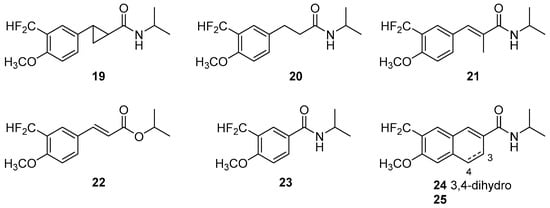
Figure 3.
Analogs of 11b with modified side chains.
Finally, all compounds active against M. smegmatis were evaluated for cytotoxicity in human lung carcinoma A549 cells and hepatoma HepG2 cells. Compound 13c was also included due to its activity against the Gram-negative bacteria S. boydii and S. flexneri (Table 3). Only compound 18a exhibited significant cytotoxicity against both cell lines, while compound 11d was moderately cytotoxic for HepG2 cells. Two of the most active compounds, 11b and 11g, had selectivity indexes of 24.9 or higher.

Table 3.
Cytotoxicity data of selected compounds on A549 and HepG2 cells.
3. Materials and Methods
3.1. General
Melting points were taken on a Fisher–Johns apparatus and are uncorrected. 1H- and 13C-NMR spectra were measured at 500.13 and 125.76 MHz, respectively, on a Bruker Avance II 500 NMR spectrometer unless noted otherwise; J values are given in Hz. All assignments were confirmed by a combination of 2D spectra (COSY, HSQC, and HMBC). Elemental analysis (C, H, and N) was performed on an Exeter Analytical Inc. CE-440 apparatus, North Chelmsford, MA, USA. The electron impact mass spectra (EIMS) were measured on a Shimadzu QP-5000 or a Thermo DSQ-II mass spectrometer at 70 eV by direct inlet. Exact mass spectra (HRMS) were measured on a Bruker micrOTOF-Q II mass spectrometer using positive electrospray ionization. Analytical thin layer chromatography (tlc) was performed on pre-coated silica gel plates (Merck F254, 0.2 mm thickness); compounds were visualized under 254 nm UV light. Flash column chromatography was performed on silica gel Merck 9385 (0.0040–0.0063 mm). All solvents were distilled and stored over 4 Å molecular sieves before use. Solvents were evaporated at ca. 45 °C under vacuum in a rotary evaporator. The homogeneity of all compounds was confirmed by tlc. Products obtained as solids or syrups were dried under high vacuum. 5-Iodo-2-methoxybenzaldehyde was obtained by iodination of 2-methoxybenzaldehyde with iodine/silver nitrate in methanol [28]. Methyl-3-[4-formyl-3-methoxyphenyl]-(E)-propenoate (14) was obtained as described previously [26]. 6-Methoxy-3,4-dihydronaphthalene-2-carboxylic acid was obtained from commercial 6-methoxytetralone [41].
3.2. Chemical Synthesis
3.2.1. 3-(3-Formyl-4-methoxyphenyl)-(E)-propenoic acid (9)
5-Iodo-2-methoxy-benzaldehyde (1.0 g, 3.82 mmol) was dissolved in 25 mL of acetonitrile and oxygen was removed by bubbling nitrogen through the solution. Triethylamine (3.71 mL, 26.7 mmol) and methyl acrylate (0.686, mL, 7.63 mmol) were added dropwise with stirring to the solution, followed by four portions of tri-o-tolylphosphine (0.0214 g, 0.095 mmol) and palladium (II) acetate (0.008 g, 0.047 mmol) at 1 h intervals. The mixture was then heated at 65–70 °C for 4 h, volatiles were removed by distillation, and the residue was dissolved in dichloromethane. The resulting solution was percolated through silica gel, eluting with a mixture of hexane–ethyl acetate (6:4). The percolate was evaporated to dryness, re-dissolved in 20 mL of methanol, and 20% aqueous potassium carbonate solution (10 mL) added. The mixture was stirred for 6 h at 20 °C and concentrated under reduced pressure to a third of its volume. The solution was acidified with conc. HCl (to pH 1) and the precipitate was filtered and recrystallized from isopropanol, to give 3-formyl-4-methoxycinnamic acid 9 as a crystalline white solid (0.472 g). A second harvest obtained by concentration of the mother liquor gave an additional 0.105 g (73%); m.p. 223–225 °C; 1H-NMR (DMSO-d6) δ: 12.35 (s, 1H, COOH), 10.33 (s, 1H, ArCHO), 8.03 (dd, J = 2.4, 8.8 Hz, 1H, 6′-H), 7.93 (d, J = 2.4 Hz, 1H, 2′-H), 7.59 (d, J = 16.0 Hz, 1H, 3-H), 7.28 (d, J = 8.8 Hz, 1H, 5′-H), 6.47 (d, J = 16.0 Hz, 1H, 2-H), 3.96 (s, 3H, CH3O); 13C-NMR (DMSO-d6) δ: 188.9 (ArCHO), 167.5 (1-C), 162.6 (4′-C), 142.6 (3-C), 135.6 (6′-C), 128.2 (2′-C), 126.9 (1′-C), 124.2 (3′-C), 118.3 (2-C), 113.4 (5′-C), 56.4 (CH3O). EIMS m/z (%): 206 (18, M+), 81 (33), 69 (100), 57 (25), 55 (33), 43 (39), 41 (58).
3.2.2. 3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenoyl fluoride (10)
A solution of Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol) was added dropwise to a suspension of 0.030 g of 3-formyl-4-methoxycinnamic acid (9, 0.030 g, 0.145 mmol) in 0.4 mL of dry dichloromethane under an argon atmosphere. The reaction mixture was stirred for 45 min at room temperature, diluted with 2 mL of dry dichloromethane and subsequently percolated through 2.5 g of silica gel under an argon atmosphere. The silica gel bed was rinsed with 8 mL of dry dichloromethane and the resulting solution was concentrated to dryness. The resulting solid was purified by flash column chromatography eluting with mixtures of ethyl acetate–hexane of increasing polarity to give 10 as a white solid (0.028 g, 84%). 1H-NMR (CDCl3, 200.13 MHz) δ: 7.80 (d, J = 16.0 Hz, 1H, 3-H), 7.79 (bs, 1H, 2′-H), 7.64 (m, 1H, 6′-H), 7.00 (m, 1H, 5′-H), 6.93 (t, J = 55.3 Hz, 1H, CF2H), 6.29 (dd, J = 7.1, 16.0 Hz, 1H, 2-H), 3.94 (s, 3H, CH3O); 13C-NMR (CDCl3, 50.32 MHz) δ: 157.2 (d, J = 338 Hz, 1-C), 159.9 (t, J = 6 Hz, 4′-C), 150.1 (d, J = 6 Hz, 3-C), 133.0 (s, 6′-C), 126.7 (t, J = 6.0 Hz, 2′-C), 125.9 (s, 1′-C), 123.7 (t, J = 22 Hz, 3′-C), 111.5 (s, 5′-C), 110.8 (t, J = 236 Hz, CF2H), 110.7 (d, J = 68 Hz, 2-C), 56.0 (s, CH3O).
3.2.3. Representative Procedure. Preparation of N-(1-Methylethyl)-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11b)
A solution of Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol) was added dropwise to a suspension of 0.030 g of 3-formyl-4-methoxycinnamic acid (9, 0.030 g, 0.145 mmol) in 0.4 mL of dry dichloromethane under an argon atmosphere. The reaction mixture was stirred for 45 min at room temperature, diluted with 2 mL of dry dichloromethane, and subsequently percolated through 2.5 g of silica gel under an argon atmosphere. The silica gel bed was rinsed with 8 mL of dry dichloromethane and the resulting solution was concentrated under a nitrogen flow to a final volume of 1 mL. Isopropylamine (0.037 mL, 0.435 mmol) and triethylamine (0.061 mL, 0.435 mmol) were added and the mixture was subsequently stirred for 1 h at room temperature. The solution was diluted with 10 mL of dichloromethane, washed twice with 1M HCl and once with water, dried with anhydrous sodium sulfate, and the solvent evaporated. The resulting solid was purified by flash column chromatography eluting with mixtures of ethyl acetate–hexane of increasing polarity to give 11b as a crystalline white solid (0.025 g, 64%). m.p. 113–114 °C; 1H-NMR (CDCl3) δ: 7.75–7.71 (m, 1H, 2′-H), 7.57 (d, J = 15.5 Hz, 1H, 3-H), 7.53–7.49 (m, 1H, 6′-H), 6.92 (t, J = 55.5 Hz, 1H, CF2H), 6.94–6.88 (m, 1H, 5′-H), 6.31 (d, J = 15.6 Hz, 1H, 2-H), 5.60–5.54 (m, NH), 4.29 – 4.16 (m, 1H, 1′′-H), 3.89 (s, 3H, CH3O), 1.22 (d, J = 6.5 Hz, 6H, 2′′-H); 13C-NMR (CDCl3) δ: 165.1 (s, 1-C), 158.3 (t, J = 6.3 Hz, 4′-C), 139.6 (s, 3-C), 132.4 (t, J = 2.1 Hz, 6′-C), 127.8 (s, 1′-C), 125.0 (t, J = 5.9 Hz, 2′-C), 123.2 (t, J = 22.2 Hz, 3′-C), 120.1 (s, 2-C), 111.3 (t, J = 236.3 Hz, CF2H), 111.3 (s, 5′-C), 56.0 (s, CH3O), 41.7 (s, 1′′-C), 23.0 (s, 2′′-C). EIMS m/z (%): 269 (57, M+), 211 (100), 183 (23), 132 (19), 58 (34). Analysis for C14H17F2NO2: Calcd: C, 62.42; H, 6.36; N, 5.20%. Found: C, 62.45; H, 6.32; N, 4.98%.
