Abstract
The synthesis of five hydroxycinnamoyl amides (HCAs) was accomplished and their identification and quantification in the green coffee bean samples of Coffea arabica, Coffea canephora, and Coffea liberica was performed. The HCAs p-coumaroyl-N-tyrosine 1b, caffeoyl-N-phenylalanine 2b, caffeoyl-N-tyrosine 3b, and p-coumaroyl-N-tryptophan 4b were characteristic of the C. canephora species while caffeoyl-N-tryptophan 5b was present in both C. canephora and C. arabica, but with higher content in C. canephora. The HCAs presence was also analyzed in C. liberica for the first time and none of the targeted compounds was found, indicating that this species is very similar to C. arabica species. Between C. canephora samples from various origins, significant differences were observed regarding the presence of all the HCAs, with C. canephora from Tanzania containing all five derivatives.
1. Introduction
Hydroxycinnamic acid conjugates are secondary metabolites widely present in the plant kingdom, such as endive (Cichorium endivia) [1], purple coneflower (Echinacea purpurea) [2], African star grass (Hypoxis hemerocallidea) [3], lady fern (Athyrium filix-femina) [4], Robusta coffee (Coffea canephora) [5], and cocoa (Theobroma cacao) [6]. Hydroxycinnamic acids can be esterified with quinic acid forming the class of chlorogenic acids, esterified with monosaccharides forming the cinnamoyl glycosides, or can give origin to amides with different amino acids, named hydroxycinnamoyl amides (HCAs) or N-phenylpropenoyl-amino acids. The hydroxycinnamoyl acids are usually conjugated with the amino acids phenylalanine, tyrosine, tryptophan, or aspartic acid, and although their role in the plant is not known, they exhibit biological activity, such as anti-neuroinflammatory activity [7], hepatoprotective activity [8], HIV-1 integrase inhibitors [9], and antidiabetic effects [10]. Moreover, from a sensory point of view, HCAs have been indicated as possible contributors to the sensory properties of some food [11].
More than 124 species are known of the genus Coffea and the two most important ones are Coffea arabica L., simply known as Arabica, and Coffea canephora Pierre ex Froehner, simply known as Robusta, which correspond to 57% and 43% of the international coffee trade, respectively, according to the International Coffee Organization (ICO, 2019). Coffea liberica Bull ex Hiern, contributing less than one per cent of the marketed coffee, is the third commercially exploited coffee species, well known for its larger cherries, when compared with those of C. arabica or C. canephora. C. liberica is less cultivated, due to its lower caffeine content (1.8% dry matter basis), and to its sensitivity to Fusarium xyloriodes, despite the fact that this species was of great economic importance during the 1930–1950 period [12]. Due to its low economic importance, C. liberica is also the less studied of the three considered species.
The HCAs can contribute to distinguish the two mainly commercially exploited coffee species, Arabica and Robusta, since in the literature it is shown that HCAs are manly present in the Robusta species [5,13,14,15,16,17,18]. Moreover, their identification could also contribute in differentiating the two species, depending on their geographical origin. To our knowledge, HCAs presence in C. liberica have not yet been evaluated and their analysis could contribute in the chemical characterization of this species. Caffeoyl-N-tryptophan was first isolated in 1987 by Takai et al. [19] from coffee beans of C. canephora from Java, while caffeoyl-N-tyrosine was first isolated in 1989 by Kellard et al. [20], from C. canephora from Angola. Successively, caffeoyl-N-phenylalanine, p-coumaroyl-N-tyrosine, and p-coumaroyl-N-tryptophan were identified in green coffee beans, analyzing both Robusta and Arabica coffee beans by LC-MS [5,14]. Since these HCAs are not commercially available, their quantification was determined by UV detection using the available standard 5-caffeoylquinic acid (5-CQA) and assuming a similar molar absorption coefficient for 5-CQA and each of the cinnamoyl amides [5]. Usually, chlorogenic acids are the major phenolic compounds present in coffee (30.0–44.4 g/kg) while caffeoyl-N-tyrosine and p-coumaroyl-N-tyrosine were estimated in lower amount (1.3–4.8 g/kg and 1.9–3.7 g/kg, respectively) [5].
HCAs are normally present in plants as enantiomerically enriched compounds, as found, for example, by Hofmann in cocoa beans [11] who isolated N-[3′,4′-dihydroxy-(E)-cinnamoyl]-L-aspartic acid and verified the absolute configuration of the amino acid by means of polarimetry. Murata et al. [17] isolated from coffee beans p-coumaroyl-N-tryptophan and demonstrated that L-tryptophan was part of the HCA.
In the present work, 23 samples of green coffee beans (10 of C. canephora, 10 of C. arabica, and 3 of C. liberica) of different geographical origin were analyzed by LC-MS using synthetized p-coumaroyl-N-tyrosine 1b, caffeoyl-N-phenylalanine 2b, caffeoyl-N-tyrosine 3b, p-coumaroyl-N-tryptophan 4b, and caffeoyl-N-tryptophan 5b. The availability of authentic standards is useful to unequivocally determine their presence in the examined green coffee samples and contribute in the identification of a chiral pool to distinguish the two commercially relevant species.
