Design, Synthesis, and Antimicrobial Activities of 1,2,3-Triazole Glycoside Clickamers
Abstract
1. Introduction
2. Results and Discussion
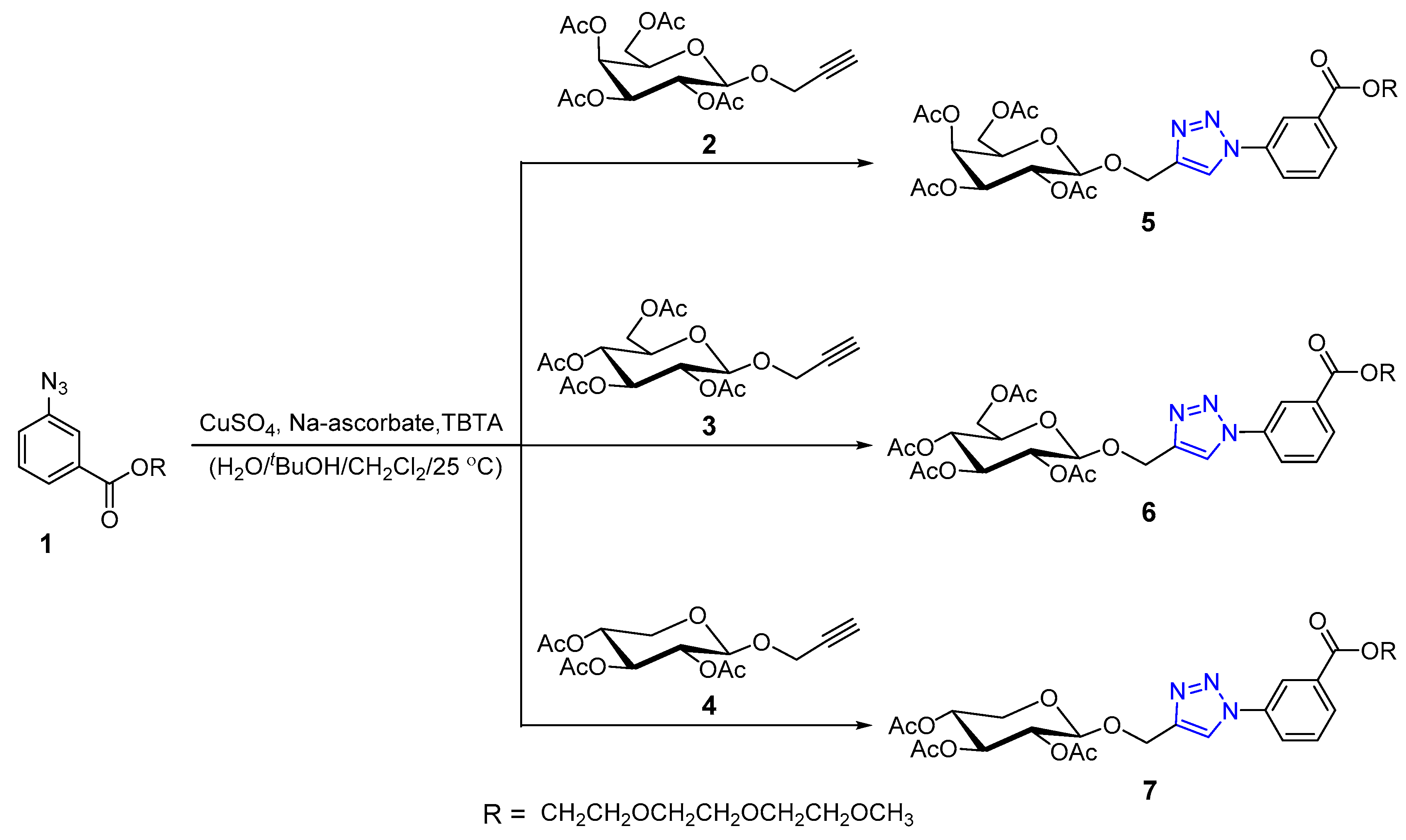
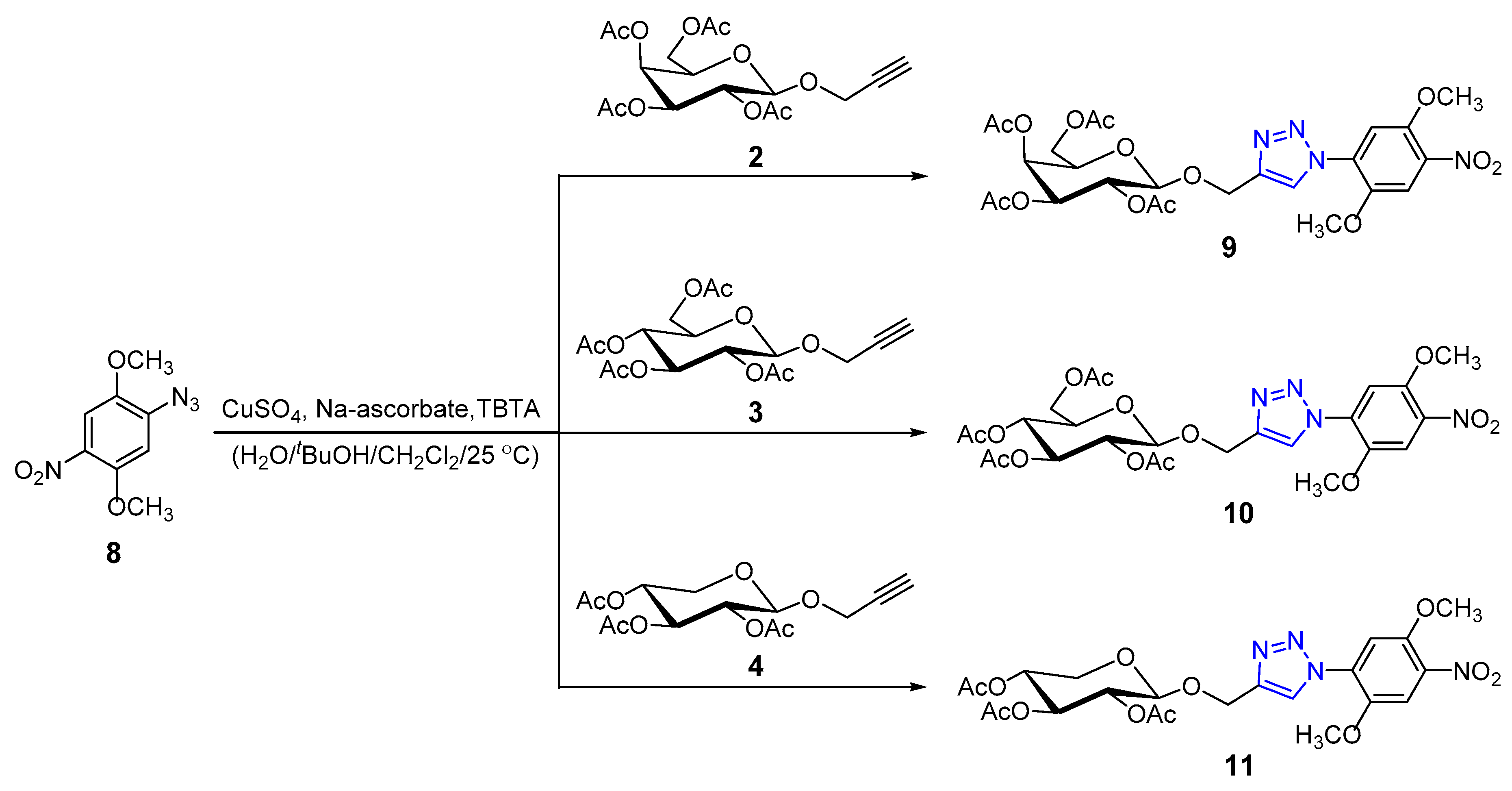
2.1. Chemistry
Synthesis and Characterization
2.2. Antimicrobial Activity
2.2.1. Toxicity Evaluation of the Studied Compounds
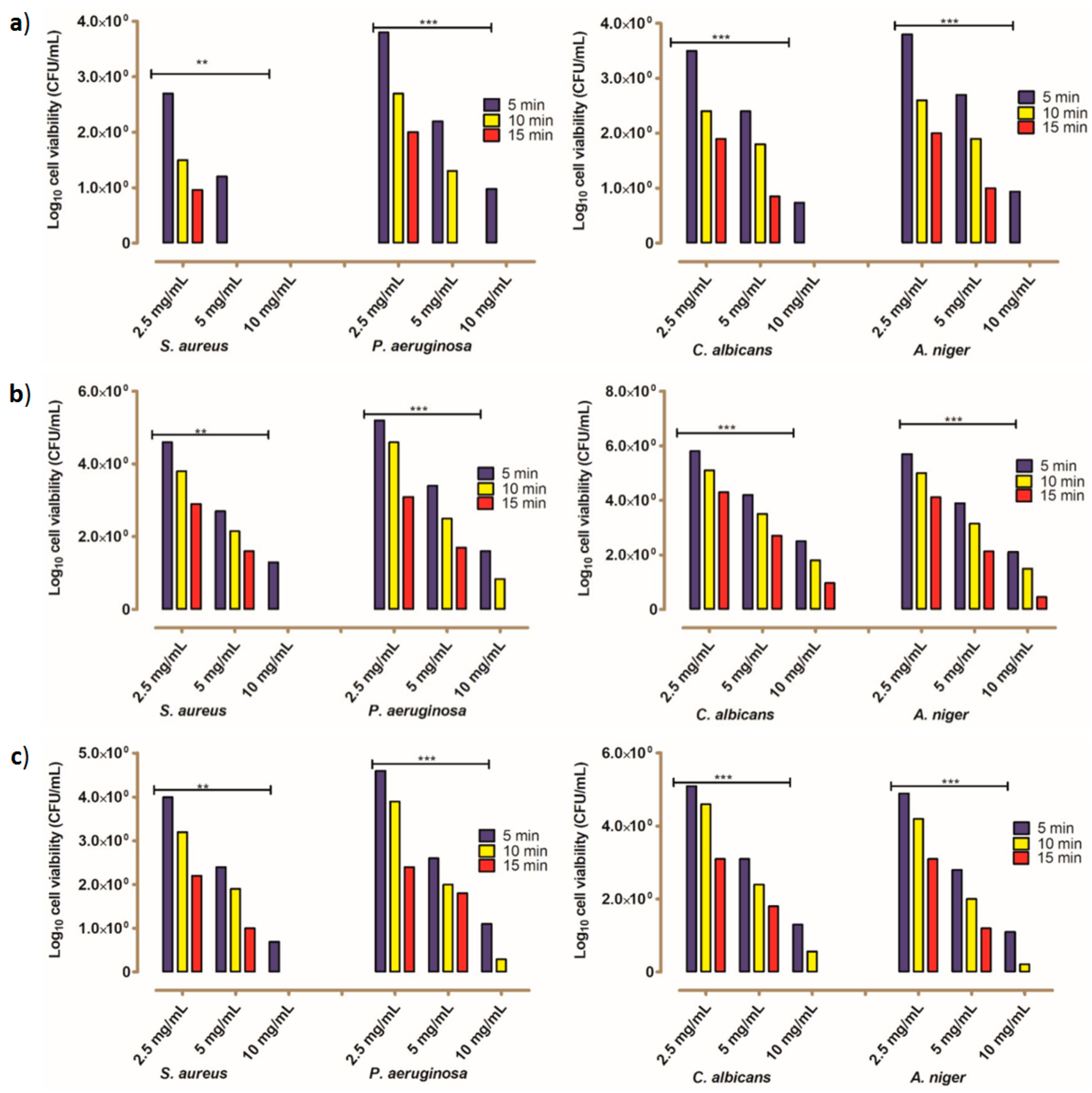
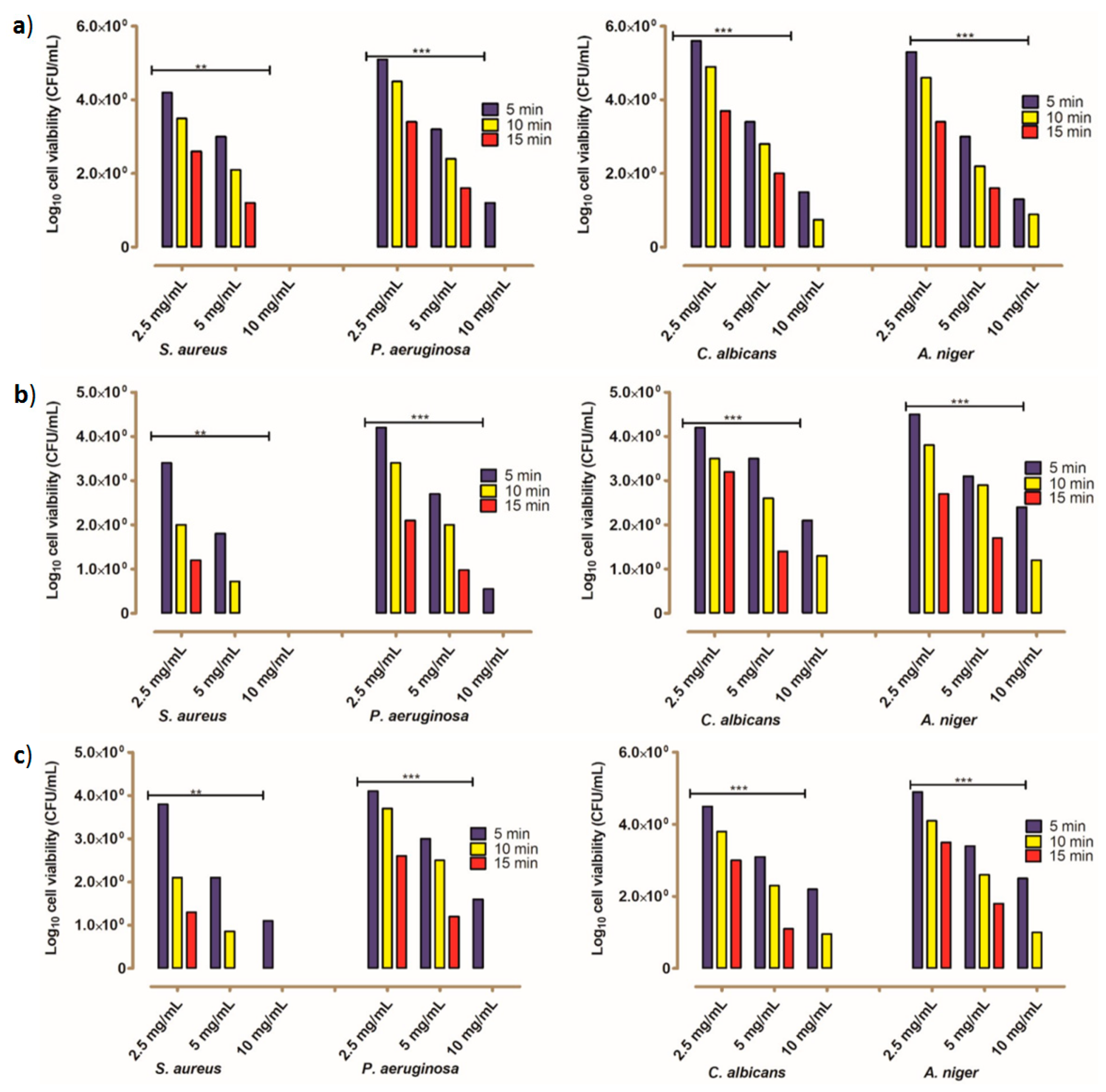
2.2.2. The Kinetic Modeling of the Killing Rate of the Tested Microbes
3. Experimental
3.1. Chemistry
3.1.1. General Procedures
3.1.2. General Click Chemistry Procedure
3.1.3. (2R,3R,4S,5S,6R)-2-((1-(3-(2,5,8,11-tetraoxadodecanoyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)-6-(a-cetoxymethyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (5)
3.1.4. (2R,3R,4S,5R,6R)-2-((1-(3-(2,5,8,11-tetraoxadodecanoyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)-6-(a-cetoxymethyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (6)
3.1.5. (2R,3R,4S,5R)-2-((1-(3-(2,5,8,11-tetraoxadodecanoyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)tetrahyd-ro-2H-pyran-3,4,5-triyl triacetate (7)
3.1.6. (2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-((1-(2,5-dimethoxy-4-nitrophenyl)-1H-1,2,3-triazol-4-yl)meth-oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9)
3.1.7. (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-((1-(2,5-dimethoxy-4-nitrophenyl)-1H-1,2,3-triazol-4-yl)meth-oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10)
3.1.8. (2R,3R,4S,5R)-2-((1-(2,5-dimethoxy-4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)tetrahydro-2H-py-ran-3,4,5-triyl triacetate (11)
3.2. Biology
3.2.1. Preparation of Stock Solutions for Each Studied Designed Compound
3.2.2. Bacterial Cultures and Growth Conditions
3.2.3. Antimicrobial Activity Assay
3.2.4. Minimum Inhibitory Concentration (MIC)
3.2.5. Estimation of Decay Rate Using Pseudo-First-Order Kinetic Modeling
3.2.6. Toxicity Valuation of the Tested Compounds
3.2.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef] [PubMed]
- von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Ochman, H.; Lawrence, J.G.; Groisman, E.A. Lateral gene transfer and the nature of bacterial innovation. Nature 2000, 405, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.; Bhattacharyya, S. Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 2019, 9, 9788. [Google Scholar] [CrossRef]
- Woodford, N.; Ellington, M.J. The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect. 2007, 13, 5–18. [Google Scholar] [CrossRef]
- Levy, S.B.; Bergman, M.M. The antibiotic paradox: How the misuse of antibiotics destroys their curative powers. In Clinical Infectious Diseases, 2nd ed.; Perseus Publishing: Boston, MA, USA, 2003. [Google Scholar]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Fuda, C.C.S.; Fisher, J.F.; Mobashery, S. β-Lactam resistance in Staphylococcus aureus: The adaptive resistance of a plastic genome. Cell. Mol. Life Sci. 2005, 62, 2617–2633. [Google Scholar] [CrossRef]
