Phytochemical and Safety Evaluations of Volatile Terpenoids from Zingiber cassumunar Roxb. on Mature Carp Peripheral Blood Mononuclear Cells and Embryonic Zebrafish
Abstract
1. Introduction
2. Results
2.1. Chemical Compositions of Essential Oil
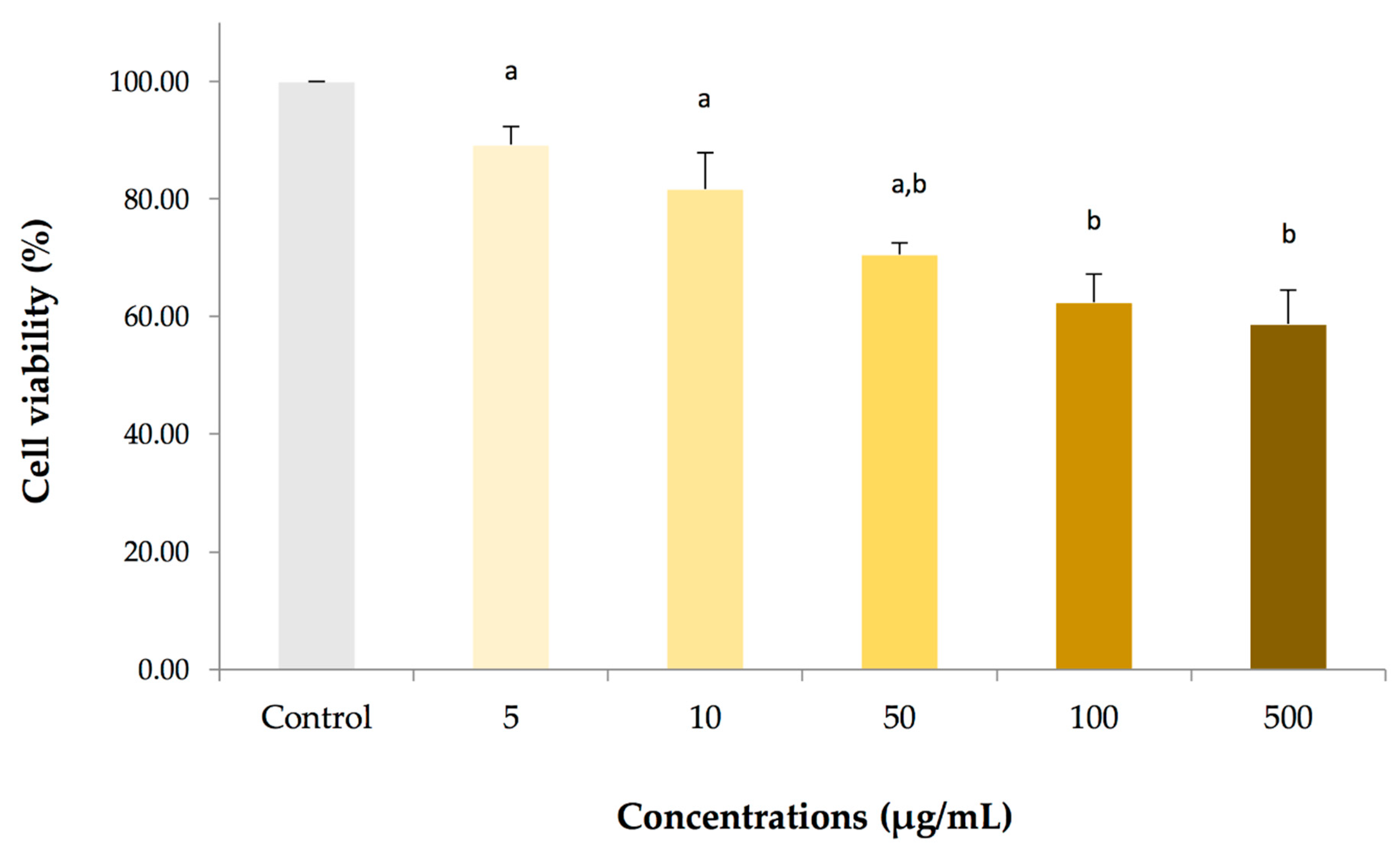
2.2. Cytotoxicity on Adult Fish PBMCS
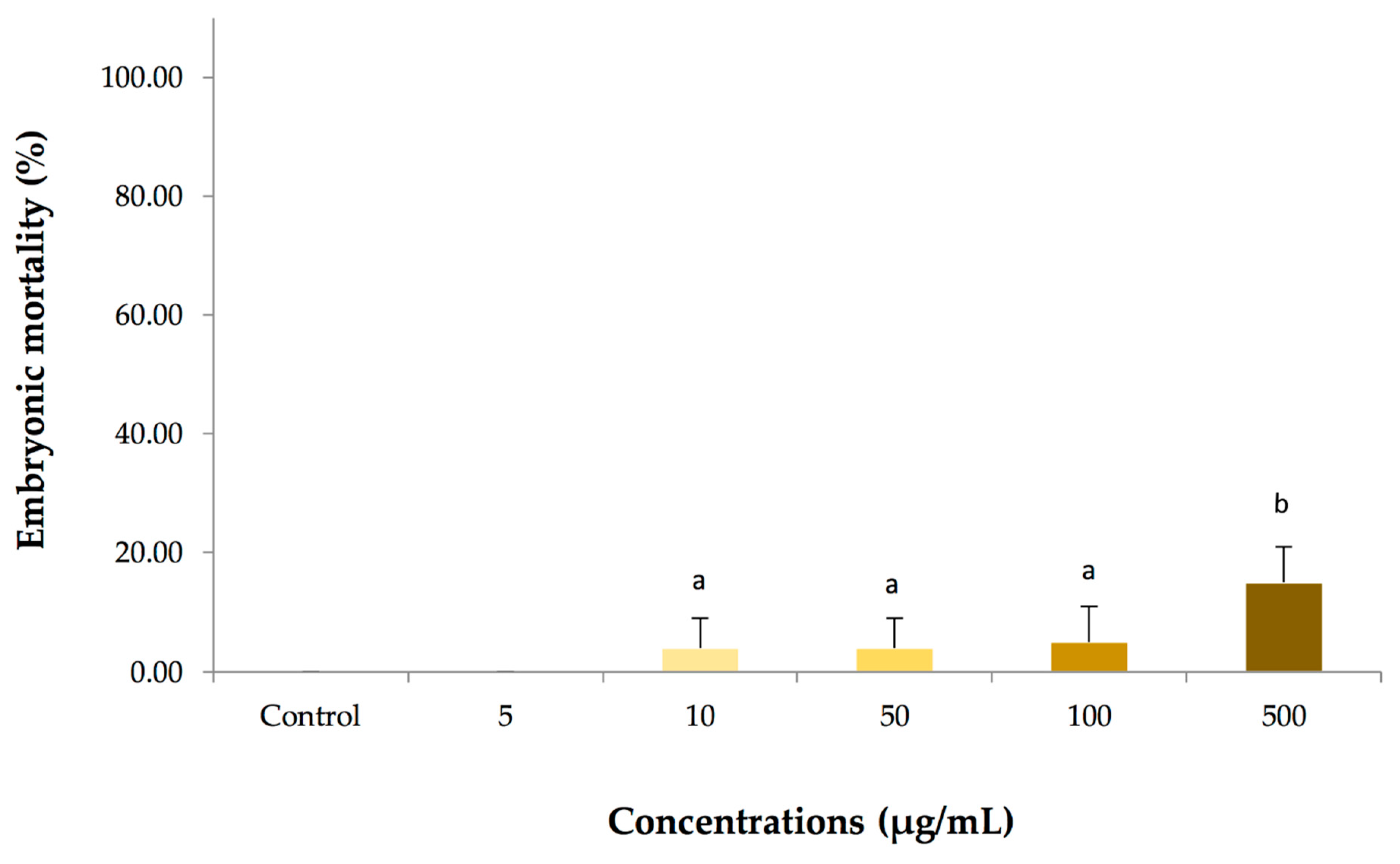
2.3. Dose-response Embryotoxicity in Zebrafish
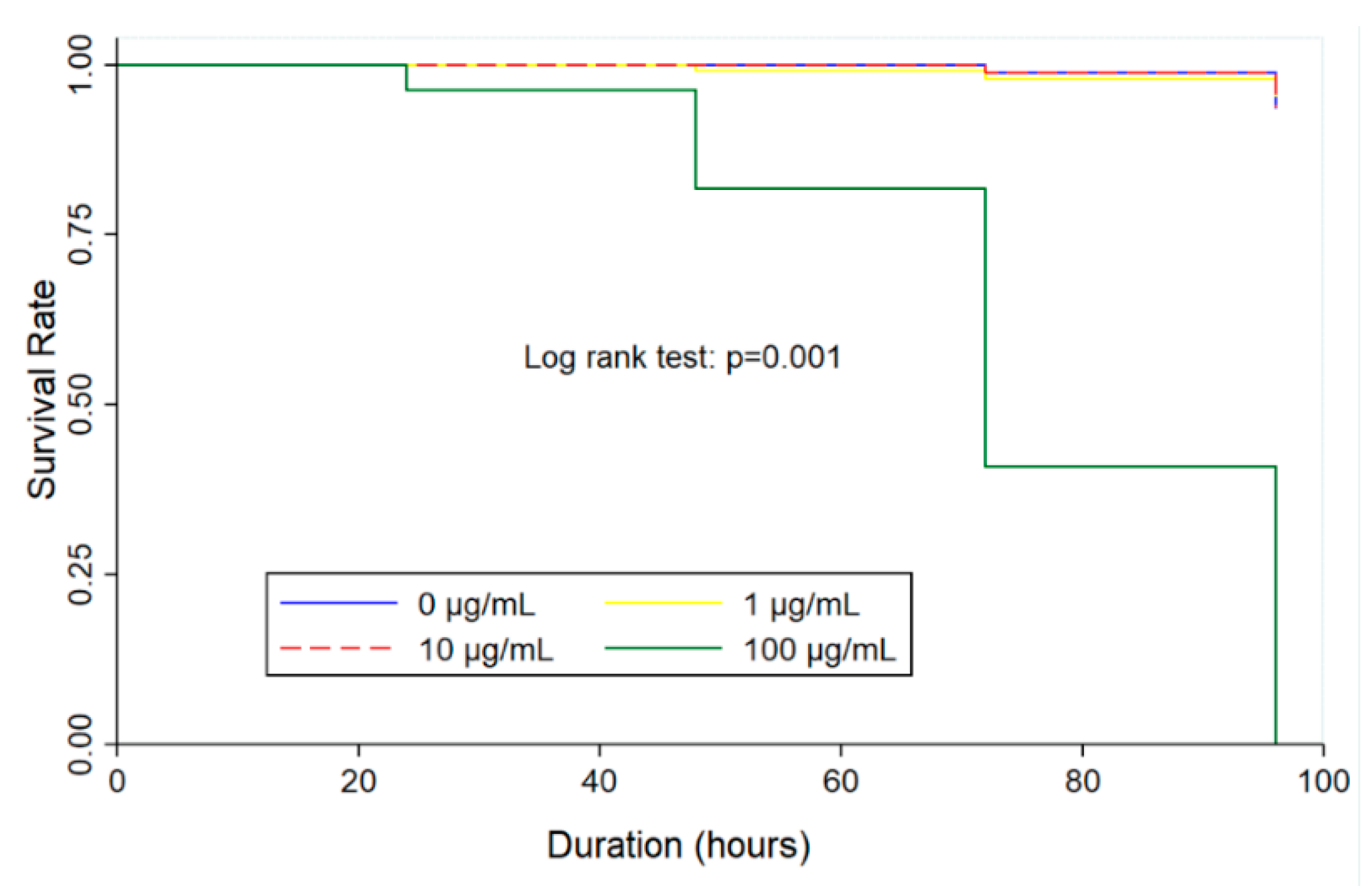
2.4. Time-kill Analysis in Zebrafish Embryos
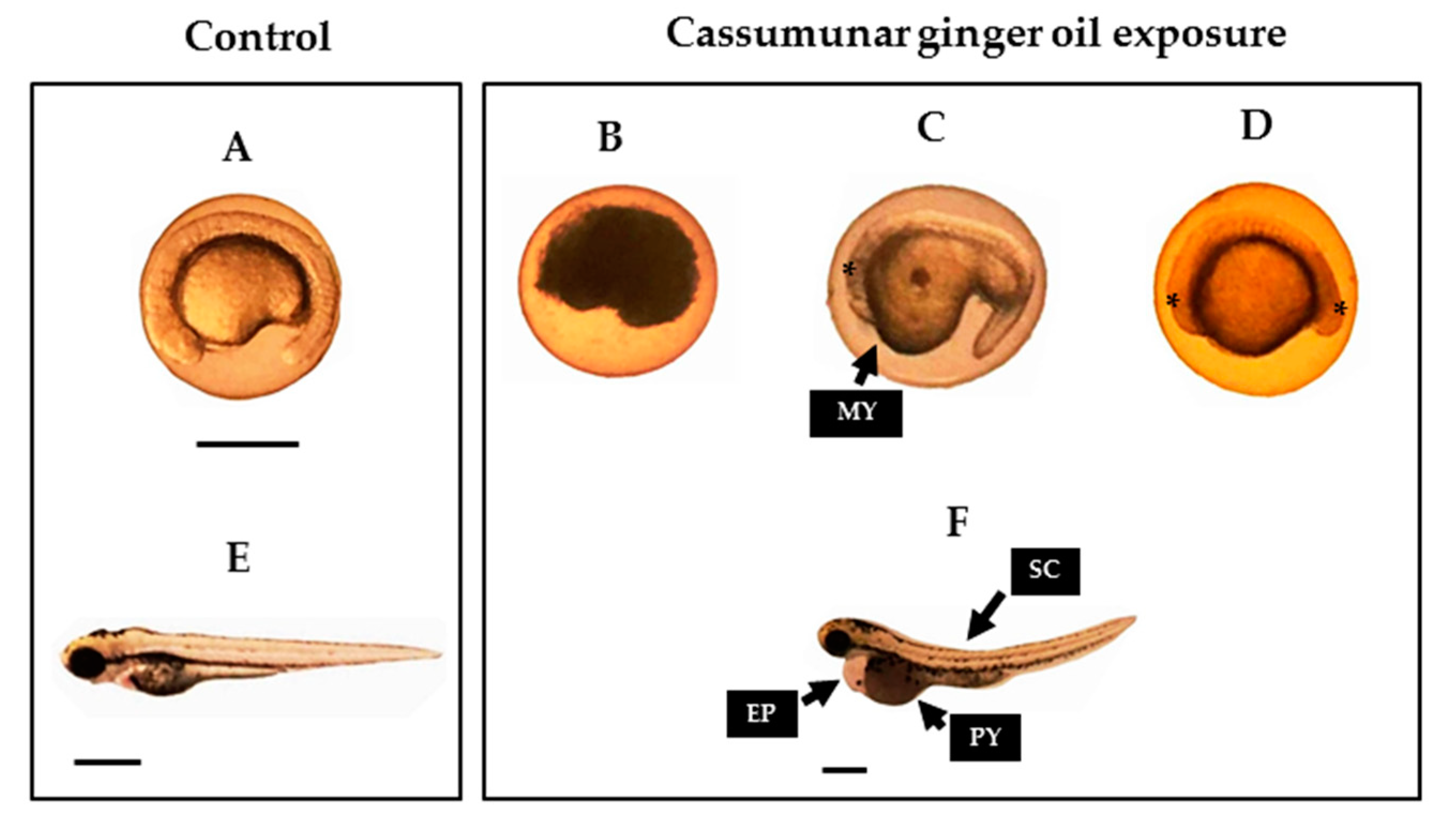
2.5. Teratogenicity in Zebrafish
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Distillation of Essential Oil and Chemical Characterization
4.3. Animal and Ethic Statement
4.4. Fish Blood Collection and PBMC Isolation
4.5. In Vitro MTT Reduction Assay
4.6. Zebrafish Breeding and Embryo Acquisition
4.7. Zebrafish Embryonic Toxicity Test
4.7.1. Dose-response Embryotoxicity
4.7.2. Time-killed Analysis
4.8. Zebrafish Teratogenicity Test
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Majumder, P.B.; Mandi, S.S. Species-specific AFLP markers for identification of Zingiber officinale, Z. montanumand, Z. zerumbet (Zingiberaceae). Genet. Mol. Res. 2011, 10, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.B.; Manglembi, N.; Swapana, N.; Chanu, S.B. Ethnobotany, Phytochemistry and Pharmacology of Zingiber cassumunar Roxb. (Zingiberaceae). J Pharmacogn. Phytochem. 2015, 4, 1–6. [Google Scholar]
