Selective Chromogenic Recognition of Copper(II) Ion by Thiacalix[4]arene Tetrasulfonate and Mechanism
Abstract
1. Introduction
2. Results and Discussion
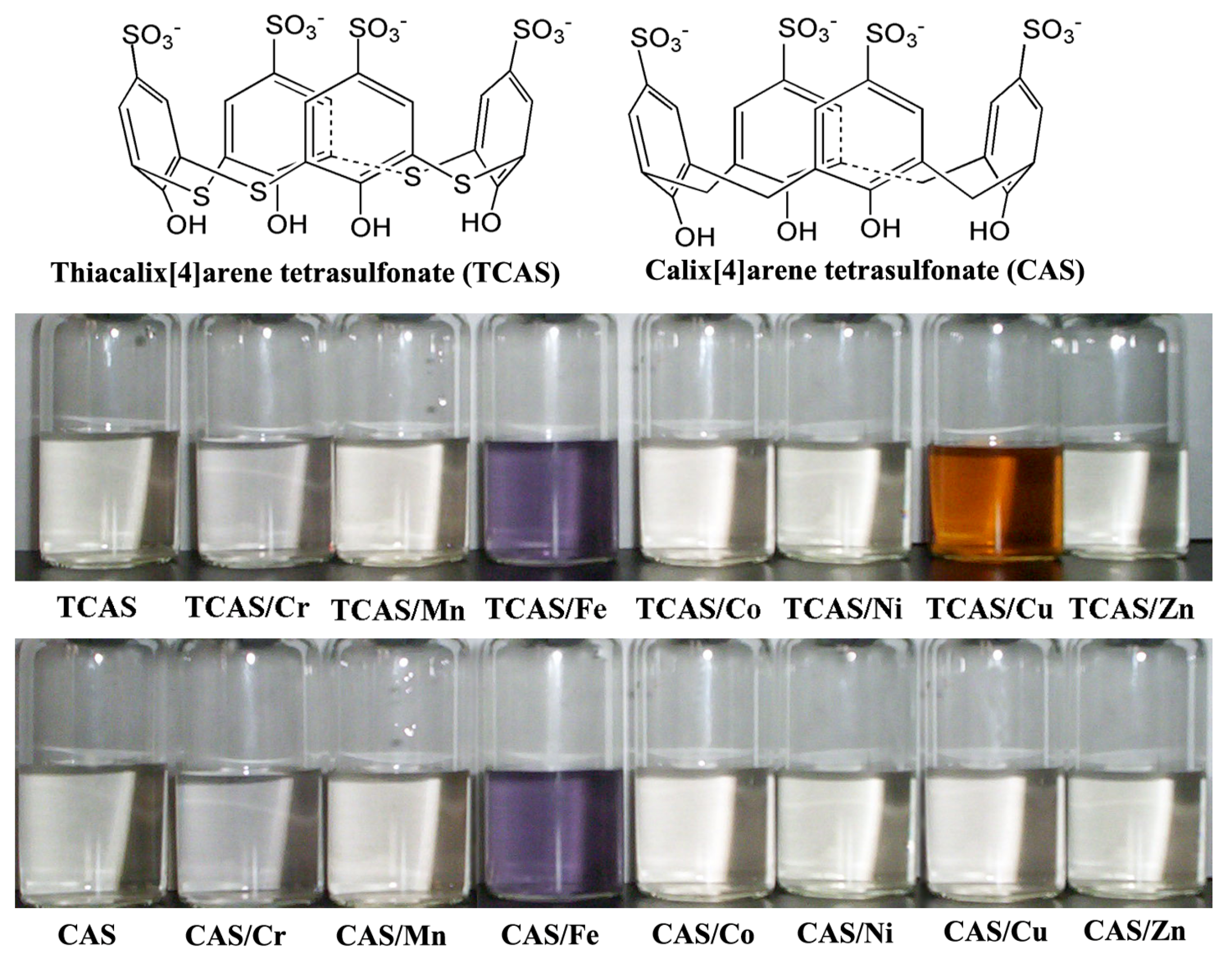
2.1. Chromogenic Response of TCAS to Transition Metal Ions in Aqueous Solution
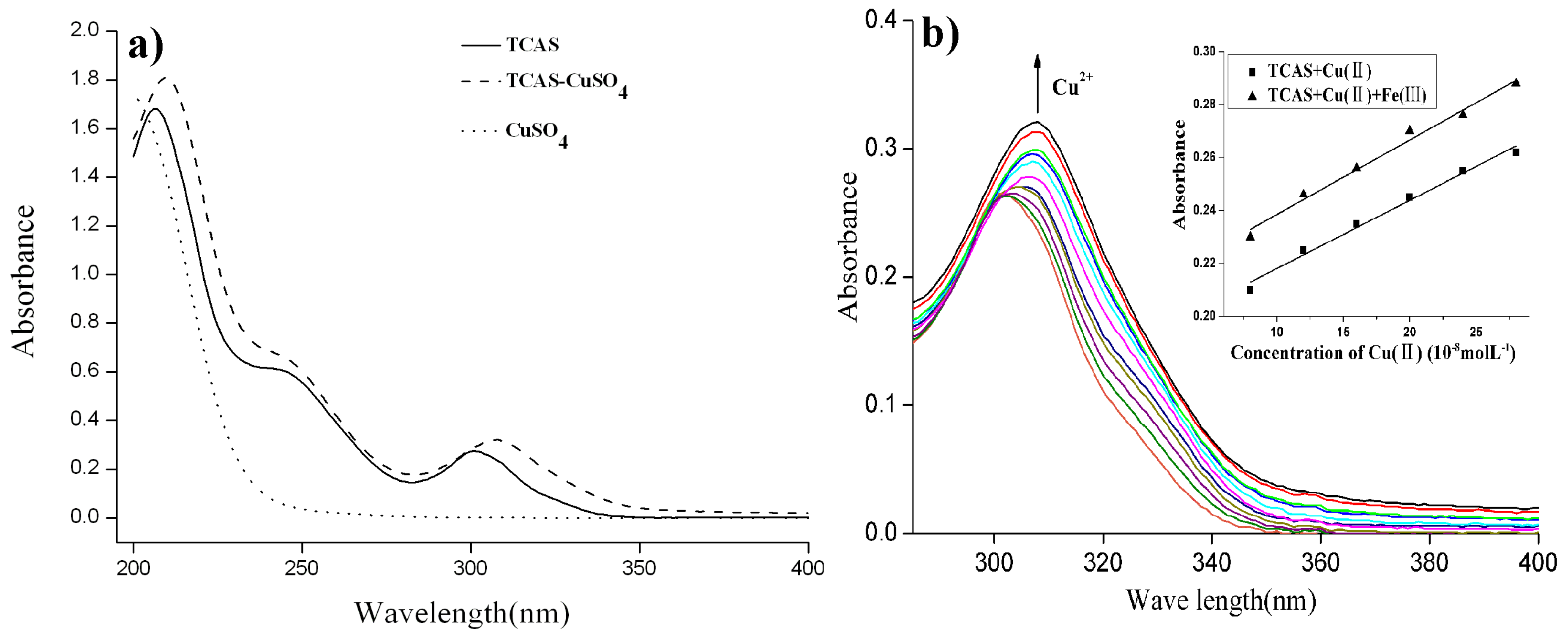
2.2. Absorption Spectra of TCAS-Cu Solution and the Effect of Copper Ion Concentration
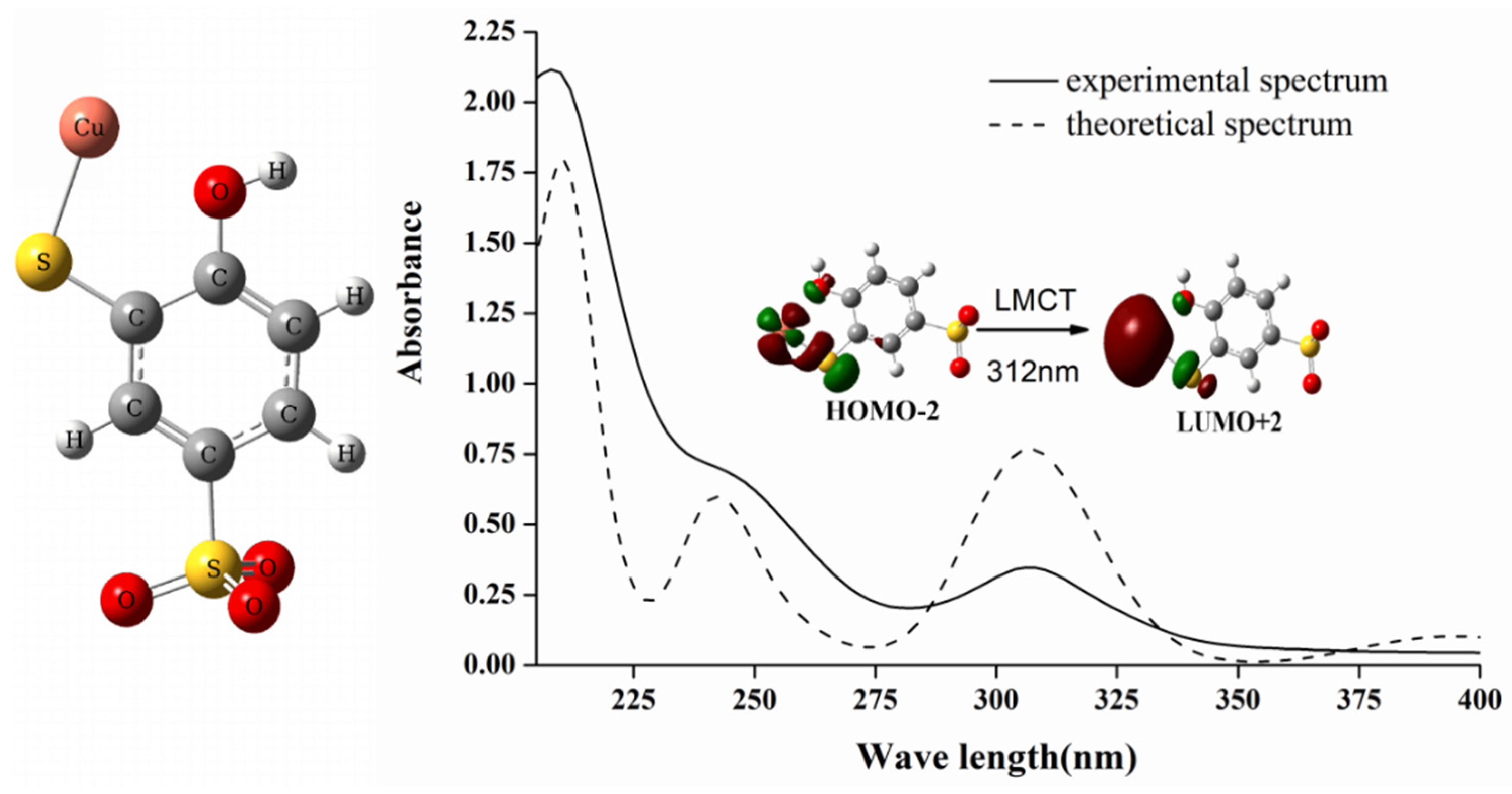
2.3. The Mechanism of Selective Chromogenic Response of TCAS to Copper Ions
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Occurrence of Copper Proteins through the Three Domains of Life: A Bioinformatic Approach. J. Proteome Res. 2008, 7, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D. Copper and iron: A Landmark Connection of Two Essential Metals. J. Trace Elem. Exp. Med. 2001, 14, 207–210. [Google Scholar] [CrossRef]
- Waggoner, D.J.; Bartnikas, T.B.; Gitlin, J.D. The Role of Copper in Neurodegenerative Disease. Neurobiol. Dis. 1999, 6, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Vulpe, C.; Evinson, B.; Whitney, S.; Packman, S.; Gitschier, J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper–transporting ATPase. Nat. Genet. 1993, 3, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.Y.; Ko, J.; Lee, J.Y.; Kim, J.S. Regioselective Complexation of Metal Ion in Chromogenic Calix[4]biscrowns. J. Org. Chem. 2004, 69, 2902–2905. [Google Scholar] [CrossRef]
