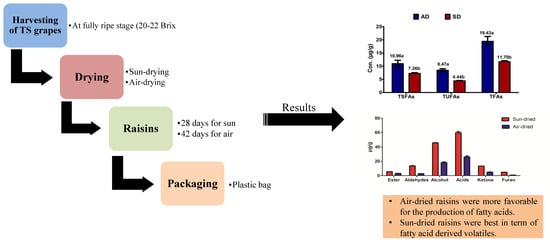
Impact of Drying Method on the Evaluation of Fatty Acids and Their Derived Volatile Compounds in ‘Thompson Seedless’ Raisins
Abstract
1. Introduction
2. Results
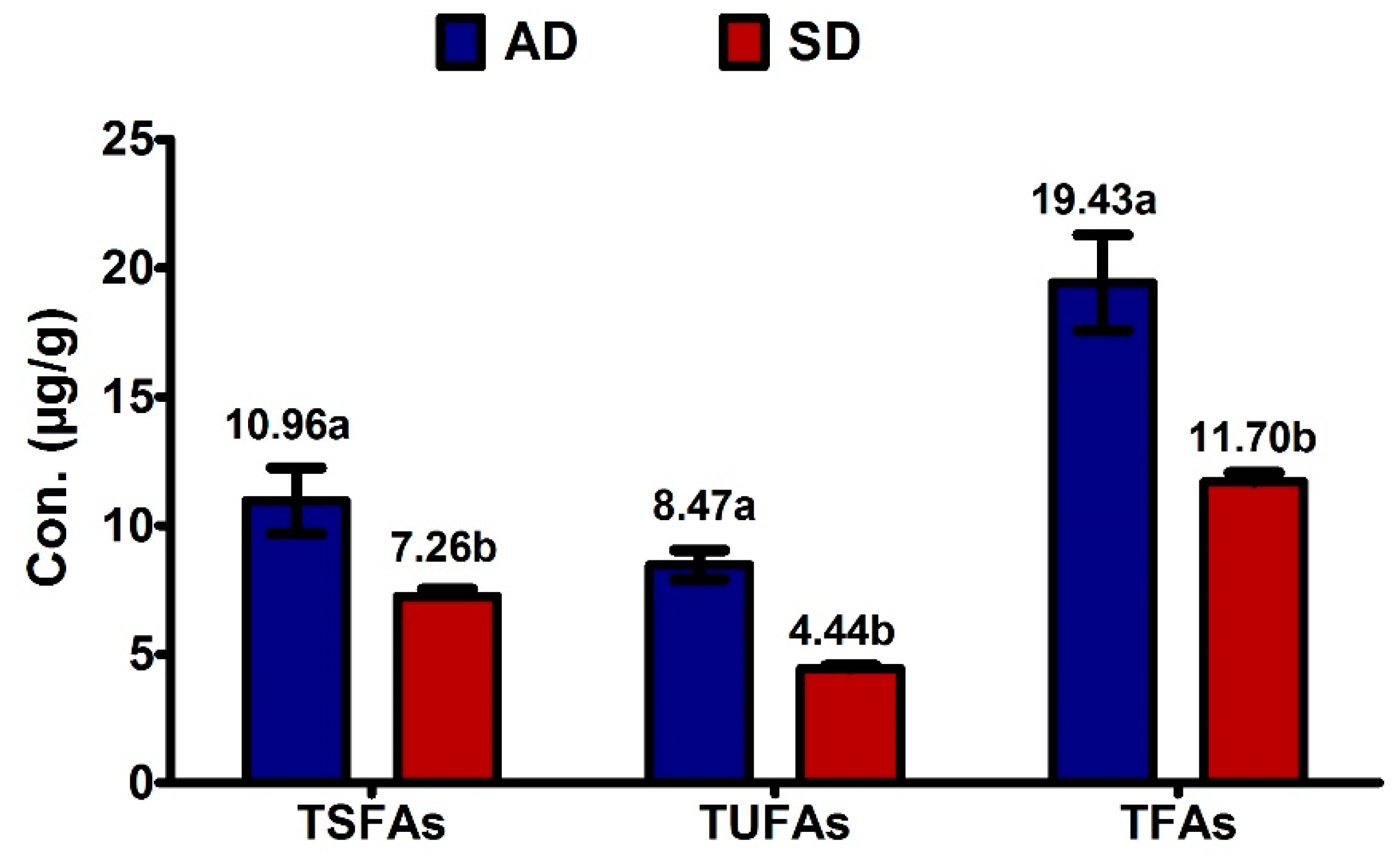
2.1. Fatty Acid Composition