- Sukatta, U.; Rugthaworn, P.; Punjee, P.; Chidchenchey, S.; Keeratinijakal, V. Chemical composition and physical properties of oil from Plai (Zingiber cassumunar Roxb.) obtained by hydrodistillation and hexane extraction. Kasetsart J. (Nat. Sci.) 2009, 43, 212–217. [Google Scholar]
- Chaiyana, W.; Anuchapreeda, S.; Leelapornpisid, P.; Phongpradist, R.; Viernstein, H.; Mueller, M. Development of microemulsion delivery system of essential oil from Zingiber cassumunar Roxb. rhizome for improvement of stability and anti-inflammatory activity. AAPS PharmSciTech 2017, 18, 1332–1342. [Google Scholar] [CrossRef]
- Boonyanugomol, W.; Kraisriwattana, K.; Rukseree, K.; Boonsam, K.; Narachai, P. In vitro synergistic antibacterial activity of the essential oil from Zingiber cassumunar Roxb against extensively drug-resistant Acinetobacter baumannii strains. J. Infect. Public Health 2017, 10, 586–592. [Google Scholar] [CrossRef]
- Koontongkaew, S.; Poachanukoon, O.; Sireeratawong, S.; Decatiwongse Na Ayudhya, T.; Khonsung, P.; Jaijoy, K.; Soawakontha, R.; Chanchai, M. Safety evaluation of Zingiber cassumunar roxb rhizome extract: Acute and chronic toxicity studies in rats. Int. Sch. Res. Not. 2014, 16, 1–14. [Google Scholar]