- Tsuda, D.; Nakdhara, Y.; Machitani, K.; Kannaka, M.; Takahashi, E.; Kimura, K. Ultra-High-Sensitive Extraction-Photometric Determination of Sodium Ion Using Flow Injection Analysis with a Chromogenic Calix[4]arene Derivative and a Laser Interferometric Photothermal Detector. Anal. Chem. 2012, 84, 3710–3715. [Google Scholar] [CrossRef]
- Nabeshima, T.; Saiki, T.; Iwabuchi, J.; Akine, S. Stepwise and Dramatic Enhancement of Anion Recognition with a Triple-Site Receptor Based on the Calix[4]arene Framework Using Two Different Cationic Effectors. J. Am. Chem. Soc. 2005, 127, 5507–5511. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, S.H.; Lee, J.Y.; Lee, J.Y.; Bartsch, R.A.; Kim, J.S. An Excimer-Based, Binuclear, On-Off Switchable Calix[4]crown Chemosensor. J. Am. Chem. Soc. 2004, 126, 16499–16506. [Google Scholar] [CrossRef]
- Halouani, H.; Bonnamour, I.D.; Perrin, M.; Lamartine, R. First Synthesis and Structure of β-Ketoimine Calix[4]arenes: Complexation and Extraction Studies. J. Org. Chem. 2004, 69, 6521–6527. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, S.; Liu, X.; Xie, Z.; Yan, X. Highly selective chromogenic ionophores for the recognition of chromium(III) based on a water-soluble azocalixarene derivative. Anal. Chim. Acta 2005, 535, 183–187. [Google Scholar] [CrossRef]