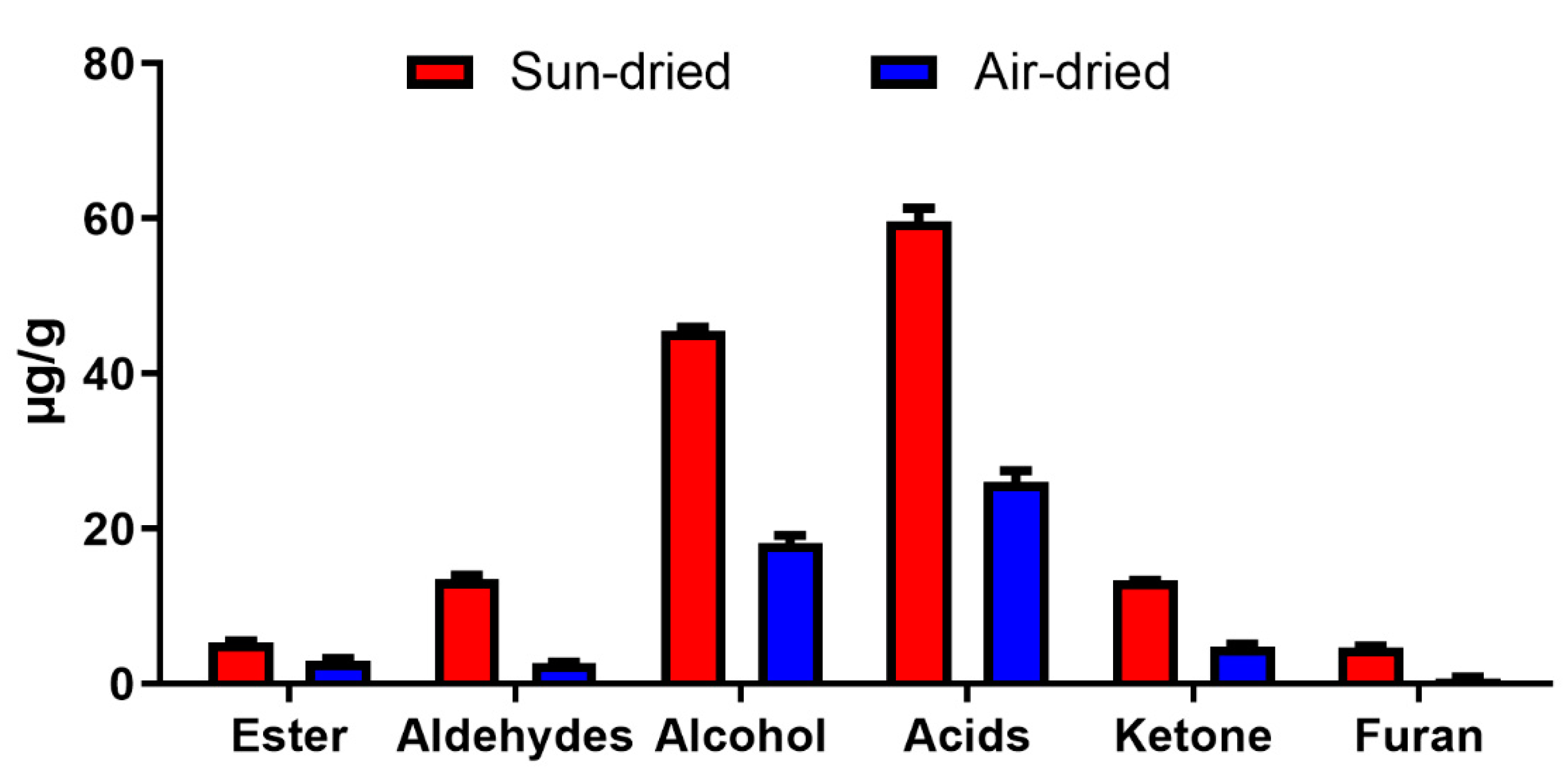
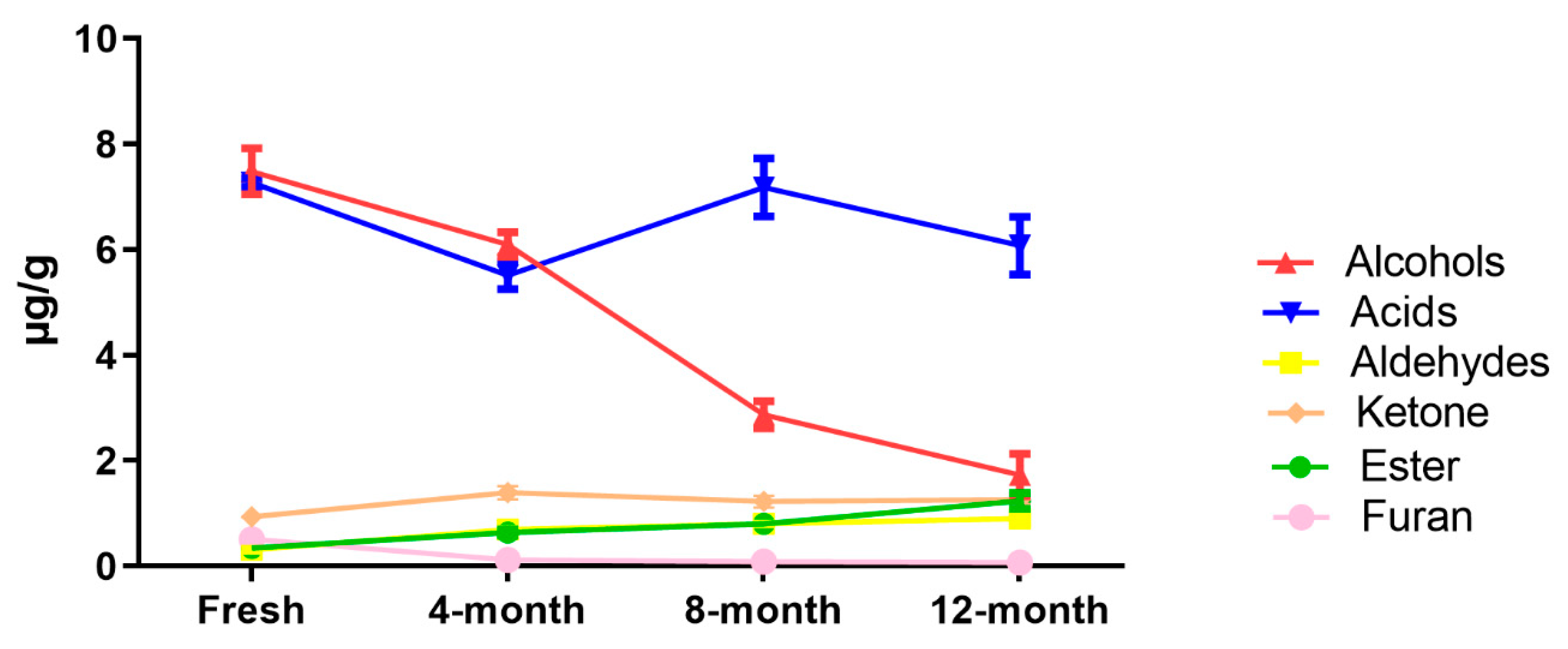
2.2. Fatty Acid-Derived Volatile Compounds
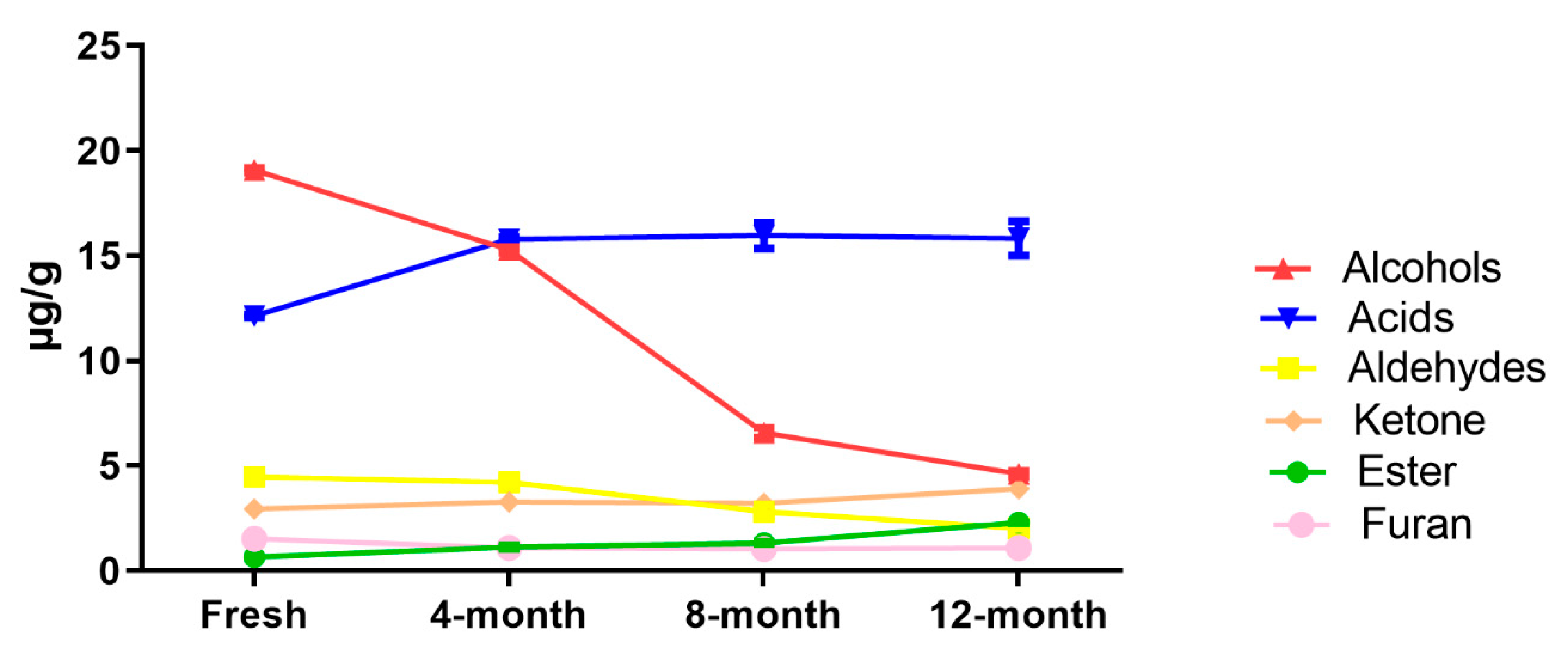
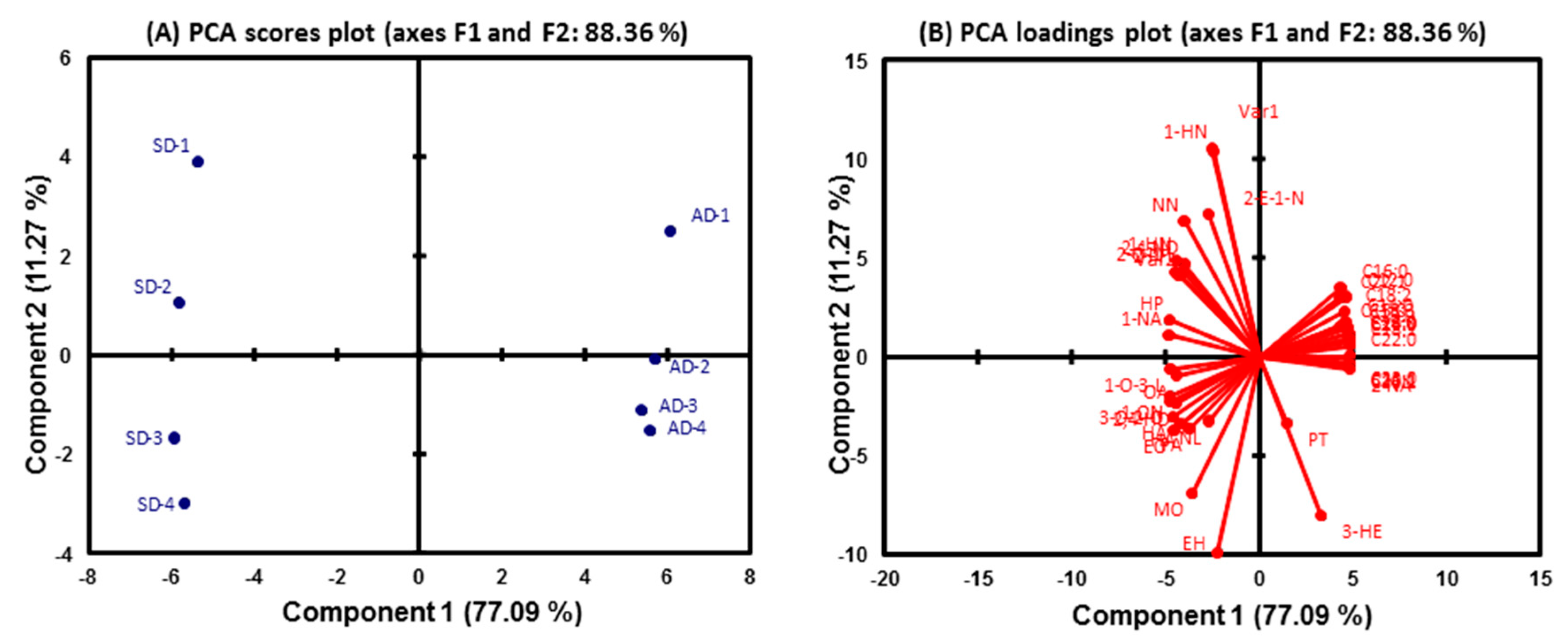
2.3. Effect of Drying Method on Fatty Acids and UFAO-Derived Volatile Compounds