- Nishima, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. (Kyoto) 2016, 56, 18–27. [Google Scholar] [CrossRef]
- Tran, S.; Facciol, A.; Gerlai, R. The Zebrafish, a Novel Model Organism for Screening Compounds Affecting Acute and Chronic Ethanol-Induced Effects. Int. Rev. Neurobiol. 2016, 126, 467–484. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmell, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Falcão, M.A.P.; de Souza, L.S.; Dolabella, S.S.; Guimarães, A.G.; Walker, C.I.B. Zebrafish as an alternative method for determining the embryo toxicity of plant products: a systematic review. Environ. Sci. Pollut. Res. Int. 2018, 25, 35015–35026. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Li, W.F.; Liu, Y.; Wang, Q.; Chen, X.L.; Wang, A.G.; Jin, H.T.; Ma, S.C. Acute toxicity screening of different extractions, components and constituents of Polygonum multiflorum Thunb. on zebrafish (Danio rerio) embryos in vivo. Biomed. Pharmacother. 2018, 99, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Alafiatayo, A.A.; Lai, K.S.; Syahida, A.; Mahmood, M.; Shaharuddin, N.A. Phytochemical evaluation, embryotoxicity, and teratogenic effects of Curcuma longa extract on zebrafish (Danio rerio). Evid. Based Complement Alternat. Med. 2019, 10, 3807207. [Google Scholar] [CrossRef] [PubMed]
