Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet
Abstract
1. Introduction
2. Biochemical Mechanisms Responsible for the Production of Volatile Flavor Compounds in Dairy Products
2.1. Metabolism of Residual Lactose, Lactate, and Citrate
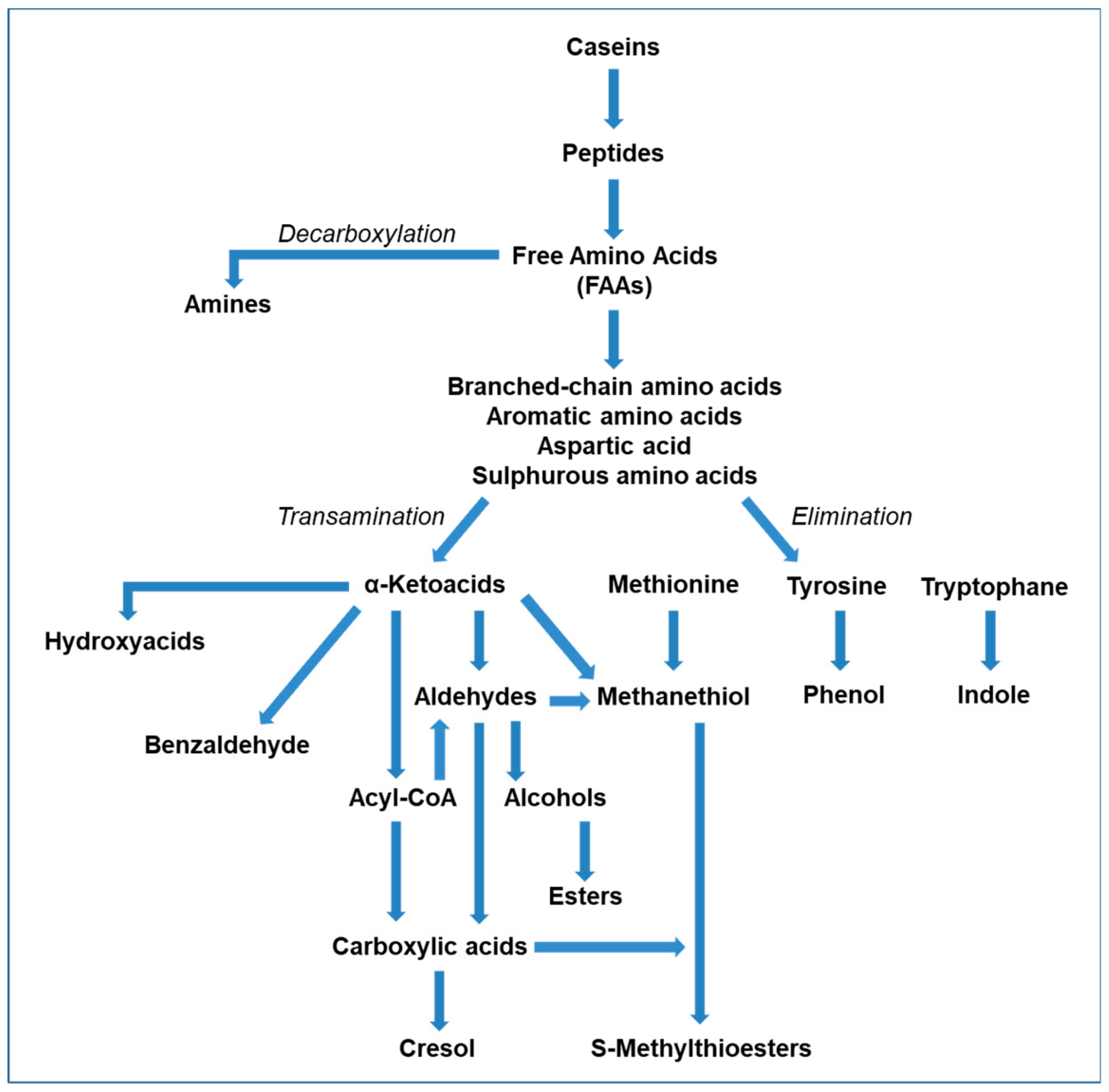
2.2. Metabolism of Free Amino Acids (FAA)
2.3. Metabolism of Free Fatty Acids (FFAs)
3. Major Volatile Flavor Compounds Found in Ripened Cheese and Influenced by Ruminant Diet
3.1. Acids
3.2. Aldehydes
3.3. Lactones
3.4. Ketones and Alcohols
3.5. Esters
3.6. Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McSweeney, P.L.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Le Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- McSweeney, P.L. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Microbiology of cheese ripening. In Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; pp. 333–390. [Google Scholar]
- Buchin, S.; Delague, V.; Duboz, G.; Berdague, J.L.; Beuvier, E.; Pochet, S.; Grappin, R. Influence of pasteurization and fat composition of milk on the volatile compounds and flavor characteristics of a semi-hard cheese. J. Dairy Sci. 1998, 81, 3097–3108. [Google Scholar] [CrossRef]
- Sutton, J.D. Altering milk composition by feeding. J. Dairy Sci. 1989, 72, 2801–2814. [Google Scholar] [CrossRef]
- DePeters, E.J.; Cant, J.P. Nutritional factors influencing the nitrogen composition of bovine milk: A review. J. Dairy Sci. 1992, 75, 2043–2070. [Google Scholar] [CrossRef]
- White, S.L.; Bertrand, J.A.; Wade, M.R.; Washburn, S.P.; Green, J.T., Jr.; Jenkins, T.C. Comparison of fatty acid content of milk from Jersey and Holstein cows consuming pasture or a total mixed ration. J. Dairy Sci. 2001, 84, 2295–2301. [Google Scholar] [CrossRef]
- De Noni, I.; Battelli, G. Terpenes and fatty acid profiles of milk fat and “Bitto” cheese as affected by transhumance of cows on different mountain pastures. Food Chem. 2008, 109, 299–309. [Google Scholar] [CrossRef]
- Walker, G.P.; Dunshea, F.R.; Doyle, P.T. Effects of nutrition and management on the production and composition of milk fat and protein: A review. Aust. J. Agric. Res. 2004, 55, 1009–1028. [Google Scholar] [CrossRef]
- Cappucci, A.; Alves, S.P.; Bessa, R.J.; Buccioni, A.; Mannelli, F.; Pauselli, M.; Viti, C.; Pastorelli, R.; Roscini, V.; Serra, A.; et al. Effect of increasing amounts of olive crude phenolic concentrate in the diet of dairy ewes on rumen liquor and milk fatty acid composition. J. Dairy Sci. 2018, 101, 4992–5005. [Google Scholar] [CrossRef]
- Ianni, A.; Di Maio, G.; Pittia, P.; Grotta, L.; Perpetuini, G.; Tofalo, R.; Cichelli, A.; Martino, G. Chemical–nutritional quality and oxidative stability of milk and dairy products obtained from Friesian cows fed with a dietary supplementation of dried grape pomace. J. Sci. Food Agric. 2019, 99, 3635–3643. [Google Scholar] [CrossRef]