2. Results and Discussion
To identify and quantify the HCAs in green coffee beans, the amides 1b–5b were synthetized, which are the compounds of interest in differentiating the two coffee species (Figure 1). Different syntheses of the compounds are reported in the literature [6,9,11], but the best results have been obtained with the procedure of Wang et al. [7], which performed the condensation reaction between the hydroxycinnamic acid and the methyl ester amino acid in pyridine with DCC as the condensing agent. The hydroxycinnamoyl amides methyl esters were obtained from 41% to 78% yield after purification by flash chromatography. These results are in line with the results reported in literature using the same synthetic procedure [7], although for other HCAs. The deprotection reaction with LiOH was a crucial step, and each deprotection was performed at room temperature and was monitored by thin layer chromatography (TLC) in order to determine the end of the reaction, while in the literature, it was performed at 40 °C overnight [7]. In fact, for longer times, we observed a rapid degradation of the products. In this case, better yields were obtained with respect to the literature ranging from 56% to 94%, depending on the purification procedure. For compounds 1b–4b a recrystallization from petroleum ether was performed, while for 5b it was necessary to purify it by flash chromatography at the expense of a lower yield (56%). All products were amorphous solids. The complete synthesis is reported in Scheme 1. All compounds 1a–5a and 1b–5b were fully characterized by 1D and 2D 1H and 13C NMR using acetone-d6 as the solvent for all the products, while in the literature, different solvents were used (Figure 1). Moreover, chiroptical properties, as optical rotatory power and circular dichroism, were determined for 1b–5b. Circular Dichroism (CD) spectra are reported in Figure 2. Although the isolation of the pure compounds from coffee beans was not the purpose of this work, it was considered important to analyze the chiroptical properties of the synthetized compounds to evaluate, in the future, the correspondence of the chirality of the products with the one isolated from plants.
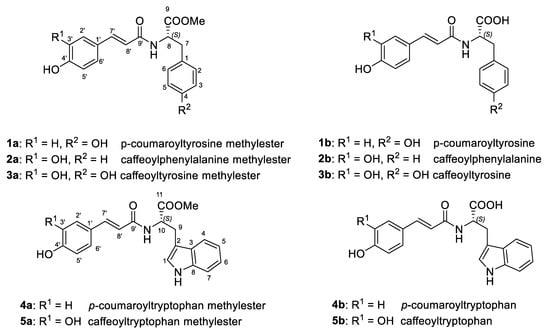
Figure 1.
Chemical structures of 1a–5a and 1b–5b.
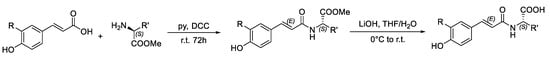
Scheme 1.
Synthesis of the hydroxycinnamoyl amides.
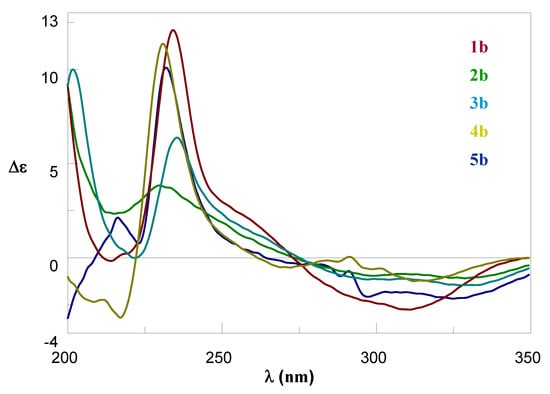
Figure 2.
CD spectra of hydroxycinnamoyl amides 1b–5b.
The synthetized HCAs where analyzed by LC-MS, both in positive and negative mode, to determine their retention time and obtain their mass spectra, in order to unequivocally determine their presence in green coffee bean extract (Table 1).

Table 1.
HPLC retention times, MS analysis, and specific rotation.
As can be seen in Figure 3, all mass spectra of HCAs 1b–5b present, besides the quasi molecular ion, an intense peak at higher m/z ratio, which can be attributed to a dimer that is formed during ionization. The structure of the dimer formed from 1b is reported in Figure 4 and the assignment of this structure is based on what was found by Yuan et al. in 2014 [8], who isolated these compounds from Abrus mollis Hance. Analogues dimers can be expected from the other cinnamoyl amides as confirmed by their m/z value.
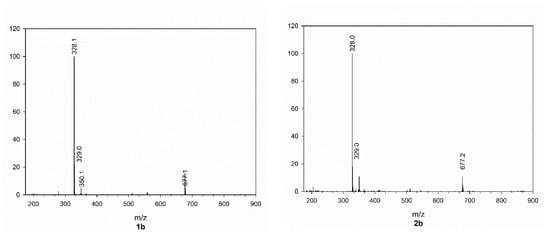
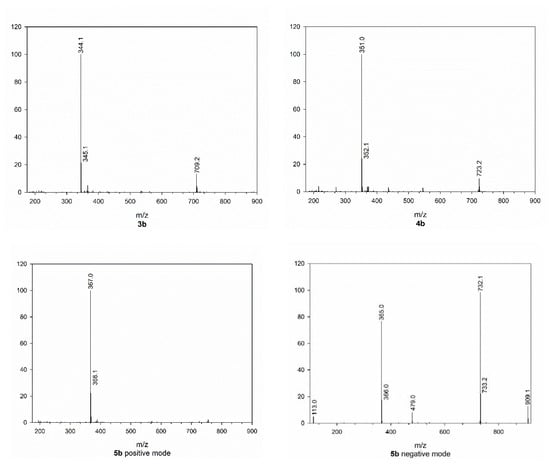
Figure 3.
Mass spectra of cinnamoyl amides 1b–5b.
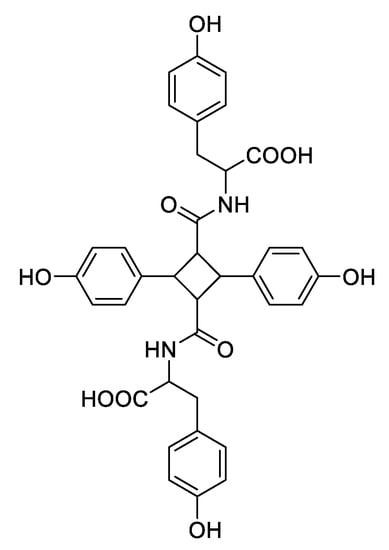
Figure 4.
