In Vitro Propagation and Variation of Antioxidant Properties in Micropropagated Vaccinium Berry Plants—A Review
Abstract
1. Introduction
2. Health Benefits of Vaccinium Berry Crops
3. Phenolics in Vaccinium Berries
4. Propagation of Vaccinium Berries
4.1. Sexual Propagation
4.2. Asexual Propagation
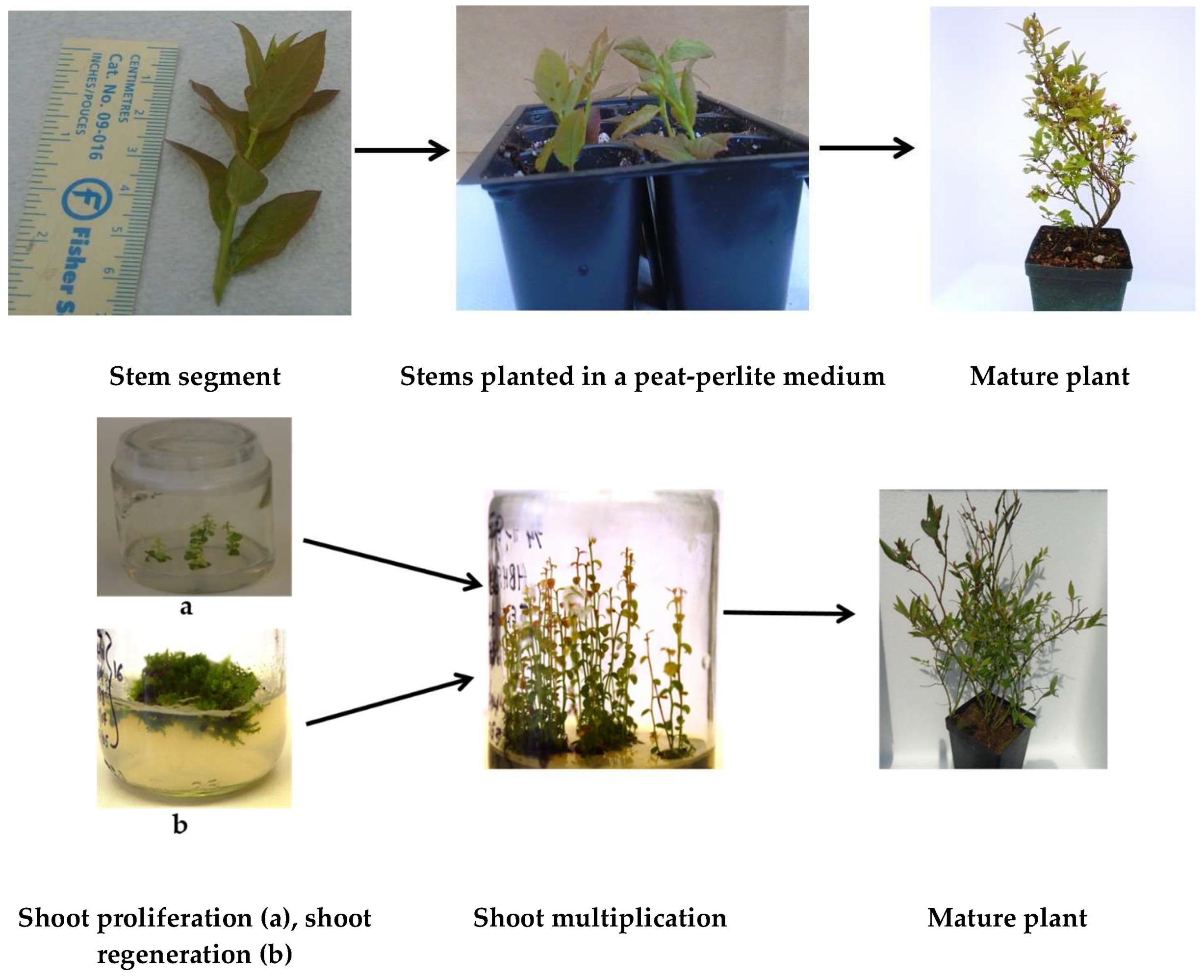
4.2.1. Propagation by Stem Cutting
4.2.2. In Vitro Propagation or Micropropagation.
5. Micropropagation, Morphology and Antioxidant Phenolic Contents in Vaccinium Berries
5.1. Genotype Specific Action of Micropropagation for Phenolics and Antioxidant Capacity
5.2. Tissue Culture Effects on Fruits vs. Leaves for Phenolics
5.3. Development-Specific Action of Tissue Culture for Phenolics
5.4. Seasonal Effect on Micropropagation for Phenolics
6. Conclusions and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, G.-Q. Blueberry (Vaccinium corymbosum L.). In Agrobacterium Protocols; Wang, K., Ed.; Springer: New York, NY, USA, 2015; Volume 2, pp. 121–131. [Google Scholar]
- Hancock, J.F.; Luby, J.J.; Beaudry, R. Fruits of temperate climates/Fruits of the Ericaceae. In Encyclopedia of Food Science, Food Technology and Nutrition, 2nd ed.; Trugo, L., Finglas, P.M., Caballero, B., Eds.; Academic Press: London, UK, 2003; pp. 2762–2768. [Google Scholar]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Sion, M. Genetic diversity, antioxidant activities, and anthocyanin contents in lingonberry. Int. J. Fruit Sci. 2009, 9, 185–199. [Google Scholar] [CrossRef]
- De Almeida Alvarenga, L.; Borges, N.A.; Moreira, L.D.S.G.; Resende Teixeira, K.T.; Carraro-Eduardo, J.C.; Dai, L.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Cranberries potential benefits in patients with chronic kidney disease. Food Funct. 2019, 10, 3103–3112. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2019, 00, 1–13. [Google Scholar] [CrossRef]
- Rowland, L.J.; Hancock, J.F.; Bassil, N.V. Blueberry. In Genetics, Genomics and Breeding of Berries; Folta, K.M., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–39. [Google Scholar]
- Akšić, M.F.; Tosti, T.; Sredojević, M.; Milivojević, J.; Meland, M.; Natić, M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants 2019, 8, 205. [Google Scholar] [CrossRef]
- Piljac-Zegarac, J.; Belscak, A.; Piljac, A. Antioxidant capacity and polyphenolic content of blueberry (Vaccinium corymbosum L.) leaf infusions. J. Med. Food 2009, 12, 608–614. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef]
- Yang, B.; Kortesniemi, M. Clinical evidence on potential health benefits of berries. Curr. Opin. Food Sci. 2015, 2, 36–42. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; De Freitas, V.; Mateus, N.; Calhau, C. Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phytother. Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef] [PubMed]
- Shafi, S. Some uncommon fruits of the amazing world. Int. J. Pharm. Pharm. Sci. 2014, 6, 54–58. [Google Scholar]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, J.; Stevenson, D.; Hedderley, D. Blueberry estimated harvest from seven new cultivars: Fruit and anthocyanins. Food Chem. 2013, 139, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Koca, I.; Karadeniz, B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea region of Turkey. Sci. Hortic. 2009, 121, 447–450. [Google Scholar] [CrossRef]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Contreras, R.; Köhler, H.; Pizarro, M.; Zúiga, G. In vitro cultivars of Vaccinium corymbosum L. (Ericaceae) are a source of antioxidant phenolics. Antioxidants 2015, 4, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S.; Finn, C.E.; Hancock, J.F. Genotypic and environmental variation in antioxidant activity, total phenolic content, and anthocyanin content among blueberry cultivars. J. Am. Soc. Hortic. Sci. 2002, 127, 89–97. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S. Variability in antioxidant activity in blueberry and correlations among different antioxidant activity assays. J. Am. Soc. Hortic. Sci. 2002, 127, 238–244. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Quantitation and distribution of simple and acylated anthocyanins and other phenolics in blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Goyali, J.C.; Igamberdiev, A.U.; Debnath, S.C. Propagation methods affect fruit morphology and antioxidant properties but maintain clonal fidelity in lowbush blueberry. HortScience 2015, 50, 888–896. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Dunlap, K.L.; Lila, M.A. Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J. Agric. Food Chem. 2014, 62, 4007–4017. [Google Scholar] [CrossRef]
- Vyas, P.; Debnath, S.C.; Igamberdiev, A.U. Metabolism of glutathione and ascorbate in lingonberry cultivars during in vitro and ex vitro propagation. Biol. Plant. 2013, 57, 603–612. [Google Scholar] [CrossRef]
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Phytochemical properties and antioxidant activities of extracts from wild blueberries and lingonberries. Plant Foods Hum. Nutr. 2017, 72, 360–364. [Google Scholar] [CrossRef]
- Debnath, S.C.; An, D. Antioxidant properties and structured biodiversity in a diverse set of wild cranberry clones. Heliyon 2019, 5, e01493. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Törrönen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Strik, B. Blueberry: An expanding world berry crop. Chronica Hortic. 2005, 45, 7–12. [Google Scholar]
- Strik, B.C.; Yarborough, D. Blueberry production trends in North America, 1992 to 2003, and predictions for growth. HortTechnology 2005, 15, 391–398. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada. Statistical overview of the Canadian fruit industry 2018. Available online: http://www.agr.gc.ca/horticulture_e (accessed on 29 July 2019).