- Chedea, V.S.; Pelmus, R.S.; Lazar, C.; Pistol, G.C.; Calin, L.G.; Toma, S.M.; Dragomir, C.; Taranu, I. Effects of a diet containing dried grape pomace on blood metabolites and milk composition of dairy cows. J. Sci. Food Agric. 2017, 97, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Elgendy, R.; Giantin, M.; Martino, C.; Giansante, D.; Ianni, A.; Dacasto, M.; Martino, G. RNA sequencing-based whole-transcriptome analysis of friesian cattle fed with grape pomace-supplemented diet. Animals 2018, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Ardö, Y.; McSweeney, P.L.; Magboul, A.A.; Upadhyay, V.K.; Fox, P.F. Biochemistry of cheese ripening: Proteolysis. In Cheese; Academic Press: Burlington, MA, USA, 2017; pp. 445–482. [Google Scholar]
- Singh, T.K.; Drake, M.A.; Cadwallader, K.R. Flavor of Cheddar cheese: A chemical and sensory perspective. Compr. Rev. Food Sci. Food Saf. 2003, 2, 166–189. [Google Scholar] [CrossRef]
- Ganesan, B.; Weimer, B.C. Amino acid catabolism and its relationship to cheese flavor outcomes. 2017. Biochemistry of cheese ripening: Proteolysis. In Cheese: Chemistry, Physics, and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 483–516. [Google Scholar]
- Kilcawley, K.N. Cheese flavour. In Fundamentals of Cheese Science; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2017; pp. 443–474. [Google Scholar]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Ardö, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Peralta, G.H.; Wolf, I.V.; Bergamini, C.V.; Perotti, M.C.; Hynes, E.R. Evaluation of volatile compounds produced by Lactobacillus paracasei I90 in a hard-cooked cheese model using solid-phase microextraction. Dairy Sci. Technol. 2014, 94, 73–81. [Google Scholar] [CrossRef]
- Morgan, M. The chemistry of some microbially induced flavor defects in milk and dairy foods. Biotechnol. Bioeng. 1976, 18, 953–965. [Google Scholar] [CrossRef]
- Klačanová, K.; Fodran, P.; Rosenberg, M. The possible production of natural flavours by amino acid degradation. Chem. Mon. 2010, 141, 823–828. [Google Scholar] [CrossRef]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Schirone, M.; Tofalo, R.; Perpetuini, G.; Manetta, A.; Di Gianvito, P.; Tittarelli, F.; Battistelli, N.; Corsetti, A.; Suzzi, G.; Martino, G. Influence of Iodine Feeding on Microbiological and Physico-Chemical Characteristics and Biogenic Amines Content in a Raw Ewes’ Milk Cheese. Foods 2018, 7, 108. [Google Scholar] [CrossRef]
- Forde, A.; Fitzgerald, G.F. Biotechnological approaches to the understanding and improvement of mature cheese flavour. Curr. Opin. Biotechnol. 2000, 11, 484–489. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Bertuzzi, A.S.; McSweeney, P.L.; Rea, M.C.; Kilcawley, K.N. Detection of volatile compounds of cheese and their contribution to the flavor profile of surface-ripened cheese. Compr. Rev. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef]
- Urbach, G. The flavour of milk and dairy products: II. Cheese: Contribution of volatile compounds. Int. J. Dairy Technol. 1997, 50, 79–89. [Google Scholar] [CrossRef]
- Helinck, S.; Spinnler, H.E.; Parayre, S.; Dame-Cahagne, M.; Bonnarme, P. Enzymatic versus spontaneous S-methyl thioester synthesis in Geotrichum candidum. FEMS Microbiol. Lett. 2000, 193, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Faccia, M.; Trani, A.; Natrella, G.; Gambacorta, G. Chemical-sensory and volatile compound characterization of ricotta forte, a traditional fermented whey cheese. J. Dairy Sci. 2018, 101, 5751–5757. [Google Scholar] [CrossRef]