3.2.4. N-Methyl-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11a)
Compound 11a was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), methylamine hydrochloride (0.030 g, 0.435 mmol), and triethylamine (0.082 mL, 0.580 mmol) following the procedure described for 11b. Compound 11a was obtained as a crystalline white solid (0.021 g, 60 %); m.p. 169–171 °C; 1H-NMR (CDCl3-CD3OD 9:1) δ: 7.76–7.71 (m, 1H, 2′-H), 7.57–7.54 (m, 1H, 6′-H), 7.54 (d, J = 15.5 Hz, 1H, 3-H), 6.97–6.93 (m, 1H, 5′-H), 6.93 (t, J = 55.5 Hz, 1H, CF2H), 6.40 (d, J = 15.7 Hz, 1H, 2-H), 3.90 (s, 3H, CH3O), 2.89 (s, 3H, NCH3); 13C-NMR (CDCl3-CD3OD 9:1) δ: 167.5 (1-C), 158.2 (t, JCF = 5.7 Hz, 4′-C), 139.4 (3-C), 132.2 (6′-C), 127.6 (1′-C), 125.0 (t, JCF = 5.9 Hz, 2′-C), 123.0 (t, JCF = 22.2 Hz, 3′-C), 119.3 (2-C), 111.2 (t, JCF = 236.1 Hz, CF2H), 111.2 (5′-C), 55.8 (CH3O), 26.2 (NCH3). EIMS m/z (%): 241 (55, M+), 240 (26), 211 (100), 183 (25), 132 (22). Analysis for C12H13F2NO2: Calcd: C, 59.75; H, 5.43; N, 5.81 %. Found: C, 59.27; H, 5.28; N, 5.66 %.
3.2.5. N-(2-Methylpropyl)-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11c)
Compound 11c was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), isobutylamine (0.044 mL, 0.435 mmol), and triethylamine (0.061 mL, 0.435 mmol) following the procedure described for 11b. Compound 11c was obtained as a crystalline white solid (0.027 g, 66 %); m.p. 145–146 °C; 1H-NMR (CDCl3) δ: 7.77–7.72 (m, 1H, 2′-H), 7.59 (d, J = 15.5 Hz, 1H, 3-H), 7.56–7.49 (m, 1H, 6′-H), 6.93 (t, J = 55.5 Hz, 1H, CF2H), 6.94–6.90 (m, 1H, 5′-H), 6.35 (d, J = 15.5 Hz, 1H, 2-H), 5.74 (t, J = 5.4 Hz, 1H, NH), 3.89 (s, 3H, CH3O), 3.23 (dd, J = 6.2, 6.7 Hz, 2H, 1′′-H), 1.92–1.77 (1 H, m, 2′′-H), 0.96 (d, J = 6.7 Hz, 6H, 3′′-H); 13C-NMR (CDCl3) δ: 166.0 (1-C), 158.3 (t, JCF = 5.7 Hz, 4′-C), 139.8 (3-C), 132.4 (6′-C), 127.8 (1′-C), 125.0 (t, JCF = 5.9 Hz, 2′-C), 123.3 (t, JCF = 22.3 Hz, 3′-C), 119.8 (2-C), 111.3 (t, JCF = 236.2 Hz, CF2H), 111.3 (5′-C), 56.0 (CH3O), 47.2 (1′′-C), 28.8 (2′′-C), 20.3 (3′′-C). EIMS m/z (%): 283 (30, M+), 226 (53), 211 (100), 183 (22), 132 (20), 43 (18). Analysis for C15H19F2NO2·0.5H2O: Calcd: C, 61.63; H, 6.90; N, 4.79%. Found: C, 61.51; H, 6.57; N, 4.76%.
3.2.6. N-(3-Methylbutyl)-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11d)
Compound 11d was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), isopentylamine (0.051 mL, 0.435 mmol), and triethylamine (0.061 mL, 0.435 mmol) following the procedure described for 11b. Compound 11d was obtained as a crystalline white solid (0.025 g, 58%); m.p. 110–111 °C; 1H-NMR (CDCl3) δ: 7.80–7.68 (m, 1H, 2′-H), 7.58 (d, J = 15.5 Hz, 1H, 3-H), 7.55–7.49 (m, 1H, 6′-H), 6.92 (t, J = 55.5 Hz, 1H, CF2H), 6.97–6.85 (m, 1H, 5′-H), 6.33 (d, J = 15.5 Hz, 1H, 2-H), 5.71 (t, J = 5.6 Hz, 1H, NH), 3.89 (s, 3H, CH3O), 3.50–3.33 (m, 2H, 1′′-H), 1.72–1.61 (1 H, m, 2′′-H), 1.46 (dt, J = 7.0, 8.5 Hz, 2H, 3′′-H), 0.94 (d, J = 6.6 Hz, 6H, 4′′-H); 13C-NMR (CDCl3) δ: 165.9 (1-C), 158.3 (t, JCF = 5.7 Hz, 4′-C), 139.7 (3-C), 132.4 (t, JCF = 1.9 Hz, 6′-C), 127.8 (1′-C), 125.0 (t, JCF = 5.9 Hz, 2′-C), 123.2 (t, JCF = 22.2 Hz, 3′-C), 119.9 (2-C), 111.3 (t, JCF = 236.2 Hz, CF2H), 111.3 (5′-C), 56.0 (CH3O), 38.7 (2′′-C), 38.2 (1′′-C), 26.0 (3′′-C), 22.6 (4′′-C). EIMS m/z (%): 297 (30, M+), 241 (44), 240 (32), 226 (22), 211 (100), 183 (27), 132 (25). Analysis for. C16H21F2NO2: Calcd: C, 64.63; H, 7.12; N, 4.71%. Found: C, 64.48; H, 7.05; N, 4.74%.
3.2.7. N-Cyclohexyl-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11e)
Compound 11e was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), cyclohexylamine (0.050 mL, 0.435 mmol), and triethylamine (0.061 mL, 0.435 mmol) Following the procedure used for 11b. Compound 11e was obtained as a crystalline white solid (0.034 g, 76%); m.p. 181–183 °C; 1H-NMR (CDCl3) δ: 7.75–7.73 (m, 1H, 2′-H), 7.58 (d, J = 15.5 Hz, 1H, 3-H), 7.52 (m, 1H, 6′-H), 6.92 (t, J = 55.5 Hz, 1H, CF2H), 6.93–6.90 (m, 1H, 5′-H), 6.32 (d, J = 15.5 Hz, 1H, 2-H), 5.61 (d, J = 6.2 Hz, 1H, NH), 3.97–3.90 (m, 1H, 1′′-H), 3.89 (s, 3H, CH3O), 2.04–1.92 (m, 2H, 2′′-Heq), 1.79–1.69 (m, 2H, 3′′-Heq), 1.68–1.59 (m, 1H, 4′′-Heq), 1.48–1.34 (m, 2H, 3′′-Hax), 1.29–1.11 (m, 3H, 2′′-Hax and 4′′-Hax); 13C-NMR (CDCl3) δ: 165.0 (1-C), 158.3 (t, JCF = 5.6 Hz, 4′-C), 139.6 (3-C), 132.4 (6′-C), 127.9 (1′-C), 125.0 (t, JCF = 5.8 Hz, 2′-C), 123.3 (t, JCF = 22.3 Hz, 3′-C), 120.2 (2-C), 111.3 (t, JCF = 236.2 Hz, CF2H), 111.3 (5′-C), 56.0 (CH3O), 48.5 (1′′-C), 33.4 (2′′-C), 25.7 (4′′-C), 25.0 (3′′-C). EIMS m/z (%): 309 (62, M+), 226 (64), 211 (100), 183 (33), 132 (25), 98 (39). Analysis for C17H21F2NO2: Calcd C, 66.00; H, 6.84; N, 4.53%. Found: C, 65.85; H, 6.86; N, 4.36%.
3.2.8. N-Phenyl-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11f)
Compound 11f was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), aniline (0.040 mL, 0.435 mmol), and triethylamine (0.061 mL, 0.435 mmol) following the procedure used for 11b. Compound 11f was obtained as a crystalline white solid (0.025 g, 57%); m.p. 138–139 °C; 1H-NMR (CDCl3) δ: 7.76 (m, 1H, 2′-H), 7.70 (d, J = 15.5 Hz, 1H, 3-H), 7.63 (d, J = 7.2 Hz, 2H, 2′′-H), 7.60 (bs, 1H, NH), 7.54–7.50 (m, 1H, 6′-H), 7.37–7.31 (m, 2H, 3′′-H), 7.14–7.10 (m, 1H, 4′′-H), 6.92 (t, J = 55.5 Hz, 1H, CF2H), 6.92–6.88 (m, 1H, 5′-H), 6.50 (d, J = 15.5 Hz, 1H, 2-H), 3.89 (s, 3H, CH3O); 13C-NMR (CDCl3) δ: 164.0 (1-C), 158.5 (t, JCF = 5.6 Hz, 4′-C), 141.2 (3-C), 138.1 (1′′-C), 132.5 (6′-C), 129.1 (3′′-C), 127.4 (1′-C), 125.2 (t, JCF = 5.7 Hz, 2′-C), 124.4 (4′′-C), 123.2 (t, JCF = 22.2 Hz, 3′-C), 120.0 (2′′-C), 119.7 (2-C), 111.1 (5′-C), 111.2 (t, JCF = 236.4 Hz, CF2H), 55.9 (CH3O). EIMS m/z (%): 303 (25, M+), 211 (100), 183 (17), 132 (15), 93 (19). Analysis for C17H15F2NO2: Calcd C, 67.32; H, 4.98; N, 4.62%. Found: C, 67.31; H, 5.00; N, 4.37%.