Structure of 1b dimer.
Although only a few works reported the presence of HCAs in coffee beans, it was evidenced in the literature that these compounds were characteristic of the Robusta species [5,13,14,15,16,17,18]. In the present work, compounds 1b–5b have been quantified in green coffee beans of C. arabica and C. canephora of different geographical origin, and for the first time of C. liberica, using an aqueous extraction procedure, as was already used in our previous works for the quantification of chlorogenic acids [21,22].
Quantification of the HCAs 1b and 4b was reported to p-coumaric acid, and quantification of HCAs 2b, 3b, and 5b was reported to caffeic acid, as suggested by Alonso-Salces [14]. Results are summarized in Table 2 and are given on dry weight basis (DW). A 10% moisture content in green coffee beans was assumed, as it has been done before by others authors [23].

Table 2.
Concentrations of cinnamoyl amides in green coffee beans (mg/kg of DW, standard deviation in brackets).
As can be seen from Table 2, the cinnamoyl amides 1b–4b were present only in the Robusta species, although their presence depended on the geographical origin. Compounds 1b and 3b are not present in Robusta green coffee beans from India, Vietnam, and Ivory Coast, while only 3b is not present in Robusta from Cameroon. Although 1b and 2b have been detected in Robusta samples, they could not be quantified, since, in the mass spectra, two other mass peaks were present, indicating that the peak corresponding to the cinnamoyl amides 1b and 2b was overlapped with the one of two unknown compounds with mass spectra reported in Figure 5 (positive mode). For this reason, their quantification is not reported in Table 2, and is indicated as not quantifiable (n.q.).
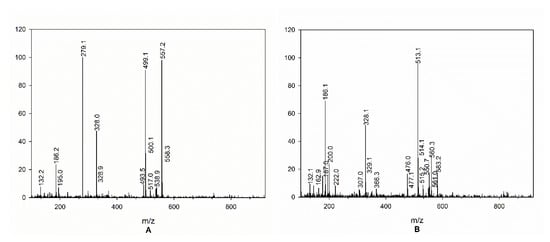
Figure 5.
Mass spectra of overlapped peaks of 1b (A) and 2b (B) with unknown compounds.
LC-MS analysis of C. liberica did not show the presence of any cinnamoyl amide, indicating that it is more similar to the Arabica species than to Robusta, but more samples of Liberica or different extraction methods are needed to verify that HCAs are completely absent in this species.
In Figure 6, the LC chromatograms referred to the Arabica sample from India, Robusta sample from Tanzania, and Liberica sample—lot 1, are reported as an example, which clearly show the differences between the three species regarding the content of HCAs.
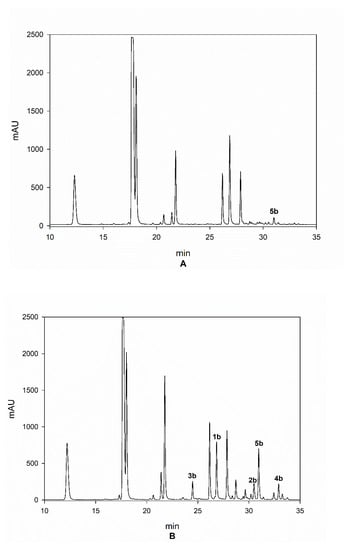
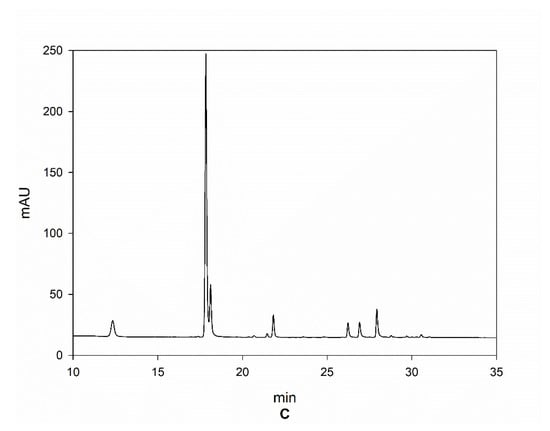
Figure 6.
Chromatograms of samples from Arabica India (A), Robusta Tanzania (B), and Liberica lot 1 (C).
In order to compare the results obtained in the present work with the ones reported in the literature, the extraction method used must be taken into consideration. All methods previously reported, regarding the extraction of HCAs, use an organic solvent, such as methanol [5,17], ethanol [16], or acetone [18], and sometimes, acidic conditions are used [13,14]. Only Rezende et al. [15] and Clifford et al. [24] used hot water for the extraction of HCAs and, in particular, Clifford analyzed the presence of 1b and 3b, also in coffee brew. Although the usage of an organic solvent can improve the extraction process, it was interesting to perform the extraction with only hot water to mimic the coffee preparation. The results obtained with this method will be compared in the future with the results obtained in the analysis of coffee brew, to highlight the presence of HCAs also in the final beverage. Nevertheless, results obtained in the present work can be related to the ones reported in the literature by Alonso-Salces [14] and Babova et al. [16] for the two varieties, Arabica and Robusta, although the extraction procedure was different. Moreover, 5-caffeoylquinic acid (5-CQA) is used as standard, assuming that there is a similar molar absorption coefficient for 5-CQA and each of the HCAs [5]; this aspect has to also be taken into consideration when comparing the results with literature data. The results obtained in the present work are in line with the results found by Alonso-Salces [14] since the same compounds for calibration curves were used. The 144 mg/kg of 4b and 789 of 5b as a mean value for Robusta samples were obtained, while Alonso-Salces found 225 mg/kg and 1557, respectively. These lower values are probably due to the extraction method, since Alonso-Salces used methanol/water/acetic acid 30:67.5:2.5. The results obtained are also in line with the results found by Babova et al. [16], who obtained 110 mg/kg for 4b and 400 mg/kg for 5b, although, using ethanol:water 50:50 as the extraction solvent.