- Beuvier, E.; Buchin, S. Raw milk cheese. In Cheese: Chemistry, Physics and Microbiology, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Academic Press: London, UK, 2004; Volume 1, pp. 319–345. [Google Scholar]
- Aprea, E.; Romanzin, A.; Corazzin, M.; Favotto, S.; Betta, E.; Gasperi, F.; Bovolenta, S. Effects of grazing cow diet on volatile compounds as well as physicochemical and sensory characteristics of 12-month-ripened Montasio cheese. J. Dairy Sci. 2016, 99, 6180–6190. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Martino, C.; Bennato, F.; Martino, G. Compositional characteristics and aromatic profile of caciotta cheese obtained from Friesian cows fed with a dietary supplementation of dried grape pomace. J. Dairy Sci. 2019, 102, 1025–1032. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Martino, C.; Di Luca, A.; Innosa, D.; Fusco, A.M.; Pomilio, F.; Martino, G. Dietary supplementation of Saanen goats with dried licorice root modifies chemical and textural properties of dairy products. J. Dairy Sci. 2020, 103, 52–62. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Martino, C.; Grotta, L.; Bennato, F.; Martino, G. Zinc supplementation of Friesian cows: Effect on chemical-nutritional composition and aromatic profile of dairy products. J. Dairy Sci. 2019, 102, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Ianni, A.; Iannaccone, M.; Martino, C.; Innosa, D.; Grotta, L.; Bennato, F.; Martino, G. Zinc supplementation of dairy cows: Effects on chemical composition, nutritional quality and volatile profile of Giuncata cheese. Int. Dairy J. 2019, 94, 65–71. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Innosa, D.; Grotta, L.; D’Onofrio, A.; Martino, G. Chemical-nutritional characteristics and aromatic profile of milk and related dairy products obtained from goats fed with extruded linseed. Asian-Austral J. Anim. Sci. 2019, 33, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ianni, A.; Martino, C.; Innosa, D.; Bennato, F.; Grotta, L.; Martino, G. Zinc supplementation of lactating dairy cows: Effects on chemical-nutritional quality and volatile profile of Caciocavallo cheese. Asian-Austral J. Anim. Sci. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Ianni, A.; Grotta, L.; Pomilio, F.; Martino, G. Influence of zinc feeding on nutritional quality, oxidative stability and volatile profile of fresh and ripened ewes’ milk cheese. Foods 2019, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Ianni, A.; Martino, C.; Pomilio, F.; Di Luca, A.; Martino, G. Dietary selenium intake in lactating dairy cows modifies fatty acid composition and volatile profile of milk and 30-day-ripened caciotta cheese. Eur. Food Res. Technol. 2019, 245, 2113–2121. [Google Scholar] [CrossRef]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Grotta, L.; Martino, G. Lipolytic volatile compounds in dairy products derived from cows fed with dried olive pomace. Eur. Food Res. Technol. 2019, 245, 159–166. [Google Scholar] [CrossRef]