3.2.9. N-(2-Phenylethyl)-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11g)
Compound 11g was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), 2-phenylethylamine (0.055 mL, 0.435 mmol), and triethylamine (0.061 mL, 0.435 mmol) following the procedure used for 11b. Compound 11g was obtained as a crystalline white solid (0.029 g, 60%); m.p. 104–105 °C; 1H-NMR (CDCl3) δ: 7.72–7.71 (m, 1H, 2′-H), 7.57 (d, J = 15.6 Hz, 1H, 3-H), 7.52–7.49 (m, 1H, 6′-H), 7.35–7.30 (m, 2H, 3′′′-H), 7.26–7.21 (m, 3H, 2′′′-H and 4′′′-H), 6.91 (t, J = 55.5 Hz, 1H, CF2H), 6.92–6.88 (m, 1H, 5′-H), 6.26 (d, J = 15.5 Hz, 1H, 2-H), 5.74 (t, J = 5.3 Hz, 1H, NH), 3.88 (s, 3H, CH3O), 3.66 (td, J = 6.9, 5.4 Hz, 2H, 1′′-H), 2.89 (t, J = 6.9 Hz, 2H, 2′′-H); 13C-NMR (CDCl3) δ: 166.0 (1-C), 158.3 (t, JCF = 5.6 Hz, 4′-C), 139.9 (3-C), 139.0 (1′′′-C), 132.4 (6′-C), 128.9 (2′′′-C), 128.8 (3′′′-C), 127.7 (1′-C), 126.7 (4′′′-C), 125.1 (t, JCF = 5.9 Hz, 2′-C), 123.2 (t, JCF = 22.2 Hz, 3′-C), 119.6 (2-C), 111.3 (t, JCF = 236.3 Hz, CF2H), 111.3 (5′-C), 56.0 (CH3O), 40.9 (1′′-C), 35.8 (2′′-C). EIMS m/z (%): 331 (34, M+), 226 (32), 211 (100), 183 (19), 132 (16), 104 (17), 91 (47). Analysis for C19H19F2NO2: Calcd C, 68.87; H, 5.78; N, 4.23%. Found: C, 68.86; H, 5.83; N, 4.10%.
3.2.10. N-Methoxy-N-methyl-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11h)
Compound 11h was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), N,O-dimethylhydroxylamine hydrochloride (0.043 g, 0.435 mmol), and triethylamine (0.082 mL, 0.580 mmol) following the procedure used for 11b. Compound 11h was obtained as a crystalline white solid (0.026 g, 66%); m.p. 101 °C; 1H-NMR (CDCl3) δ: 7.83–7.80 (m, 1H, 2′-H), 7.69 (d, J = 15.8 Hz, 1H, 3-H), 7.62–7.58 (m, 1H, 6′-H), 6.96 (d, J = 15.8 Hz, 1H, 2-H), 6.96–6.93 (m, 1H, 5′-H), 6.94 (t, J = 55.5 Hz, 1H, CF2H), 3.91 (s, 3H, CH3OAr), 3.78 (s, 3H, N(CH3)OCH3), 3.31 (s, 3H, N(CH3)OCH3); 13C-NMR (CDCl3) δ: 167.1 (1-C), 158.5 (t, JCF = 5.7 Hz, 4′-C), 142.4 (3-C), 132.7 (6′-C), 128.1 (1′-C), 125.5 (t, JCF = 5.9 Hz, 2′-C), 123.3 (t, JCF = 22.1 Hz, 3′-C), 114.8 (2-C), 111.4 (t, JCF = 236.3 Hz, CF2H), 111.3 (5′-C), 62.1 (N(CH3)OCH3), 56.0 (CH3OAr), 32.7 (N(CH3)OCH3). EIMS m/z (%): 271 (3.4, M+), 211 (100), 183 (18), 132 (12). Analysis for C13H15F2NO3: Calcd C, 57.56; H, 5.57; N, 5.16%. Found: C, 57.84; H, 5.65; N, 5.24%.
3.2.11. N,N-Bis(2-methoxyethyl)-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11i)
A solution of Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol) was added dropwise to a suspension of 0.030 g of 3-formyl-4-methoxycinnamic acid (9, 0.030 g, 0.145 mmol) in 0.4 mL of dry dichloromethane under an argon atmosphere. The reaction mixture was stirred for 45 min at room temperature. Triethylamine (0.061 mL, 0.435 mmol) was added and the mixture was subsequently stirred for 1 h at room temperature. The solution was diluted with 10 mL of dichloromethane, washed twice with 1M HCl and once with water, dried with anhydrous sodium sulfate, and the solvent evaporated. The resulting solid was purified by flash column chromatography eluting with mixtures of ethyl acetate–hexane of increasing polarity to give 11i as a crystalline white solid (0.042 g, 84%); m.p. 60–61 °C; 1H-NMR (CDCl3) δ: 7.76–7.71 (m, 1H, 2′-H), 7.64 (d, J = 15.4 Hz, 1H, 3-H), 7.58–7.52 (m, 1H, 6′-H), 6.93 (t, J = 55.5 Hz, 1H, CF2H), 6.95–6.91 (m, 1H, 5′-H), 6.89 (d, J = 15.4 Hz, 1H, 2-H), 3.90 (s, 3H, CH3OAr), 3.72 (t, J = 5.8 Hz, 2H, NCH2), 3.68 (t, J = 5.3 Hz, 2H, NCH2), 3.59 (t, J = 5.4 Hz, 2H, CH3OCH2), 3.56 (t, J = 5.8 Hz, 2H, CH3OCH2), 3.35 (s, 3H, CH3OCH2), 3.34 (s, 3H, CH3OCH2); 13C-NMR (CDCl3) δ: 167.0 (1-C), 158.3 (t, JCF = 5.60 Hz, 4′-C), 141.3 (3-C), 132.3 (t, JCF = 2.05 Hz, 6′-C), 128.4 (1′-C), 125.3 (t, JCF = 5.8 Hz, 2′-C), 123.2 (t, JCF = 22.1 Hz, 3′-C), 117.0 (2-C), 111.4 (t, JCF = 236.2 Hz, CF2H), 111.3 (5′-C), 71.4 (×2, CH3OCH2), 59.3 and 59.0 (CH3OCH2), 56.0 (CH3OAr), 49.2 and 47.5 (NCH2). EIMS m/z (%): 344 (5.4, M + 1), 343 (3.2, M+), 211 (100), 183 (9), 132 (3). Analysis for C17H23F2NO4: Calcd C, 59.46; H, 6.75; N, 4.08%. Found: C, 59.08; H, 6.55; N, 4.10%.
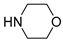
3.2.12. (E)-3-[3-(Difluoromethyl)-4-methoxyphenyl]-1-(morpholin-4-yl)propenone (11j)
Compound 11j was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), morpholine (0.0376 mL, 0.435 mmol), and triethylamine (0.061 mL, 0.435 mmol) following the procedure used for 11b. Compound 11j was obtained as a crystalline white solid (0.024 g, 56%); m.p. 120–121 °C; 1H-NMR (CDCl3) δ: 7.82–7.75 (m, 1H, 2′-H), 7.68 (d, J = 15.4 Hz, 1H, 3-H), 7.56–7.54 (m, 1H, 6′-H), 6.95 (t, J = 55.5 Hz, 1H, CF2H), 6.95–6.93 (m, 1H, 5′-H), 6.78 (d, J = 15.4 Hz, 1H, 2-H), 3.91 (s, 3H, CH3O), 3.79–3.66 (m, 8H, 2′′-H, 3′′-H, 5′′-H and 6′′-H); 13C-NMR (CDCl3) δ: 165.7 (1-C), 158.4 (t, JCF = 5.7 Hz, 4′-C), 142.2 (3-C), 132.7 (6′-C), 128.0 (1′-C), 124.9 (t, JCF = 5.8 Hz, 2′-C), 123.3 (t, JCF = 22.2 Hz, 3′-C), 115.4 (2-C), 111.3 (t, JCF = 237.0 Hz, CF2H), 111.3 (5′-C), 67.0 (2′′-C and 6′′-C), 56.0 (CH3O), 46.4 and 42.6 (br s, 3′′-C and 5′′-C). EIMS m/z (%): 297 (53, M+), 211 (100), 183 (22), 132 (15), 86 (12). Analysis for C15H17F2NO3: Calcd C, 60.60; H, 5.76; N, 4.71%. Found: C, 61.02; H, 5.74; N, 4.42%.
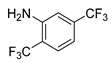
3.2.13. N-(2,5-Bis(trifluoromethyl)phenyl)-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (11k)
A solution of Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol) was added dropwise to a suspension of 3-formyl-4-methoxycinnamic acid (9, 0.030 g, 0.145 mmol) in 0.4 mL of dry dichloromethane under an argon atmosphere. The reaction mixture was stirred for 45 min at room temperature, diluted with 2 mL of dry dichloromethane, and percolated through 2.5 g of silica gel under an argon atmosphere. The silica gel bed was rinsed with 8 mL of dry dichloromethane and the resulting solution was dried under a nitrogen flow to give a white solid. Anhydrous THF (1.0 mL), 2,5-bis(trifluoromethyl)aniline (0.068 mL, 0.435 mmol) and 2.0 M lithium diisopropylamide in THF-heptane-ethylbenzene (0.145 mL, 0.29 mmol) were added and the mixture was stirred for 2 h at 25 °C under an argon atmosphere. The solution was diluted with 10 mL of dichloromethane, quenched with ice-water, washed twice with 1M HCl and once with water, dried with anhydrous sodium sulfate, and the solvent evaporated. The resulting oil was purified by flash column chromatography eluting with mixtures hexane–ethyl acetate of increasing polarity to give 11k as a white solid (0.030 g, 47%); m.p. 168–169 °C; 1H-NMR (CDCl3) δ: 8.83 (bs, 1H, 6′′-H), 7.85–7.82 (m, 1H, 2′-H), 7.78 (d, J = 15.5 Hz, 1H, 3-H), 7.76 (d, J = 8.3 Hz, 1H, 3′′-H), 7.64 (s, 1H, NH), 7.66–7.59 (m, 1H, 6′-H), 7.49 (d, J = 8.2 Hz, 1H, 4′′-H), 7.00–6.96 (m, 1H, 5′-H), 6.96 (t, J = 55.41 Hz, 1H, CF2H), 6.47 (d, J = 15.4 Hz, 1H, 2-H), 3.93 (s, 3H, CH3O); 13C-NMR (CDCl3) δ: 164.1 (1-C), 159.1 (t, JCF = 5.57 Hz, 4′-C), 143.3 (3-C), 136.6 (1′′-C), 135.2 (q, JCF = 33.4 Hz, 5′′-C), 133.0 (6′-C), 127.0 (q, JCF = 5.40 Hz, 3′′-C), 127.0 (1′-C), 125.8 (t, JCF = 5.78 Hz, 2′-C), 123.6 (q, JCF = 273.1 Hz, 2′′-CF3), 123.6 (t, JCF = 22.2 Hz, 3′-C), 123.3 (q, JCF = 273.2 Hz, 5′′-CF3), 122.3 (q, JCF = 30.1 Hz, 2”-C), 121.0 (q, JCF = 3.8 Hz, 6”-C), 120.9 (q, JCF = 3.7 Hz, 4”-C), 118.5 (2-C), 111.5 (5′-C), 111.2 (t, JCF = 236.6 Hz, CF2H), 56.1 (CH3O). EIMS m/z (%): 439 (5, M+), 420 (2), 212 (12), 211 (100), 183 (15), 132 (12). Analysis for C19H13F8NO2·0.5H2O: Calcd C, 50.90; H, 3.15; N, 3.12%. Found: C, 51.20; H, 3.10; N, 3.50%.
