Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances
Abstract
:1. Introduction
2. Results and Discussion
2.1. Weather Conditions, Crop Growth and Yield
2.2. Fatty Acid Composition
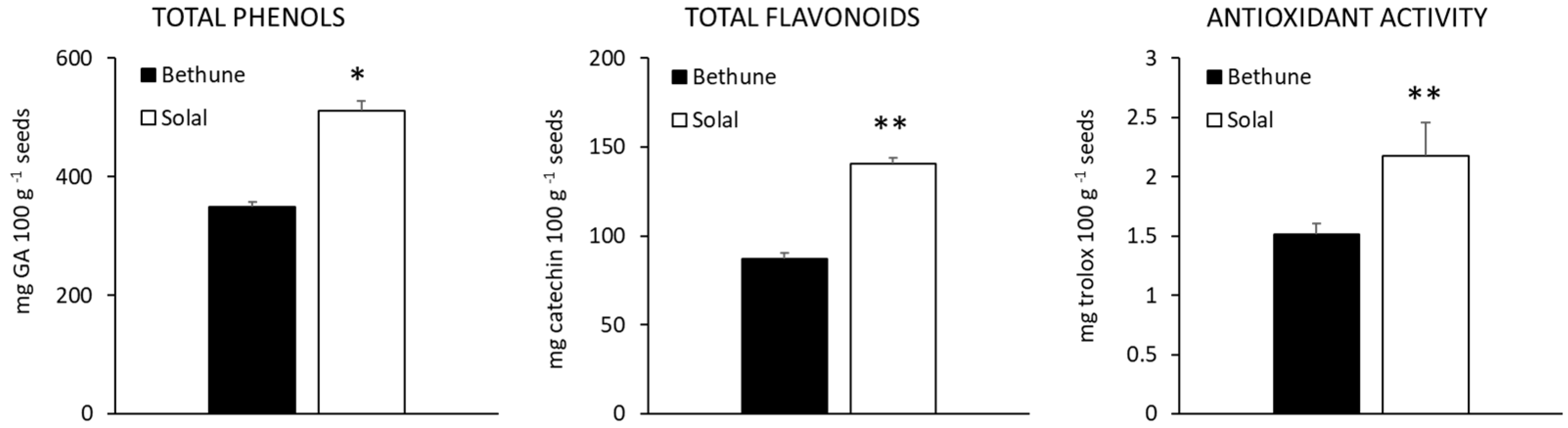
2.3. Phenolic Compounds and Antioxidant Activity
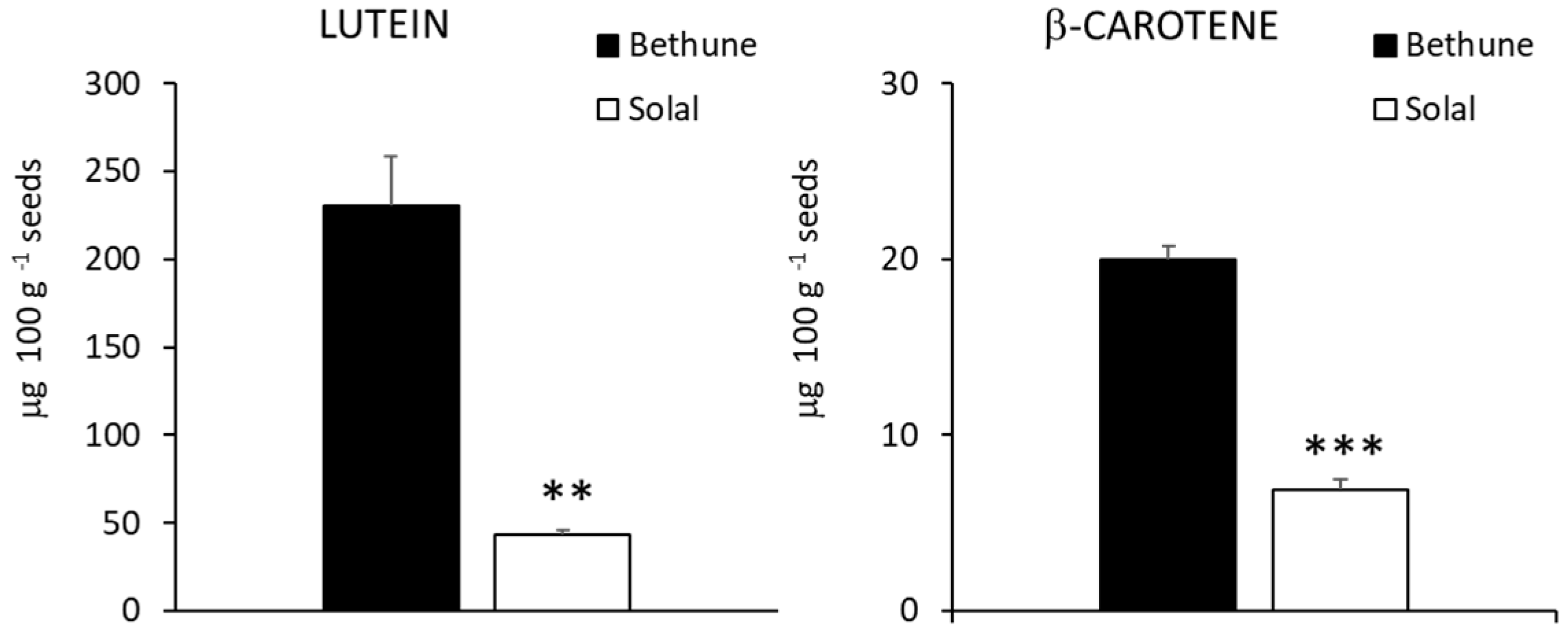
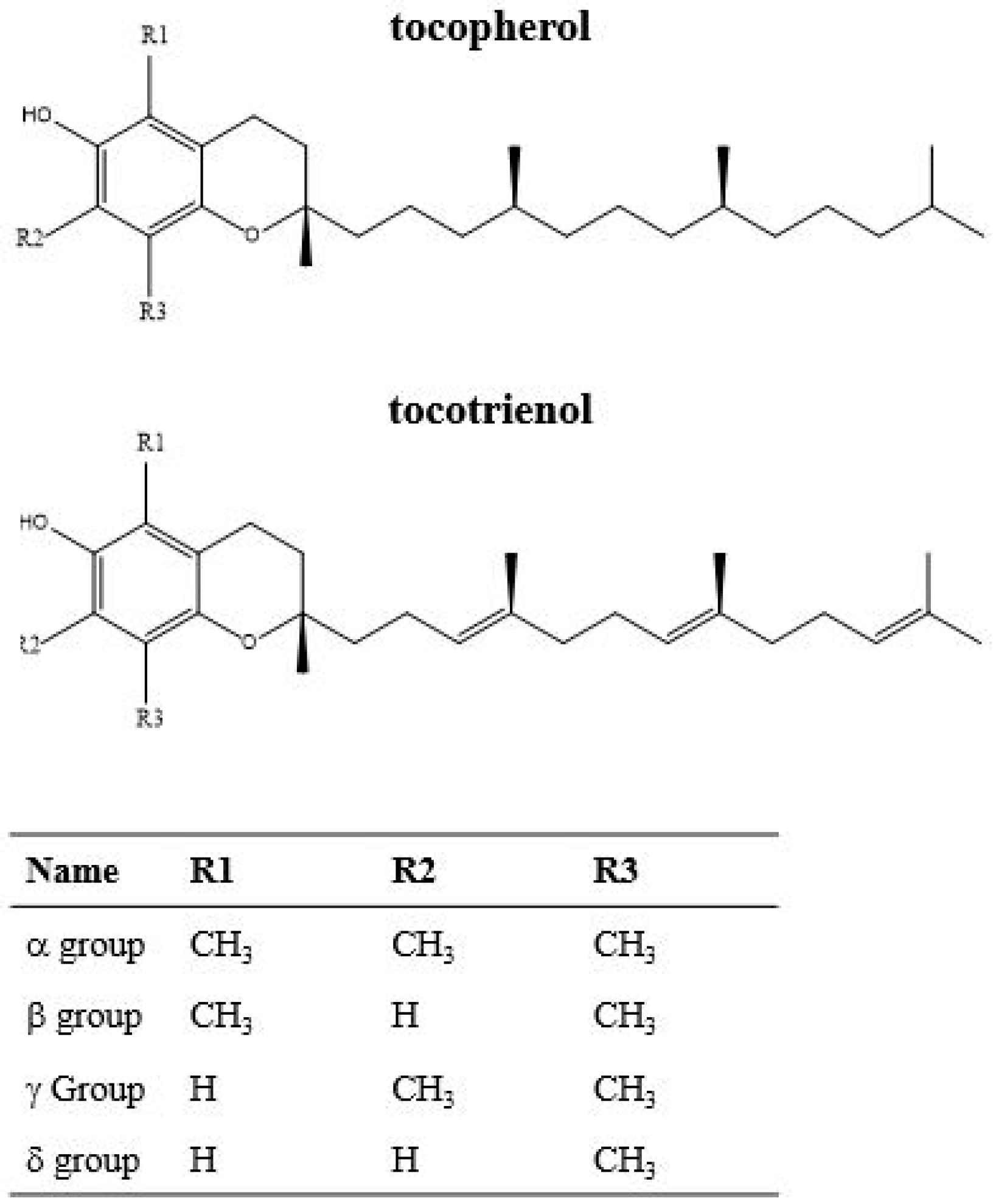
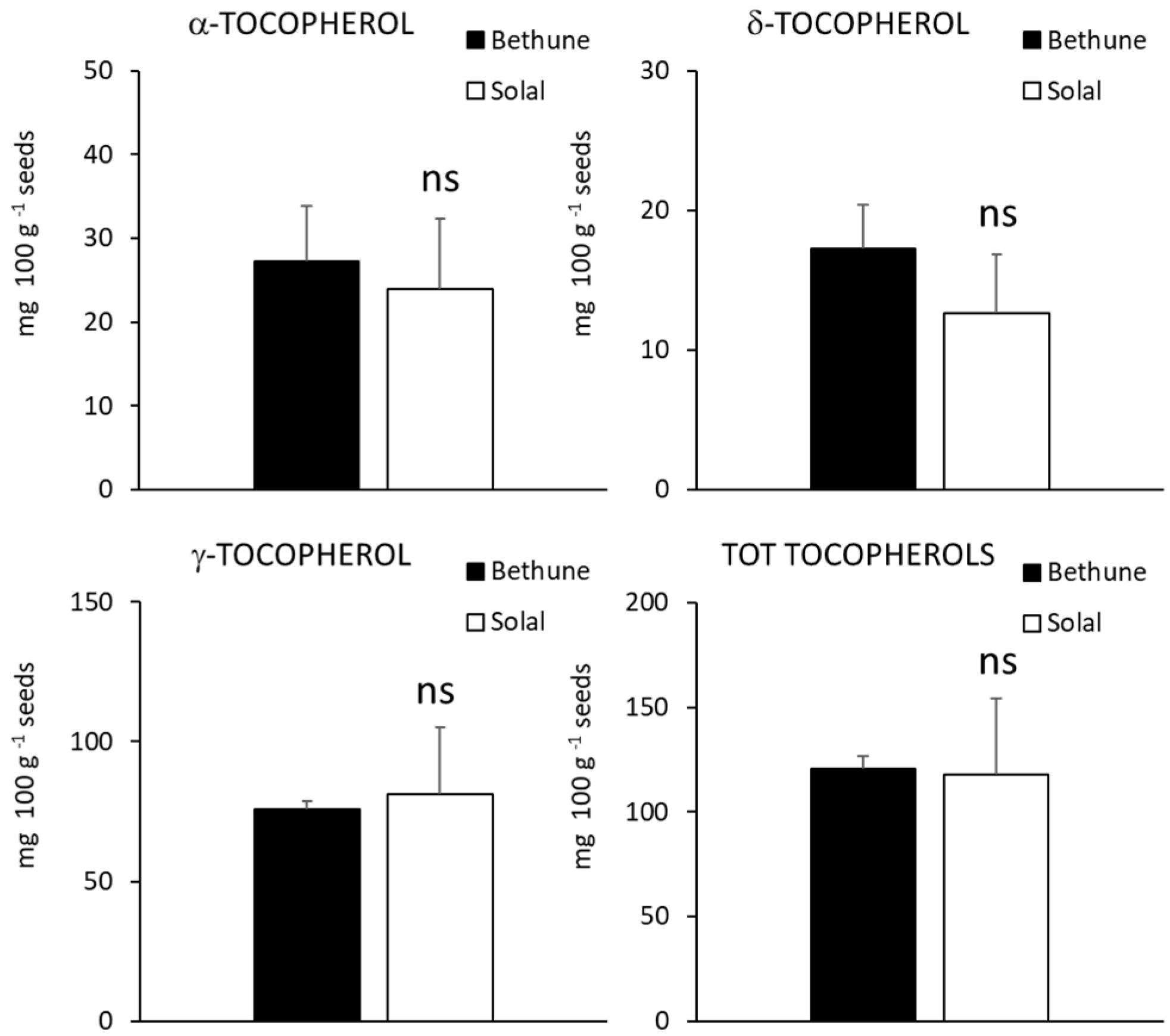
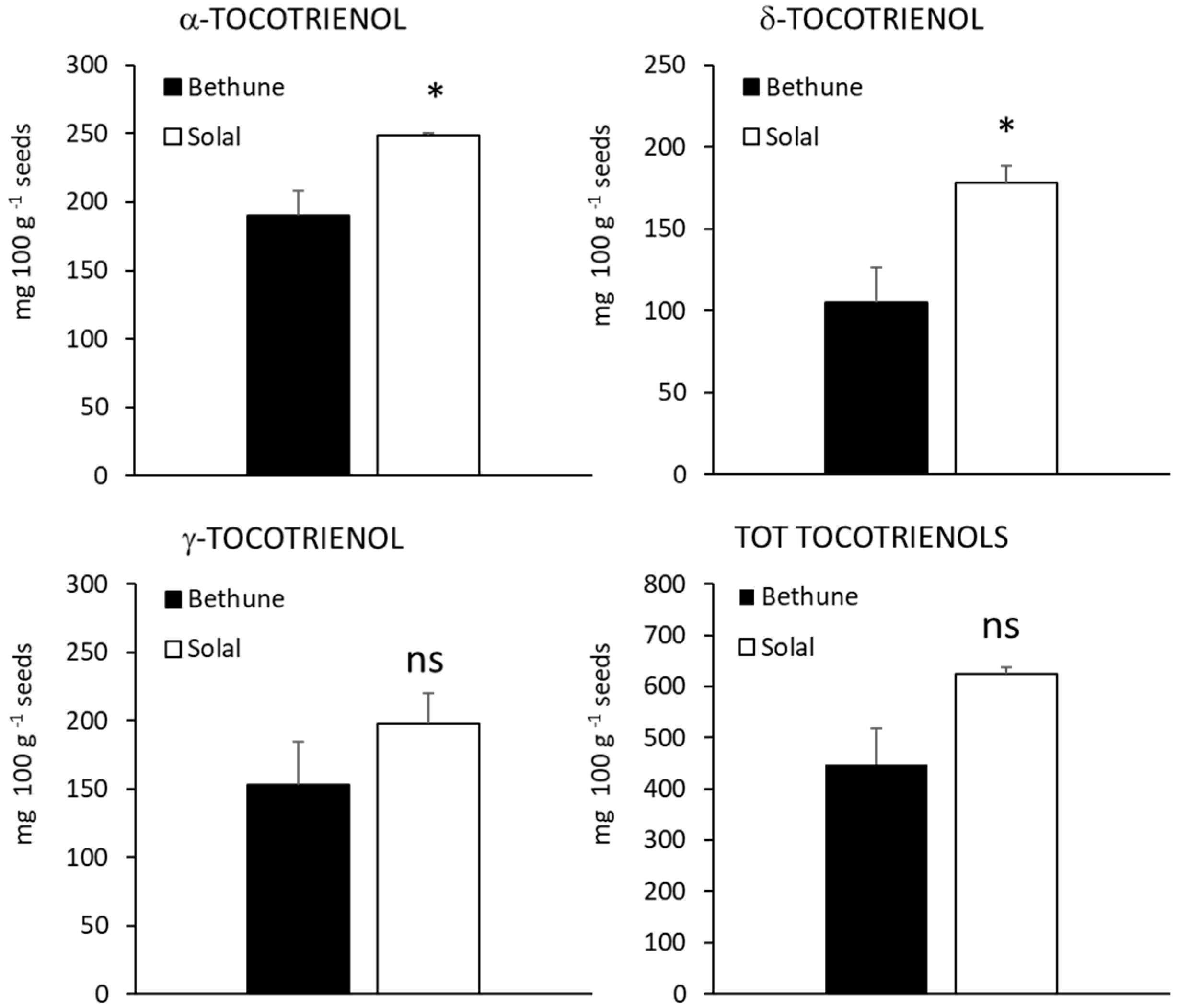
2.4. Carotenoids, Tocopherols and Tocotrienols
3. Materials and Methods
3.1. Reagents and Standards
3.2. Field Experiment and Plant Material
3.3. Plant Sampling and Yield Evaluation
3.4. Crude Protein Content
3.5. Fatty Acid Composition and Oil Content Determination
3.6. Content of Total Phenols and Flavonoids
3.7. Determination of Antioxidant Activity
3.8. Quantification of Carotenoids, Tocopherols and Tocotrienols
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [PubMed]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Morris, D.H. Flax-A Health and Nutrition Primer, 4th ed.; Flax Council of Canada: Winnipeg, MB, Canada, 2007. [Google Scholar]
- Toure, A.; Xueming, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef]
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A potential source of food, feed and fiber. Crit. Rev. Food Sci. Nutr. 2001, 51, 210–222. [Google Scholar] [CrossRef]
- Carter, J.F. Potential of flaxseeds and flaxseed oil in baked goods and other products in human nutrition. Cereal Foods World 1993, 38, 754–759. [Google Scholar]