- USDA. Noncitrus Fruits and Nuts 2018 Summary; USDA: Washington, DC, USA, 2019; pp. 90–91.
- Hall, I.V. Genetic improvement of the lowbush blueberry, Vaccinium angustifolium. Can. J. Plant. Sci. 1983, 63, 1091–1092. [Google Scholar] [CrossRef]
- Yarborough, D.E. Establishment and management of the cultivated lowbush blueberry (Vaccinium angustifolium). Int. J. Fruit Sci. 2012, 12, 14–22. [Google Scholar] [CrossRef]
- Wetzel, S.; Duchesne, L.C.; Laporte, M.F. Bioproducts from Canada’s Forests: New Partnerships in the Bioeconomy., 1st ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 89–112. [Google Scholar]
- Debnath, S.C. Strategies to propagate Vaccinium nuclear stocks for the Canadian berry industry. Can. J. Plant. Sci. 2007, 87, 911–922. [Google Scholar] [CrossRef]
- Debnath, S.C. Bioreactors and molecular analysis in berry crop micropropagation—A review. Can. J. Plant. Sci. 2011, 91, 147–157. [Google Scholar] [CrossRef]
- Debnath, S.C.; McRae, K.B. An efficient in vitro shoot propagation of cranberry (Vaccinium macrocarpon Ait.) by axillary bud proliferation. In Vitro Cell. Dev. Biol. Plant 2001, 37, 243–249. [Google Scholar] [CrossRef]
- Morrison, S.; Smagula, J.M.; Litten, W. Morphology, growth, and rhizome development of Vaccinium angustifolium Ait. seedlings, rooted softwood cuttings, and micropropagated plantlets. HortScience 2000, 35, 738–741. [Google Scholar] [CrossRef]
- Bomser, J.L.; Madhavi, D.A.L.; Singletary, K.A.L.; Smith, M.A.L. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996, 62, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, D.L.; Bomser, J.; Smith, M.A.L.; Singletary, K. Isolation of bioactive constituents from Vaccinium myrtillus (bilberry) fruits and cell cultures. Plant Sci. 1998, 131, 95–103. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Oliveira, J.; Neves, P.; Gameiro, P.; Santos-Buelga, C.; de Freitas, V.; Mateus, N. Antioxidant properties of prepared blueberry (Vaccinium myrtillus) extracts. J. Agric. Food Chem. 2005, 53, 6896–6902. [Google Scholar] [CrossRef] [PubMed]
- Skupień, K.; Oszmiański, J.; Kostrzewa-Nowak, D.; Tarasiuk, J. In vitro antileukaemic activity of extracts from berry plant leaves against sensitive and multidrug resistant HL60 cells. Cancer Lett. 2006, 236, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Akoh, C.C.; Fischer, J.; Krewer, G. Effects of phenolic compounds in blueberries and muscadine grapes on HepG2 cell viability and apoptosis. Food Res. Int. 2006, 39, 628–638. [Google Scholar] [CrossRef]
- Matchett, M.D.; MacKinnon, S.L.; Sweeney, M.I.; Gottschall-Pass, K.; Hurta, R.A.R. Blueberry flavonoids inhibit matrix metalloproteinase activity in DU145 human prostate cancer cells. Biochem. Cell Biol. 2005, 83, 637–643. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Erdman Jr, J.W.; Lila, M.A. Differential effects of blueberry proanthocyanidins on androgen sensitive and insensitive human prostate cancer cell lines. Cancer Lett. 2006, 231, 240–246. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Howell, A.B.; McEniry, B.; Knight, C.T.; Seigler, D.; Erdman Jr, J.W.; Lila, M.A. Effective separation of potent antiproliferation and antiadhesion components from wild blueberry (Vaccinium angustifolium Ait.) fruits. J. Agric. Food Chem. 2004, 52, 6433–6442. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.; Sanchez, K.; Betts, N.; Wu, M.; Aston, C.; Lyons, J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome 1-3. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Carey, A.N.; Jenkins, D.; Rabin, B.M.; Joseph, J.A. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol. Aging 2007, 28, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Racicot, K.; Pilkenton, S.; Apostolidis, E. In-vitro evaluation of bioactive fractions of blueberry extract for phenolic-mediated inhibition of carbohydrate hydrolyzing enzymes. Int. J. Appl. Res. Nat. Prod. 2016, 9, 33–38. [Google Scholar]
- Zhong, Y.; Wang, Y.; Guo, J.; Chu, H.; Gao, Y.; Pang, L. Blueberry improves the therapeutic effect of etanercept on patients with juvenile idiopathic arthritis: Phase III study. Tohoku J. Exp. Med. 2015, 237, 183–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.-H.; Vijayagopal, P.; Juma, S. Blueberries improve pain, gait performance, and inflammation in individuals with symptomatic knee osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, D.; Tong, C.; Zhang, Y.; Xu, Y.; Liu, X.; Gao, Y.; Hou, M. Blueberry anthocyanins ameliorate radiation-induced lung injury through the protein kinase RNA-activated pathway. Chem. Biol. Interact. 2015, 242, 363–371. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, M.A.; Lim, B.O. Antibacterial and antioxidant activities of Vaccinium corymbosum L. leaf extract. Asian Pac. J. Trop. Dis. 2013, 3, 444–453. [Google Scholar] [CrossRef]
- Takeshita, M.; Ishida, Y.I.; Akamatsu, E.; Ohmori, Y.; Sudoh, M.; Oto, H.; Tsubouchi, H.; Kataoka, H. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J. Biol. Chem. 2009, 284, 21165–21176. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, M.A.; Dimakopoulou, A.; Linardaki, Z.I.; Cordopatis, P.; Klimis-Zacas, D.; Margarity, M.; Lamari, F.N. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav. Brain Res. 2009, 198, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.I.; Kalt, W.; Mackinnon, S.L.; Ashby, J.; Gottschall-Pass, K.T. Feeding rats diets enriched in lowbush blueberries for six weeks decreases ischemia-induced brain damage. Nutr. Neurosci. 2002, 5, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.; Kalea, A.Z.; Harris, P.D.; Klimis-Zacas, D.J. Wild blueberry-rich diets affect the contractile machinery of the vascular smooth muscle in the sprague–dawley rat. J. Med. Food 2005, 8, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Le, P.M.; et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Leduc, C.; Coonishish, J.; Haddad, P.; Cuerrier, A. Plants used by the Cree Nation of Eeyou Istchee (Quebec, Canada) for the treatment of diabetes: A novel approach in quantitative ethnobotany. J. Ethnopharmacol. 2006, 105, 55–63. [Google Scholar] [CrossRef]
- Vyas, P.; Kalidindi, S.; Chibrikova, L.; Igamberdiev, A.U.; Weber, J.T. Chemical analysis and effect of blueberry and lingonberry fruits and leaves against glutamate-mediated excitotoxicity. J. Agric. Food Chem. 2013, 61, 7769–7776. [Google Scholar] [CrossRef]