- Bingol, H.; Kocabas, E.; Zor, E.; Coskun, A. Spectrophotometric and electrochemical behaviour of a novel azocalix[4]arene derivative as a highly selective chromogenic chemosensor for Cr3+. Electrochim. Acta 2011, 56, 2057–2061. [Google Scholar] [CrossRef]
- Shaabani, B.; Shaghaghi, Z.; Khandar, A.A. Optical spectroscopy studies of the complexation of bis(azaophenol)calix[4]arene possessing chromogenic donors with Ni2+, Co2+, Cu2+, Pb2+ and Hg2+. Spectrochim. Acta Part A Molecular Biomol. Spectr. 2012, 98, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Chen, C.F.; Zheng, Q.Y.; Huang, Z.T. A selective fluorescent probe for La3+ and Y3+ based on calix[6]arene. Tetrahedron Lett. 2004, 45, 6071–6074. [Google Scholar] [CrossRef]
- Beer, P.D.; Szemes, F.; Passaniti, P.; Maestri, M. Luminescent Ruthenium(II) Bipyridine−Calix[4]arene Complexes as Receptors for Lanthanide Cations. Inorg. Chem. 2004, 43, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Ebdelli, R.; Rouis, A.; Mlika, R.; Bonnamour, I.; Ouada, H.B.; Davenas, J. Photo-physical and complexation properties of chromogenic azo-calix[4]arene: Application to the detection of Eu3+. J. Mol. Struct. 2011, 1006, 210–215. [Google Scholar] [CrossRef]