- Butler, M.S.; Hansford, K.A.; Blaskovich, M.A.T.; Halai, R.; Cooper, M.A. Glycopeptide antibiotics: Back to the future. J. Antibiot. 2014, 67, 631–644. [Google Scholar] [CrossRef]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of resistance to aminoglycoside antibiotics: Overview and perspectives. Med. Chem. Commun. 2016, 7, 11–27. [Google Scholar] [CrossRef]
- Gade, N.D.; Qazi, M.S. Fluoroquinolone therapy in Staphylococcus aureus infections: Where do we stand? J. Lab. Physicians 2013, 5, 109–112. [Google Scholar] [CrossRef]
- Singh, A.; Grover, S.; Sinha, S.; Das, M.; Somvanshi, P.; Grover, A. Mechanistic principles behind molecular mechanism of Rifampicin resistance in mutant RNA polymerase beta subunit of myco- bacterium tuberculosis. J. Cell. Biochem. 2017, 118, 4594–4606. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.A.; Manson, A.L.; Desjardins, C.A.; Abeel, T.; Earl, A.M. Deciphering drug resistance in Mycobacterium tuberculosis using whole-genome sequencing: Progress, promise, and challenges. Genome Med. 2019, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.C.; Martin, A. Drug resistance mechanisms in Mycobacterium tuberculosis. Antibiotics 2014, 3, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Singh, H.; Thangaraj, P.; Chakrabarti, A. Acinetobacter baumannii: A brief account of mechanisms of multidrug resistance and current and future therapeutic management. J. Clin. Diagn. Res. 2013, 7, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents–How P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G.; Bathini, R.; Manga, V. Dimers of coumarin-1,2,3-triazole hybrids bearing alkyl spacer: Design, microwave-assisted synthesis, molecular docking and evaluation as antimycobacterial and antimicrobial agents. J. Mol. Struct. 2018, 1157, 312–321. [Google Scholar] [CrossRef]
- Goud, G.L.; Ramesh, S.; Ashok, D.; Reddy, V.P.; Yogeeswari, P.; Sriram, D.; Saikrishna, B.; Manga, V. Design, synthesis, molecular-docking and antimycobacterial evaluation of some novel 1,2,3-triazolyl xanthenones. Med. Chem. Commun. 2017, 8, 559–570. [Google Scholar] [CrossRef]
- Ashok, D.; Chiranjeevi, P.; Kumar, A.V.; Sarasija, M.; Krishna, V.S.; Sriram, D.; Balasubramanian, S. 1,2,3-Triazole-fused spirochromenes as potential anti-tubercular agents: Synthesis and biological evaluation. RSC Adv. 2018, 8, 16997–17007. [Google Scholar] [CrossRef]
- Pogaku, V.; Krishna, V.S.; Balachandran, C.; Rangan, K.; Sriram, D.; Aoki, S.; Basavoju, S. The design and green synthesis of novel benzotriazoloquinolinyl spirooxindolopyrrolizidines: Antimycobacterial and antiproliferative studies. New J. Chem. 2019, 43, 17511–17520. [Google Scholar] [CrossRef]
- Phatak, P.S.; Bakale, R.D.; Dhumal, S.T.; Dahiwade, L.K.; Choudhari, P.B.; Krishna, V.S.; Sriram, D.; Haval, K.P. Synthesis, antitubercular evaluation and molecular docking studies of phthalimide bearing 1,2,3-triazoles. Synth. Commun. 2019, 49, 2017–2028. [Google Scholar] [CrossRef]