- Wang, S.Y.; Feng, R.; Bowman, L.; Penhallegon, R.; Ding, M.; Lu, Y. Antioxidant activity in lingonberries (Vaccinium vitis-idaea L.) and its inhibitory effect on activator protein-1, nuclear factor-kappaB, and mitogen-activated protein kinases activation. J. Agric. Food Chem. 2005, 53, 3156–3166. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; Leppänen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European cranberry (Vaccinium microcarpon) proanthocyanidins: Isolation, identification, and bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef]
- Brown, E.M.; Nitecki, S.; Pereira-Caro, G.; McDougall, G.J.; Stewart, D.; Rowland, I.; Crozier, A.; Gill, C.I. Comparison of in vivo and in vitro digestion on polyphenol composition in lingonberries: Potential impact on colonic health. Biofactors 2014, 40, 611–623. [Google Scholar] [CrossRef]
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of beneficial metabolic effects of berries in high-fat fed C57BL/6J mice. J. Nutr. Metab. 2014, 2014, 403041. [Google Scholar] [CrossRef]
- Heyman-Lindén, L.; Kotowska, D.; Sand, E.; Bjursell, M.; Plaza, M.; Turner, C.; Holm, C.; Fåk, F.; Berger, K. Lingonberries alter the gut microbiota and prevent low-grade inflammation in high-fat diet fed mice. Food Nutr. Res. 2016, 60, 29993. [Google Scholar] [CrossRef] [PubMed]
- Isaak, C.K.; Wang, P.; Prashar, S.; O, K.; Brown, D.C.W.; Debnath, S.C.; Siow, Y.L. Supplementing diet with Manitoba lingonberry juice reduces kidney ischemia-reperfusion injury. J. Sci. Food Agric. 2017, 97, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Simao, T.N.; Lozovoy, M.A.; Simao, A.N.; Oliveira, S.R.; Venturini, D.; Morimoto, H.K.; Miglioranza, L.H.; Dichi, I. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br. J. Nutr. 2013, 110, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Caldas, A.P.S.; Coelho, O.G.L.; Bressan, J. Cranberry antioxidant power on oxidative stress, inflammation and mitochondrial damage. Int. J. Food Prop. 2018, 21, 582–592. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.C.; Desjardins, Y.; Furtos, A.; Marcil, V.; Dudonne, S.; Montoudis, A.; Garofalo, C.; Delvin, E.; Marette, A.; Levy, E. Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clin. Sci. 2015, 128, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liska, D.; Talan, D.; Chung, M. Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: A systematic review and meta-analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr. Rev. 2007, 65, 490–502. [Google Scholar] [CrossRef]
- Sun, J.; Hai Liu, R. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells. Cancer Lett. 2006, 241, 124–134. [Google Scholar] [CrossRef]
- Mykkänen, O.T.; Huotari, A.; Herzig, K.-H.; Dunlop, T.W.; Mykkänen, H.; Kirjavainen, P.V.; Müller, M. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PLoS ONE 2014, 9, e114790. [Google Scholar]
- Mykkänen, O.T.; Kalesnykas, G.; Adriaens, M.; Evelo, C.T.; Törrönen, R.; Kaarniranta, K. Bilberries potentially alleviate stress-related retinal gene expression induced by a high-fat diet in mice. Mol. Vision 2012, 18, 2338–2351. [Google Scholar]
- Calò, R.; Marabini, L. Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B 2014, 132, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food 2012, 15, 818–823. [Google Scholar] [CrossRef]
- Leonardi, M. Treatment of fibrocystic disease of the breast with Myrtillus anthocyanins. Our experience. Minerva Ginecol. 1993, 45, 617–621. [Google Scholar] [PubMed]
- Uysal, U.; Seremet, S.; Lamping, J.W.; Adams, J.M.; Liu, D.Y.; Swerdlow, R.H.; Aires, D.J. Consumption of polyphenol plants may slow aging and associated diseases. Curr. Pharm. Des. 2013, 19, 6094–6111. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.; Badger, T.; Nagarajan, S. Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J. Nutr. 2010, 140, 1628–1632. [Google Scholar] [CrossRef]
- Haddad, P.S.; Depot, M.; Settaf, A.; Chabli, A.; Cherrah, Y. Comparative study on the medicinal plants most recommended by traditional practitioners in Morocco and Canada. J. Herbs, Spices Med. Plants 2003, 10, 25–45. [Google Scholar] [CrossRef]
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Lab. Invest. 2012, 92, 102–109. [Google Scholar] [CrossRef]
- Shen, C.L.; von Bergen, V.; Chyu, M.C.; Jenkins, M.R.; Mo, H.; Chen, C.H.; Kwun, I.S. Fruits and dietary phytochemicals in bone protection. Nutr. Res. 2012, 32, 897–910. [Google Scholar] [CrossRef]
- Heinonen, M. Antioxidant activity and antimicrobial effect of berry phenolics – a Finnish perspective. Mol. Nutr. Food Res. 2007, 51, 684–691. [Google Scholar] [CrossRef]
- Kay, C.D.; Holub, B.J. The effect of wild blueberry (Vaccinium angustifolium) consumption on postprandial serum antioxidant status in human subjects. Br. J. Nutr. 2002, 88, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Nichenametla, S.; Taruscio, T.; Barney, D.; Exon, J. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci. Nutr. 2006, 46, 161–183. [Google Scholar] [CrossRef]
- King, A.M.Y.; Young, G. Characteristics and occurrence of phenolic phytochemicals. J. Am. Diet. Assoc. 1999, 99, 213–218. [Google Scholar] [CrossRef]
- Weidner, S.; Amarowicz, R.; Karamać, M.; Frączek, E. Changes in endogenous phenolic acids during development of Secale cereale caryopses and after dehydration treatment of unripe rye grains. Plant Physiol. Biochem. 2000, 38, 595–602. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.-T.; Shetty, K. Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac. J. Clin. Nutr. 2004, 13, 295–307. [Google Scholar]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘Northblue’ blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Harris, C.S.; Burt, A.J.; Saleem, A.; Le, P.M.; Martineau, L.C.; Haddad, P.S.; Bennett, S.A.L.; Arnason, J.T. Single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem. Anal. 2007, 18, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Gavrilova, V.; Kajdžanoska, M.; Gjamovski, V.; Stefova, M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC−DAD−ESI-MS. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef] [PubMed]