- Ho, I.-T.; Lee, G.-H.; Chung, W.-S. Synthesis of upper-rim allyl- and p-methoxyphenylazocalix[4]arenes and their efficiencies in chromogenic sensing of Hg2+ ion. J. Org. Chem. 2007, 72, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Bingol, H.; Kocabas, E.; Zor, E.; Coskun, A. A novel benzothiazole based azocalix[4]arene as a highly selective chromogenic chemosensor for Hg2+ ion: A rapid test application in aqueous environment. Talanta 2010, 82, 1538–1542. [Google Scholar] [CrossRef]
- Chawla, H.M.; Gupta, T. New chromogenic bis(isatin hydrazonyl)calix[4]arenes for dual recognition of fluoride and silver ions. Tetra. Lett. 2013, 54, 1794–1797. [Google Scholar] [CrossRef]
- Qazi, M.A.; Qureshi, I.; Memon, S. A highly copper selective chromogenic calix[4]arene dervivative. New J. Chem. 2010, 34, 2579–2586. [Google Scholar] [CrossRef]
- Kumar, M.; Babu, J.N.; Bhalla, V.; Dhir, A. Chromogenic sensing of Cu(II) by imino linked thiacalix[4]arene in mixed aqueous environment. Inorg. Chem. Commun. 2009, 12, 332–335. [Google Scholar] [CrossRef]
- Chawla, H.M.; Goel, P.; Shukla, R.; Black, D.S.; Kumar, N. New lower rim looped calix[4]arene for ratiometric and chromogenic recognition of Cu2+. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 201–207. [Google Scholar] [CrossRef]