- Payne, T.J. Promoting better health with flaxseed in bread. Cereal Foods World 2000, 45, 102–104. [Google Scholar]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Beejmohun, V.; Fliniaux, O.; Grand, E.; Lamblin, F.; Bensaddek, L.; Christen, P.; Kovensky, J.; Fliniaux, M.A.; Mesnard, F. Microwave-assisted extraction of the main phenolic compounds in flaxseed. Phytochem. Anal. 2007, 18, 275–282. [Google Scholar] [CrossRef]
- Mazza, G. Production, Processing and Uses of Canadian Flax. In Proceedings of the First CGNA International Workshop, Temuco, Chile, 3–6 August 2008. [Google Scholar]
- Morris, M.C.; Evans, D.A.; Tangney, C.C. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am. J. Clin. Nutr. 2005, 81, 508–514. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Rowland, G.G.; Bhirud, P.R.; Dyck, J.H.; Tyler, R.T. Chemical composition and physicochemical and hydrogenation characteristics of high-palmitic acid solin (low-linolenic acid flaxseed) oil. J. Am. Oil Chem. Soc. 2004, 81, 185–188. [Google Scholar] [CrossRef]
- Wiesenborn, D.; Tostenson, K.; Kangas, N.; Zheng, Y.L.; Hall, C.; Niehaus, M.; Jarvis, P.; Schwarz, J.; Twombly, W. Processing flaxseed for food and feed uses. Food Sci. Biotechnol. 2005, 14, 305–310. [Google Scholar]
- Dribnenki, J.C.P.; Green, A.G. LinolaTM ‘947’ low linolenic flax. Can. J. Plant Sci. 1995, 75, 201–202. [Google Scholar] [CrossRef]
- Bathia, C.R.; Nichterlein, K.; Maluszynski, M. Oilseed cultivars developed from induced mutations and mutations altering fatty acid composition. Mut. Breed. Rev. 1999, 11, 1–36. [Google Scholar]
- Berti, M.; Fischer, S.; Wilckens, R.; Hevia, F.; Johnson, B. Adaptation and genotype × environment interaction of flaxseed (Linum usitatissimum L.) genotypes in South Central Chile. Chil. J. Agric. Res. 2010, 70, 345–356. [Google Scholar]