- Cardeñosa, V.; Girones-Vilaplana, A.; Muriel, J.L.; Moreno, D.A.; Moreno-Rojas, J.M. Influence of genotype, cultivation system and irrigation regime on antioxidant capacity and selected phenolics of blueberries (Vaccinium corymbosum L.). Food Chem. 2016, 202, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Percival, D.; MacKenzie, J.L. Use of plant growth regulators to increase polyphenolic compounds in the wild blueberry. Can. J. Plant. Sci. 2007, 87, 333–336. [Google Scholar] [CrossRef]
- Zifkin, M.; Jin, A.; Ozga, J.A.; Zaharia, L.I.; Schernthaner, J.P.; Gesell, A.; Abrams, S.R.; Kennedy, J.A.; Constabel, C.P. Gene expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 2012, 158, 200–224. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Sanoner, P.; Guyot, S.; Marnet, N.; Molle, D.; Drilleau, J.F. Polyphenol profiles of french cider apple varieties (Malus domestica sp.). J. Agric. Food Chem. 1999, 47, 4847–4853. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.; Fry, S.C. Phenolic components of the plant cell wall. Int. Rev. Cytol. 1994, 151, 229–267. [Google Scholar]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants. Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Graham, T.L. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 1991, 95, 594–603. [Google Scholar] [CrossRef]
- Taulavuori, E.; Tahkokorpi, M.; Taulavuori, K.; Laine, K. Anthocyanins and glutathione S-transferase activities in response to low temperature and frost hardening in Vaccinium myrtillus (L.). J. Plant Physiol. 2004, 161, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Christie, P.; Alfenito, M.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 71, 1085–1097. [Google Scholar]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef] [PubMed]
- Ranger, C.M.; Singh, A.P.; Johnson-Cicalese, J.; Polavarapu, S.; Vorsa, N. Intraspecific variation in aphid resistance and constitutive phenolics exhibited by the wild blueberry Vaccinium darrowi. J. Chem. Ecol. 2007, 33, 711–729. [Google Scholar] [CrossRef]
- Golan, K.; Sempruch, C.; Górska-Drabik, E.; Czerniewicz, P.; Łagowska, B.; Kot, I.; Kmieć, K.; Magierowicz, K.; Leszczyński, B. Accumulation of amino acids and phenolic compounds in biochemical plant responses to feeding of two different herbivorous arthropod pests. Arthropod-Plant Interact. 2017, 11, 675–682. [Google Scholar] [CrossRef]
- Strack, D.; Wray, V. The anthocyanins. In The Flavonoids Advances in Research Since 1986, 1st ed.; Harborne, J.B., Ed.; Chapman & Hall: New York, NY, USA, 1994; pp. 1–22. [Google Scholar]
- Hicks, B.J. Pollination of lowbush blueberry (Vaccinium angustifolium) in Newfoundland by native and introduced bees. J. Acad. Entomol. Soc. 2011, 7, 108–118. [Google Scholar]
- Solecka, D. Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol. Plant. 1997, 19, 257–268. [Google Scholar] [CrossRef]
- Strack, D. Phenolic metabolism. In Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: London, UK, 1997; pp. 387–416. [Google Scholar]
- Xuan, T.D.; Shinkichi, T.; Khanh, T.D.; Chung, I.M. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: An overview. Crop Protect. 2005, 24, 197–206. [Google Scholar] [CrossRef]
- Li, Y.; Hong, Y. The current status and future of the blueberry industry in China. Acta Hortic. 2009, 810, 445–456. [Google Scholar]
- Jamieson, A.R.; Nickerson, N.L. Field performance of the lowbush blueberry propagated by seed, stem cuttings and micropropagation. Acta Hortic. 2003, 626, 431–436. [Google Scholar] [CrossRef]
- Goyali, J.C.; Igamberdiev, A.U.; Debnath, S.C. Morphology, phenolic content and antioxidant capacity of lowbush blueberry (Vaccinium angustifolium Ait.) plants as affected by in vitro and ex vitro propagation methods. Can. J. Plant. Sci. 2013, 93, 1001–1008. [Google Scholar] [CrossRef]
- Wood, G.W. Self-fertility in the lowbush blueberry. Can. J. Plant. Sci. 1968, 48, 431–433. [Google Scholar] [CrossRef]
- Bell, D.J.; Rowland, L.J.; Drummond, F.A. Does pollen ‘Neighborhood’ affect berry yield in lowbush blueberry (Vaccinium angustifolium Ait)? Int. J. Fruit Sci. 2012, 12, 65–74. [Google Scholar] [CrossRef]
- Aalders, L.E.; Hall, I.V.; Brydon, A.C. A comparison of fruit yields of lowbush blueberry clonal lines and related seedling progenies. Can. J. Plant. Sci. 1979, 59, 875–877. [Google Scholar] [CrossRef]
- Shorthouse, J.D.; Bagatto, G. Potential role of lowbush blueberry (Vaccinium angustifolium) in colonizing metal-contaminated ecosystems. In Restoration and Recovery of an Industrial Region; Gunn, J.M., Ed.; Springer: New York, NY, USA, 1995; pp. 247–255. [Google Scholar]
- Debnath, S.C. Influence of propagation method and indole-3-butyric acid on growth and development of in vitro- and ex vitro-derived lingonberry plants. Can. J. Plant. Sci. 2006, 86, 235–243. [Google Scholar] [CrossRef]
- Meiners, J.; Schwab, M.; Szankowski, I. Efficient in vitro regeneration systems for Vaccinium species. Plant Cell Tissue Organ Cult. 2007, 89, 169–176. [Google Scholar] [CrossRef]
- Litwińczuk, W. Micropropagation of Vaccinium sp. by in vitro axillary shoot proliferation. Methods Mol. Biol. 2013, 994, 63–76. [Google Scholar]
- Debnath, S.C. Influence of indole-3-butyric acid and propagation method on growth and development of in vitro- and ex vitro-derived lowbush blueberry plants. Plant Growth Regul. 2007, 51, 245–253. [Google Scholar] [CrossRef]
- Debnath, S.C.; McRae, K.B. In vitro culture of lingonberry (Vaccinium vitis-idaea L.). Small Fruits Rev. 2001, 1, 3–19. [Google Scholar] [CrossRef]
- Steward, F.C.; Ammirato, P.V.; Mapes, M.O. Growth and development of totipotent cells: Some problems, procedures, and perspectives. Ann. Bot. 1970, 34, 761–787. [Google Scholar] [CrossRef]
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–131. [Google Scholar] [PubMed]
- Rani, V.; Raina, S. Genetic fidelity of organized meristem-derived micropropagated plants: A critical reappraisal. In Vitro Cell. Dev. Biol. Plant 2000, 36, 319–330. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Farkya, S.; Srivastava, A.; Bisaria, V. Bioprocess considerations for production of secondary metabolites by plant cell suspension cultures. Biotechnol. Bioprocess Eng. 2002, 7, 138–149. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. BioAllied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.C. Propagation of Vaccinium in vitro: A review. Int. J. Fruit Sci. 2007, 6, 47–71. [Google Scholar] [CrossRef]