- Manochai, B.; Paisooksantivatana, Y.; Choi, H.; Hong, J.H. Variation in DPPH scavenging activity and major volatile oil components of cassumunar ginger, Zingiber montanum (Koenig), in response to water deficit and light intensity. Sci. Hortic. 2010, 126, 462–466. [Google Scholar] [CrossRef]
- Kamazeri, T.S.A.T.; Samah, O.A.; Taher, M.; Susanti, D.; Qaralleh, H. Antimicrobial activity and essential oils of Curcuma aeruginosa, Curcuma mangga, and Zingiber cassumunar from Malaysia. Asian Pac. J. Trop. Med. 2012, 5, 202–209. [Google Scholar] [CrossRef]
- Younis, A.; Riaz, A.; Khan, M.A.; Khan, A.A. Effect of Time of Growing Season and Time of Day for Flower Harvest on Flower Yield and Essential Oil Quality and Quantity of Four Rosa Cultivars. Floric. Ornam. Biotech. 2009, 3, 98–103. [Google Scholar]
- Akram, A.; Younis, A.; Akhtar, G.; Ameer, K.; Farooq, A.; Hanif, M.A.; Saeed, M.; Lim, K. Comparative Efficacy of Various Essential Oil Extraction Techniques on Oil Yield and Quality of Jasminum sambac L. Sci. Int. 2017, 5, 84–95. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Gupta, J.; Prabhakar, P.K.; Gupta, P.; Solanki, P.; Rajput, A. Phytochemical Repurposing of Natural Molecule: Sabinene for Identification of Novel Therapeutic Benefits Using In Silico and In Vitro Approaches. Assay Drug Dev. Technol. 2019, 17, 339–351. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Li, M.X.; Bai, X.; Ma, Y.P.; Zhang, H.X.; Nama, N.; Pei, S.J.; Du, Z.Z. Cosmetic potentials of extracts and compounds from Zingiber cassumunar Roxb. rhizome. Ind. Crop Prod. 2019, 141, 111764. [Google Scholar] [CrossRef]
- Suksaeree, J.; Charoenchai, L.; Madaka, F. Zingiber cassumunar blended patches for skin application: formulation, physicochemical properties, and in vitro studies. Asian J. Pharm. Sci. 2015, 10, 341–349. [Google Scholar] [CrossRef]
- Priprem, A.; Janpim, K.; Nualkaew, S.; Mhakunakorn, P. Topical niosome gel of Zingiber cassumunar Roxb. extract for anti-inflammatory activity enhanced skin permeation and stability of compound D. AAPS Pharm. Sci. Tech. 2016, 173, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Thaweboon, S.; Thaweboon, B.; Kaypetch, R. Antifungal, Anti-Inflammatory and Cytotoxic Effects of Zingiber cassumunar Gel. Key Eng. Mater. 2018, 773, 360–364. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: the MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Taechowisan, T.; Suttichokthanakorn, S.; Phutdhawong, W.S. Antibacterial and cytotoxicity activities of phenylbutanoids from Zingiber cassumunar Roxb. J. App. Pharm. Sci. 2018, 8, 122–128. [Google Scholar]