- Adugna, W.; Labuschagne, M.T. Association of linseed characters and its variability in different environments. J. Agric. Sci. 2003, 140, 285–296. [Google Scholar] [CrossRef]
- Tavarini, S.; Angelini, L.G.; Casadei, N.; Spugnoli, P.; Lazzeri, L. Agronomical evaluation and chemical characterization of Linum usitatissimum L. as oilseed crop for bio-based products in two environments of Central and Northern Italy. Ital. J. Agron. 2016, 11, 122–132. [Google Scholar] [CrossRef]
- Casa, R.; Russell, G.; Lo Cascio, B.; Rossini, F. Environmental effects on linseed (Linum usitatissimum L.) yield and growth of flax at different stand densities. Eur. J. Agron. 1999, 11, 267–278. [Google Scholar] [CrossRef]
- Khan, M.A.; Bradshaw, A.D. Adaptation to heterogeneous environments. II. Phenotypic plasticity in response to spacing in Linum. Aust. J. Agric. Res. 1976, 27, 519–531. [Google Scholar] [CrossRef]
- Gubbels, G.H. Interaction of cultivar and seeding rate on various agronomic characteristics of flax. Can. J. Plant Sci. 1978, 58, 303–309. [Google Scholar] [CrossRef]
- Hassan, F.U.; Leitch, M.H. Influence of seeding density on contents and uptake of N, P and K in linseed (Linum usitatissimum L.). J. Agron. Crop Sci. 2000, 185, 193–199. [Google Scholar] [CrossRef]
- Leitch, M.H.; Sahi, F. The effect of plant spacing on growth and development of linseed. Ann. Appl. Biol. 1999, 135, 529–534. [Google Scholar] [CrossRef]