- Zimmerman, R.H. Micropropagation of woody plants: Post tissue culture aspects. Acta Hortic. 1988, 227, 489–499. [Google Scholar] [CrossRef]
- Barker, W.G.; Collins, W.B. The blueberry rhizome: In vitro culture. Can. J. Bot. 1963, 41, 1325–1329. [Google Scholar] [CrossRef]
- White, P.R. A Handbook of Plant Tissue Culture; The Jacques Cattell Press: Lancaster, PA, USA, 1943; Volume 56, p. 151. [Google Scholar]
- Boxus, P.H. The production of strawberry plants by in vitro micro-propagation. J. Hortic. Sci. 1974, 49, 209–210. [Google Scholar] [CrossRef]
- Anderson, W.C. Propagation of rhododendrons by tissue culture. 1. Development of a culture medium for multiplication of shoots. Proc. Int. Plant Prop. Soc. 1975, 25, 129–135. [Google Scholar]
- Zhao, X.; Zhan, L.; Zou, X. In vitro high-frequency regeneration of half-highbush ‘Northland’ blueberry. N. Z. J. Crop Hortic. Sci. 2011, 39, 51–59. [Google Scholar] [CrossRef]
- Nickerson, N.L.; Hall, I.V. Callus formation in stem internode sections of lowbush blueberry (Vaccinium angustifolium Ait.) cultured on a medium containing plant growth regulators. Hortic. Res. 1976, 16, 29–35. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nickerson, N.L. In vitro shoot formation in lowbush blueberry seedling explants. HortScience 1978, 13, 698. [Google Scholar]
- Nickerson, N.L. Callus formation in lowbush blueberry fruit explants cultured in vitro. Hortic. Res. 1978, 18, 85–91. [Google Scholar]
- Frett, J.J.; Smagula, J.M. In vitro shoot production of lowbush blueberry. Can. J. Plant. Sci. 1983, 63, 467–472. [Google Scholar] [CrossRef]
- Debnath, S.C. In vitro culture of lowbush blueberry (Vaccinium angustifolium Ait.). Small Fruits Rev. 2004, 3, 393–408. [Google Scholar] [CrossRef]
- Debnath, S.C. A two-step procedure for adventitious shoot regeneration on excised leaves of lowbush blueberry. In Vitro Cell. Dev. Biol. Plant 2009, 45, 122–128. [Google Scholar] [CrossRef]
- Cohen, D. Application of micropropagation methods for blueberries and tamarillos. Proc. Int. Plant Prop. Soc. 1980, 30, 144–146. [Google Scholar]
- Grout, J.M.; Read, P.E.; Wildung, D.K. Influence of tissue culture and leaf-bud propagation on the growth habit of ‘Northblue’ blueberry. J. Am. Soc. Hortic. Sci. 1986, 111, 372–375. [Google Scholar]
- Read, P.E.; Wildung, D.K.; Hanky, C.A. Field performance of in vitro-propagated ‘Northblue’ blueberries. Acta Hortic. 1989, 241, 191–194. [Google Scholar] [CrossRef]
- Debnath, S.C. Temporary immersion and stationary bioreactors for mass propagation of true-to-type highbush, half-high, and hybrid blueberries (Vaccinium spp.). J. Hortic. Sci. Biotechnol. 2017, 92, 72–80. [Google Scholar] [CrossRef]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Thidiazuron-induced somatic embryogenesis and changes of antioxidant properties in tissue cultures of half-high blueberry plants. Sci. Rep. 2018, 8, 16978. [Google Scholar] [CrossRef]
- Debnath, S.C. A scale-up system for lowbush blueberry micropropagation using a bioreactor. HortScience 2009, 44, 1962–1966. [Google Scholar] [CrossRef]
- Debnath, S.C. Adventitious shoot regeneration in a bioreactor system and EST-PCR based clonal fidelity in lowbush blueberry (Vaccinium angustifolium Ait.). Sci. Hortic. 2011, 128, 124–130. [Google Scholar] [CrossRef]
- Debnath, S.C. A two-step procedure for adventitious shoot regeneration from in vitro-derived lingonberry leaves: Shoot induction with TDZ and shoot elongation using zeatin. HortScience 2006, 40, 189–192. [Google Scholar] [CrossRef]
- Smith, M.A.L.; Spomer, L.A. Vessels, gels, liquid media, and support systems. In Automation and Environmental Control in Plant Tissue Culture; Aitken-Christie, J., Kozai, T., Smith, M.A.L., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 371–404. [Google Scholar]
- Zimmerman, R.H.; Broome, O.C. Blueberry micropropagation. In Proceedings of the Conference on Nursery Production of Fruit Plants through Tissue Culture—Applications and Feasibility, Beltsville, MD, USA, 21–22 April 1980; U.S. Department of Agriculture: Beltsville, MD, USA, 1980; pp. 44–47. [Google Scholar]
- Litwińczuk, W.; Wadas, M. Auxin-dependent development and habituation of highbush blueberry (Vaccinium × covilleanum But. et Pl.) ‘Herbert’ in vitro shoot cultures. Sci. Hortic. 2008, 119, 41–48. [Google Scholar] [CrossRef]
- Kaldmäe, H.; Karp, K.; Starast, M.; Paal, T. Effect of donor plant physiological condition on in vitro establishment of Vaccinium angustifolium shoot explants. Acta Hortic. 2006, 715, 433–438. [Google Scholar] [CrossRef]
- Dweikat, I.M.; Lyrene, P.M. Adventitious shoot production from leaves of blueberry cultured in vitro. HortScience 1988, 23, 629. [Google Scholar]
- Brissette, L.; Tremblay, L.; Lord, D. Micropropagation of lowbush blueberry from mature field-grown plants. HortScience 1990, 25, 349–351. [Google Scholar] [CrossRef]
- Georgieva, M. Micropropagation of lowbush blueberry (Vaccinium angustifolium). Banat’s J. Biotechnol. 2013, 4, 42. [Google Scholar] [CrossRef]
- Hruskoci, J.D.; Read, P.E. In vitro shoot regeneration from internode segments and internode-derived callus of blueberry (Vaccinium spp.). Acta Hortic. 1993, 346, 125–130. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.-H.; Kim, S.-K.; Lee, K.-H.; Park, J.-Y.; Dung, C.D.; Nam, M.-W.; Choi, D.-H.; Lee, H.-I. In vitro proliferation and ex vitro rooting of microshoots of commercially important rabbiteye blueberry (Vaccinium ashei Reade) using spectral lights. Sci. Hortic. 2016, 211, 248–254. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Nam, M.W.; Choi, D.H.; Lee, H.I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Tsuda, H.; Kunitake, H.; Aoki, Y.; Oyama, A.; Tetsumura, T.; Komatsu, H.; Yoshioka, K. Efficient in vitro screening for higher soil pH adaptability of intersectional hybrids in blueberry. HortScience 2014, 49, 141–144. [Google Scholar] [CrossRef]
- Ružić, D.; Vujović, T.; Libiakova, G.; Cerović, R.; Gajdošova, A. Micropropagation in vitro of highbush blueberry (Vaccinium corymbosum L.). J. Berry Res. 2012, 2, 97–103. [Google Scholar] [CrossRef]