3.2.14. N,N’-Bis [3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenoyl]-1,3-propanediamine (11l)
Compound 11l was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), 1,3-diaminopropane (0.0055 mL, 0.065 mmol), and triethylamine (0.061 mL, 0.435 mmol) following the procedure used for 11b. Compound 11l was obtained as a white solid (0.019 g, 59%); m.p. 174–175 °C; 1H-NMR (CDCl3-CD3OD 9:1) δ: 7.74 (m, 2H, 2”-H), 7.56 (d, J = 15.8 Hz, 2H, 3′-H), 7.57–7.53 (m, 2H, 6”-H), 6.94–6.90 (m, 2H, 5”-H), 6.92 (t, J = 55.5 Hz, 2H, CF2H), 6.43 (d, J = 15.7 Hz, 2H, 2′-H), 3.90 (s, 6H, CH3O), 3.45–3.35 (m, 4H, 1-H and 3-H), 1.80–1.74 (m, 2H, 2-H); 13C-NMR (CDCl3-CD3OD 9:1) δ: 167.2 (1′-C), 158.3 (t, JCF = 5.6 Hz, 4”-C), 139.8 (3′-C), 132.1 (6”-C), 127.6 (1”-C), 125.4 (t, JCF = 5.8 Hz, 2”-C), 123.1 (t, JCF = 22.1 Hz, 3”-C), 119.6 (2′-C), 111.3 (t, JCF = 236.2 Hz, CF2H), 111.3 (5”-C), 55.9 (CH3O), 36.5 (1-C and 3-C), 29.3 (2-C). HRMS: calcd for C25H27F4N2O4+ (M + H)+: 495.1902, found: 495.1893; HRMS/MS (33 eV) from (M + H)+ m/z (%): 268.1155 (4), 211.0570 (100), 183.0614 (13). Analysis for C25H26F4N2O4: Calcd C, 60.72; H, 5.30; N, 5.67%. Found: C, 60.46; H, 5.41; N, 5.23%.
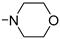
3.2.15. 1,4-Bis[3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenoyl]-piperazine (11m)
Compound 11m was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), piperazinium diacetate (0.015 g, 0.073 mmol), and triethylamine (0.102 mL, 0.730 mmol) following the procedure used for 11b. Compound 11m was obtained as a white solid (0.017 g, 46%); m.p. 259–261 °C; 1H-NMR (CDCl3-CD3OD 9:1) δ: 7.80 (m, 2H, 2′-H), 7.66 (d, J = 15.3 Hz, 2H, 3-H), 7.65–7.61 (m, 2H, 6′-H), 7.03–6.99 (m, 2H, 5′-H), 6.96 (t, J = 55.5 Hz, 2H, CF2H), 6.88 (d, J = 15.4 Hz, 2H, 2-H), 3.93 (s, 6H, CH3O), 3.86–3.76 (m, 8H, N(CH2CH2)2N); 13C-NMR (CDCl3-CD3OD 9:1) δ: 166.3 (1-C), 158.5 (t, JCF = 5.6 Hz, 4′-C), 142.8 (3-C), 132.3 (br s, 6′-C), 127.4 (1′-C), 125.2 (br s, 2′-C), 123.1 (t, JCF = 22.2 Hz, 3′-C), 114.8 (2-C), 111.2 (5′-C), 111.1 (t, JCF = 236.1 Hz, CF2H), 55.7 (CH3O), 45.4 and 42.2 (br s, N(CH2CH2)2N). HRMS calcd for C26H27F4N2O4+ (M + H)+: 507.1902, found: 507.1892; HRMS/MS (33 eV) from (M + H)+ m/z (%): 211.0558 (100), 183.0610 (31), 160.0515 (4). Analysis for C26H26F4N2O4·0.5H2O: Calcd C, 60.58; H, 5.28; N, 5.43%. Found: C, 60.13; H, 5.15; N, 5.19%.
3.2.16. N-(1-Methylethyl)-3-(4-methoxyphenyl)-(E)-propenamide (13a)
To a solution of 4-methoxycinnamic acid (12a, 50 mg, 0.28 mmol) in dry DMF (0.6 mL), triethylamine (0.059 mL, 0.42 mmol), isopropylamine (0.048 mL, 0.56 mmol) and a solution of (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (0.186 g, 0.42 mmol) in dry dichloromethane (0.6 mL) were added at 0 °C. The reaction mixture was stirred at 0 °C for 30 min and then at 25 °C for 2 h. Water (15 mL) was added and the mixture was extracted with dichloromethane (30 mL). The extract was washed with 1M HCl and water, dried over anhydrous sodium sulfate, and the solvent evaporated. The residue was recrystallized from hexane to give 13a as a crystalline white solid (0.052 g, 85%); m.p. 131 °C; 1H-NMR (CDCl3) δ: 7.57 (d, J = 15.6 Hz, 1H, 3-H), 7.44 (d, J = 8.6 Hz, 2H, 2′-H and 6′-H), 6.88 (d, J = 8.7 Hz, 2H, 3′-H and 5′-H), 6.24 (d, J = 15.6 Hz, 1H, 2-H), 5.49 (d, J = 8.2 Hz, 1 H, NH), 4.28–4.17 (m, 1H, 1”-H), 3.82 (s, 3 H, CH3O), 1.22 (d, J = 6.6 Hz, 6 H, 2”-H); 13C-NMR (CDCl3) δ: 165.5 (1-C), 160.9 (4′-C), 140.5 (3-C), 129.4 (2′-C and 6′-C), 127.8 (1′-C), 118.8 (2-C), 114.3 (3′-C and 5′-C), 55.5 (CH3O), 41.7 (1”-C), 23.0 (2”-C). EIMS m/z (%): 220 (18, M + 1), 219 (51, M+), 161 (100), 134 (28), 133 (40), 118 (14). Analysis for C13H17NO2: C, 71.21; H, 7.81; N, 6.39%. Found: C, 70.93; H, 7.82; N, 6.36%.
3.2.17. N-(1-Methylethyl)-3-(4-methoxy-3-methylphenyl)-(E)-propenamide (13b)
Compound 13b was prepared from acid 12b (50 mg, 0.26 mmol), triethylamine (0.054 mL, 0.39 mmol), isopropylamine (0.045 mL, 0.52 mmol), and (benzotriazol-1-yloxy)-tris(dimethylamino)-phosphonium hexafluorophosphate (0.173 g, 0.39 mmol) following the procedure used for 13a. The reaction product was purified by flash column chromatography eluting with mixtures of hexane–ethyl acetate of increasing polarity to give 13c as a pale yellow solid (0.049 g, 80%); m.p. 124–125 °C; 1H-NMR (CDCl3) δ: 7.54 (d, J = 15.5 Hz, 1H, 3-H), 7.33–7.29 (m, 2H, 2′-H and 6′-H), 6.80 (d, J = 9.0 Hz, 1H, 5′-H), 6.22 (d, J = 15.5 Hz, 1H, 2-H), 5.38 (d, J = 6.6 Hz, 1H, NH), 4.30–4.16 (m, 1H, 1”-H), 3.85 (s, 3H, CH3O), 2.21 (s, 3H, CH3Ar), 1.22 (d, J = 6.5 Hz, 6H, 2”-H); 13C-NMR (CDCl3) δ: 165.6 (1-C), 159.2 (4′-C), 140.8 (3-C), 129.8 (2′-C), 127.5 (6′-C), 127.2 (1′-C and 3′-C), 118.4 (2-C), 110.0 (5′-C), 55.5 (CH3O), 41.6 (1”-C), 23.1 (2”-C), 16.4 (CH3Ar). EIMS m/z (%): 234 (13, M + 1), 233 (36, M+), 175 (100), 148 (32), 147 (26), 115 (17). Analysis for C14H19NO2·0.25H2O: Calcd: C, 70.71; H, 8.26; N, 5.89%. Found: C, 70.77; H, 8.15; N, 5.86%.
3.2.18. N-(1-Methylethyl)-3-(3-hydroxy-4-methoxyphenyl)-(E)-propenamide (13c)
Compound 13c was prepared from isoferulic acid (8, 54 mg, 0.28 mmol), triethylamine (0.059 mL, 0.42 mmol), isopropylamine (0.048 mL, 0.56 mmol), and (benzotriazol-1-yloxy)-tris(dimethylamino)-phosphonium hexafluorophosphate (0.186 g, 0.42 mmol) following the procedure used for 13a. The reaction product was purified by flash column chromatography eluting with mixtures of hexane–ethyl acetate of increasing polarity to give 13b as a white solid (0.048 g, 73%); m.p. 164–165 °C; 1H-NMR (CDCl3-CD3OD 9:1) δ: 7.46 (d, J = 15.6 Hz, 1H, 3-H), 7.08 (d, J = 2.1 Hz, 1H, 2′-H), 6.98 (dd, J = 2.0, 8.6 Hz, 1H, 6′-H), 6.83 (d, J = 8.3 Hz, 1H, 5′-H), 6.25 (d, J = 15.6 Hz,1H, 2-H), 4.21–4.12 (m, 1H, 1”-H), 3.90 (s, 3H, CH3O), 1.21 (d, J = 6.6 Hz, 6H, 2”-H); 13C-NMR (CDCl3-CD3OD 9:1) δ: 166.1 (1-C), 148.7 (4′-C), 146.0 (3′-C), 140.6 (3-C), 128.4 (1′-C), 121.4 (6′-C), 118.8 (2-C), 112.9 (2′-C), 111.0 (5′-C), 55.9 (CH3O), 41.5 (1”-C), 22.6 (2”-C). EIMS m/z (%): 235 (43, M+), 178 (35), 177 (98),150 (27), 145 (28), 134 (27), 117 (43), 89 (69), 58 (100). Analysis for C13H17NO3: Calcd C, 66.36; H, 7.28; N, 5.95%. Found: C, 66.28; H, 7.17; N, 5.69%.