- Lafond, G.P.; Irvine, B.; Johnston, A.M.; May, W.E.; McAndrew, D.W.; Shirtliffe, S.J.; Stevenson, F.C. Impact of agronomic factors on seed yield formation and quality in flax. Can. J. Plant Sci. 2008, 88, 485–500. [Google Scholar] [CrossRef]
- Andruszczak, S.; Gawlik-Dziki, U.; Kraska, P.; Kwiecińska-Poppe, E.; Różyło, K.; Pałys, E. Yield and quality traits of two linseed (Linum usitatissimum L.) cultivars as affected by some agronomic factors. Plant Soil Environ. 2015, 61, 247–252. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Pihlava, J.M.; Eurola, M.; Klemola, A.; Jauhiainen, L.; Hietaniemi, V. Yield, SDG lignan, cadmium, lead, oil and protein contents of linseed (Linum usitatissimum L.) cultivated in trials and at different farm conditions in the southwestern part of Finland. Agric. Food Sci. 2013, 22, 296–306. [Google Scholar] [CrossRef]
- D’Antuono, L.F.; Rossini, F. Experimental estimation of linseed crop parameters. Ind. Crop Prod. 1995, 3, 261–271. [Google Scholar] [CrossRef]
- Dybing, C.D.; Zimmerman, D.C. Temperature effects on flax (Linum usitatissimum L.) growth, seed production and oil quality in controlled environments. Crop Sci. 1965, 5, 184–187. [Google Scholar] [CrossRef]
- Mirshekari, M.; Amiri, R.; Nezhad, H.I.; Noori, S.S.; Zandvakili, O.R. Effects of planting date and water deficit on quantitative and qualitative traits of flax seed. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 901–913. [Google Scholar]
- Pandey, R.K.; Maranville, J.W.; Admon, A. Tropical wheat response to irrigation and nitrogen in Sahelian environment. I. Grain yield, yield components and water use efficiency. Eur. J. Agron. 2001, 15, 93–105. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Functional properties and uses of flaxseed protein. Inform 1995, 6, 1246–1252. [Google Scholar]