3. Materials and Methods
3.1. Chemicals
L-tyrosine methyl ester, L-phenylalanine methyl ester, L-tryptophan methyl ester, caffeic acid, p-coumaric acid, N,N′-Dicyclohexylcarbodiimide (DCC), LiOH, silica gel were from Sigma-Aldrich (St. Louis, MO, USA). All solvents were used without further purification.
3.2. Instrumentation
NMR spectra were recorded on a Varian 400 (Agilent Technologies, Santa Clara, CA, USA) spectrometer using acetone-d6 as the solvent. Coupling constants are given in Hz. LC-MS analyses were run on an Agilent Infinity 1200 (Agilent Tech.), with autosampler and a diode array detector set at 320 nm, coupled with an Agilent Technologies 6120 Quadrupole (Agilent Tech.) equipped with an electrospray ionization source (ESI) operating in both positive and negative mode. The column was a Kinetex C18 (150 × 2.1 mm, 100 Å, 5 μm) (Phenomenex, Torrance, CA, USA), and the eluent was a mixture of water + 0.1% formic acid (FA) (v/v) (solvent A) and acetonitrile + 0.1% FA (v/v) (solvent B) with the gradient reported in Table 3. The flow was 0.25 mL/min.

Table 3.
HPLC gradient method.
Optical rotations were determined on a Jasco P2000 (Jasco, Tokyo, Japan) polarimeter at the wavelength of sodium D (λ = 589 nm) using a quartz cell of 1 dm length. Circular dichroism spectra were recorded on a Jasco J-710 spectropolarimeter using a quartz cell of 0.1 cm optical path.
3.3. Samples
A total of 23 samples of green coffee beans from different geographical origins and commercial lots, 10 of C. arabica, 10 of C. canephora, and 2 of C. liberica were analyzed. A wild C. liberica (named lot 3) was also used. In particular, all C. arabica samples were “wet processed” with zero primary and secondary defects, and the beverages prepared by using the samples were not affected by off-flavors (clean cup). C. canephora from India, all “in parchment”, were kindly supplied by Allanasons Private Limited (India), C. canephora var. Conilon “dry processed” from Brazil was kindly supplied by Fazendas Dutra (Brazil); all of the other C. canephora samples were kindly provided by Sandalj Trading Company S.p.A., Trieste (Italy). Two different lots of commercial C. liberica “dry processed” from India were kindly provided by Allanasons Private Limited (India), whereas C. liberica, named lot 3 from the Costa Rica CATIE germplasm (code T.03476) “in parchment”, was kindly provided by Dr. William Solano Sánchez (Agriculture, Livestock and Agroforestry Program, CATIE, Turrialba, Costa Rica).
3.4. General Procedure for the Preparation of the Hydroxycinnamoyl Amides Esters 1a–5a
The syntheses of all hydroxycinnamoyl amides were performed according to a literature procedure [7].
In a three-necked round bottom flask, 1.58 mmol of the amino acid were dissolved in 12.8 mL of anhydrous pyridine. 2.35 mmol of the hydroxycinnamic acid, and 2.28 mmol of DCC were subsequently added. The reaction mixture was stirred under argon atmosphere for 72 h. At the end of the reaction, the crude was filtered, and the liquid phase was acidified with H2SO4 3 M till pH = 2 and washed with a NaHCO3 5% solution. After extraction with ethyl acetate, the organic phase was dried over anhydrous Na2SO4 and finally evaporated in vacuum. The crude product was purified by silica gel column chromatography using mixtures of petroleum ether/ethyl acetate as eluent.
N-[4′-Hydroxy-(E)-cinnamoyl]-L-tyrosine methyl ester 1a: Yield 67%. 1H NMR (400 MHz, Acetone-d6) δ 8.84 (1H, s, OH), 8.27 (1H, s, OH), 7.49 (1H, d, J 15.7, H-7′), 7. 47 (1H, NH), 7.43 (2H, d, J = 8.6, H-2′, H-6′), 7.06 (2H, d, J 8.5, H-2, H-6), 6.86 (2H, d, J 8.6, H-3′, H-5′), 6.76 (2H, d, J = 8.5, H-3, H-5), 6.62 (1H, d, J 15.7, H-8′), 4.81 (1H, dt, J 7.7, 5.8, H-8), 3.66 (s, 3H, CH3), 3.08 (1H, dd, J 13.9, 5.8, H-7), 2.98 (1H, dd, J 13.9, 7.7, H-7); 13C NMR (100 MHz, Acetone-d6) δ 172.06 (C-9), 165.8 (C-9′), 159.0 (C-4′), 156.3 (C-4), 140.4 (C-7′), 130.2 (C-2, C-6), 129.5 (C-2′, C-6′), 127.6 (C-1), 126.7 (C-1′), 117.9 (C-8′), 115.7 (C-3′, C-5′), 115.2 (C-3, C-5), 54.1 (C-8), 51.4 (CH3), 36.8 (C-7).
N-[3′,4′-Dihydroxy-(E)-cinnamoyl]-L-phenylalanine methyl ester 2a: Yield 41%. 1H NMR and 13C NMR are in accordance with the literature [9].