- Rouis, A.; Darbost, U.; Bonnamour, I.; Quada, H.B. Development and characterization of a copper ion-selective optical sensor based on a novel calix[4]dicyano-diimidazole thin film. Mater. Chem. Phys. 2015, 164, 145–149. [Google Scholar] [CrossRef]
- Kumagai, H.; Hasegawa, M.; Miyanari, S.; Sugawa, Y.; Sato, Y.; Hori, T.; Ueda, S.; Kamiyama, H.; Miyano, S. Facile synthesis of p-tert-butylthiacalix[4]arene by the reaction of p-tert-butylphenol with elemental sulfur in the presence of a base. Tetra. Lett. 1997, 38, 3971–3972. [Google Scholar] [CrossRef]
- Iki, N.; Fujimoto, T.; Miyano, S. A New Water-Soluble Host Molecule Derived from Thiacalixarene. Chem. Lett. 1998, 27, 625–626. [Google Scholar] [CrossRef]
- Mislin, G.; Graf, E.; Hosseini, M.W.; Bilyk, A.; Hall, A.K.; Harrowfield, J.M.; Skelton, B.W.; White, A.H. Thiacalixarenes as cluster keepers: Synthesis and structural analysis of a magnetically coupled tetracopper(II) square. Chem. Comm. 1999, 4, 373–374. [Google Scholar] [CrossRef]
- Guo, Q.L.; Zhu, W.X.; Gao, S.; Ma, S.L.; Dong, S.J.; Xu, M.Q. A novel 2D coordination polymer based on a copper(II) tetramer with p-sulfonated thiacalix[4]arene. Inorg. Chem. Commun. 2004, 7, 467–470. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 03; Revision B.05; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
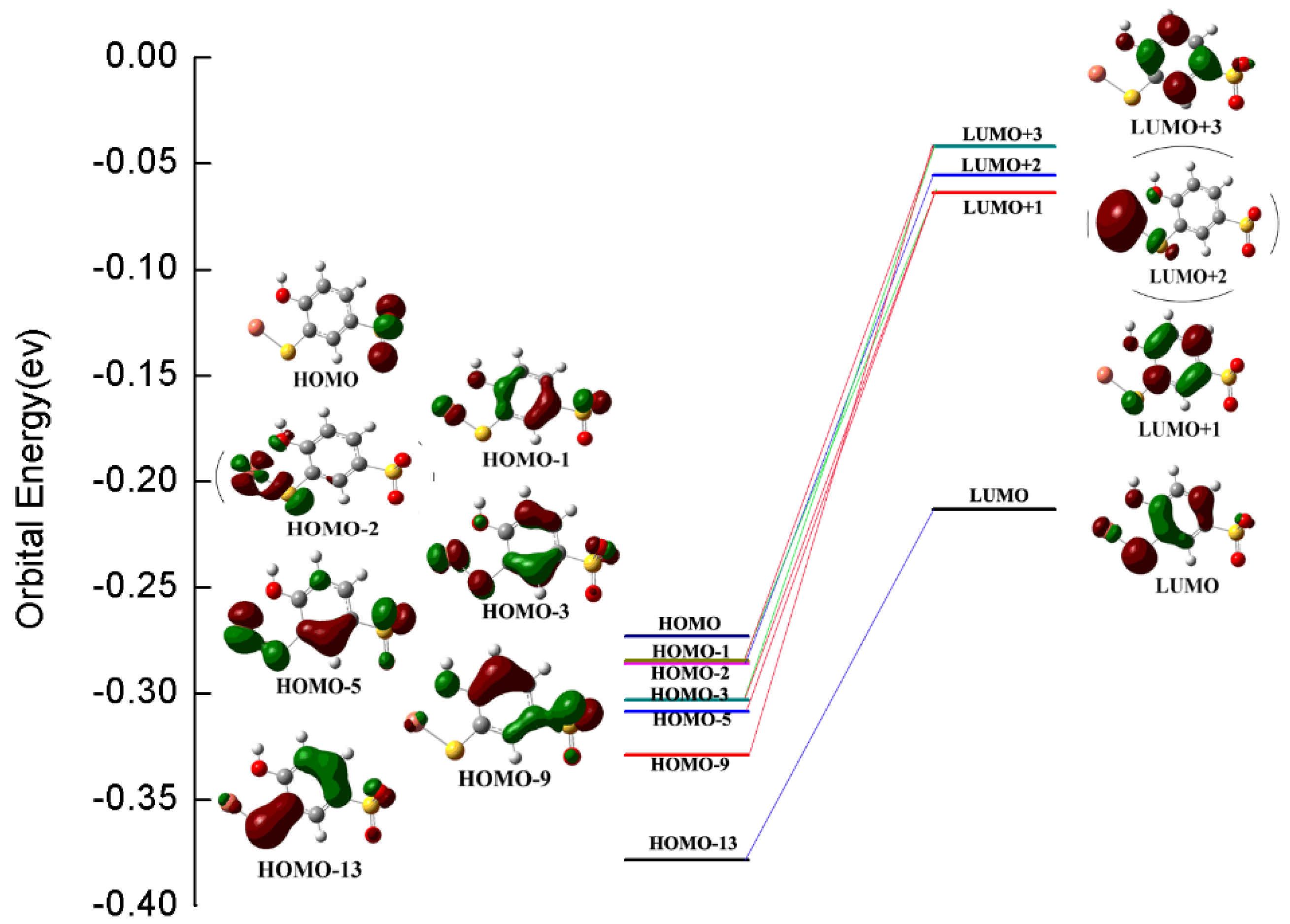