- Vescan, L.A.; Pamfil, D.; Clapa, D.; Fira, A.; Sisea, C.R.; Pop, I.F.; Petricele, I.V.; Ciuzan, O.; Pop, R. Efficient micropropagation protocol for highbush blueberry (Vaccinium corymbosum L.) cv. ‘Elliot’. Rom. Biotechnol. Lett. 2012, 17, 6893–6902. [Google Scholar]
- Tetsumura, T.; Matsumoto, Y.; Sato, M.; Honsho, C.; Yamashita, K.; Komatsu, H.; Sugimoto, Y.; Kunitake, H. Evaluation of basal media for micropropagation of four highbush blueberry cultivars. Sci. Hortic. 2008, 119, 72–74. [Google Scholar] [CrossRef]
- Pathirana, R.; Wiedow, C.; Pathirana, S.; Hedderley, D.; Morgan, E.; Scalzo, J.; Frew, T.; Timmerman-Vaughan, G. Ovule culture and embryo rescue facilitate interspecific hybridisation in blueberry (Vaccinium spp.). Acta Hortic. 2015, 1083, 123–132. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L. Use of a cytokinin conjugate for efficient shoot regeneration from leaf sections of highbush blueberry. HortScience 1992, 27, 1127–1129. [Google Scholar] [CrossRef]
- Cao, X.; Hammerschlag, F.A. Improved shoot organogenesis from leaf explants of highbush blueberry. HortScience 2000, 35, 945–947. [Google Scholar] [CrossRef]
- Cao, X.; Hammerschlag, F.A.; Douglass, L. A two-step pretreatment significantly enhances shoot organogenesis from leaf explants of highbush blueberry cv. Bluecrop. HortScience 2002, 37, 819–821. [Google Scholar] [CrossRef]
- Tetsumura, T.; Kajiwara, Y.; Honsho, C.; Sato-Yamauchi, M.; Sugimoto, Y.; Kunitake, H. Effective micropropagation of rabbiteye blueberries for leaf tea production. Environ. Control Biol. 2012, 50, 289–296. [Google Scholar] [CrossRef]
- Gonzalez, M.V.; Lopez, M.; Valdes, A.E.; Ordas, R.J. Micropropagation of three berry fruit species using nodal segments from field-grown plants. Ann. Appl. Biol. 2000, 137, 73–78. [Google Scholar] [CrossRef]
- Reed, B.M.; Abdelnouresquivel, A. The use of zeatin to initiate in vitro cultures of Vaccinium species and cultivars. HortScience 1991, 26, 1320–1322. [Google Scholar] [CrossRef]
- Graham, J.; Greig, K.; McNicol, R.J. Transformation of blueberry without antibiotic selection. Ann. Appl. Biol. 1996, 128, 557–564. [Google Scholar] [CrossRef]
- Liu, C.; Callow, P.; Rowland, L.; Hancock, J.; Song, G.-Q. Adventitious shoot regeneration from leaf explants of southern highbush blueberry cultivars. Plant Cell Tissue Organ Cult. 2010, 103, 137–144. [Google Scholar] [CrossRef]
- Debnath, S.C. Zeatin-induced one-step in vitro cloning affects the vegetative growth of cranberry (Vaccinium macrocarpon Ait.) micropropagules over stem cuttings. Plant Cell Tissue Organ Cult. 2008, 93, 231–240. [Google Scholar] [CrossRef]
- Debnath, S.C.; McRae, K.B. A one-step in vitro cloning procedure for cranberry (Vaccinium macrocarpon Ait.). Small Fruits Rev. 2005, 4, 57–75. [Google Scholar] [CrossRef]
- Debnath, S.C. Effects of carbon source and concentration on development of lingonberry (Vaccinium vitis-idaea L.) shoots cultivated in vitro from nodal explants. In Vitro Cell. Dev. Biol. Plant 2005, 41, 145–150. [Google Scholar] [CrossRef]
- Debnath, S.C.; Vyas, P.; Igamberdiev, A.U. Characteristics of lingonberry plants propagated by in vitro and ex vitro propagation methods. Acta Hortic. 2012, 926, 259–264. [Google Scholar] [CrossRef]
- Debnath, S.C.; McRae, K.B. An efficient adventitious shoot regeneration system on excised leaves of micropropagated lingonberry (Vaccinium vitis- idaea L.). J. Hortic. Sci. Biotechnol. 2002, 77, 744–752. [Google Scholar] [CrossRef]
- Georgieva, M.; Badjakov, I.; Dincheva, I.; Yancheva, S.; Kondakova, V. In vitro propagation of wild Bulgarian small berry fruits (bilberry, lingonberry, raspberry and strawberry). Bul. J. Agric. Sci. 2016, 22, 46–51. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Marino, S.R.; Williamson, J.G.; Olmstead, J.W.; Harmon, P.F. Vegetative growth of three Southern highbush blueberry cultivars obtained from micropropagation and softwood cuttings in two Florida locations. HortScience 2014, 49, 556–561. [Google Scholar] [CrossRef]
- El-Shiekh, A.; Wildung, D.K.; Luby, J.J.; Sargent, K.L.; Read, P.E. Long-term effects of propagation by tissue culture or softwood single-node cuttings on growth habit, yield, and berry weight of ‘Northblue’ blueberry. J. Am. Soc. Hortic. Sci. 1996, 121, 339–342. [Google Scholar] [CrossRef]
- Litwińczuk, W.; Szczerba, G.; Wrona, D. Field performance of highbush blueberries (Vaccinium x corymbosum L.) cv. ‘Herbert’ propagated by cuttings and tissue culture. Sci. Hortic. 2005, 106, 162–169. [Google Scholar] [CrossRef]
- Debnath, S.C.; Vyas, P.; Goyali, J.C.; Igamberdiev, A.U. Morphological and molecular analyses in micropropagated berry plants acclimatized under ex vitro condition. Can. J. Plant. Sci. 2012, 92, 1065–1073. [Google Scholar] [CrossRef]
- Gustavsson, B.A.; Stanys, V. Field performance of ‘Sanna’ lingonberry derived by micropropagation vs. stem cuttings. HortScience 2000, 35, 742–744. [Google Scholar] [CrossRef]
- Foley, S.L.; Debnath, S.C. Influence of in vitro and ex vitro propagation on anthocyanin content and anti-oxidant activity of lingonberries. J. Hortic. Sci. Biotechnol. 2007, 82, 114–118. [Google Scholar] [CrossRef]
- Goyali, J.C.; Igamberdiev, A.U.; Debnath, S.C. Micropropagation affects not only the fruit morphology of lowbush blueberry (Vaccinium angustifolium Ait.) but also its medicinal properties. Acta Hortic. 2015, 1098, 137–142. [Google Scholar] [CrossRef]
- Giri, C.C.; Zaheer, M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: Recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult. 2016, 126, 1–8. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Sakakibara, H.; Takei, K.; Hirose, N. Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci. 2006, 11, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Amoo, S.O.; Aremu, A.O.; Van Staden, J. In vitro plant regeneration, secondary metabolite production and antioxidant activity of micropropagated Aloe arborescens Mill. Plant Cell Tissue Organ Cult. 2012, 111, 345–358. [Google Scholar] [CrossRef]
- Deikman, J. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol. 1995, 108, 47–57. [Google Scholar] [CrossRef]