- Stefanon, B.; Procida, G. Effects of including silage in the diet on volatile compound profiles in Montasio cheese and their modification during ripening. J. Dairy Res. 2004, 71, 58–65. [Google Scholar] [CrossRef]
- Bovolenta, S.; Romanzin, A.; Corazzin, M.; Spanghero, M.; Aprea, E.; Gasperi, F.; Piasentier, E. Volatile compounds and sensory properties of Montasio cheese made from the milk of Simmental cows grazing on alpine pastures. J. Dairy Sci. 2014, 97, 7373–7385. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Innosa, D.; Grotta, L.; Martino, G. Effects of selenium supplementation on chemical composition and aromatic profiles of cow milk and its derived cheese. J. Dairy Sci. 2019, 102, 6853–6862. [Google Scholar] [CrossRef]
- Carpino, S.; Mallia, S.; La Terra, S.; Melilli, C.; Licitra, G.; Acree, T.E.; Barbano, D.M.; Van Soest, P.J. Composition and aroma compounds of Ragusano cheese: Native pasture and total mixed rations. J. Dairy Sci. 2004, 87, 816–830. [Google Scholar] [CrossRef]
- Villeneuve, M.P.; Lebeuf, Y.; Gervais, R.; Tremblay, G.F.; Vuillemard, J.C.; Fortin, J.; Chouinard, P.Y. Milk volatile organic compounds and fatty acid profile in cows fed timothy as hay, pasture, or silage. J. Dairy Sci. 2013, 96, 7181–7194. [Google Scholar] [CrossRef]
- King, R.A.; Mano, M.M.; Head, R.J. Assessment of isoflavonoid concentrations in Australian bovine milk samples. J. Dairy Res. 1998, 65, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Harper, W.J.; Kristoffersen, T.; Wang, J.Y. Formation of free fatty acids during the ripening of fat modified cheese slurries. Milchwissenschaft 1978, 33, 604–608. [Google Scholar]
- Zabaleta, L.; Gourrat, K.; Barron, L.J.R.; Albisu, M.; Guichard, E. Identification of odour-active compounds in ewes’ raw milk commercial cheeses with sensory defects. Int. Dairy J. 2016, 58, 23–30. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.; Cogan, T.M.; Guinee, T.P. Cheese: Chemistry, Physics and Microbiology, General Aspects; Elsevier: Oxford, UK, 2004; Volume 1. [Google Scholar]
- Huard, C.; Miranda, G.; Wessner, F.; Bolotin, A.; Hansen, J.; Foster, S.J.; Chapot-Chartier, M.P. Characterization of AcmB, an N-acetylglucosaminidase autolysin from Lactococcus lactis. Microbiology 2003, 149, 695–705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Attaie, R.; Richter, R.L. Formation of volatile free fatty acids during ripening of Cheddar-like hard goat cheese. J. Dairy Sci. 1996, 79, 717–724. [Google Scholar] [CrossRef]
- Woo, A.H.; Kollodge, S.; Lindsay, R.C. Quantification of major free fatty acids in several cheese varieties. J. Dairy Sci. 1984, 67, 874–878. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ntambi, J.M. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 113–121. [Google Scholar] [CrossRef]