N-Caffeoyl-tyrosine methyl ester 3a: Yield 58%. 1H NMR is in accordance with the literature [9]; 13C NMR (100 MHz, Acetone-d6) δ 172.0 (C-9), 166.0 (C-9′), 156.3 (C-4), 147.2 (C-4′), 145.4 (C-3′), 140.9 (C-7′), 130.2 (C-2 + C-6), 127.5 (C-1), 127.3 (C-1′), 120.9 (C-6′), 117.8 (C-8′), 115.4 (C-5′), 115.2 (C-3 + C-5), 114.1 (C-2′), 54.9 (C-8), 52.2 (COOCH3), 36.8 (C-7).
N-[4′-Hydroxy-(E)-cinnamoyl]-L-tryptophan methyl ester 4a: Yield 72%. 1H NMR (400 MHz, Acetone-d6) δ 10.15 (1H, s, OH), 8.82 (1H, s, NH), 7.65 (1H, d, J 7.8, H-4), 7.54 (1H, d, J 15.7, H-7′), 7.50 (2H, d, J 8.5, H-2′ + H-6′), 7.45 (1H, d, J 8.1, H-7), 7.27 (1H, s, H-1), 7.17 (1H, t, J 7.9, H-6), 7.09 (1H, t, J 7.9, H-5), 6.93 (2H, d, J 8.6, H-3′ + H-5′), 6.67 (1H, d, J 15.7, H-8′), 5.01 (1H, m, H-10), 3.72 (3H, s, OCH3), 3.36 (2H, dd, J1 20.0, J2 6.4, H-9); 13C NMR (100 MHz, Acetone-d6) δ 173.2 (COOCH3), 166.5 (C-9′), 159.8 (C-4′), 141.0 (C-7′), 137.5 (C-8), 130.2 (C-2′ + C-6′), 128.5 (C-1′), 127.6 (C-3), 124.3 (C-1), 122.2 (C-6), 119.6 (C-5), 119.1 (C-4), 118.9 (C-8′), 116.5 (C-3′ + C-5′), 112.2 (C-7), 110.8 (C-2), 54.1 (C-10), 52.2 (COOCH3), 28.5 (C-9).
N-[3′,4′-Dihydroxy-(E)-cinnamoyl]-L-tryptophan methyl ester 5a: Yield 78%. 1H NMR (400 MHz, Acetone-d6) δ 8.33 (1H, s, OH), 8.11 (1H, s, OH), 7.57 (1H, d, J 7.6, H-4), 7.50 (1H, NH), 7.45 (1H, d, J 15.6, H-7′), 7.37 (1H, d, J 8.1, H-7), 7.20 (1H, s, H-1), 7.08 (2H, m, H-2′ + H-5), 7.03 (1H, t, J 8.1, H-6), 6.92 (1H, dd, J1 8.2, J2 2.0, H-6′), 6.82 (1H, d, J 8.2, H-5′), 6.56 (1H, d, J 15.6, H-8′), 4.93 (1H, m, H-10), 3.65 (3H, s, COOCH3), 3.30 (2H, ddd, J1 21.8, J2 14.7, J3 6.4, H-9); 13C NMR (100 MHz, Acetone-d6) δ 172.4 (C-11), 165.9 (C-9′), 147.3 (C-7′), 145.4 (C-3′), 140.7 (C-4′), 136.7 (C-8), 127.6 (C-3), 127.6 (C-1′), 123.5 (C-1), 121.3 (C-4), 121.3 (C-6′), 120.9 (C-5), 118.8 (C-6), 118.8 (C-8′), 115.4 (C-5′), 114.0 (C-2′), 111.3 (C-7), 109.9 (C-2), 53.4 (C-10), 51.42 (COOCH3), 27.6 (C-9).
3.5. General Procedure for the Preparation of the Hydroxycinnamoyl Amides 1b–5b
The 1.11 mmol of the cinnamoylamide ester were dissolved in 68 mL of a THF/H2O 1:1 v/v solution and cooled in an ice bath. Subsequently, 5.53 mmol of LiOH∙H2O were added and the mixture was stirred at room temperature. TLC analyses were performed to check the end of the reaction. The mixture was diluted with 50 mL of distilled water and acidified with HCl 3 M till pH 2. After extraction with ethyl acetate, the organic phase was dried over anhydrous Na2SO4. Evaporation of the solvent under vacuum gave the crude product which was first purified by column chromatography on silica gel using a 99:1 mixture of ethyl acetate: acetic acid as the eluent or by recrystallization from petroleum ether.
(-)-N-[4′-Hydroxy-(E)-cinnamoyl]-L-tyrosine 1b: Yield 85%. 1H NMR (400 MHz, Acetone-d6) δ 8.80 (1H, bs, OH), 8.20 (1H, bs, OH), 7.50 (1H, d, J 15.7, H-7′), 7.43 (2H, d, J 8.6, H-2′, H-6′), 7.31 (1H, d, NH), 7.10 (2H, d, J 8.5, H-2, H-6), 6.85 (2H, d, J 8.6, H-3′, H-5′), 6.75 (2H, d, J 8.5, H-3, H-5), 6.45 (1H, d, J 15.7, H-8′), 4.81 (1H, dt, J = 7.8, 5.3, H-8), 3.17 (1H, dd, J 14.0, 5.3, H-7), 3.00 (1H, dd, J 14.0, 7.8, H-7); 13C NMR (100 MHz, Acetone-d6) δ 172.3 (C-9), 165.9 (C-9′), 158.9 (C-4′), 156.1 (C-4), 140.4 (C-7′), 130.3 (C-2, C-6), 129.5 (C-2′, C-6′), 127.8 (C-1), 126.7 (C-1′), 117.9 (C-8′), 115.6 (C-3′, C-5′), 115.1 (C-3, C-5), 53.8 (C-8), 36.5 (C-7); −39.9 (c 0.1 MeOH) (lit. −26.9) [7]; CD (CH3OH) Δε309nm = −2.94, Δε258nm = +2.17, Δε234 nm = +13.05; LC-MS (ESI+), m/z 328 ([M + H]+), (ESI−), m/z 326 ([M-H]−).