- Daun, J.; Barthet, V.; Chornick, T.; Duguid, S. Structure, composition, and variety development of flaxseed. In Flaxseed in Human Nutrition, 2nd ed.; Thompson, L., Cunanne, S., Eds.; AOCS Press: Champaign, IL, USA, 2003; pp. 1–40. [Google Scholar]
- Kaur, R.; Kaur, M.; Singh Gill, B. Phenolic acid composition of flaxseed cultivars by ultra-performance liquid chromatography (UPLC) and their antioxidant activities: Effect of sand roasting and microwave heating. J. Food Process. Preserv. 2017, 41, e13181. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Qiu, C.; Ye, Y.; Guo, X.; Chen, G.; Li, T.; Wang, Y.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles and health benefits in fiber and oil flaxseeds (Linum usitatissimum L.). Food Chem. 2017, 214, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Alu’datt, M.H.; Rababah, T.; Ereifej, K.; Alli, I. Distribution, antioxidant and characterisation of phenolic compounds in soybeans, flaxseed and olives. Food Chem. 2013, 139, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Franke, S.; Fröhlich, K.; Werner, S.; Böhm, V.; Schöne, F. Analysis of carotenoids and vitamin E in selected oilseeds, press cakes and oils. Eur. J. Lipid Sci. Technol. 2010, 112, 1122–1129. [Google Scholar] [CrossRef]
- Fujisawa, M.; Watanabe, M.; Choi, S.K.; Teramoto, M.; Ohyama, K.; Misawa, N. Enrichment of carotenoids in flaxseed (Linum usitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtB. J. Biosci. Bioeng. 2008, 105, 636–641. [Google Scholar] [CrossRef]