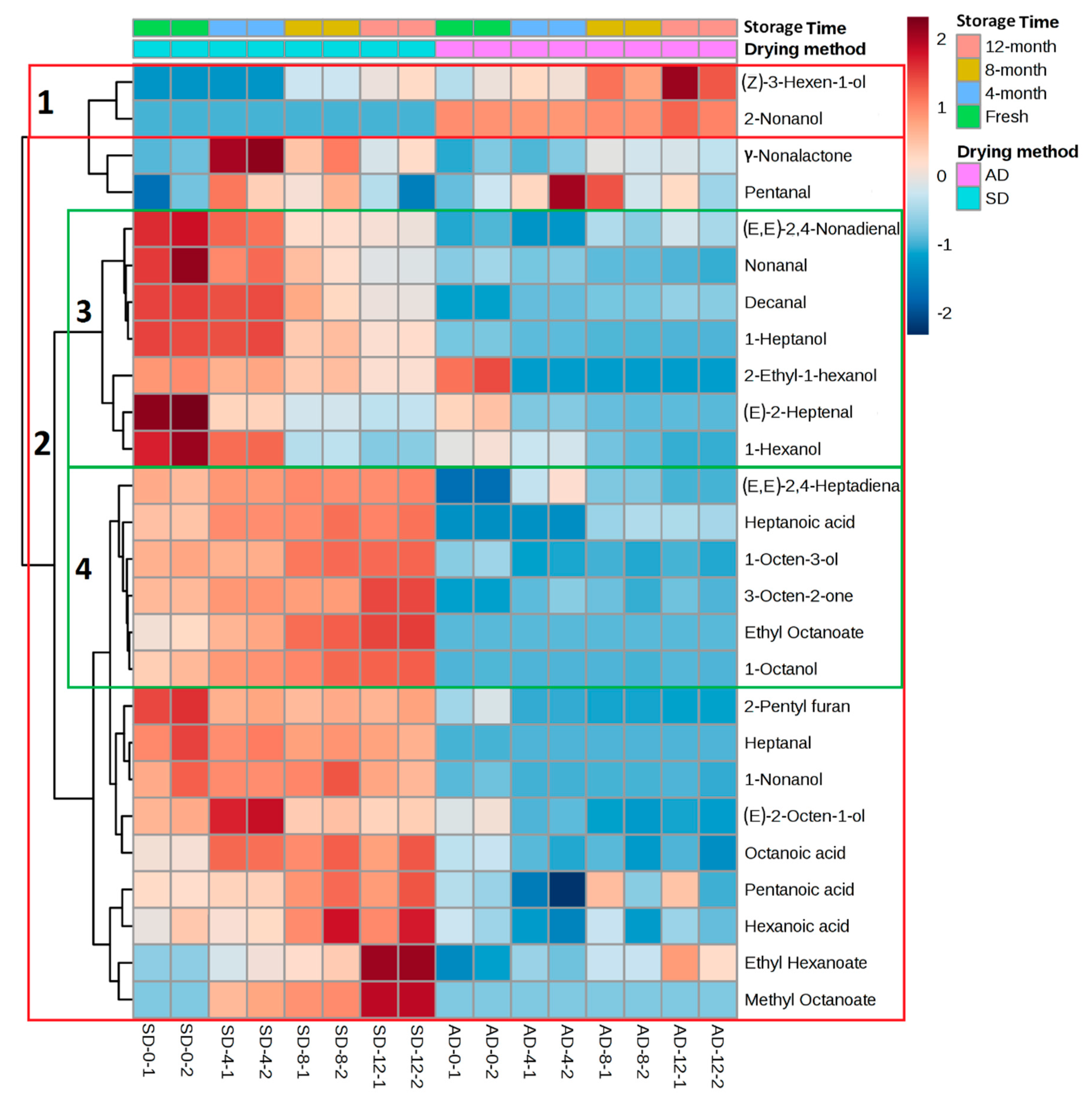
2.4. Hierarchical Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Analysis of Fatty Acids
4.1.1. Sample Preparation
4.1.2. Extraction and Methylation
4.1.3. GC-MS Condition
4.2. Fatty Acid-Derived Volatile Compounds
4.2.1. Sample Preparation
4.2.2. SPME Condition
4.2.3. GC-MS Condition
4.2.4. Quantification Method
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| USFAs | Unsaturated Fatty Acids |
| SFAs | Saturated Fatty Acids |
| FAs | Fatty Acids |
| TS | Thompson Seedless |
| GC/MS | Gas Chromatography/Mass Spectrometry |
References
- Raisins: World Markets and Trade; USDA: Washington, DC, USA, 2017. Available online: https://apps.fas.usda.gov/psdonline/circulars/raisins.pdf (accessed on 20 January 2020).