- Smith, S.B.; Lunt, D.K.; Chung, K.Y.; Choi, C.B.; Tume, R.K.; Zembayashi, M. Adiposity, fatty acid composition, and delta-9 desaturase activity during growth in beef cattle. Anim. Sci. J. 2006, 77, 478–486. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Zimmerman, M.T.; Bayse, C.A.; Ramoutar, R.R.; Brumaghim, J.L. Sulphur and selenium antioxidants: Challenging radical scavenging mechanisms and developing structure-activity relationships based on metal binding. J. Inorg. Biochem. 2015, 145, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Castada, H.Z.; Hanas, K.; Barringer, S.A. Swiss Cheese flavor variability based on correlations of volatile flavor compounds, descriptive sensory attributes, and consumer preference. Foods 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Collins, Y.F.; Mukdsi, M.A.; McSweeney, P.L.; Wilkinson, M.G.; Spinnler, H.E. Lipolysis and metabolism of fatty acids in cheese. In Cheese; Academic Press: Burlington, MA, USA, 2017; pp. 423–444. [Google Scholar]
- Urbach, G. The chemical and biochemical basis of cheese and milk aroma. In Microbiology and Biochemistry of Cheese and Fermented Milk; Springer: Boston, MA, USA, 1997; pp. 253–298. [Google Scholar]
- Belviso, S.; Giordano, M.; Dolci, P.; Zeppa, G. Degradation and biosynthesis of terpenoids by lactic acid bacteria isolated from cheese: First evidence. Dairy Sci. Technol. 2011, 91, 227. [Google Scholar] [CrossRef][Green Version]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. J. Dairy Sci. 2017, 100, 8658–8669. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Dicke, M.; Gols, R.; Ludeking, D.; Posthumus, M.A. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J. Chem. Ecol. 1999, 25, 1907–1922. [Google Scholar] [CrossRef]
- Pereira, D.; Valentão, P.; Pereira, J.; Andrade, P. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Han, J.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Salmieri, S.; Lacroix, M. Polyphenolic compounds as functional ingredients in cheese. Food Chem. 2011, 124, 1589–1594. [Google Scholar] [CrossRef]
- Kajikawa, H.; Kudo, H.; Kondo, T.; Jodai, K.; Honda, Y.; Kuwahara, M.; Watanabe, T. Degradation of benzyl ether bonds of lignin by ruminal microbes. FEMS Microbiol. Lett. 2000, 187, 15–20. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Raes, K.; Balcaen, A.; Dirinck, P.; De Winne, A.; Claeys, E.; Demeyer, D.; De Smet, S. Meat quality, fatty acid composition and flavour analysis in Belgian retail beef. Meat Sci. 2003, 65, 1237–1246. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Castellani, F.; Bernardi, N.; Vitali, A.; Marone, E.; Grotta, L.; Martino, G. Proteolytic volatile compounds in milk and cheese of cows fed dried olive pomace supplementation. J. Anim. Feed Sci. 2018, 27, 361–365. [Google Scholar] [CrossRef]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Anim. Feed Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M.; Leardi, R.; Toppino, P.M. Influence of heat treatment on the volatile compounds of milk. J. Agric. Food Chem. 1997, 45, 3171–3177. [Google Scholar] [CrossRef]

| VOC Family | Dietary Supplement (Ruminant) | Type of Dairy Product | Effects | Ref. |
|---|---|---|---|---|
| Carboxylic acids | Dried grape pomace (Friesian cows) | Fresh and 28-day ripened Caciotta cheese | ↓ Acetic acid | [32] |
| Nutrient-rich pasture (Simmental cows) | 12-month ripened Montasio cheese | ↓ Butanoic acid ↓ Hexanoic acid | [33] | |