3.2.19. N-(1-Methylethyl)-3-[4-(difluoromethyl)-3-methoxyphenyl]-(E)-propenamide (15)
Methyl-3-[4-formyl-3-methoxyphenyl]-(E)-propenoate (14, 33 mg, 0.150 mmol) was dissolved in a mixture of methanol (2 mL) and 20% aqueous potassium carbonate solution (1 mL). The reaction mixture was stirred for 4 h at 20 °C and concentrated under reduced pressure to a third of its volume. The solution was acidified with conc. HCl (to pH 1) and the precipitate was filtered and used without further purification. The crude product was treated with Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), isopropylamine (0.044 mL, 0.435 mmol) and triethylamine (0.061 mL, 0.435 mmol) following the procedure described for 11b. Compound 15 was obtained as a crystalline white solid (0.025 g, 62% 2 steps); m.p. 128–130 °C; 1H-NMR (CDCl3) δ: 7.62 (d, J = 15.5 Hz, 1H, 3-H), 7.59–7.56 (m, 1H, 5′-H), 7.22–7.18 (m, 1H, 6′-H), 7.05–7.01 (m, 1H, 2′-H), 6.95 (t, J = 55.5 Hz, 1H, CF2H), 6.41 (d, J = 15.5 Hz, 1H, 2-H), 5.48 (d, J = 6.5 Hz, 1H NH), 4.32–4.20 (m, 1H, 1′′-H), 3.92 (s, 3H, CH3O), 1.26 (d, J = 6.6 Hz, 6H, 2”-H); 13C-NMR (CDCl3) δ: 164.6 (s, 1-C), 157.6 (t, J = 6.0 Hz, 3′-C), 140.0 (s, 3-C), 138.9 (t, J = 1.8 Hz, 1′-C), 126.8 (t, J = 5.8 Hz, 5′-C), 123.8 (t, J = 22.3 Hz, 4′-C), 122.9 (s, 2-C), 120.0 (s, 6′-C), 111.4 (t, J = 236.4 Hz, CF2H), 110.2 (s, 2′-C), 55.9 (s, CH3O), 41.9 (s, 1”-C), 23.0 (s, 2”-C). EIMS m/z (%): 269 (55, M+), 211 (100), 183 (24), 132 (18), 58 (33). Analysis for C14H17F2NO2: Calcd C, 62.42; H, 6.36; N, 5.20%. Found: C, 62.44; H, 6.35; N, 4.99%.
3.2.20. 3-(4-Acetyloxy-3-formylphenyl)-(E)-propenoic acid (17)
To a solution of 5-iodosalicylaldehyde 16 (2.68 g, 10.8 mmol) in dry acetone (50 mL) was added K2CO3 (3.0 g, 21.6 mmol) and then acetic anhydride (2.15 mL, 21.6 mmol) was added dropwise to the suspension with vigorous stirring. Stirring was continued at room temperature for 2.5 h and the reaction mixture was percolated through a silica gel pad using acetone as eluent. The percolate was evaporated to dryness and the residue recrystallized from n-hexane to give 5-iodo-2-acetyloxy-benzaldehyde as pale yellow needles (2.9 g, 92%); m.p. 89–90 °C; 1H-NMR (CDCl3) δ: 10.01 (s, 1H, ArCHO), 8.16 (d, J = 2.2 Hz, 1H, 2-H), 7.91 (dd, J = 2.3, 8.5 Hz, 1H, 6-H), 6.96 (d, J = 8.5 Hz, 1H, 5-H), 2.38 (s, 3H, CH3C(O)Ar); 13C-NMR (CDCl3) δ: 187.0 (ArCHO), 168.7 (CH3C(O)Ar), 151.3 (2-C), 143.8 (4-C), 139.5 (6-C), 129.5 (1-C), 125.5 (3-C), 90.2 (5-C), 20.8 (CH3C(O)Ar).
The 5-iodo-2-acetyloxybenzaldehyde obtained above (0.2 g, 0.69 mmol) was dissolved in 5 mL of acetonitrile and oxygen was removed by bubbling nitrogen through the solution. Triethylamine (0.673 mL, 4.82 mmol) and acrylic acid (0.095 mL, 1.38 mmol) were added dropwise with stirring to the solution, followed by tri-o-tolylphosphine (16 mg, 0.069 mmol) and palladium (II) acetate (8.0 mg, 0.047 mmol). The mixture was then heated at 65–70 °C for 4 h, volatiles were removed by distillation, and the residue was dissolved in dichloromethane. The resulting solution was percolated through silica gel eluting with a mixture of hexane–ethyl acetate (6:4). The percolate was evaporated to dryness and purified by flash column chromatography eluting with mixtures of hexane–ethyl acetate of increasing polarity to give compound 17 as a white solid (0.095 g, 54%); m.p. 205–207 °C; 1H-NMR (DMSO-d6) δ: 12.53 (s, 1H, COOH), 10.07 (s, 1H, ArCHO), 8.22 (d, J = 2.3 Hz, 1H, 2′-H), 8.08 (dd, J = 8.6, 2.3 Hz, 1H, 6′-H), 7.67 (d, J = 16.0 Hz, 1H, 3-H), 7.36 (d, J = 8.5 Hz, 1H, 5′-H), 6.63 (d, J =16.1 Hz, 1H, 2-H), 2.35 (s, 3H, CH3CO); 13C-NMR (DMSO-d6) δ: 189.9 (ArCHO), 169.1 (CH3CO), 167.3 (1-C), 151.7 (4′-C), 141.8 (3-C), 134.7 (6′-C), 132.8 (1′-C), 131.2 (2′-C), 128.2 (3′-C), 124.5 (5′-C), 120.9 (2-C), 20.7 (CH3CO). EIMS m/z (%): 234 (1, M+), 192 (23), 146 (9), 91 (20), 89 (45), 63 (19), 43 (100).
3.2.21. N-(1-Methylethyl)-3-[4-acetyloxy-3-(difluoromethyl)phenyl]-(E)-propenamide (18a)
Compound 18a was prepared from acid 17 (30.0 mg, 0.128 mmol), Deoxofluor® 50% in toluene (0.095 mL, 0.386 mmol), isopropylamine (0.033 mL, 0.386 mmol), and triethylamine (0.054 mL, 0.386 mmol) following the procedure used for 11b. Compound 18a was obtained as a white solid (0.018 g, 47%); m.p. 131–133 °C; 1H-NMR (CDCl3) δ: 7.76–7.71 (m, 1 H, 2′-H), 7.60 (d, J = 15.5 Hz, 1H, 3-H), 7.61–7.55 (m, 1H, 6′-H), 7.23–7.18 (m, 1H, 5′-H), 6.74 (t, J = 55.2 Hz, 1H, CF2H), 6.36 (d, J = 15.6 Hz, 1H, 2-H), 5.50 (d, J = 7.8 Hz, 1 H, NH), 4.28–4.17 (m, 1H, 1”-H), 2.34 (s, 3 H, CH3CO), 1.23 (d, J = 6.6 Hz, 6 H, 2”-H); 13C-NMR (CDCl3) δ: 168.7 (CH3CO), 164.5 (1-C), 149.1 (t, JCF = 5.2 Hz, 4′-C), 138.9 (3-C), 133.4 (1′-C), 131.2 (br s, 6′-C), 127.0 (t, JCF = 22.6 Hz, 3′-C), 125.5 (t, JCF = 6.4 Hz, 2′-C), 123.8 (s, 5′-C), 122.6 (s, 2-C), 111.6 (t, J = 238.9 Hz, CF2H), 41.9 (s, 1′′-C), 23.0 (s, 2”-C), 21.0 (CH3CO). EIMS m/z (%): 298 (23, M + 1), 297 (18, M+), 255 (88), 197 (68), 177 (44), 101 (30), 58 (100), 43 (60). Analysis for C15H17F2NO3: Calcd C, 60.60; H, 5.76; N, 4.71%. Found: C, 60.21; H, 5.76; N, 4.64%.
3.2.22. N-(1-Methylethyl)-3-[3-(difluoromethyl)-4-hydroxyphenyl]-(E)-propenamide (18b)
To a solution of 18a (10.0 mg, 0.034 mmol) in MeOH (1.0 mL) was added conc. H2SO4 (50 µL) and the mixture stirred at 60 °C for 2 h. The reaction mixture was diluted with water (15 mL) and concentrated under reduced pressure. The milky suspension was extracted with ethyl acetate (30 mL), the organic layer was dried with anhydrous sodium sulfate, and the solvent evaporated. The residue was purified by flash column chromatography eluting with mixtures of hexane–ethyl acetate of increasing polarity to give compound 18b as a white amorphous solid (0.007 g, 82%); m.p. 153–154 °C; 1H-NMR (CDCl3-CD3OD 9:1) δ: 7.70–7.65 (m, 1H, 2′-H), 7.50 (d, J 15.6, 1H, 3-H), 7.41–7.35 (m, 1H, 6′-H), 6.95 (t, 1H, J = 55.7 Hz, CF2H), 6.87–6.82 (m, 1H, 5′-H), 6.29 (d, J = 15.6 Hz, 1H, 2-H), 4.23–4.13 (m, 1H, 1”-H), 1.21 (d, J = 6.5 Hz, 6H, 2”-H); 13C-NMR (CDCl3-CD3OD 9:1) δ: 165.9 (1-C), 156.6 (t, JCF = 5.6 Hz, 4′-C), 140.0 (3-C), 132.0 (6′-C), 126.6 (1′-C), 125.3 (t, JCF = 5.6 Hz, 2′-C), 121.5 (t, JCF = 22.3 Hz, 3′-C), 118.9 (2-C), 116.1 (5′-C), 111.8 (t, JCF = 235.5 Hz, CF2H), 41.6 (1”-C), 22.7 (2”-C). EIMS m/z (%): 255 (13, M+), 197 (36), 177 (45), 121 (23), 101 (77), 58 (100), 43 (33). HRMS: calcd for C13H16F2NO2+ (M + H)+: 256.1144, found: 256.1145.