| Experimental | Theoretical | |
|---|---|---|
| Absorption Bands | Orbital Contributions | |
| 210 nm (f = 0.2851) | ||
| HOMO − 1→LUMO + 3 | 0.4233 | |
| HOMO − 3→LUMO +1 | 0.3141 | |
| 210 nm | HOMO − 9→LUMO + 3 | 0.3011 |
| HOMO − 5→LUMO + 1 | 0.2744 | |
| 242 nm (f = 0.0950) | ||
| 242 nm | HOMO − 1→LUMO + 1 | 0.6658 |
| HOMO − 3→LUMO + 3 | 0.1144 | |
| 312 nm (f = 0.0778) | ||
| 312 nm | HOMO − 2→LUMO + 2 | 0.5067 |
| HOMO − 13→LUMO | 0.4104 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Lu, L. Selective Chromogenic Recognition of Copper(II) Ion by Thiacalix[4]arene Tetrasulfonate and Mechanism. Molecules 2020, 25, 612. https://doi.org/10.3390/molecules25030612
Zhu S, Lu L. Selective Chromogenic Recognition of Copper(II) Ion by Thiacalix[4]arene Tetrasulfonate and Mechanism. Molecules. 2020; 25(3):612. https://doi.org/10.3390/molecules25030612
Chicago/Turabian StyleZhu, Shufang, and Lilin Lu. 2020. "Selective Chromogenic Recognition of Copper(II) Ion by Thiacalix[4]arene Tetrasulfonate and Mechanism" Molecules 25, no. 3: 612. https://doi.org/10.3390/molecules25030612
APA StyleZhu, S., & Lu, L. (2020). Selective Chromogenic Recognition of Copper(II) Ion by Thiacalix[4]arene Tetrasulfonate and Mechanism. Molecules, 25(3), 612. https://doi.org/10.3390/molecules25030612