- Reddyrajula, R.; Dalimba, U.; Kumar, S.M. Molecular hybridization approach for phenothiazine incorporated 1,2,3-triazole hybrids as promising antimicrobial agents: Design, synthesis, molecular docking and in silico ADME studies. Eur. J. Med. Chem. 2019, 168, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Sajja, Y.; Vanguru, S.; Vulupala, H.R.; Bantu, R.; Yogeswari, P.; Sriram, D.; Nagarapu, L. Design, synthesis and in vitro antituberculosis activity of benzo [6,7]cyclohepta [1–b]pyridine-1,2,3-triazole derivatives. Bioorg. Med. Chem. 2017, 27, 5119–5121. [Google Scholar] [CrossRef]
- Shaikh, M.H.; Subhedar, D.D.; Nawale, L.; Sarkar, D.; Khan, F.A.K.; Sangshettic, J.N.; Shingate, B.B. 1,2,3-Triazole derivatives as antitubercular agents: Synthesis, biological evaluation and molecular docking study. Med. Chem. Commun. 2015, 6, 1104–1116. [Google Scholar] [CrossRef]
- El Malah, T.; Nour, H.F.; Dehbi, O.; Abdel-Megeid, F.M.E.; Essam El-Din Mahmoud, A.; Ali, M.M.; Soliman, S.M. Click synthesis, anticancer activity and molecular docking studies on pyridazinone scaffolds. Curr. Org. Chem. 2018, 22, 2300–2307. [Google Scholar] [CrossRef]
- El Malah, T.; Nour, H.F.; Nayl, A.A.; Elkhashab, R.A.; Abdel-Megeid, F.M.E.; Ali, M.M. Anticancer evaluation of tris(triazolyl)triazine derivatives generated via click chemistry. Aust. J. Chem. 2016, 69, 905–910. [Google Scholar] [CrossRef]
- Huang, R.-Z.; Liang, G.-B.; Li, M.-S.; Fang, Y.-L.; Zhao, S.-F.; Zhou, M.-M.; Liao, Z.-X.; Sun, J.; Wang, H.-S. Synthesis and discovery of asiatic acid based 1,2,3-triazole derivatives as antitumor agents blocking NF-κB activation and cell migration. Med. Chem. Commun. 2019, 10, 584–597. [Google Scholar] [CrossRef]
- Ruddarraju, R.R.; Murugulla, A.C.; Kotla, R.; Tirumalasetty, M.C.B.; Wudayagiri, R.; Donthabakthuni, S.; Maroju, R. Design, synthesis, anticancer activity and docking studies of theophylline containing 1,2,3-triazoles with variant amide derivatives. Med. Chem. Commun. 2017, 8, 176–183. [Google Scholar] [CrossRef]
- Khalil, H.; El Malah, T.; Abd El Maksoud, A.I.; El Halfawy, I.; El Rashedy, A.A.; El Hefnawy, M. Identification of novel and efficacious chemical compounds that disturb influenza A virus entry in vitro. Front. Cell. Infect. Microbiol. 2017, 7, 304. [Google Scholar] [CrossRef]
- Głowacka, I.E.; Andrei, G.; Schols, D.; Snoeck, R.; Piotrowska, D.G. Phosphonylated acyclic guanosine analogues with the 1,2,3-triazole linker. Molecules 2015, 20, 18789–18807. [Google Scholar] [CrossRef]
- Cheng, H.; Wan, J.; Lin, M.-I.; Liu, Y.; Lu, X.; Liu, J.; Xu, Y.; Chen, J.; Tu, Z.; Cheng, Y.-S.E.; et al. Design, synthesis, and in vitro biological evaluation of 1H-1,2,3-triazole-4-carboxamide derivatives as new anti-influenza A agents targeting virus nucleoprotein. J. Med. Chem. 2012, 55, 2144–2153. [Google Scholar] [CrossRef]
- He, Y.-W.; Dong, C.-Z.; Zhao, J.-Y.; Ma, L.-L.; Li, Y.-H.; Aisa, H.A. 1,2,3-Triazole-containing derivatives of rupestonic acid: Click-chemical synthesis and antiviral activities against influenza viruses. Eur. J. Med. Chem. 2014, 76, 245–255. [Google Scholar] [CrossRef]
- Kaoukabi, H.; Kabri, Y.; Curti, C.; Taourirte, M.; Rodriguez-Ubis, J.C.; Snoeck, R.; Andrei, G.; Vanelle, P.; Lazrek, H.B. Dihydropyrimidinone/1,2,3-triazole hybrid molecules: Synthesis and anti-varicella-zoster virus (VZV). Eur. J. Med. Chem. 2018, 155, 772–781. [Google Scholar] [CrossRef]