- Serifi, I.; Tzima, E.; Bardouki, H.; Lampri, E.; Papamarcaki, T. Effects of the Essential Oil from Pistacia lentiscus Var. chia on the Lateral Line System and the Gene Expression Profile of Zebrafish (Danio rerio). Molecules 2019, 24, 3919. [Google Scholar] [CrossRef]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of terpenes as skin penetration enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef]
- Ali, M.K.; Saber, S.P.; Taite, D.R.; Emadi, S.; Irving, R. The protective layer of zebrafish embryo changes continuously with advancing age of embryo development (AGED). J. Toxicol. Pharmacol. 2017, 1, 9. [Google Scholar]
- Chongmelaxme, B.; Sruamsiri, R.; Dilokthornsakul, P.; Dhippayam, T.; Kongkaew, C.; Saokaew, S.; Chuthapultti, A.; Chaiyakunapruk, N. Clinical effects of Zingiber cassumunar (Plai): A systematic review. Complement. Ther. Med. 2017, 35, 70–77. [Google Scholar] [CrossRef]
- Chooluck, K.; Singh, R.P.; Sathirakul, K.; Derendorf, H. Dermal Pharmacokinetics of Terpinen-4-ol Following Topical Administration of Zingiber cassumunar (plai) Oil. Planta Med. 2012, 78, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Chooluck, K.; Singh, R.P.; Sathirakul, K.; Derendorf, H. Plasma and dermal pharmacokinetics of terpinen-4-ol in rats following intravenous administration. Die Pharmazie 2013, 68, 135–140. [Google Scholar] [PubMed]