(-)-N-[3′,4′-Dihydroxy-(E)-cinnamoyl]-L-phenylalanine 2b: Yield 94%. 1H NMR (400 MHz, Acetone-d6) δ 8.30 (OH), 7.44 (1H, d, NH), 7.42 (1H, d, J 15.6 Hz, H-7′), 7.35–7.19 (5H, m), 7.08 (1H, d, J 2.0 Hz, H-2′), 6.95 (1H, dd, J 8.2, 2.0 Hz, H-6′), 6.84 (1H, d, J 8.2 Hz, H-5′), 6.56 (1H, d, J 15.6 Hz, H-8′), 4.88 (1H, dt, J 8.0, 5.4 Hz, H-8), 3.27 (1H, dd, J 13.9, 5.4 Hz, H-7), 3.09 (1H, dd, J 13.9, 8.0 Hz, H-7); 13C NMR (100 MHz, Acetone-d6) δ 172.2 (C-9), 165.7 (C-9′), 147.0 (, 145.2, 140.6 (H-7′), 137.4 (Ph), 129.3 (Ph), 128.3 (Ph), 127.4 (Ph), 126.6 (Ph), 120.9 (C-6′), 118.0 (C-8′), 115.4 (C-5′), 114.0 (C-2′), 53.5 (C-8), 37.3 (C-7); −48.8 (c 0.1, MeOH); CD (MeOH) Δε330nm = −1.1, Δε297nm = −1.0, Δε250nm = +1.8, Δε230nm = +3.9; LC-MS (ESI+), m/z 328 ([M + H]+), (ESI−), m/z 326 ([M-H]−).
(-)-N-[3′,4′-Dihydroxy-(E)-cinnamoyl]-L-tyrosine 3b: yield 84%. 1H NMR (400 MHz, Acetone-d6) δ 7.36 (1H, d, J 15.6, H-7′), 7.26 (1H, NH), 7.05 (2H, d, J 8.6, H-2 + H-6), 7.03 (1H, d, J 2.0, H-2′), 6.90 (1H, dd, J1 8.2, J2 2.0, H-6′), 6.78 (1H, d, J 8.2 Hz, H-5′), 6.70 (2H, d, J 8.6, H-3 + H-5), 6.51 (1H, d, J 15.6, H-8′), 4.75 (1H, dt, J1 7.8, J2 5.3, H-8), 3.10 (1H, dd, J1 14.0, J2 5.3, H-7), 2.95 (1H, dd, J1 14.0, J2 7.8, H-7); 13C NMR (100 MHz, Acetone-d6) δ 173.2 (C-9), 166.6 (C-9′), 157.0 (C-4), 147.9 (C-4′), 146.1 (C-3′), 141.4 (C-7′), 131.1 (C-2 + C-6), 128.7 (C-1), 128.3 (C-1′), 121.7 (C-6′), 119.0 (C-8′), 116.3 (C-5′), 115.9 (C-3 + C-5), 114.9 (C-2′), 54.7 (C-8), 37.7 (C-7); −41.7 (c 0.1, MeOH) (lit. −35.6) [11]; CD (MeOH): Δε331 = −1.5, Δε269 = +0.4, Δε235 = +6.5, Δε221 = −0.2; LC-MS (ESI+), m/z 344.1 ([M + H]+), (ESI−), m/z 342.0 ([M-H]−).
(-)-N-[4′-Hydroxy-(E)-cinnamoyl]-L-tryptophan 4b: yield 91%. 1H NMR (400 MHz, Acetone-d6) δ 10.05 (1H, s, OH), 8.90 (1H, bs, COOH), 7.61 (1H, d, J 7.9, H-4), 7.51 (2H, m, H-7′+NH), 7.37 (2H, d, J 8.6, H-6′ + H-2′), 7.33 (1H, d, J 8.1, H-7), 7.20 (1H, s, H-1), 7.04 (1H, t, J 7.9, H-6), 6.96 (1H, t, J 7.9, H-5), 6.82 (2H, d, J 8.6, H-5′ + H-3′), 6.60 (1H, d, J 15.7, H-8′), 4.99 (1H, m, H-10), 3.42 (1H, dd, J1 22.2, J2 14.8, H-9), 3.28 (1H, dd, J1 22.2, J2 6.3, H-9); 13C NMR (100 MHz, Acetone-d6) δ 172.9 (COOH), 166.4 (C-9′), 159.2 (C-4′), 140.6 (C-7′), 136.7 (C-8), 129.6 (C-2′ + C-6′), 127.9 (C-1′), 126.7 (C-3), 123.6 (C-1), 121.4 (C-6), 118.8 (C-5), 118.5 (C-4), 117.9 (C-8′), 115.8 (C-3′ + C-5′), 111.4 (C-7), 110.1 (C-2), 53.4 (C-10), 27.6 (C-9); −27.8 (c 0.1, MeOH); CD (MeOH): Δε323 = −1.4, Δε292 = −0.1, Δε270 = −0.5, Δε250 = +1.2, Δε231 = +11.7, Δε217 = −3.5; LC-MS (ESI+), m/z 351.0 ([M + H]+), (ESI−), m/z 349.1 ([M-H]−).