- Saeedi, M.; Mohammadi-Khanaposhtani, M.; Pourrabia, P.; Razzaghi, N.; Ghadimi, R.; Imanparast, S.; Faramarzi, M.A.; Bandarian, F.; Esfahani, E.N.; Safavi, M.; et al. Design and synthesis of novel quinazolinone-1,2,3-triazole hybrids as new antidiabetic agents: In vitro α-glucosidase inhibition, kinetic, and docking study. Bioorg. Chem. 2019, 83, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Avula, S.K.; Khan, A.; Rehman, N.U.; Anwar, M.U.; Al-Abri, Z.; Wadood, A.; Riaz, M.; Csuk, R.; Al-Harrasi, A. Synthesis of 1H-1,2,3-triazole derivatives as new α-glucosidase inhibitors and their molecular docking studies. Bioorg. Chem. 2018, 81, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.B.; Sodero, A.C.R.; Cardoso, M.F.C.; Lima, E.S.; Kaiser, C.R.; Silva, F.P., Jr.; Ferreira, V.F. Synthesis, biological activity, and molecular modeling studies of 1H-1,2,3-triazole derivatives of carbohydrates as α-glucosidases inhibitors. J. Med. Chem. 2010, 53, 2364–2375. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, D.R.; Santos, W.C.; Lima, E.S.; Ferreira, V.F. Synthesis of 1,2,3-triazole glycoconjugates as inhibitors of α-glucosidases. Carbohydr. Res. 2012, 350, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, C.P.; Luxmi, R.; Kumar, M.; Singh, D.; Kumar, K.; Pahwa, A. One-pot facile synthesis, crystal structure and antifungal activity of 1,2,3-triazoles bridged with amine-amide functionalities. Synth. Commun. 2019, 49, 118–128. [Google Scholar] [CrossRef]
- Dai, Z.-C.; Chen, Y.-F.; Zhang, M.; Li, S.-K.; Yang, T.-T.; Shen, L.; Wang, J.-X.; Qian, S.-S.; Zhue, H.-L.; Ye, Y.-H. Synthesis and antifungal activity of 1,2,3-triazole phenylhydrazone derivatives. Org. Biomol. Chem. 2015, 13, 477–486. [Google Scholar] [CrossRef]
- Akolkar, S.V.; Nagargoje, A.A.; Krishna, V.S.; Sriram, D.; Sangshetti, J.N.; Damaled, M.; Shingate, B.B. New N-phenylacetamide-incorporated 1,2,3-triazoles: [Et3NH][OAc]-mediated efficient synthesis and biological evaluation. RSC Adv. 2019, 9, 22080–22091. [Google Scholar] [CrossRef]
- Huo, X.-Y.; Guo, L.; Chen, X.-F.; Zhou, Y.-T.; Zhang, J.; Han, X.-Q.; Dai, B. Design, synthesis, and antifungal activity of novel aryl-1,2,3-triazole-β-carboline hybrids. Molecules 2018, 23, 1344. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Kummetha, I.R.; Singh, G.; Sharova, N.; Lichinchi, G.; Dang, J.; Stevenson, M.; Rana, T.M. 1,2,3-Triazoles as amide bioisosteres: Discovery of a new class of potent HIV-1 Vif antagonists. J. Med. Chem. 2016, 59, 7677–7682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, T.; Wu, G.; Kang, D.; Fu, Z.; Wang, Z.; De Clercq, E.; Pannecouque, C.; Zhan, P.; Liu, X. Targeting the hydrophobic channel of NNIBP: Discovery of novel 1,2,3-triazole-derived diarylpyrimidines as novel HIV-1 NNRTIs with high potency against wild-type and K103N mutant virus. Org. Biomol. Chem. 2019, 17, 3202–3321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, G.; Zalloum, W.A.; Meuser, M.E.; Dick, A.; Sun, L.; Chen, C.-H.; Kang, D.; Jing, L.; Jia, R.; et al. Discovery of novel 1,4-disubstituted 1,2,3-triazole phenyl- alanine derivatives as HIV-1 capsid inhibitors. RSC Adv. 2019, 9, 28961–28986. [Google Scholar] [CrossRef]