- Nordstrom, A.; Tarkowski, P.; Tarkowska, D.; Norbaek, R.; Astot, C.; Dolezal, K.; Sandberg, G. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: A factor of potential importance for auxin-cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 2004, 101, 8039–8044. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wu, J.; Wang, H.-X.; Li, S.-S.; Zheng, X.-C.; Du, H.; Xu, Y.-J.; Wang, L.-S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Alam, Z.; Morales, H.R.; Roncal, J. Environmental conditions affect phenolic content and antioxidant capacity of leaves and fruit in wild partridgeberry (Vaccinium vitis-idaea). Botany 2016, 94, 509–521. [Google Scholar] [CrossRef]
- Yildirim, A.B.; Turker, A.U. Effects of regeneration enhancers on micropropagation of Fragaria vesca L. and phenolic content comparison of field-grown and in vitro-grown plant materials by liquid chromatography-electrospray tandem mass spectrometry (LC-ESI-MS/MS). Sci. Hortic. 2014, 169, 169–178. [Google Scholar] [CrossRef]
- Nawa, Y.; Asano, S.; Motoori, S.; Ohtani, T. Production of anthocyanins, carotenoids, and proanthocyanidins by cultured cells of rabbiteye blueberry (Vaccinium ashei Reade). Biosci. Biotechnol. Biochem. 1993, 57, 770–774. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Inostroza-Blancheteau, C.; Millaleo, R.; Cruces, E.; Wulff-Zottele, C.; Alberdi, M.; Mora, M.d.l.L. Long-term aluminum exposure effects on physiological and biochemical features of highbush blueberry cultivars. J. Am. Soc. Hortic. Sci. 2010, 135, 212–222. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Phyisiology; Sinauer Associated Inc: Sunderland, MA, USA, 2006; pp. 315–344. [Google Scholar]
- Ulloa-Inostroza, E.M.; Alberdi, M.; Meriño-Gergichevich, C.; Reyes-Díaz, M. Low doses of exogenous methyl jasmonate applied simultaneously with toxic aluminum improve the antioxidant performance of Vaccinium corymbosum. Plant Soil 2017, 412, 81–96. [Google Scholar] [CrossRef]
- Khalil, S.A.; Kamal, N.; Sajid, M.; Ahmad, N.; Zamir, R.; Ahmad, N.; Ali, S. Synergism of polyamines and plant growth regulators enhanced morphogenesis, stevioside content, and production of commercially important natural antioxidants in Stevia rebaudiana Bert. In Vitro Cell. Dev. Biol. Plant 2016, 52, 174–184. [Google Scholar] [CrossRef]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit phenolics; CRC Press: Boca Raton, FL, USA, 1990; pp. 149–238. [Google Scholar]
- Dewick, P.M. Isoflavonoids. In The Flavonoids: Advances in Research since 1986, 1st Ed.; Harborne, J.B., Ed.; Chapman & Hall: New York, NY, USA, 1994; pp. 117–189. [Google Scholar]
- Makowczynska, J.; Grzegorczyk, K.I.; Wysokinska, H. Antioxidant activity of tissue culture-raised Ballota nigra L. plants grown ex vitro. Acta Pol. Pharm. 2015, 72, 769–775. [Google Scholar]
- Goyali, J.C.; Igamberdiev, A.U.; Debnath, S.C. DNA methylation in lowbush blueberry (Vaccinium angustifolium Ait.) propagated by softwood cutting and tissue culture. Can. J. Plant Sci. 2018, 98, 1035–1044. [Google Scholar] [CrossRef]
| Berry Types | Phenolics | Flavonoids | Anthocyanins | Proanthocyanidins | References |
|---|---|---|---|---|---|
| Highbush blueberries | 77.0–820.0 | 155.2–512.3 | 18.0–249.0 | 179.8 | [11,18,19,20,21,22,23,24,25] |
| Half-high blueberries | 110.0–668.0 | 161.7–492.1 | 94.5–310.0 | – | [22,26,27,28] |
| Lowbush blueberries | 299.0–840.0 | 260.0–320.0 | 59.0–344.0 | 190.0–331.9 | [11,18,20,21,29,30,31] |
| Rabbiteye blueberries | 230.8–929.6 | – | 12.7–410.0 | – | [11,18,19,23] |
| Lingonberries | 489.1–760.0 | 692.0–1047.0 | 35.0–708.8 | 278.8–1294.7 | [31,32,33] |
| Cranberries | 328.0–915.0 | 278.0–751.0 | 13.0–227.0 | 11.2–418.8 | [31,34,35] |
| Bilberries | 458.0–570.0 | 374.0–418.0 | 301.0–393.0 | 85.5 | [21,31,33,36] |
| Berry Types | Bioactive Compounds | Biological Properties | References |
|---|---|---|---|
| Highbush blueberries | Polyphenols, anthocyanin, tannins; β-carotene, lutein and zeaxanthin | Anticancer, anti-inflammatory, anti-microbial activities; retard and reverse age-related deficits in behaviour; reduce cardiovascular risks; ameliorate radiation-induced lung injury; retard type II diabetes, juvenile idiopathic arthritis and osteoarthritis. | [3,52,55,57,58,59,60,61,62,63,64,65] |
| Lowbush blueberries | Phenolics, flavonoids, anthocyanin and proanthocyanidin fractions | Retard liver and prostate cancer; inhibit urinary tract infections; reverse signs of aging; protect brain against ischemia-damage; strengthen blood vessels and arteries; neuroprotective effect. | [48,54,55,56,66,67,68,69,70,71] |
| Rabbiteye blueberries | Polyphenols, anthocyanin, tannins | Inhibit colon and liver cancer. | [50,53] |
| Lingonberries | Polyphenols, anthocyanin and proanthocyanidin fractions | Prevent the detrimental metabolic effects induced by high-fat diet; protect kidney against ischemia–reperfusion induced kidney injury; anti-inflammatory, anticarcinogenic, antimicrobial, antiadhesion activities; prevent leukemia and colon cancer. | [31,48,71,72,73,74,75,76,77] |
| Cranberries | Polyphenols, anthocyanin and proanthocyanidin fractions | Antibacterial, anticarcinogenic activities; reduce cardiovascular risk in patients with metabolic syndrome; protect from diet-induced obesity and insulin resistance; prevent intestinal oxidative stress, inflammation and urinary tract infection. | [3,31,48,73,78,79,80,81,82,83,84,85] |
| Bilberries | Anthocyanins, flavonols, carotenoid, lutein, and zeaxanthin | Anticarcinogenic; reduce inflammation and progression of chronic hypertension; prevent development of glaucoma, cataract and macular degeneration. | [15,48,49,51,86,87,88,89,90] |
| Species | Media Types 1 | Micropropagation Via | Explants Used | Rooting In Vitro/Ex Vitro | References |
|---|---|---|---|---|---|
| V. corymbosum × V. angustifolium cv. ‘St. Cloud’, ‘Patriot’, ‘Northblue’, ‘Chippewa | MBM-C | Somatic embryogenesis | Leaf segments | In vitro & ex vitro | [163] |
| V. angustifolium wild clones | MBM-C | Shoot proliferation | Single nodes, axillary buds | Ex vitro | [157] |
| V. angustifolium cv. ‘Fundy’ and wild clones | MBM-C | Shoot proliferation | Shoot tip and segments | Ex vitro | [138,158] |
| V. angustifolium wild clones | MBM-C | Shoot regeneration | Leaf segments | Ex vitro | [158,165] |