- Inouye, A. China—Peoples Republic of Raisin Annual; Global Agricultural Information Network (GAIN): Turpan, China, 2017. [Google Scholar]
- Christensen, L.P. Raisin Production Manual; UCANR Publication: Oakland, CA, USA, 2000; ISBN 978-1879906440. [Google Scholar]
- Javed, H.U.; Wang, D.; Shi, Y.; Wu, G.F.; Xie, H.; Pan, Y.Q.; Duan, C.Q. Changes of free-form volatile compounds in pre-treated raisins with different packaging materials during storage. Food Res. Int. 2018, 107, 649–659. [Google Scholar] [CrossRef]
- Javed, H.U.; Wang, D.; Wu, G.; Muhammad, Q.; Duana, C.-Q.; Shi, Y. Post-storage changes of volatile compounds in air- and sun-dried raisins with different packaging materials using HS-SPME with GC/MS. Food Res. Int. 2019, 119, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cai, J.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q.; Chen, G.; Shi, Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using HS-SPME with GC-MS. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Jelen, H.; Wa, E. Lipid-derived flavor compounds. In Food Flavors: Chemical, Sensory, and Technological Properties; Jelen, H., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 65–89. [Google Scholar]
- Feussner, I.; Wasternack, C. Te lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Xu, X.Q.; Wu, Y.W.; Duan, C.Q.; Pan, Q.H. Isolation and characterization of two hydroperoxide lyase genes from grape berries HPL isogenes in Vitis vinifera grapes. Mol. Biol. Rep. 2012, 39, 7443–7455. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Mottram, D.S. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef]
- Meeting, A.C.S.; Ho, C.-T.; Hartman, T.G. Lipids in Food Flavors; ACS Publications; American Chemical Society: Washington, DC, USA, 1994. [Google Scholar]
- Rizzo, V.; Muratore, G. Effects of packaging on shelf life of fresh celery. J. Food Eng. 2009, 90, 124–128. [Google Scholar] [CrossRef]
- Sebranek, J.; Neel, S. Rancidity and antioxidants. WFLO Commod. Storage Man 2008, 1–3. [Google Scholar]
- Quezada-Gallo, J.A.; Debeaufor, F. Mechanism of aroma transfer through edible and plastic packaging. In Food Packaging Testing Method and Applications; Risch, S.J., Ed.; American Chemical Society: Washington, DC, USA, 2000; pp. 125–140. [Google Scholar]
- Khiari, R.; Zemni, H.; Mihoubi, D. Raisin processing: Physicochemical, nutritional and microbiological quality characteristics as affected by drying process. Food Rev. Int. 2018, 35, 246–298. [Google Scholar] [CrossRef]
- Aydin, A. Determination of fatty acid compositions of some raisin cultivars in Turkey. Asian J. Chem 2011, 23, 1819–1821. [Google Scholar]
- Núñez, V.; Monagas, M.; Gomez-Cordovés, M.C.; Bartolomé, B. Vitis vinifera L. cv. Graciano grapes characterized by its anthocyanin profile. Postharvest Biol. Technol. 2004, 31, 69–79. [Google Scholar] [CrossRef]
- Han, Y.; Barringer, S. Formation of volatiles in the lipoxygenase pathway as affected by fruit type and temperature. J. Exp. Food Chem. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Wang, D.; Duan, C.-Q.; Shi, Y.; Zhu, B.-Q.; Javed, H.U.; Wang, J. Free and glycosidically bound volatile compounds in sun-dried raisins made from different fragrance intensities grape varieties using a validated HS-SPME with GC–MS method. Food Chem. 2017, 228, 125–135. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 5th ed.; Springer: Berlin, Germany, 2004; ISBN 9783540408185. [Google Scholar]
- Frankel, E.N. Lipid oxidation. Prog. Lipid Res. 1980, 19, 1–22. [Google Scholar] [CrossRef]