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical composition and health benefits of flaxseed. Austin J. Nutr. Food Sci. 2014, 2, 1045. [Google Scholar]
- Mannucci, A.; Castagna, A.; Santin, M.; Serra, A.; Mele, M.; Ranieri, A. Quality of flaxseed oil cake under different storage conditions. LWT 2019, 104, 84–90. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Dong, S.; Li, Y.; Wei, L.; Zhao, C.; Li, J.; Liu, X.; Wang, Y. Effects of germination on tocopherol, secoisolarlciresinol diglucoside, cyanogenic glycosides and antioxidant activities in flaxseed (Linum usitatissimum L.). Int. J. Food Sci. Technol. 2019, 54, 2346–2354. [Google Scholar] [CrossRef]
- Mathur, P.; Ding, Z.; Saldeen, T.; Mehta, J.L. Tocopherols in the prevention and treatment of atherosclerosis and related cardiovascular disease. Clin. Cardiol. 2015, 38, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G. The discovery of the antioxidant function of vitamin E: The contribution of Henry A. Mattill. J. Nutr. 2005, 135, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Traber, M.G. γ-Tocopherol, the new vitamin E? Am. J. Clin. Nutr. 2003, 77, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Benaksas, E.J.; Bolli, R.; Comp, P.; Grammas, P.; Hamdheydari, L.; Mou, S.; Pye, Q.N.; Stoddard, M.F.; Wallis, G.; et al. New perspectives on vitamin E: Gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004, 36, 1–15. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef]
- Soil Survey Staff USA. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; USDA-SCS Agricultural Handbook, 436; U.S. Gov. Print. Office: Washington, DC, USA, 1975.