3.2.23. N-(1-Methylethyl)-trans-2-[3-(difluoromethyl)-4-methoxyphenyl]-cyclopropanecarboxamide (19)
Compound S2 (see the Supplementary Materials) (0.018 g, 0.066 mmol) was dissolved in diethyl ether (0.1 mL) and water (0.003 mL) and potassium t-butoxide (40 mg, 0.036 mmol) were added. The mixture was stirred at room temperature for 3 h. After this, the reaction mixture was diluted with 2M HCl (2 mL) and extracted with dichloromethane, the organic layer was dried with anhydrous sodium sulfate, and the solvent evaporated. The resulting crude product was treated with BOP and isopropylamine following the same procedure used for compound 13a. The residue was purified by flash column chromatography eluting with mixtures of hexane–ethyl acetate of increasing polarity to give compound 19 as a white solid (0.015 g, 80%); m.p. 166–167 °C; 1H-NMR (CDCl3) δ: 7.22 (bs, 1H, 2′-H), 7.24–7.19 (m, 1H, 6′-H), 6.91 (t, J = 55.7 Hz, 1H, CF2H), 6.86 (m, 1H, 5′-H), 5.47 (d, J = 7.2 Hz, 1H, NH), 4.20–4.04 (m, 1H, 1′′-H), 3.84 (s, 3H, CH3OAr), 2.47 (ddd, J = 4.1, 6.3, 9.1 Hz, 1H, 2-H), 1.58 (ddd, J = 4.1, 5.2, 9.1 Hz, 1H, 3a-H), 1.49 (ddd, J = 4.1, 5.2, 8.2 Hz, 1H, 1-H), 1.17 (d, J = 6.6 Hz, 3H, 2a’’-H), 1.19–1.14 (m, 1H, 3b-H), 1.17 (d, J = 6.6 Hz, 3H, 2b’’-H); 13C-NMR (CDCl3) δ: 170.8 (C(O)N), 155.9 (t, JCF = 6.0 Hz, 4′-C), 133.4 (1′-C), 130.4 (t, JCF = 2.1 Hz, 6′-C), 123.3 (t, JCF = 5.7 Hz, 2′-C), 122.8 (t, JCF = 22.0 Hz, 3′-C), 111.6 (t, JCF = 235.6 Hz, CF2H), 111.2 (5′-C), 55.9 (CH3OAr), 41.8 (1′′-C), 26.6 (1-C), 24.1 (1-C), 23.1 (2b’’-C), 23.0 (2a’’-C), 15.8 (3-C). EIMS m/z (%): 284 (39, M + 1), 283 (100, M+), 264 (29), 224 (21), 197 (30), 178 (21), 146 (42), 43 (15). Analysis for C15H19F2NO2: Calcd C, 63.59; H, 6.76; N, 4.94%. Found: C, 63.29; H, 6.50; N, 4.82%.
3.2.24. N-(1-Methylethyl)-3-[3-(difluoromethyl)-4-methoxyphenyl]-propanamide (20)
Compound 11b (0.030 g, 0.111 mmol) was dissolved in ethyl acetate (10 mL), 10% wt. Pd/C (0.003 g) added and the mixture hydrogenated at 3 bar and room temperature for 8 h. The catalyst was filtered and the residue crystallized from n-hexane to give compound 20 as a crystalline white solid (0.028 g, 93%); m.p. 84 °C; 1H-NMR (CDCl3) δ: 7.38 (m, 1H, 2′-H), 7.30 – 7.23 (m, 1H, 6′-H), 6.92 (t, J = 55.7 Hz, 1H, CF2H), 6.86–6.83 (m, 1H, 5′-H), 5.16 (d, J = 6.4 Hz, 1H, NH), 4.05 (d heptet, J = 6.6, 7.9 Hz, 1H, 1′′-H), 3.84 (s, 3H, CH3OAr), 2.94 (t, J = 7.6 Hz, 2H, 3-H), 2.40 (t, J = 7.6 Hz, 2H, 2-H), 1.08 (d, J = 6.6 Hz, 6H, 2′′-H); 13C-NMR (CDCl3) δ: 171.1 (1-C), 155.9 (t, JCF = 6.1 Hz, 4′-C), 133.3 (1′-C), 132.1 (6′-C), 126.0 (t, JCF = 5.7 Hz, 2′-C), 122.7 (t, JCF = 22.2 Hz, 3′-C), 111.7 (t, JCF = 235.3 Hz, CF2H), 111.2 (5′-C), 55.9 (CH3OAr), 41.5 (1′′-C), 38.9 (2-C), 31.0 (3-C), 22.9 (2′′-C). EIMS m/z (%): 272 (70, M + 1), 271 (100, M+), 252 (21), 184 (46), 171 (46), 100 (19). Analysis for C14H19F2NO2: Calcd C, 61.98; H, 7.06; N, 5.16%. Found: C, 62.04; H, 7.02; N, 5.11%.
3.2.25. N-(1-Methylethyl)-2-methyl-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenamide (21)
Compound 21 was prepared from 2-Methyl-3-(3-formyl-4-methoxyphenyl)-(E)-propenoic acid (S3, see the Supplementary Materials) (24.0 mg, 0.109 mmol), Deoxofluor® 50% in toluene (0.160 mL, 0.327 mmol), isopropylamine (0.028 mL, 0.327 mmol), and triethylamine (0.046 mL, 0.327 mmol) following the procedure used for 11b. Compound 21 was obtained as a crystalline white solid (0.020 g, 65%); m.p. 96–97 °C; 1H-NMR (CDCl3) δ: 7.57–7.53 (m, 1H, 2′-H), 7.47–7.37 (m, 1H, 6′-H), 7.25 (bs, 1H, 3-H), 6.97 – 6.93 (m, 1H, 5′-H), 6.95 (t, J = 55.6 Hz, 1H, CF2H), 5.66 (d, J = 7.3 Hz, 1H, NH), 4.20 (dhept, J = 6.6, 7.7 Hz, 1′′-H), 3.90 (s, 3H, CH3O), 2.09 (d, J = 1.4 Hz, 3H, C=CCH3), 1.24 (d, J = 6.6 Hz, 6H, 2”-H); 13C-NMR (CDCl3) δ: 168.8 (s, 1-C), 156.8 (t, J = 5.9 Hz, 4′-C), 133.2 (t, J = 2.1 Hz, 6′-C), 132.4 (s, 3-C), 131.9 (s, 2-C), 128.9 (s, 1′-C), 127.4 (t, J = 5.8 Hz, 2′-C), 122.7 (t, J = 22.2 Hz, 3′-C), 111.4 (t, J = 236.1 Hz, CF2H), 111.0 (s, 5′-C), 55.9 (s, CH3O), 41.9 (s, 1”-C), 23.0 (s, 2”-C), 14.4 (s, C=CCH3). EIMS m/z (%): 284 (55, M + 1), 283 (100, M+), 225 (36), 197 (30), 146 (97), 131 (16), 58 (11). Analysis for C15H19F2NO2: Calcd C, 63.59; H, 6.76; N, 4.94%. Found: C, 63.45; H, 6.76; N, 4.94%.
3.2.26. (1-Methylethyl)-3-[3-(difluoromethyl)-4-methoxyphenyl]-(E)-propenoate (22)
Compound 22 was prepared from acid 9 (30.0 mg, 0.145 mmol), Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), isopropanol (0.034 mL, 0.435 mmol), and triethylamine (0.061 mL, 0.435 mmol) following the procedure used for 11b. Compound 22 was obtained as a white solid (0.033 g, 84%); m.p. 62 °C; 1H-NMR (CDCl3) δ: 7.77–7.74 (m, 1H, 2′-H), 7.63 (d, J = 16.0 Hz, 1H, 3-H), 7.60–7.56 (m, 1H, 6′-H), 6.97–6.93 (m, 1H, 5′-H), 6.92 (t, J = 55.5 Hz, 1H, CF2H), 6.35 (d, J = 16.0 Hz, 1H, 2-H), 5.13 (hept, J = 6.3 Hz, 1′′-H), 3.91 (s, 3H, CH3O), 1.31 (d, J = 6.3 Hz, 6H, 2”-H); 13C-NMR (CDCl3) δ: 166.6 (s, 1-C), 158.7 (t, J = 5.7 Hz, 4′-C), 143.1 (s, 3-C), 132.1 (t, J = 1.9 Hz, 6′-C), 127.5 (s, 1′-C), 126.0 (t, J = 5.9 Hz, 2′-C), 123.4 (t, J = 22.3 Hz, 3′-C), 117.8 (s, 2-C), 111.4 (s, 5′-C), 111.2 (t, J = 236.3 Hz, CF2H), 67.9 (s, 1”-C), 56.0 (s, CH3O), 22.1 (s, 2”-C). HRMS: calcd for C14H17F2O3+ (M + H)+ requires m/z 271.1140, found m/z 271.1141.