| V. angustifolium | WPM | Shoot proliferation | Single node | N/R | [170] |
| V. angustifolium | ANM | Shoot regeneration | Hypocotyl and cotyledons | N/R | [154] |
| V. angustifolium | MSM | Callus formation | Internodes and fruits | N/R | [152,155] |
| V. angustifolium | ZBM | Shoot proliferation | Shoot | Ex vitro | [156] |
| V. angustifolium | ZBM | Shoot proliferation | Young shoot | Ex vitro | [171,172] |
| V. angustifolium | ZBM | Shoot regeneration | Leaf | Ex vitro | [171] |
| V. angustifolium cv. ‘Dwarf Tophat’ | WPM | Shoot proliferation | Single node | In vitro on WPM | [173] |
| V. angustifolium | ZBM | Shoot regeneration | Internodes | N/R | [174] |
| V. ashei cv. ‘Titan’ | MSM & WPM | Shoot proliferation | Multiple shoots | Ex vitro | [175] |
| V. corymbosum cv. ‘Polaris’, ‘St. Cloud’ | MBM-C | Shoot proliferation | AxillaryShoots | Ex vitro | [162] |
| V. corymbosum cv. ‘Huron’ | MSM & WPM | Shoot proliferation | Nodal segments | Ex vitro | [176] |
| Hybrid of V. corymbosum ‘Spartan’ × V. bracteatum | MSM & WPM | Shoot proliferation | Axillary buds | In vitro | [177] |
| V. corymbosum cv. ‘Berkeley’, ‘Bluecrop’ ‘Goldtraube’ | MSM & ANM | Shoot multiplication | Shoots | In vitro on ANM | [178] |
| V. corymbosum cv. ‘Elliot’ | WPM | Shoot regeneration and proliferation | Buds, leaves, microshoots | Ex vitro | [179] |
| V. corymbosum cv. ‘Bluecrop’ ‘Berkeley’, ‘Earliblue’ | MSM & WPM | Shoot proliferation | Nodal segments | In vitro | [180] |
| V. corymbosum × V. angustifolium cv. ‘Northland’ | WPM | Shoot regeneration | Nodal and leaf segments | In vitro | [151] |
| Interspecific hybrids of Vaccinium spp. | MSM & ZBM | Shoot regeneration | Ovule | Ex vitro | [181] |
| V. corymbosum cv. ‘Ozarkblue’ | WPM | Shoot proliferation and regeneration | Nodal and leaf segments | In vitro & ex vitro | [136] |
| V. corymbosum cv. ‘Bluecrop’, ‘Duke’, ‘Sunrise’. | WPM | Adventitious shoot regeneration | Leaf | Ex vitro | [182,183] |
| V. corymbosum cv. ‘Bluecrop’ | WPM | Shoot regeneration | Leaf | Ex vitro | [184] |
| V. virgatum cv. ‘Kunisato 35 Gou’ | MSM & WPM | Shoot multiplication | Nodal segments | In vitro | [185] |
| V. corymbosum cv. ‘Berkeley’ | WPM | Shoot proliferation | Nodal segments | Ex vitro | [186] |
| V. corymbosum cv. ‘Herbert’ | ZBM | Shoot proliferation and regeneration | Nodal segments | Ex vitro | [169] |
| V. corymbosum | WPM | Shoot proliferation | Single node | N/R | [187] |
| V. corymbosum × V. angustifolium cv. ‘Northblue’ | ZBM | Shoot proliferation | Shoot tips | Ex vitro | [159,160,161] |
| V. corymbosum × V. angustifolium cv. ‘North Country’ | WPM | Shoot proliferation and regeneration | Leaf segments | N/R | [188] |
| V. corymbosum (southern highbush) | MSM & WPM | Shoot regeneration | Leaf segments | Ex vitro | [189] |
| V. macrocarpon cv. ‘Ben Lear’ ‘Pilgrim’ ‘Stevens’ | MBM-C | Shoot proliferation | Nodal segments, shoot tips | In vitro & ex vitro | [46,190] |
| V. macrocarpon wild clones | MBM-C | Shoot proliferation | Nodal segments | In vitro & ex vitro | [190,191] |
| V. vitis-idaea ssp. minus wild clones | MBM-C | Shoot proliferation | Nodal segments | Ex vitro | [192,193] |
| V. vitis-idaea ssp. vitis-idaea cv. ‘Regal’, ‘Splendor’ ‘Erntedank’ | MBM-C | Shoot proliferation | Nodal segments | Ex vitro | [192,193] |
| V. vitis-idaea ssp. vitis-idaea cv. ‘Regal’, ‘Splendor’ ‘Erntedank’ | MBM-C | Shoot regeneration | Leaf segments | Ex vitro | [166,194] |
| V. myrtillus | WPM | Shoot proliferation | Auxiliary buds | Ex vitro | [195] |
| Berry Types | Stem Cutting | Shoot Proliferation | Shoot Regeneration | References |
|---|---|---|---|---|
| Highbush blueberries | Compared to TC plants, SC plants grow slower, produce less and shorter shoots, greater number of flowers, larger berries and develop flowers a year earlier. | Plants grow faster with taller and more shoots, higher plant dry weight, less flowers and smaller berries than SC plants. | Plants grow faster with taller and more shoots, less flowers and smaller berries than SC plants. | [197,199] |
| Half-high blueberries | Less and shorter shoots, low berry yield than TC plants. | Grow faster with taller and more shoots and higher fruit yield than SC plants. | - | [198] |
| Lowbush blueberries | Number of flowers and berries, size and weight berries were greater than TC plant. | Faster vegetative growth with more stems, branches, bigger leaves and larger canopy than SC plants. | - | [30,130,173] |
| Lingonberries | Higher berry weight, diameter and number per plant than TC plants. | Taller plant, more rhizomes and leaves per plant than SC plants. | Taller plant, more rhizomes and leaves per plant than SC and node culture. | [32,193,202] |
| Cranberries | Less runners and uprights than TC plants. | More runners, uprights, leaves per upright than the SC plants. | - | [190] |
| Blueberries | Less phenolic and flavonoid content than TC plant. | Higher contents of phenolics and flavonoids and their antioxidant activity than SC plants. | - | [30,163,203] |
| Lingonberries | Less total phenolics, anthocyanins, tannins and antioxidant activities than TC plants. | Higher total phenolics, flavonoids, tannins and antioxidant activity in berries of TC plants. | Higher total phenolic flavonoids tannins and antioxidant activity in berries. | [32,195,202] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debnath, S.C.; Goyali, J.C. In Vitro Propagation and Variation of Antioxidant Properties in Micropropagated Vaccinium Berry Plants—A Review. Molecules 2020, 25, 788. https://doi.org/10.3390/molecules25040788
Debnath SC, Goyali JC. In Vitro Propagation and Variation of Antioxidant Properties in Micropropagated Vaccinium Berry Plants—A Review. Molecules. 2020; 25(4):788. https://doi.org/10.3390/molecules25040788
Chicago/Turabian StyleDebnath, Samir C., and Juran C. Goyali. 2020. "In Vitro Propagation and Variation of Antioxidant Properties in Micropropagated Vaccinium Berry Plants—A Review" Molecules 25, no. 4: 788. https://doi.org/10.3390/molecules25040788
APA StyleDebnath, S. C., & Goyali, J. C. (2020). In Vitro Propagation and Variation of Antioxidant Properties in Micropropagated Vaccinium Berry Plants—A Review. Molecules, 25(4), 788. https://doi.org/10.3390/molecules25040788