- Venglat, P.; Xiang, D.; Qiu, S.; Stone, S.L.; Tibiche, C.; Cram, D.; Alting-Mees, M.; Nowak, J.; Cloutier, S.; Deyholos, M.; et al. Gene expression analysis of flax seed development. BMC Plant Biol. 2011, 11, 74. [Google Scholar] [CrossRef]
- Ragupathy, R.; Rathinavelu, R.; Cloutier, S. Physical mapping and BAC-end sequence analysis provide initial insights into the flax (Linum usitatissimum L.) genome. BMC Genom. 2011, 12, 217. [Google Scholar] [CrossRef]
- Kumar, S.; You, F.M.; Cloutier, S. Genome wide SNP discovery in ax through next generation sequencing of reduced representation libraries. BMC Genom. 2012, 13, 684–695. [Google Scholar] [CrossRef]
- Wang, Z.; Hobson, N.; Galindo, L.; Zhu, S.; Shi, D.; McDill, J.; Yang, L.; Hawkins, S.; Neutelings, G.; Datla, R.; et al. The genome of flax (Linum usitatissimum) assembled de novo from short shotgun sequence reads. Plant J. 2012, 72, 461–473. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen total. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph 9; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Mehlich, A. Determination of cation- and anion-exchange properties of soils. Soil Sci. 1948, 66, 429–446. [Google Scholar] [CrossRef]
- Nelson, P.W.; Sommers, C.E. Total Carbon, organic Carbon and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Dreimanis, A. Quantitative determination of calcite and dolomite by using Chittick apparatus. J. Sediment. Petrol. 1962, 32, 520–529. [Google Scholar]
- Smith, J.M.; Froment, M.A. A growth stage key for winter linseed (Linum usitatissimum). Ann. Appl. Biol. 1998, 133, 297–306. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- The International Seed Testing Association (ISTA). International Rules for Seed Testing; Edition 2005; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2005. [Google Scholar]
- Christie, W.W. Preparation of lipid extracts from tissues. In Advances in Lipid Methodology—Two; Christie, W.W., Ed.; Oily Press: Dundee, UK, 1993; pp. 195–213. [Google Scholar]
- Alonso-Borbolan, M.A.; Zorro, L.; Guilleen, D.A.; Barroso, C.G. Study of the polyphenol content of red and white grape varieties by liquid chromatography-mass spectrometry and its relationship to antioxidant power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| PGS | Duration | GDD (°C) | Cumulate Rainfall (mm) | T Min (°C) | T Max (°C) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bethune | Solal | Bethune | Solal | Bethune | Solal | Bethune | Solal | Bethune | Solal | |
| Emergence—start of flowering | 42 | 49 | 385.30 | 478.90 | 53.20 | 66.40 | 7.42 | 7.92 | 20.50 | 21.23 |
| Start-End of flowering | 8 | 11 | 109.90 | 126.55 | 13.40 | 10.60 | 11.23 | 10.29 | 26.25 | 22.72 |
| End of flowering—seed development * | 33 | 27 | 494.95 | 451.65 | 19.20 | 21.20 | 13.32 | 14.69 | 26.68 | 28.76 |
| Seed development *—seed maturity | 8 | 9 | 133.80 | 166.65 | 12.80 | 1.00 | 13.69 | 15.52 | 29.76 | 31.51 |
| Total growing cycle | 91 | 96 | 1123.95 | 1223.75 | 98.60 | 99.20 | 10.41 | 10.78 | 24.02 | 24.45 |
| Vegetative cycle (BF **) | 42 | 49 | 385.30 | 478.90 | 53.20 | 66.40 | 7.42 | 7.92 | 20.50 | 21.23 |
| Reproductive cycle (AF ***) | 49 | 47 | 738.65 | 744.85 | 45.40 | 32.80 | 13.04 | 13.82 | 27.11 | 27.87 |
| Bethune | Solal | p-Value 1 | |
|---|---|---|---|
| Plant density (n. plant m−2) | 240.00 ± 32.66 | 140.63 ± 10.18 | ** |
| Plant height (m) | 0.45 ± 0.04 | 0.50 ± 0.03 | n.s. |
| Total above-ground biomass (Mg ha−1) | 1.90 ± 0.28 | 2.33 ± 0.36 | n.s. |