- da Silva, F.D.C.; de Souza, M.C.B.V.; Frugulhetti, I.I.P.; Castro, H.C.; Silmara, L.D.O.; de Souza, T.M.L.; Rodrigues, D.Q.; Souza, A.M.T.; Abreu, P.A.; Passamani, F.; et al. Synthesis, HIV-RT inhibitory activity and SAR of 1-benzyl-1H-1,2,3-triazole derivatives of carbohydrates. Eur. J. Med. Chem. 2009, 44, 373–383. [Google Scholar] [CrossRef]
- Rao, P.S.; Kurumurthy, C.; Veeraswamy, B.; Kumar, G.S.; Poornachandra, Y.; Kumar, C.G.; Vasamsetti, S.B.; Kotamraju, S.; Narsaiah, B. Synthesis of novel 1,2,3-triazole substituted-N-alkyl/aryl nitrone derivatives, their anti-inflammatory and anticancer activity. Eur. J. Med. Chem. 2014, 80, 184–191. [Google Scholar]
- Naaz, F.; Pallavi, M.C.P.; Shafic, S.; Mulakayala, N.; Yar, M.S.; Kumar, H.M.S. 1,2,3-triazole tethered Indole-3-glyoxamide derivatives as multiple inhibitors of 5-LOX, COX-2 & tubulin: Their anti-proliferative & antiinflammatory activity. Bioorg. Chem. 2018, 81, 1–20. [Google Scholar]
- Haider, S.; Alam, M.S.; Hamid, H.; Shafi, S.; Nargotra, A.; Mahajan, P.; Nazreen, S.; Kalle, A.M.; Kharbanda, C.; Ali, Y.; et al. Synthesis of novel 1,2,3-triazole based benzoxazolinones: Their TNF-α based molecular docking with in-vivo anti-inflammatory, antinociceptive activities and ulcerogenic risk evaluation. Eur. J. Med. Chem. 2013, 70, 579–588. [Google Scholar] [CrossRef]
- Ahmadi, F.; Ghayahbashi, M.R.; Sharifzadeh, M.; Alipoiur, E.; Ostad, S.N.; Vosooghi, M.; Khademi, H.R.; Amini, M. Synthesis and evaluation of anti-inflammatory and analgesic activities of new 1,2,4-triazole derivatives. Med. Chem. 2015, 11, 69–76. [Google Scholar] [CrossRef]
- Chu, X.-M.; Wang, C.; Wang, W.-L.; Liang, L.-L.; Liu, W.; Gong, K.-K.; Sun, K.-L. Triazole derivatives and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2019, 166, 206–223. [Google Scholar] [CrossRef]
- Batra, N.; Rajendran, V.; Agarwal, D.; Wadi, I.; Ghosh, P.C.; Gupta, R.D.; Nath, M. Synthesis and antimalarial evaluation of [1,2,3]-triazole-tethered sulfonamide-berberine hybrids. ChemistrySelect 2018, 3, 9790–9793. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Verdaguer, I.B.; Perez, C.; Banchio, C.; Ferreira de Azevedo, M.; Katzin, A.M.; Labadie, G.R. Repositioning Salirasib as a new antimalarial agent. Med. Chem. Commun. 2019, 10, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, C.P.; Pahwa, A. Convenient synthesis, antimalarial and antimicrobial potential of thioethereal 1,4-disubstituted 1,2,3-triazoles with ester functionality. Med. Chem. Res. 2018, 27, 458–469. [Google Scholar] [CrossRef]
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G. Microwave-assisted synthesis, antioxidant and antimicrobial evaluation of 2-indolinone-based bis-1,2,3-triazole derivatives. Mol. Divers. 2018, 22, 57–70. [Google Scholar] [CrossRef]