3.2.27. N-(1-Methylethyl)-3-(difluoromethyl)-4-methoxybenzamide (23)
p-Methoxybenzoic acid (91 mg, 0.60 mmol) was dissolved in anhydrous dichloromethane (0.90 mL) and cooled to −40 °C. Then, dichloromethylmethyl ether (0.49 mL, 0.54 mmol) and a solution of TiCl4 (0.144 mL, 1.30 mmol) in anhydrous dichloromethane (0.3 mL) were added dropwise under continuous stirring. The deep red solution was stirred at −40 °C for 1.5 h and then 1M HCl (3 mL) added. The resulting emulsion was stirred at room temperature for 0.5 h and extracted with dichloromethane. The organic layer was dried with anhydrous sodium sulfate and the solvent evaporated. The resulting product was treated with Deoxofluor® 50% in toluene (0.107 mL, 0.435 mmol), isopropyl amine (0.037 mL, 0.435 mmol), and triethylamine (0.061 mL, 0.435 mmol) following the procedure used for 11b. Compound 23 was obtained as a white solid (0.025 g, 52% for 2 steps); m.p. 99–100 °C; 1H-NMR (CDCl3) δ: 7.97–7.93 (m, 1H, 6-H), 7.90 – 7.87 (m, 1H, 2-H), 7.00–6.95 (m, 1H, 5-H), 6.94 (t, J = 55.4 Hz, 1H, CF2H), 5.91 (d, J = 6.7 Hz, 1H, NH), 4.28 (dhept, J = 6.6, 7.6 Hz, 1′-H), 3.92 (s, 3H, CH3O), 1.28 (d, J = 6.6 Hz, 6H, 2′-H); 13C-NMR (CDCl3) δ: 165.5 (s, C(O)N), 159.6 (t, J = 5.6 Hz, 4-C), 131.8 (s, 6-C), 127.4 (s, 1-C), 124.7 (t, J = 5.9 Hz, 2-C), 122.5 (t, J = 22.3 Hz, 3-C), 111.3 (t, J = 236.4 Hz, CF2H), 111.0 (s, 5-C), 56.1 (s, CH3O), 42.1 (s, 1′-C), 23.0 (s, 2′-C). EIMS m/z (%): 243 (13, M+), 185 (100), 157 (4), 127 (4), 109 (2). Analysis for C12H15F2NO2: Calcd C, 59.25; H, 6.22; N, 5.76%. Found: C, 59.14; H, 6.23; N, 5.77%.
3.2.28. N-(1-Methylethyl)-7-(difluoromethyl)-6-methoxy-3,4-dihydronaphthalene-2-carboxamide (24)
6-Methoxy-3,4-dihydronaphthalene-2-carboxylic acid (50 mg, 0.25 mmol) was dissolved in anhydrous dichloromethane (0.38 mL) and cooled to −40 °C. Then, dichloromethylmethyl ether (0.49 mL, 0.54 mmol) and a solution of TiCl4 (60 µL, 0.54 mmol) in anhydrous dichloromethane (0.12 mL) were added dropwise under continuous stirring. The deep red solution was stirred at −40 °C for 1.5 h and then 1M HCl (3 mL) was added. The resulting emulsion was stirred at room temperature for 0.5 h and then extracted with dichloromethane. The organic layer was dried with anhydrous sodium sulfate and the solvent evaporated. The resulting product was treated with Deoxofluor® 50% in toluene (0.192 mL, 0.779 mmol), isopropylamine (0.066 mL, 0.779 mmol), and triethylamine (0.109 mL, 0.779 mmol) following the procedure used for 11b. Compound 24 was obtained as a white solid (0.027 g, 35% for 2 steps); mp 150–152 °C; 1H-NMR (CDCl3) δ: 7.36 (m, 1H, 8-H), 7.10–7.06 (m, 1H, 1-H), 6.90 (t, J = 55.7 Hz, 1H, CF2H), 6.75 (s, 1H, 5-H), 5.65 (d, J = 7.8 Hz, 1H, NH), 4.20 (dhept, J = 6.5, 7.1 Hz, 1′-H), 3.88 (s, 3H, CH3O), 2.89 (t, J = 8.2 Hz, 2H, 4-H), 2.57 (td, J = 1.5, 8.2 Hz, 2H, 3-H), 1.23 (d, J = 6.5 Hz, 6H, 2′-H); 13C-NMR (CDCl3) δ: 167.2 (s, C(O)N), 157.6 (t, J = 6.0 Hz, 6-C), 141.0 (t, J = 1.7 Hz, 10-C), 132.0 (s, 2-C), 129.6 (s, 1-C), 125.8 (s, 9-C), 125.8 (t, J = 5.7 Hz, 8-C), 121.1 (t, J = 22.4 Hz, 7-C), 111.5 (t, J = 235.5 Hz, CF2H), 110.7 (s, 5-C), 56.0 (s, CH3O), 41.7 (s, 1′-C), 28.5 (s, 4-C), 23.0 (s, 2′-C), 22.7 (s, 3-C). HRMS: calcd for C16H20F2NO2+ (M + H)+ requires m/z 296.1456, found m/z 296.1457.
3.2.29. N-(1-Methylethyl)-7-(difluoromethyl)-6-methoxy-naphthalene-2-carboxamide (25)
Compound 24 (10 mg, 0.034 mmol) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (11.5 mg, 0.05 mmol) were dissolved in dry toluene (0.8 mL) and the mixture was heated under reflux in an argon atmosphere for 1 h. Then, 5% NaHCO3 was added and the mixture extracted with dichloromethane, the organic layer was dried with anhydrous sodium sulfate, and the solvent evaporated. The residue was purified by flash column chromatography eluting with mixtures of hexane–ethyl acetate of increasing polarity to give compound 25 as a white solid (0.0085 g, 86%); m.p. 173–174 °C; 1H-NMR (CDCl3) δ: 8.22 (bs, 1H, 1-H), 8.11 (m, 1H, 8-H), 7.88 (dd, J = 1.8, 8.5 Hz, 1H, 3-H), 7.79 (d, J = 8.5 Hz, 1H, 4-H), 7.19 (s, 1H, 5-H), 7.02 (t, J = 55.3 Hz, 1H, CF2H), 6.06 (d, J = 7.6 Hz, 1H, NH), 4.35 (dhept, J = 6.6, 7.7 Hz, 1′-H), 4.00 (s, 3H, CH3O), 1.31 (d, J = 6.6 Hz, 6H, 2′-H); 13C-NMR (CDCl3) δ: 166.6 (s, C(O)N), 156.2 (t, J = 4.7 Hz, 6-C), 137.0 (s, 10-C), 131.0 (s, 2-C), 127.9 (t, J = 6.9 Hz, 8-C), 127.7 (s, 1-C), 127.1 (s, 4-C), 127.1 (s, 9-C), 125.9 (s, 3-C), 125.0 (t, J = 21.6 Hz, 7-C), 111.6 (t, J = 236.9 Hz, CF2H), 105.9 (s, 5-C), 55.9 (s, CH3O), 42.2 (s, 1′-C), 23.1 (s, 2′-C). HRMS: calcd for C16H18F2NO2+ (M + H)+ requires m/z 294.1307, found m/z 294.1300.
3.3. Biological Activity
3.3.1. Determination of the Minimum Inhibitory Concentration
The Minimum Inhibitory Concentration (MIC), defined as the lowest antimicrobial agent concentration inhibiting the visible growth of a microorganism following its incubation, was subsequently determined. For the purpose of such determination, the compounds under study were tested against Acinetobacter guillouiae ATCC 11171, Clostridium sporogenes ATCC 19404, Enterobacter cloacae ATCC 35587, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 8739, Klebsiella pneumoniae ATCC 10273, Mycobacterium smegmatis ATCC 607, Proteus mirabilis ATCC 10005, Pseudomonas aeruginosa ATCC 9027, Shigella boydii ATCC 12027, Shigella flexneri ATCC 11836, Staphylococcus aureus ATCC 29737, Streptococcus pneumoniae ATCC 10015, and Streptococcus pyogenes ATCC 8133 using Mueller–Hinton (MH) broth.
The MICs of the tested compounds were determined by microdilution with MH broth supplemented with Mg+2 and Ca+2 cations. A stock solution of the synthesized compounds (1 mg/mL) in DMSO was prepared and graded quantities of the test compounds were incorporated in specified quantity of sterilized liquid medium (MH, supplemented with defibrinated horse blood for S. pneumoniae and Tween 80 for M. smegmatis) to test different concentrations of the compounds. The test was conducted on a plate on the basis of a 1 × 105 CFU/mL inoculum concentration, with negative, positive, and sterility controls being simultaneously performed. Different concentrations of the compounds under study were tested. Following incubation at 32.5 ± 2.5 °C for a 24 h period (48 h in the case of C. sporogenes and 72 h in the case of M. smegmatis), the plates were visually read. After incubation, the MICs were visually determined by the presence or absence of visible growth [42,43].
3.3.2. Cytotoxicity Assay
Cytotoxicity of compounds 11b–11d, 11f–11g, 13a–13c, and 18a was measured using the MTT (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide; Sigma-Aldrich, USA) method [44], in A549 and HepG2 cells, respectively. Confluent cultures in 96-well plates were exposed to two-fold dilutions of the compounds, with three wells for each dilution, during 48 h of incubation at 37 °C. Then, 10 µL of Maintenance Medium (MM) containing MTT (final concentration 5 mg/mL) was added to each well. After 2 h of incubation, the supernatant was decanted and 200 µL ethanol were added to each well to solubilize the formazan crystals. After vigorous shaking, absorbance was measured in a microplate reader and cytotoxicity was calculated as the cytotoxic concentration 50% (CC50), i.e., the compound concentration required to reduce the MTT signal by 50% compared to controls.
4. Conclusions
Our results show that the combination of the difluoromethyl group and the p-methoxycinamyl scaffold provided both selectivity and increased activity towards M. smegmatis, with a high dependency on the characteristics of the substituent on the amide nitrogen. The most active compounds (11b, 11d, and 11g) had an activity similar to that of the reference antibiotic, exhibited very low cytotoxicity, and were inactive against the other bacteria assayed. This makes them potential leads for the development of narrow spectrum antibiotics against M. tuberculosis, where long-term treatments with broad spectrum antibiotics entail an increased risk of damaging the gut microbiota and promoting the appearance of resistant genes [45].
5. Patents
The following patents have been granted: Compounds having antibacterial activity process for their preparation and pharmaceutical compositions comprising them, G. Burton, F. J. Durán, M. D. Martinez, E. Zini, V. Mora Muñoz, L. Bertoncello, (CONICET—Laboratorios Richmond), US 9,255,071 B2 (2016); EP 2,802,558 B1 (2016).