- Khemawoot, P.; Hunsakunachai, N.; Anukunwithaya, T.; Bangphumi, K.; Ongpipattanakul, B.; Jiratchariyakul, W. Pharmacokinetics of compound D, the major bioactive component of Zingiber cassumunar, in rats. Planta Med. 2016, 82, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidelines for the Testing of Chemicals. Section 2-Effects on Biotic Systems Test (No 236 Fish Embryo Acute Toxicity (FET) Test); Organization for Economic Cooperation and Development: Paris, France, 2013. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
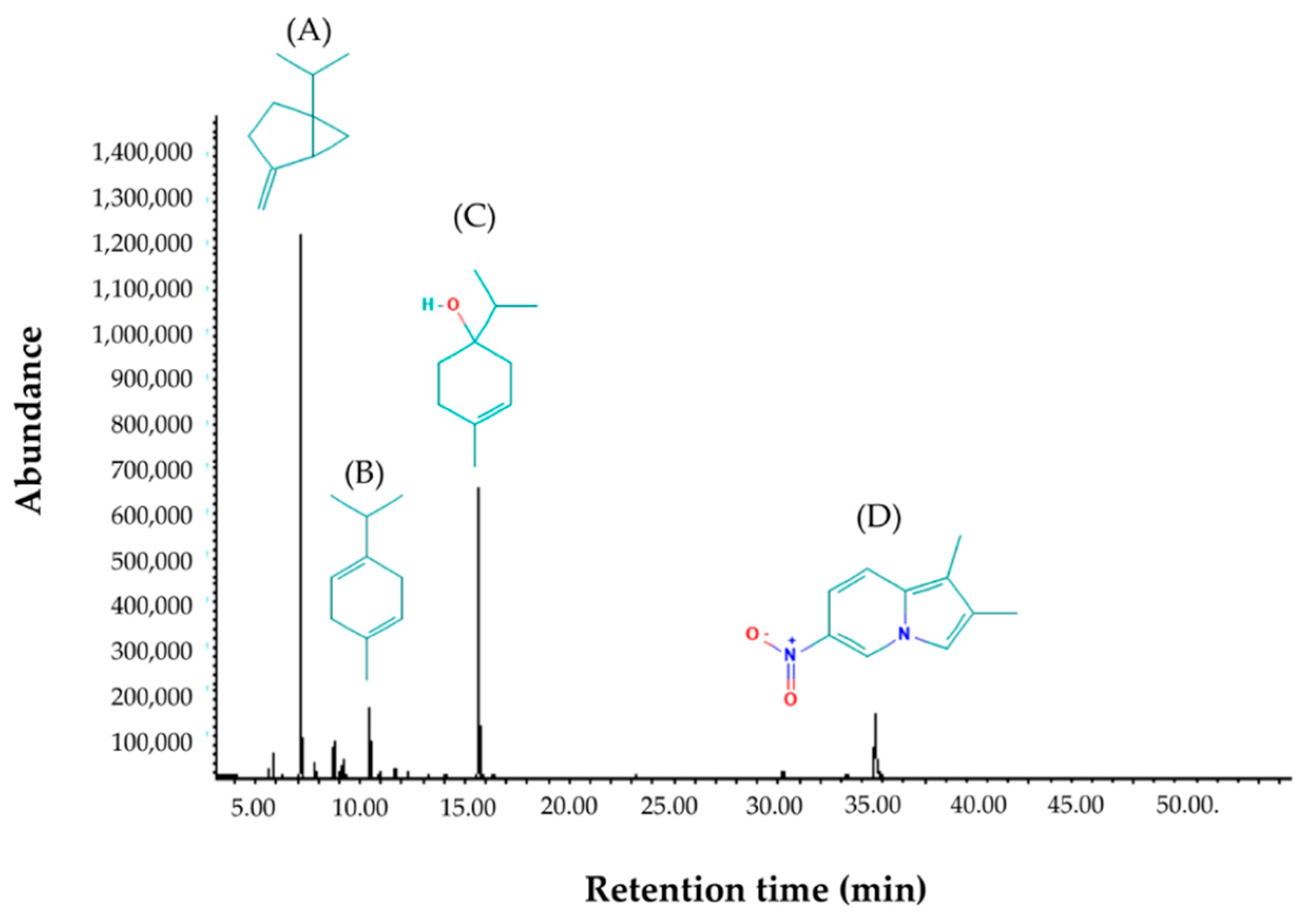
| Peak | RT (min) | Component | Formula | MW (g/mol) | Amount (%) |
|---|---|---|---|---|---|
| 1 | 5.64 | α-Thujene | C10H16 | 136.23 | 0.60 |
| 2 | 5.83 | α-Pinene | C10H16 | 136.23 | 1.58 |
| 3 | 7.16 | Sabinene | C10H16 | 136.23 | 43.54 |
| 4 | 7.84 | Myrcene | C10H16 | 136.23 | 1.17 |
| 5 | 8.74 | α-Terpinene | C10H16 | 136.23 | 2.82 |
| 6 | 9.09 | Benzene | C6H6 | 78.11 | 0.94 |
| 7 | 10.46 | γ-Terpinene | C10H16 | 136.23 | 7.38 |
| 8 | 11.69 | α-Terpinolene | C10H16 | 136.23 | 0.84 |
| 9 | 15.68 | Terpinen-4-ol | C10H18O | 154.25 | 29.52 |
| 10 | 30.24 | β-Sesquiphellandrene | C15H24 | 204.35 | 0.52 |
| 11 | 34.63 | 1,2-Dimethyl-6-nitroindolizine | C10H10O2N2 | 190.20 | 11.10 |
| Concentrations (µg/mL) | Times of Exposure (h) | |||
|---|---|---|---|---|
| 24 | 48 | 72 | 96 | |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 |
| 10 | 1.0 ± 0.8 | 0 | 0 | 0 |