| Dried grape pomace (Friesian cows) | 28-day ripened Caciotta cheese | [34] | ||
| Dried licorice root (Saanen goats) | Fresh and 30-day ripened Caciotta cheese | ↓ Hexanoic acid | [35] | |
| Organic zinc (Friesian cows) | 30-day ripened Caciotta cheese | ↑ Butanoic acid ↑ Hexanoic acid | [36] | |
| 5-day stored Giuncata cheese | [37] | |||
| Extruded linseed (Saanen goats) | 60-day ripened Caciotta cheese | ↓ Dodecanoic acid | [38] | |
| Aldehydes | Organic zinc (Friesian cows) | 120-day ripened Caciocavallo cheese | ↑ Nonanal | [39] |
| Organic zinc (half-breed ewes) | 90-day ripened Pecorino cheese | ↑ Hexanal | [40] | |
| Organic selenium (Friesian cows) | 30-day ripened Caciotta cheese | ↓ Hexanal ↓ Heptanal | [41] | |
| Lactones | Organic zinc (Friesian cows) | 120-day ripened Caciocavallo cheese | ↑ γ-nonalactone ↑ γ-dodecalactone ↑ δ-nonalactone ↑ δ-decalactone ↑ δ-dodecalactone ↑ δ-tetralactone | [39] |
| 30-day ripened Caciotta cheese | ↑ δ-octalactone ↑ δ-decalactone | [36] | ||
| Organic selenium (Friesian cows) | 30-day ripened Caciotta cheese | [41] | ||
| Dried olive pomace (Friesian cows) | 30-day ripened Caciotta cheese | ↑ γ-dodecalactone ↑ δ-octalactone | [42] | |
| Ketones and Alcohols | Silages (Simmental cows) | 68-day, 200-day and 360-day ripened Montasio cheese | ↑ acetone ↑ 2-3-butanedione ↑ 2-butanone ↑ 2-hexanone ↑ 2-heptanone ↑ 2-methyl-1-butanol | [43] |
| Nutrient-rich vs nutrient-poor pasture (Simmental cows) | 60-day ripened Montasio cheese | ↑ 2-Propanone 1 ↑ 2-Hepta-none 1 ↑ 2-Undecanone 1 ↑ 3-Methyl-1-butanol 2 | [44] | |
| Organic selenium (Friesian cows) | 120-day ripened Caciocavallo cheese | ↑ 2-pentanone ↑ 2-nonan-2-one ↓ Hexanol | [45] | |
| Esters | TMR + native pasture (dairy cows) | Ragusano Cheese | Geranyl acetate 3 [E]-Methyl-jasmonate3 | [46] |
| Organic zinc (Friesian cows) | 120-day ripened Caciocavallo cheese | ↑ Ethyl butanoate ↑ Ethyl hexanoate ↑ Ethyl octanoate ↑ Ethyl nonanoate ↑ Ethyl decanoate ↑ Ethyl dodecanoate ↑ Ethyl tetradecanoate ↑ Ethyl hexadecanoate | [39] | |
| 30-day ripened Caciotta cheese | ↑ Ethyl hexanoate ↑ Ethyl hexadecanoate | [36] | ||
| Phenolic compounds | Pasture (dairy cows) | Raw milk | ↑ Toluene | [47] |
| Crops (dairy cows) | ↑ Ptaquiloside ↑ Genistein ↑ Daidzein | [48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianni, A.; Bennato, F.; Martino, C.; Grotta, L.; Martino, G. Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet. Molecules 2020, 25, 461. https://doi.org/10.3390/molecules25030461
Ianni A, Bennato F, Martino C, Grotta L, Martino G. Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet. Molecules. 2020; 25(3):461. https://doi.org/10.3390/molecules25030461
Chicago/Turabian StyleIanni, Andrea, Francesca Bennato, Camillo Martino, Lisa Grotta, and Giuseppe Martino. 2020. "Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet" Molecules 25, no. 3: 461. https://doi.org/10.3390/molecules25030461
APA StyleIanni, A., Bennato, F., Martino, C., Grotta, L., & Martino, G. (2020). Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet. Molecules, 25(3), 461. https://doi.org/10.3390/molecules25030461