| Seed yield (Mg ha−1) | 0.80 ± 0.09 | 0.73 ± 0.04 | n.s. |
| Capsules per plant (n.) | 8.50 ± 0.04 | 14.13 ± 0.53 | *** |
| Seeds per capsule (n.) | 7.91 ± 1.20 | 6.34 ± 1.63 | n.s. |
| Thousand seed weight (g) | 4.81 ± 0.02 | 5.69 ± 0.07 | *** |
| Harvest Index (HI) | 0.38 ± 0.06 | 0.31 ± 0.01 | n.s. |
| Oil content (% dry matter) | 34.23 ± 0.89 | 41.33 ± 0.86 | *** |
| Crude protein content (%) | 20.84 ± 0.22 | 20.49 ± 0.09 | * |
| Oil yield (kg ha−1) | 272.73 ± 50.99 | 302.19 ± 41.33 | n.s. |
| Fatty Acids (g × 100 g−1 of Seed) | Fatty Acids (g × 100 g−1 of Total FA) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bethune | Solal | SE 1 | p-Value 2 | Bethune | Solal | SE 1 | p-Value 2 | |
| C16:0 | 0.84 | 2.00 | 0.06 | *** | 4.20 | 5.50 | 0.24 | *** |
| C16:1c9 | 0.02 | 0.04 | 0.01 | * | 0.09 | 0.12 | 0.02 | n.s. |
| C17:0 | 0.01 | 0.05 | 0.01 | * | 0.07 | 0.13 | 0.03 | n.s. |
| C18:0 | 0.28 | 0.46 | 0.07 | n.s. | 1.40 | 1.27 | 0.15 | n.s. |
| C18:1c9 | 2.77 | 3.78 | 0.18 | *** | 13.91 | 10.42 | 1.50 | * |
| C18:1c11 | 0.11 | 0.24 | 0.03 | * | 0.55 | 0.66 | 0.05 | n.s. |
| C18:2c9c12 | 3.06 | 28.24 | 2.10 | *** | 15.39 | 77.82 | 1.87 | *** |
| C18:3c9c12c15 | 22.74 | 1.44 | 0.89 | *** | 64.02 | 3.96 | 1.64 | *** |
| SFA 3 | 1.14 | 2.54 | 0.07 | *** | 5.72 | 6.99 | 0.24 | *** |
| MUFA 4 | 2.91 | 4.08 | 0.13 | *** | 14.55 | 11.20 | 1.75 | * |
| PUFA 5 | 25.86 | 29.68 | 0.95 | *** | 79.40 | 81.78 | 1.63 | n.s. |
| PUFA/SFA | 22.68 | 11.69 | 0.84 | *** | 13.88 | 11.69 | 1.63 | n.s. |
| n6/n3 | 0.13 | 19.61 | 2.34 | *** | 0.24 | 19.65 | 2.44 | *** |
| Clay (%) | 18.4 |
| Silt (%) | 39.1 |
| Sand (%) | 42.5 |
| pH (1:25) | 8.15 |
| Electrical conductivity (μS cm−1) | 71.2 |
| Total N (‰) | 1.18 |
| Organic matter (%) | 1.8 |
| Assimilable P (mg kg−1) | 3.6 |
| Cation exchange capacity (mEq 100 g−1) | 11.9 |
| Total CaCO3 (%) | 33.3 |
| Active CaCO3 (%) | 2.8 |
| Code a | Definition | Date | |
|---|---|---|---|
| 0: Germination and emergence (sub-code 05: emergence) | Emergence b | 29 March 2015 | |
| 5: Inflorescence emergence (sub-code 59: first flower formed) | Beginning of flowering | Bethune 10 May 2015 | Solal 17 May 2015 |
| 6: Flowering and capsule formation (sub-code 69: end of flowering, all pedicles bearing capsules) | End of flowering | Bethune 18 May 2015 | Solal 28 May 2015 |
| 8: Capsule and seed ripening (sub-code 85: capsules all yellow brown, but soft) | Seed development (seed plump and pliable) | Bethune 20 June 2015 | Solal 24 June 2015 |
| 8: Capsule and seed ripening (sub-code 89: capsules brown, seed rattle in the capsule) | Seed maturity | Bethune 28 June 2015 | Solal 3 July 2015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 3729. https://doi.org/10.3390/molecules24203729
Tavarini S, Castagna A, Conte G, Foschi L, Sanmartin C, Incrocci L, Ranieri A, Serra A, Angelini LG. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules. 2019; 24(20):3729. https://doi.org/10.3390/molecules24203729
Chicago/Turabian StyleTavarini, Silvia, Antonella Castagna, Giuseppe Conte, Lara Foschi, Chiara Sanmartin, Luca Incrocci, Annamaria Ranieri, Andrea Serra, and Luciana G. Angelini. 2019. "Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances" Molecules 24, no. 20: 3729. https://doi.org/10.3390/molecules24203729
APA StyleTavarini, S., Castagna, A., Conte, G., Foschi, L., Sanmartin, C., Incrocci, L., Ranieri, A., Serra, A., & Angelini, L. G. (2019). Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules, 24(20), 3729. https://doi.org/10.3390/molecules24203729