| 100 | 24.5 ± 6.6 | 0 | 10.5 ± 1.9 | NE |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mektrirat, R.; Yano, T.; Okonogi, S.; Katip, W.; Pikulkaew, S. Phytochemical and Safety Evaluations of Volatile Terpenoids from Zingiber cassumunar Roxb. on Mature Carp Peripheral Blood Mononuclear Cells and Embryonic Zebrafish. Molecules 2020, 25, 613. https://doi.org/10.3390/molecules25030613
Mektrirat R, Yano T, Okonogi S, Katip W, Pikulkaew S. Phytochemical and Safety Evaluations of Volatile Terpenoids from Zingiber cassumunar Roxb. on Mature Carp Peripheral Blood Mononuclear Cells and Embryonic Zebrafish. Molecules. 2020; 25(3):613. https://doi.org/10.3390/molecules25030613
Chicago/Turabian StyleMektrirat, Raktham, Terdsak Yano, Siriporn Okonogi, Wasan Katip, and Surachai Pikulkaew. 2020. "Phytochemical and Safety Evaluations of Volatile Terpenoids from Zingiber cassumunar Roxb. on Mature Carp Peripheral Blood Mononuclear Cells and Embryonic Zebrafish" Molecules 25, no. 3: 613. https://doi.org/10.3390/molecules25030613
APA StyleMektrirat, R., Yano, T., Okonogi, S., Katip, W., & Pikulkaew, S. (2020). Phytochemical and Safety Evaluations of Volatile Terpenoids from Zingiber cassumunar Roxb. on Mature Carp Peripheral Blood Mononuclear Cells and Embryonic Zebrafish. Molecules, 25(3), 613. https://doi.org/10.3390/molecules25030613