- Frankel, E.N.; Neff, W.E.; Edward, S. Analysis of autoxidized fats by gas chromatography-mass spectrometry: VII. volatile thermal decomposition products of pure hydroperoxides from autoxidized and photosensitized oxidized methyl oleate, linoleate and linolenate. Lipids 1981, 16, 279–285. [Google Scholar] [CrossRef]
- Horvat, R.I.; Mcfadden, W.H.; Ng, H.; Lane, W.G.; Lee, A.; Lundin, R.E.; Scherer, R. Identification of some acids from autoxidation of methyl linoleate. J. Am. Oil Chem. Soc. 1968, 46, 94–96. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef]
- Arancibia, M.Y.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Release of volatile compounds and biodegradability of active soy protein lignin blend films with added citronella essential oil. Food Control 2014, 44, 7–15. [Google Scholar] [CrossRef]
- Xu, X.Q.; Cheng, G.; Duan, L.L.; Jiang, R.; Pan, Q.H.; Duan, C.Q.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in Cabernet Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| S/No | RT | Fatty Acids | Common Name | ID | Linear Equation | R | Air-Dried | Sun-Dried | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated Fatty Acid | 0-M | 4-M | 8-M | 12-M | 0-M | 4-M | 8-M | 12-M | ||||||
| 1 | 10.4 | C12:0 | Lauric acid | 1 | y = 0.1282x + 0.4898 | 0.999 | 2.39a | 0.56b | 0.56b | 0.56b | 0.51b | 0.49b | 0.50b | 0.50b |
| 2 | 11.8 | C14:0 | Myristic acid | 1 | y = 0.1344x + 0.919 | 0.992 | 1.16a | 1.07b | 1.09b | 1.09b | 0.99c | 0.95d | 0.95d | 0.94d |
| 3 | 12.6 | C15:0 | Pentadecylic acid | 1 | y = 0.1395x + 1.8572 | 0.991 | 1.89b | 1.90b | 1.91a | 1.91a | 1.88c | 1.86d | 1.87d | 1.86d |
| 4 | 13.4 | C16:0 | Palmitic acid | 1 | y = 0.0148x + 0.8343 | 0.990 | 1.68a | 1.37b | 1.26c | 1.27c | 0.97d | 0.91e | 0.91e | 0.92e |
| 5 | 14.4 | C17:0 | Margaric acid | 1 | y = 0.1428x + 0.5216 | 0.993 | 0.63a | 0.62a | 0.62a | 0.62a | 0.55b | 0.53b | 0.54b | 0.53b |
| 6 | 15.4 | C18:0 | Stearic acid | 1 | y = 0.1428x + 0.5216 | 0.993 | 4.41a | 3.75b | 3.45c | 3.38c | 1.14d | 1.10d | 1.04d | 1.10d |
| 7 | 18.2 | C20:0 | Arachidic acid | 1 | y = 0.2098x + 0.6079 | 0.991 | 0.99a | 0.96a | 0.95a | 0.96a | 0.69b | 0.65b | 0.67b | 0.66b |
| 8 | 21.9 | C22:0 | Behenic acid | 1 | y = 0.1511x + 0.2175 | 0.995 | 0.70a | 0.68a | 0.69a | 0.68a | 0.29b | 0.27b | 0.28b | 0.28b |
| 9 | 24.4 | C23:0 | Tricosylic acid | 1 | y = 0.1259x + 0.2172 | 0.995 | 0.28ab | 0.28ab | 0.29a | 0.29a | 0.23c | 0.23c | 0.23c | 0.23c |
| 10 | 27.6 | C24:0 | Lignoceric acid | 1 | y = 0.0953x + 0.1182 | 0.997 | 0.26a | 0.24a | 0.24a | 0.24a | 0.15b | 0.14b | 0.14b | 0.14b |
| Unsaturated Fatty Acid | 0-M | 4-M | 8-M | 12-M | 0-M | 4-M | 8-M | 12-M | ||||||
| 11 | 13.8 | C16:1 | Palmitoleic acid | 1 | y = 0.1042x + 0.7286 | 0.991 | 0.73a | 0.74a | 0.74a | 0.74a | NF | NF | NF | NF |
| 12 | 15.8 | C18:1 | Oleic acid | 1 | y = 0.1283x + 1.5322 | 0.993 | 2.03a | 1.51b | 1.58b | 1.62b | 0.70c | 0.66d | 0.64d | 0.66d |
| 13 | 16.4 | C18:2 | Linoleic acid | 1 | y = 0.0883x + 1.3784 | 0.993 | 3.52a | 2.78b | 2.66bc | 2.53c | 1.61d | 1.47d | 1.50d | 1.52d |
| 14 | 17.3 | C18:3 | Linolenic acid | 1 | y = 0.1006x + 0.5955 | 0.997 | 1.96a | 1.93ab | 1.90b | 1.92ab | 1.59c | 1.57c | 1.57c | 1.59c |
| 15 | 18.6 | C20:1 | Paullinic acid | 1 | y = 0.053x + 0.2264 | 0.994 | 0.24a | 0.24a | 0.24a | 0.24a | NF | NF | NF | NF |
| 16 | 22.4 | C22:1 | Erucic acid | 2 | 1.54a | 1.10b | 1.04b | 1.10b | 0.68c | 0.63c | 0.67c | 0.66c | ||
| S/No | RI | Volatile Compounds | Source | ID-M | Ion (m/z) | Formula | Precursor | Aroma Descriptor |
|---|---|---|---|---|---|---|---|---|