(-)-N-[3′,4′-Dihydroxy-(E)-cinnamoyl]-L-tryptophan 5b: Yield 56%. 1H NMR (400 MHz, Acetone-d6) δ 10.06 (1H, s, OH), 7.59 (1H, d, J 7.6, H-4), 7.40 (1H, d, J 15.6, H-7′), 7.36 (1H, NH), 7.32 (1H, d, J 8.2, H-7), 7.17 (1H, d, J 2.0, H-1), 7.02 (2H, m, H-5 + H-2′), 6.96 (1H, t, J 7.6, H-6), 6.88 (1H, dd, J1 8.2, J2 2.0, H-6′), 6.77 (1H, d, J 8.2, H-5′), 6.53 (1H, d, J 15.6, H-8′), 4.94 (1H, m, H-10), 3.37 (1H, dd, J1 14.8, J2 5.3, H-9), 3.24 (1H, dd, J114.8, J2 7.3, H-9); 13C NMR (100 MHz, Acetone-d6) δ 173.5 (C-11), 166.8 (C-9′), 147.9 (C-7′), 146.1 (C-3′), 141.4 (C-4′), 137.5 (C-8), 128.7 (C-3), 128.3 (C-1′), 124.3 (C-1), 122.1 (C-4), 121.7 (C-6′), 119.5 (C-5), 119.3 (C-6), 119.0 (C-8′), 116.2 (C-5′), 114.9 (C-2′), 112.1 (C-7), 111.0 (C-2), 54.0 (C-10), 28.3 (C-9). −33.2 (c 1.0, MeOH); CD (MeOH): Δε325 = −2.3, Δε298 = −2.1, Δε232 = +10.2, Δε216 = +2.4; LC-MS (ESI+), m/z 367.1 ([M + H]+), (ESI−), m/z 365.1 ([M-H]−).
3.6. Extraction of Hydroxycinnamoyl Amides and Sample Preparation
Green coffee beans were ground to a fine powder in a mixer ball mill MM400 (Retsch, Haan, Germany) and extraction was performed in duplicate by dynamic heat-assisted water extraction [15,16]. For this purpose, 1 g of powdered green coffee from each species was added to 100 mL of boiling water and the mixture was stirred for 10 min at 200 rpm on a heated plate (Arex Velp Scientifica, Usmate, Italy) and filtered. The aqueous extract was frozen with liquid nitrogen and freeze dried for 3 days.
HPLC samples were prepared dissolving the lyophilized extracts in MilliQ water (obtained with a Milli-Q Reference Water Purification System, Merck, Darmstadt, Germany) at a concentration of 6 mg/mL. Subsequently the solutions were filtered with 0.2 μm PTFE syringe filters (from VWR International Ltd., Radnor, PA, USA), preconditioned with methanol and injected to LC-MS.
3.7. Quantitative Analyses
To quantify the hydroxycinnamoyl amides, caffeic acid and p-coumaric acid were used as standard compounds, exploiting their UV absorption at 320 nm for detection, as already reported in the literature [14]. Calibration curves of caffeic acid and p-coumaric acid were built reporting the integration signal (mAu*s) versus concentration (μg/mL) as mean value of triplicate analyses in the range of concentration 0.035–3.5 μg/mL and 0.041–4.1 μg/mL, respectively. Both calibration curves showed a good response linearity with a coefficient of correlation (R2) of 0.997. Limits of detection (LOD) and quantification (LOQ) were, respectively, 0.14 μg/mL and 0.20 μg/mL calculated as signal-to-noise ratio with S:N = 3 for LOD and S:N = 10 for LOQ.
4. Conclusions
The results obtained in the present work are in accordance with the literature demonstrating that the HCAs p-coumaroyl-N-tyrosine 1b, caffeoyl-N-phenylalanine 2b, caffeoyl-N-tyrosine 3b, and p-coumaroyl-N-tryptophan 4b, and caffeoyl-N-tryptophan 5b, are characteristic of the Robusta green coffee beans, although their distribution between the samples depends on the geographical origin. Samples from Tanzania and Uganda contain all five HCAs while Robusta from Indonesia has only p-coumaroyl-N-tryptophan 4b and caffeoyl-N-tryptophan 5b. The only HCA present in Arabica is caffeoyl-N-tryptophan 5b, although in a very small amount with respect to Robusta. Liberica didn’t show the presence of any HCAs, although a small number of samples were considered. The investigation on more samples of Liberica of different origins is necessary to confirm the absence of HCAs. Preliminary studies on roasted coffee beans of both Robusta and Arabica were carried out by Kim et al. [13], where it was evidenced that the HCAs content decreased. A future development of the HCAs analysis could consider coffee brews from different commercial sources to evaluate if the HCAs content can discriminate on the coffee species used.