Supplementary Materials
The following are available online. Synthetic procedures and characterization of precursors 12b, S1, S2, and S3. Table S1: Calculated LogP of compounds 11a–11m, 13a–11c, 15, 18a, 18b, and 19–25. Mass spectra for compounds 9, 11a–11m, 13a–13c, 15, 17, 18a, 18b, 19–25, and S2. 1H and 13C-NMR spectra of compounds 9, 10, 11a–11m, 13a–13c, 15, 17, 18a, 18b, 19–25, S2, and S3.
Author Contributions
Conceptualization, F.J.D., and G.B.; Data curation, M.D.M.; Formal analysis, M.D.M., D.A.R., and C.G.; Funding acquisition, F.J.D. and G.B.; Investigation, M.D.M., D.A.R., and C.G; Methodology, M.D.M., C.G., F.J.D., and G.B.; Project administration, G.B.; Resources, G.B.; Supervision, F.J.D. and G.B.; Visualization, M.D.M. and G.B.; and Writing—review and editing, M.D.M., F.J.D., and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONICET (PIP 11220110100896CO) and a research agreement “CONICET (Argentina)—Laboratorios Richmond”.
Acknowledgments
We thank Verónica Mora Muñoz (Laboratorios Richmond) for performing the antibacterial assays.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The antibacterial assays were performed by Laboratorios Richmond (see acknowledgments).
References
- Theuretzbacher, U. Global antibacterial resistance: The never-ending story. J. Glob. Antimicrob. Resist. 2013, 1, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr. Med. Chem. 2015, 22, 2590–2614. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.F.; Mobashery, S. Endless resistance. Endless antibiotics? MedChemComm 2016, 7, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Cars, O.; Hedin, A.; Heddini, A. The global need for effective antibiotics—Moving towards concerted action. Drug Resist. Update 2011, 14, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Freire-Moran, L.; Aronsson, B.; Manz, C.; Gyssens, I.C.; So, A.D.; Monnet, D.L.; Cars, O. Critical shortage of new antibiotics in development against multidrug-resistant bacteria—Time to react is now. Drug Resist. Update 2011, 14, 118–124. [Google Scholar] [CrossRef]
- Spagnuolo, L.A.; Tonge, P.J.; Fisher, S.L. Narrow Spectrum Antibacterial Agents. In Antibiotic Drug Discovery: New Targets and Molecular Entities; Firestine, S.M., Lister, T., Eds.; The Royal Society of Chemistry: London, UK, 2017; Volume 58, pp. 76–102. [Google Scholar]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Budryn, G.; Rachwal-Rosiak, D. Interactions of Hydroxycinnamic Acids with Proteins and Their Technological and Nutritional Implications. Food Rev. Int. 2013, 29, 217–230. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.; Benfeito, S.; Soares, P.; Magalhães e Silva, D.; Loureiro, J.; Borges, A.; Borges, F.; Simões, M. Fine-tuning of the hydrophobicity of caffeic acid: studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. RSC Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef]
- Neelam, A.K.; Sharma, K.K. Phenylpropanoids and its derivatives: biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2019, 1–21. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Yoya, G.K.; Bedos-Belval, F.; Constant, P.; Duran, H.; Daffé, M.; Baltas, M. Synthesis and evaluation of a novel series of pseudo-cinnamic derivatives as antituberculosis agents. Bioorg. Med. Chem. Lett. 2009, 19, 341–343. [Google Scholar] [CrossRef]
- Fu, J.; Cheng, K.; Zhang, Z.m.; Fang, R.q.; Zhu, H.l. Synthesis, structure and structure-activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur. J. Med. Chem. 2010, 45, 2638–2643. [Google Scholar] [CrossRef]
- Yingyongnarongkul, B.e.; Apiratikul, N.; Aroonrerk, N.; Suksamrarn, A. Solid-phase synthesis and antibacterial activity of hydroxycinnamic acid amides and analogues against methicillin-resistant Staphylococcus aureus and vancomycin-resistant S. aureus. Bioorg. Med. Chem. Lett. 2006, 16, 5870–5873. [Google Scholar] [CrossRef]
- De, P.; Koumba Yoya, G.; Constant, P.; Bedos-Belval, F.; Duran, H.; Saffon, N.; Daffé, M.; Baltas, M. Design, synthesis, and biological evaluation of new cinnamic derivatives as antituberculosis agents. J. Med. Chem. 2011, 54, 1449–1461. [Google Scholar] [CrossRef]
- Carvalho, S.A.; da Silva, E.F.; de Souza, M.V.N.; Lourenço, M.C.S.; Vicente, F.R. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 538–541. [Google Scholar] [CrossRef]
- De, P.; De, K.; Veau, D.; Bedos-Belval, F.; Chassaing, S.; Baltas, M. Recent advances in the development of cinnamic-like derivatives as antituberculosis agents. Expert Opin. Ther. Pat. 2012, 22, 155–168. [Google Scholar] [CrossRef]
- Erickson, J.A.; McLoughlin, J.I. Hydrogen bond donor properties of the difluoromethyl group. J. Org. Chem. 1995, 60, 1626–1631. [Google Scholar] [CrossRef]
- Gu, Y.; Kar, T.; Scheiner, S. Fundamental properties of the CH ··· O interaction: Is it a true hydrogen bond? J. Am. Chem. Soc. 1999, 121, 9411–9422. [Google Scholar] [CrossRef]
- Zafrani, Y.; Sod-Moriah, G.; Yeffet, D.; Berliner, A.; Amir, D.; Marciano, D.; Elias, S.; Katalan, S.; Ashkenazi, N.; Madmon, M.; et al. CF2H, a Functional Group-Dependent Hydrogen-Bond Donor: Is It a More or Less Lipophilic Bioisostere of OH, SH, and CH3? J. Med. Chem. 2019, 62, 5628–5637. [Google Scholar] [CrossRef] [PubMed]
- Dalvit, C.; Invernizzi, C.; Vulpetti, A. Fluorine as a Hydrogen-Bond Acceptor: Experimental Evidence and Computational Calculations. Chem. Eur. J. 2014, 20, 11058–11068. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.D.; Luna, L.; Tesio, A.Y.; Feresin, G.E.; Duran, F.J.; Burton, G. Antioxidant properties in a non-polar environment of difluoromethyl bioisosteres of methyl hydroxycinnamates. J. Pharm. Pharmacol. 2016, 68, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Toteva, M.M.; Richard, J.P. The generation and reactions of quinone methides. In Advances in Physical organic Chemistry; Richard, J., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 45, pp. 39–91. [Google Scholar]
- Hathaway, B.A.; White, K.L.; McGill, M.E. Comparison of iodination of methoxylated benzaldehydes and related compounds using iodine/silver nitrate and iodine/periodic acid. Synth. Commun. 2007, 37, 3855–3860. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, Y.J.; Bae, J.W.; Yoon, C.M. An efficient and convenient synthesis of 5-formylsalicylaldehyde. Synth. Commun. 2000, 30, 1003–1008. [Google Scholar] [CrossRef]
- Mahé, O.; Desroches, J.; Paquin, J.F. Amide formation using in situ activation of carboxylic acids with [Et 2NSF2]BF4. Eur. J. Org. Chem. 2013, 4325–4331. [Google Scholar] [CrossRef]
- Tunoori, A.R.; White, J.M.; Georg, G.I. A one-flask synthesis of weinreb amides from chiral and achiral carboxylic acids using the deoxo-fluor fluorinating reagent. Org. Lett. 2000, 2, 4091–4093. [Google Scholar] [CrossRef]
- Singh, R.P.; Shreeve, J.M. One-pot route to new α,α-difluoroamides and α-ketoamides. J. Org. Chem. 2003, 68, 6063–6065. [Google Scholar] [CrossRef]
- White, J.M.; Tunoori, A.R.; Turunen, B.J.; Georg, G.I. [Bis(2-methoxyethyl)amino]sulfur Trifluoride, the Deoxo-Fluor Reagent: Application toward One-Flask Transformations of Carboxylic Acids to Amides. J. Org. Chem. 2004, 69, 2573–2576. [Google Scholar] [CrossRef]
- Kangani, C.O.; Kelley, D.E.; Day, B.W. One-pot synthesis of aldehydes or ketones from carboxylic acids via in situ generation of Weinreb amides using the Deoxo-Fluor reagent. Tetrahedron Lett. 2006, 47, 6289–6292. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Minrovic, B.M.; Melander, R.J.; Melander, C. Identification of Anti-Mycobacterial Biofilm Agents Based on the 2-Aminoimidazole Scaffold. ChemMedChem 2019, 14, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M.; Lieutaud, A.; Lome, V.; Pagès, J.-M.; Bolla, J.-M. Polyamino geranic derivatives as new chemosensitizers to combat antibiotic resistant Gram-negative bacteria. Bioorg. Med. Chem. 2013, 21, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Doherty, E.M.; Fotsch, C.; Bo, Y.; Chakrabarti, P.P.; Chen, N.; Gavva, N.; Han, N.; Kelly, M.G.; Kincaid, J.; Klionsky, L.; et al. Discovery of Potent, Orally Available Vanilloid Receptor-1 Antagonists. Structure−Activity Relationship of N-Aryl Cinnamides. J. Med. Chem. 2004, 48, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Rodriques, K.E. A novel route to cyclopropyl ketones, aldehydes, and carboxylic acids. Tetrahedron Lett. 1991, 32, 1275–1278. [Google Scholar] [CrossRef]
- Rieche, A.; Gross, H.; Höft, E. Über α-Halogenäther, IV. Synthesen aromatischer Aldehyde mit Dichlormethyl-alkyläthern. Chem. Ber. 1960, 93, 88–94. [Google Scholar] [CrossRef]
- Yakubov, A.P.; Tsyganov, D.V.; Belen’kii, L.I.; Krayushkin, M.M. Formylation and dichloromethylation as alternative directions of Rieche reaction. A novel to the synthesis of sterically hindered aromatic dialdehydes. Tetrahedron 1993, 49, 3397–3404. [Google Scholar] [CrossRef]
- Calderwood, E.F.; England, D.B.; Gould, A.E.; Harrison, S.J.; Ma, L. Substituted Hydroxamic Acids and Uses Thereof. US 20120015942A, 19 January 2012. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-spectrum antibacterial agents. MedChemComm 2018, 9, 12–21. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).