| 1 | 1227 | Ethyl hexanoate | Ester | 1 | 88 | C8H16O2 | Linoleic acid F | Fruity, apple-like e |
| 2 | 1432 | Ethyl octanoate | Ester | 2 | 74 | C10H20O2 | Linoleic acid, Linolenic acid F | Apple, fruity, sweet e |
| 3 | 1378 | Methyl octanoate | Ester | 2 | 88 | C9H18O2 | Oleic acid A | Fruity, citrus like e |
| 4 | 2035 | γ-Nonalactone | Ester | 2 | 85 | C9H16O2 | Linoleic acid F | Coconut, peach e |
| 5 | 975 | Pentanal | Aldehyde | 2 | 44 | C5H10O | Linoleic acid, Arachidonic acid E | Fat, Green e |
| 6 | 1178 | Heptanal | Aldehyde | 2 | 44 | C7H14O | Linoleic acid D,E, Oleic acid E | Dry fish, solvent, smoky e |
| 7 | 1325 | (E)-2-Heptenal | Aldehyde | 2 | 41 | C7H12O | Linoleic acid D,E | Fatty, soapy, tallow e |
| 8 | 1393 | Nonanal | Aldehyde | 1 | 57 | C9H18O | Linoleic acid B | Green, Fruity e |
| 9 | 1501 | Decanal | Aldehyde | 1 | 43 | C10H20O | Oleic acid D,E | Sweet, citrus, green c |
| 10 | 1497 | (E,E)-2,4-Heptadienal | Aldehyde | 2 | 81 | C7H10O | Linolenic acid E | Fatty, hay a |
| 11 | 1705 | (E,E)-2,4-Nonadienal | Aldehyde | 2 | 81 | C9H14O | Linoleic acid D,E | Fatty, oily a |
| 12 | 1349 | 1-Hexanol | Alcohol | 2 | 56 | C6H14O | NF | green a |
| 13 | 1395 | (Z)-3-Hexen-1-ol | Alcohol | 2 | 67 | C6H12O | NF | Fruity, green a |
| 14 | 1449 | 1-Octen-3-ol | Alcohol | 1 | 57 | C8H16O | Arachidonic acid D, Linoleic acid A | Mushroom, fruity e |
| 15 | 1453 | 1-Heptanol | Alcohol | 2 | 70 | C7H16O | Oleic acid A | Grape, sweet b |
| 16 | 1487 | 2-Ethyl-1-hexanol | Alcohol | 1 | 57 | C8H18O | NF | Floral, sweet fruity b |
| 17 | 1555 | 1-Octanol | Alcohol | 1 | 56 | C8H18O | Methyl Oleate B | Citrus, rose c |
| 18 | 1614 | (E)-2-Octen-1-ol | Alcohol | 2 | 57 | C8H16O | Oleic acid A | Fatty, rancid a |
| 19 | 1657 | 1-Nonanol | Alcohol | 1 | 56 | C9H20O | NF | Floral a |
| 20 | 1488 | 2-Nonanol | Alcohol | 2 | 45 | C9H20O | NF | NF |
| 21 | 1740 | Pentanoic acid | Acid | 2 | 60 | C5H10O2 | Methyl Linoleic acid C | Sweet a |
| 22 | 1847 | Hexanoic acid | Acid | 1 | 60 | C6H12O2 | Methyl Linoleic acid C | Rancid, Cheese, Fatty e |
| 23 | 1953 | Heptanoic acid | Acid | 1 | 60 | C7H14O2 | Methyl Linoleic acid C | Sweety, cheesy d |
| 24 | 2060 | Octanoic acid | Acid | 1 | 60 | C8H16O2 | Methyl Linoleic acid C | Rancid, Cheese, Fatty e |
| 25 | 1416 | 3-Octen-2-one | Ketone | 2 | 55 | C8H14O | Arachidonic acid E | Green, fruity a |
| 26 | 1224 | 2-Pentyl furan | Furan | 2 | 81 | C9H14O | Linoleic acid B,E | Fruity, green, sweet e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Javed, H.U.; Shi, Y.; Naz, S.; Ali, S.; Duan, C.-Q. Impact of Drying Method on the Evaluation of Fatty Acids and Their Derived Volatile Compounds in ‘Thompson Seedless’ Raisins. Molecules 2020, 25, 608. https://doi.org/10.3390/molecules25030608
Wang D, Javed HU, Shi Y, Naz S, Ali S, Duan C-Q. Impact of Drying Method on the Evaluation of Fatty Acids and Their Derived Volatile Compounds in ‘Thompson Seedless’ Raisins. Molecules. 2020; 25(3):608. https://doi.org/10.3390/molecules25030608
Chicago/Turabian StyleWang, Dong, Hafiz Umer Javed, Ying Shi, Safina Naz, Sajid Ali, and Chang-Qing Duan. 2020. "Impact of Drying Method on the Evaluation of Fatty Acids and Their Derived Volatile Compounds in ‘Thompson Seedless’ Raisins" Molecules 25, no. 3: 608. https://doi.org/10.3390/molecules25030608
APA StyleWang, D., Javed, H. U., Shi, Y., Naz, S., Ali, S., & Duan, C.-Q. (2020). Impact of Drying Method on the Evaluation of Fatty Acids and Their Derived Volatile Compounds in ‘Thompson Seedless’ Raisins. Molecules, 25(3), 608. https://doi.org/10.3390/molecules25030608