- Savegnago, L.; do Sacramento, M.; Brod, L.M.P.; Fronza, M.G.; Seus, N.; Lenardão, E.J.; Paixão, M.W.; Alves, D. Phenylselanyl-1H-1,2,3-triazole-4-carbonitriles: Synthesis, antioxidant properties and use as precursors to highly functionalized tetrazoles. RSC Adv. 2016, 6, 8021–8031. [Google Scholar] [CrossRef]
- Settypalli, T.; Chunduri, V.R.; Maddineni, A.K.; Begari, N.; Allagadda, R.; Kotha, P.; Chippada, A.R. Design, synthesis, in silico docking studies and biological evaluation of novel quinoxalinehydrazide hydrazone-1,2,3-triazole hybrids as α-glucosidase inhibitors and antioxidants. New J. Chem. 2019, 43, 15435–15452. [Google Scholar] [CrossRef]
- Saraei, M.; Ghasemi, Z.; Dehghan, G.; Hormati, M.; Ojaghi, K. Synthesis of some novel 1,2,3-triazole derivatives containing kojic acid moiety and evaluation for their antioxidant activity. Mon. Chem. 2017, 148, 917–923. [Google Scholar] [CrossRef]
- Singh, K.; Gangrade, A.; Jana, A.; Mandal, B.B.; Das, N. Design, synthesis, characterization, and anti- proliferative activity of organoplatinum compounds bearing a 1,2,3-triazole ring. ACS Omega 2019, 4, 835–841. [Google Scholar] [CrossRef]
- Kapkoti, D.S.; Singh, S.; Luqman, S.; Bhakuni, R.S. Synthesis of novel 1,2,3-triazole based artemisinin derivatives and their antiproliferative activity. New J. Chem. 2018, 42, 5978–5995. [Google Scholar] [CrossRef]
- Singh, A.; Saha, S.T.; Perumal, S.; Kaur, M.; Kumar, V. Azide-alkyne cycloaddition en route to 1H- 1,2,3-triazole-tethered isatin-ferrocene, ferrocenylmethoxy-isatin, and isatin-ferrocenylchalcone conjugates: Synthesis and antiproliferative evaluation. ACS Omega 2018, 3, 1263–1268. [Google Scholar] [CrossRef]
- Fu, D.-J.; Zhang, S.-Y.; Liu, Y.-C.; Yue, X.-X.; Liu, J.-J.; Song, J.; Zhao, R.-H.; Li, F.; Sun, H.-H.; Zhang, Y.-B.; et al. Design, synthesis and antiproliferative activity studies of 1,2,3-triazole-chalcones. Med. Chem. Commun. 2016, 7, 1664–1671. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Ay, K.; Ispartaloğlu, B.; Halay, E.; Ay, E.; Yaşa, İ.; Karayıldırım, T. Synthesis and antimicrobial evaluation of sulfanilamide- and carbohydrate-derived 1,4-disubstitued-1,2,3-triazoles via click chemistry. Med. Chem. Res. 2017, 26, 1497–1505. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-Triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Alminderej, F.M.; Elganzory, H.H.; El-Bayaa, M.N.; Awad, H.M.; El-Sayed, W.A. Synthesis and cytotoxic activity of new 1,3,4-thiadiazole thioglycosides and 1,2,3-triazolyl-1,3,4-thiadiazole N-glycosides. Molecules 2019, 24, 3738. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Abdel-Rahman, A.A.-H. Copper-catalyzed synthesis and antimicrobial activity of disubstituted 1,2,3-triazoles starting from 1-propargyluracils and ethyl (4-azido-1,2,3-trihydroxy- butyl)furan-3-carboxylate. Z. Naturforsch B. 2010, 65, 57–66. [Google Scholar] [CrossRef]
- Tolan, H.E.M.; El-Sayed, W.A.; Tawfek, N.; Abdel-Megeid, F.M.E.; Kutkat, O.M. Synthesis and anti-H5N1 virus activity of triazole- and oxadiazole-pyrimidine hybrids and their nucleoside analogs. Nucleosides Nucleotides Nucleic Acids 2019. [Google Scholar] [CrossRef]