Author Contributions
Conceptualization: C.F.; Investigation: C.F.; Formal analysis: C.F., F.B.; Methodology: C.F.; Validation and supervision: C.F., F.B., L.N.; Writing—original draft, C.F.; Writing—review and editing, C.F., F.B., L.N., S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CD | Circular Dichroism |
| 5-CQA | 5-caffeoylquinic acid |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DW | Dry weight |
| FA | Formic acid |
| HCA | Hydroxycinnamoyl amide |
References
- Papetti, A.; Daglia, M.; Aceti, C.; Sordelli, B.; Spini, V.; Carazzone, C.; Gazzani, G. Hydroxycinnamic acid derivatives occuring in Cichorium endivia vegetables. J. Pharm. Biomed. Anal. 2008, 48, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Abbasi, B.H.; Gao, M.; Murch, S.J. Caffeic Acid Derivatives Production By Hairy Root Cultures Of Echinacea purpurea. J. Agric. Food Chem. 2006, 54, 8456–8460. [Google Scholar] [CrossRef] [PubMed]
- Moyo, M.; Amoo, S.O.; Aremu, A.O.; Gruz, J.; Šubrtova, M.; Doležal, K.; Van Staden, J. Plant regeneration and biochemical accumulation of hydroxybenzoic acid and hydroxycinnamic acid derivatives in Hypoxis hemerocallidea organ and callus cultures. Plant Sci. 2014, 227, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Adam, K.P. Caffeic acid derivatives in fronds of the lady fern. Phytochemistry 1995, 40, 1577–1578. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S. The cinnamoyl-ammino acid conjugates of green robusta coffee beans. Food Chem. 2004, 87, 457–463. [Google Scholar] [CrossRef]
- Stark, T.; Justus, H.; Hofmann, T. Quantitative analysis of N-phenylpropenoyl- L-amino acids in roasted coffee and cocoa powder by means of a stable isotope dilution assay. J. Agric. Food Chem. 2006, 54, 2859–2867. [Google Scholar] [CrossRef]
- Hu, X.L.; Lin, J.; Lv, X.-Y.; Feng, J.-H.; Zhang, X.-Q.; Wang, H. Synthesis and biological evaluation of clovamide analogues as potent anti-neuroinflammatory agents in vitro and in vivo. Eur. J. Med. Chem. 2018, 151, 261–271. [Google Scholar] [CrossRef]
- Yuan, X.; Lin, L.; Zhang, X.; Deng, S. Abrusamide A and B, two hepatoprotective isomeric compounds from Abrus mollis Hance. Phytochem. Lett. 2014, 7, 137–142. [Google Scholar] [CrossRef]
- Lee, S.U.; Shin, C.-G.; Lee, C.-K.; Lee, Y.S. Caffeoylglycolic and caffeoylamino acid derivatives, halmers of L-chicoric acid, as new HIV-1 integrase inhibors. Eur. J. Med. Chem. 2007, 42, 1309–1315. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Sookkongwaree, K.; Roengsumran, S.; Petsom, A.; Ngamrojnavanich, N.; Chavasiri, W.; Deesamer, S.; Yibchok-anun, S. Structure-activity relationships of trans-cinnamic acid derivatives on alpha-glucosidase inhibition. Bioorg. Med. Chem. Lett. 2004, 14, 2893–2896. [Google Scholar] [CrossRef]
- Stark, T.; Hofmann, T. Isolation, structure determination, synthesis and sensory activity of N-phenylpropenoyl-L-amino acids from Cocoa (Theobroma cacao). J. Agric. Food Chem. 2005, 53, 5419–5428. [Google Scholar] [CrossRef] [PubMed]
- Patay, E.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Asamenew, G.; Kim, H.-W.; Lee, M.-K.; Lee, S.-H.; Lee, S.; Cha, Y.-S.; Lee, S.H.; Yoo, S.M.; Kim, J.-B. Comprehensive characterization of hydroxycinnamoyl derivatives in green and roasted beans: A new group of methyl hydroxycinnamoyl quinate. Food Chem. 2019, 2, 100033. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Salces, R.M.; Serra, F.; Reniero, F.; Heberger, K. Botanical and Geographical Characterization of green coffee (Coffea arabica and Coffea canephora): Chemometric evaluation of phenolic and methylxanthine contents. J. Agric. Food Chem. 2009, 57, 4224–4235. [Google Scholar] [CrossRef]
- Garrett, R.; Vaz, B.G.; Hovell, A.M.C.; Eberlin, M.N.; Rezende, C.M. Arabica and Robusta coffees: Identification of major polar compounds and quantification of blends by direct-infusion electrospray ionization-mass spectrometry. J. Agric. Food Chem. 2012, 60, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Babova, O.; Occhipinti, A.; Maffei, M.E. Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin. Phytochemistry 2016, 123, 33–39. [Google Scholar] [CrossRef]
- Murata, M.; Okada, H.; Homma, S. Hydroxycinnamic acid derivatives and p-coumaroyl-N-tryptophan, a novel hydroxycannamic acid derivative from coffee beans. Biosci. Biotechnol. Biochem. 1995, 59, 1887–1890. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; de Jesus Garcia Salva, T.; Bragagnolo, N. Influence of coffee genotype on bioactive compounds and the in vitro capacity to scavenge reactive oxygen and nitrogen species. J. Agric. Food Chem. 2015, 63, 4815–4826. [Google Scholar] [CrossRef]
- Morishita, H.; Takai, Y.; Yamada, H.; Fukuda, F.; Sawada, M.; Iwahashi, H.; Kido, R. Caffeoyltryptophan from green robusta coffee beans. Phytochemistry 1987, 26, 1195–1196. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kellard, B.; Ah-Sing, E. Caffeoyltyrosine from green robusta coffee beans. Phytochemistry 1989, 28, 1989–1990. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, A.L.; Berti, F.; Solano Sánchez, W.; Navarini, L.; Colomban, S.; Crisafulli, P.; Forzato, C. Distribution of p-coumaroylquinic acids in commercial Coffea spp. of different geographical origin and in other wild coffee species. Food Chem. 2019, 286, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Ortiz, A.L.; Berti, F.; Navarini, L.; Crisafulli, P.; Colomban, S.; Forzato, C. Aqueous extracts of walnut (Juglans regia L.) leaves: Quantitative analyses of hydroxycinnamic and chlorogenic acids. J. Chromatogr. Sci. 2018, 56, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Anthony, F.; Clifford, M.N.; Noirot, M. Biochemical diversity in the genus Coffea L.: Chlorogenic acids, caffeine and mozambioside contents. Genet. Resour. Crop. Evol. 1993, 40, 61–70. [Google Scholar] [CrossRef]
- Correia, A.M.N.G.; Leittio, M.C.A.; Clifford, M.N. Caffeoyl-tyrosine and Angola II as characteristic markers for Angolan robusta coffees. Food Chem. 1995, 53, 309–313. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).