- Kassem, A.F.; Abbas, E.M.H.; El-Kady, D.S.; Awad, H.M.; El-Sayed, W.A. Synthesis, docking studies and anticancer activity of new tetrazolyl- and (triazolyl)thiazole glycosides and acyclic analogs. Mini-Rev. Med. Chem. 2019, 19, 933–948. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Khalaf, H.S.; Mohamed, S.F.; Hssien, H.A.; Kutkat, O.M.; Amr, A.-E.E. Synthesis and antiviral activity of 1,2,3-triazole glycosides based substituted pyridine via click cycloaddition. Russ. J. Gen. Chem. 2017, 87, 2444–2453. [Google Scholar] [CrossRef]
- Elkanzia, N.A.A.; El-Sofany, W.I.; Gaballah, S.T.; Mohamed, A.M.; Kutkat, O.; El-Sayed, W.A. Synthesis, molecular modeling, and antiviral activity of novel triazole nucleosides and their analogs. Russ. J. Gen. Chem. 2019, 89, 1896–1904. [Google Scholar] [CrossRef]
- Elbatanony, M.M.; El-Feky, A.M.; Hemdan, B.A.; El-Liethy, M.A. Assessment of the antimicrobial activity of the lipoidal and pigment extracts of Punica granatum L. leaves. Acta Ecol. Sin. 2019, 39, 89–94. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- El Nahrawy, A.M.; Abou Hammad, A.B.; Bakr, A.M.; Hemdan, B.A.; Wassel, A.R. Decontamination of ubiquitous harmful microbial lineages in water using an innovative Zn2Ti0.8Fe0.2O4 nanostructure: Dielectric and terahertz properties. Heliyon 2019, 5, e02501. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.W.; El-Hotaby, W.; Hemdan, B.; Abdel-Fattah, W.I. Thermosensitive chitosan/phosphate hydrogel-composites fortified with Ag versus Ag@Pd for biomedical applications. Life Sci. 2018, 194, 185–195. [Google Scholar] [CrossRef]
- Flokstra, B.R.; Aken, B.V.; Shcnoor, J.L. Microtox® toxicity test: Detoxification of TNT and RDX contaminated solutions by poplar tissue cultures. Chemospher 2008, 71, 1970–1976. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Tested Compounds | Diffusion Assays | Inhibition Zone Diameters (mm) | |||
|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | C. albicans | A. niger | ||
| 5 | Disc | 21 ± 0.25 | 17 ± 0.21 | 15 ± 0.22 | 14 ± 0.25 |
| Well | 25 ± 0.15 | 19 ± 0.32 | 17 ± 0.15 | 16 ± 0.18 | |
| 6 | Disc | 10 ± 0.23 | 8 ± 0.25 | 7 ± 0.23 | 6 ± 0.15 |
| Well | 12 ± 0.21 | 10 ± 0.27 | 9 ± 0.17 | 8 ± 0.24 | |
| 7 | Disc | 13 ± 0.11 | 10 ± 0.21 | 8 ± 0.26 | 9 ± 0.22 |
| Well | 16 ± 0.23 | 13 ± 0.24 | 10 ± 0.18 | 11 ± 0.27 | |
| 9 | Disc | 14 ± 0.28 | 10 ± 0.15 | 8 ± 0.18 | 9 ± 0.24 |
| Well | 16 ± 0.19 | 12 ± 0.31 | 10 ± 0.26 | 10 ± 0.29 | |
| 10 | Disc | 16 ± 0.28 | 12 ± 0.15 | 11 ± 0.18 | 10 ± 0.24 |
| Well | 19 ± 0.21 | 16 ± 0.31 | 13 ± 0.26 | 12 ± 0.29 | |
| 11 | Disc | 18 ± 0.23 | 15 ± 0.11 | 13 ± 0.31 | 11 ± 0.17 |
| Well | 21 ± 0.18 | 18 ± 0.27 | 16 ± 0.28 | 14 ± 0.21 | |
| Ampicillin | Disc | 22 ± 0.23 | 18 ± 0.23 | 0 | 0 |
| Well | 24 ± 0.19 | 20 ± 0.19 | 0 | 0 | |
| Nystatin | Disc | 0 | 0 | 19 ± 0.15 | 14 ± 0.21 |
| Well | 0 | 0 | 22 ± 0.20 | 16 ± 0.23 | |
| Studied Designed Compounds | Targeted Microbes | Estimated Values of MIC in mg/mL | |
|---|---|---|---|
| Concentration (mg/mL) | Contact Time (min) | ||
| 5 | S. aureus | 5 | 10 |
| P. aeruginosa | 5 | 15 | |
| C. albicans | 10 | 10 | |
| A. niger | 10 | 10 | |
| 6 | S. aureus | 10 | 10 |
| P. aeruginosa | 10 | 15 | |
| C. albicans | 10 | >15 | |
| A. niger | 10 | >15 | |
| 7 | S. aureus | 10 | 10 |
| P. aeruginosa | 10 | 15 | |
| C. albicans | 10 | 15 | |
| A. niger | 10 | 15 | |
| 9 | S. aureus | 10 | 5 |
| P. aeruginosa | 10 | 10 | |
| C. albicans | 10 | 15 | |
| A. niger | 10 | 15 | |
| 10 | S. aureus | 5 | 15 |
| P. aeruginosa | 10 | 10 | |
| C. albicans | 10 | 15 | |
| A. niger | 10 | 15 | |
| 11 | S. aureus | 5 | 15 |
| P. aeruginosa | 10 | 10 | |
| C. albicans | 10 | 15 | |
| A. niger | 10 | 15 | |
| Ampicillin | S. aureus | 2.5 | 10 |
| P. aeruginosa | 5 | 10 | |
| C. albicans | >10 | >15 | |
| A. niger | >10 | >15 | |
| Nystatin | S. aureus | >10 | >15 |
| P. aeruginosa | >10 | >15 | |
| C. albicans | 2.5 | 15 | |
| A. niger | 5 | 15 | |
| Studied Compounds | EC50% Concentrations | EC50% Degree | Toxicity Level |
|---|---|---|---|
| 5 | 204 | 0–19 | Extremely toxic |
| 6 | 320 | 20–39 | Very toxic |
| 7 | 245 | 40–59 | Toxic |
| 9 | 285 | 60–79 | Moderately toxic |
| 10 | 195 | ≥100 | Nontoxic and safe |
| 11 | 232 | ||
| Ampicillin | 292 | ||
| Nystatin | 254 |
| Studied Designed Compounds | Targeted Microbes | Kinetic Modeling | |
|---|---|---|---|
| K1 (min−1) | R2 | ||
| 5 | S. aureus | 0.0918 | 0.9983 |
| P. aeruginosa | 0.0743 | 0.9912 | |
| C. albicans | 0.0657 | 0.9818 | |
| A. niger | 0.0613 | 0.9824 | |
| 6 | S. aureus | 0.0412 | 0.9947 |
| P. aeruginosa | 0.0215 | 0.9915 | |
| C. albicans | 0.0201 | 0.9832 | |
| A. niger | 0.0198 | 0.9872 | |
| 7 | S. aureus | 0.0515 | 0.9963 |
| P. aeruginosa | 0.0269 | 0.9950 | |
| C. albicans | 0.0315 | 0.9867 | |
| A. niger | 0.0342 | 0.9836 | |
| 9 | S. aureus | 0.0558 | 0.9737 |
| P. aeruginosa | 0.0247 | 0.9931 | |
| C. albicans | 0.0379 | 0.9812 | |
| A. niger | 0.0354 | 0.9841 | |
| 10 | S. aureus | 0.0816 | 0.9912 |
| P. aeruginosa | 0.0686 | 0.9894 | |
| C. albicans | 0.0548 | 0.9881 | |
| A. niger | 0.0526 | 0.9862 | |
| 11 | S. aureus | 0.0845 | 0.9986 |
| P. aeruginosa | 0.0676 | 0.9927 | |
| C. albicans | 0.0593 | 0.9880 | |
| A. niger | 0.0517 | 0.9832 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Malah, T.; Nour, H.F.; Satti, A.A.E.; Hemdan, B.A.; El-Sayed, W.A. Design, Synthesis, and Antimicrobial Activities of 1,2,3-Triazole Glycoside Clickamers. Molecules 2020, 25, 790. https://doi.org/10.3390/molecules25040790
El Malah T, Nour HF, Satti AAE, Hemdan BA, El-Sayed WA. Design, Synthesis, and Antimicrobial Activities of 1,2,3-Triazole Glycoside Clickamers. Molecules. 2020; 25(4):790. https://doi.org/10.3390/molecules25040790
Chicago/Turabian StyleEl Malah, Tamer, Hany F. Nour, Amira A. E. Satti, Bahaa A. Hemdan, and Wael A. El-Sayed. 2020. "Design, Synthesis, and Antimicrobial Activities of 1,2,3-Triazole Glycoside Clickamers" Molecules 25, no. 4: 790. https://doi.org/10.3390/molecules25040790
APA StyleEl Malah, T., Nour, H. F., Satti, A. A. E., Hemdan, B. A., & El-Sayed, W. A. (2020). Design, Synthesis, and Antimicrobial Activities of 1,2,3-Triazole Glycoside Clickamers. Molecules, 25(4), 790. https://doi.org/10.3390/molecules25040790






