Abstract
We previously demonstrated that the silk protein sericin promotes pigmentation of retinal pigment epithelium (RPE) by activating the NF-κB pathway. Among numerous agents, NF-κB can be activated by hydrogen peroxide. In the present study, we explored possible associations between reactive oxygen species and sericin-induced melanogenesis in RPE. The proteome of human fetal RPE cultured for seven days with or without 1% sericin was analyzed using ingenuity pathway analysis (IPA). The proteomic data was verified by immunofluorescence and immunoblotting. Light microscopy and scanning electron microscopy were used to assess morphology. Dihydroethidium (DHE) and dihydrorhodamine (DHR) assays were used to measure superoxide and hydrogen peroxide species. Expression levels of proteins related to inflammation, differentiation, cell survival and cell adhesion were higher in cells cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 1% sericin, whereas cells cultured in DMEM alone showed higher expression levels of proteins associated with Bruch’s membrane and cytoskeleton. Despite upregulation of inflammatory proteins, sericin co-cultured RPE yielded significantly higher cell viability compared to cells cultured without sericin. Addition of sericin to culture media significantly increased hydrogen peroxide-levels without significantly affecting superoxide-levels. We suggest that sericin-induced melanogenesis in cultured RPE is associated with elevated levels of superoxide dismutase, hydrogen peroxide and inflammatory proteins.
1. Introduction
Research on retinal pigment epithelial (RPE) morphology and pigmentation is motivated by (1) RPE transplantation, (2) the quest to understand melanogenesis, and (3) a desire to modulate the RPE phenotype in pathological states (e.g., age-related macular degeneration (AMD)). With regards to transplantation, cutting down RPE production time, sometimes necessitating a total culture period of up to three months [1], could reduce the costs of therapy and the risk of infection associated with prolonged culture periods. Understanding RPE morphology and pigmentation could also aid in our ability to pharmacologically control cellular signaling pathways to restore a healthy RPE layer in diseases where RPE dysfunction is thought to play a key role.
Sericin is a major component of raw silk produced by Bombyx mori (silkworm). It has been widely investigated for potential applications in biomedicine. In 1995, Minoura et al. reported that sericin has a mitogenetic effect on murine fibroblasts [2]. Later, it was found that sericin also acts as an antioxidant [3] and promotes corneal wound healing in rats [4]. Moreover, it has been shown to display moisturizing, antiwrinkle, and antitumor properties when used in the form of lotion, cream and dietary supplement, respectively [5]. Furthermore, its application in wound dressings appeared more effective than control in treatment of split-thickness skin graft donor sites in a clinical trial [6].
We have previously reported that the addition of sericin to cell preservation medium improves RPE phenotype following a seven-day preservation period [7]. We have also shown that sericin has pro-pigmentation effects on cultured RPE, and that these effects are associated with the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway [8].
Since sericin is considered to be a potent antioxidant [5,9], and antioxidants generally are thought to inhibit NF-κB [10,11], our previous results showing that sericin activates the NF-κB pathway [8] were unexpected. The present study was designed to follow-up on our previous transcription analysis by investigating global changes at the protein level. We herein report results of proteomic analysis of the effect of amendment of culture medium with sericin on RPE cells.
2. Materials and Methods
2.1. Materials
Primary human fetal RPE cells, complete epithelial cell medium (EpiCM), fetal bovine serum (FBS) and epithelial cell growth supplement (EpiCGS) were obtained from ScienCell Research Laboratories (San Diego, CA, USA). Dulbecco’s Modified Eagle’s Medium (high glucose, with pyruvate; hereafter named DMEM), sericin (product number: S5201), and penicillin-streptomycin were obtained from Sigma Aldrich (St Louis, MO, USA). Nunclon Δ surface 96-well plates, pipettes and other routine plastics came from VWR International (West Chester, PA, USA).
2.2. Cell Culture
Third passage primary human fetal RPE cells were seeded (7000 cells/cm2) in EpiCM consisting of 20 mL/L FBS, 10 mL/L EpiCGS, 100 U/mL penicillin and 0.1 mg/mL streptomycin (hereafter named complete EpiCM) on Nunclon Δ surface 96-well plates and cultured under routine conditions of 95% air and 5% CO2 at 37 °C until confluence. Thereafter, complete EpiCM was replaced by differentiation media, i.e., either: (1) DMEM or (2) DMEM with 1% (10 mg/mL) sericin. Both media contained 100 U/mL penicillin and 0.1 mg/mL streptomycin. No vehicle was used for sericin. Sterile filtered sericin powder from Sigma Aldrich was dissolved directly in DMEM (i.e., the control differentiation medium) to obtain sericin-containing differentiation medium. The differentiation media were changed every other day and the cells were maintained in differentiation media for a total of seven days.
2.3. In-Solution Digestion and Nano LC-Q Exactive Orbitrap Mass Spectrometry
First, cells were mechanically scraped off from culture dishes and centrifuged to make cell pellets. Then, 800 µL of SILAC phosphoprotein lysis buffer A and B (Invitrogen, Oslo, Norway) was added to make a cell slurry, which was homogenized with a pestle (20×) for mechanical breakage. An ultrasonic processor (Vibra-Cell, Sonics & Materials Inc., Newtown, CT, USA) was employed for sonication and samples were subsequently centrifuged at 16,000× g for 20 min at 4 °C (Centrifuge 5415R, Eppendorf, Hamburg, Germany). To 40 µL, four volumes of ice-cold acetone were added, vortexed and precipitated at −20 °C overnight. Samples were centrifuged at 16,000× g for 20 min at 4 °C (Centrifuge 5415R, Eppendorf, Hamburg, Germany) and the supernatant was discarded. Proteins were re-dissolved in 50 µL 6 M urea and 100 mM ammonium bicarbonate, pH 7.8. 2.5 µL of 200 mM dithiothreitol (DTT) in 100 mM Tris-HCl, pH 8 was then added for reduction and alkylation of cysteines. Following this, samples were incubated at 37°C for 1 h followed by addition of 7.5 µL 200 mM iodoacetamide for 1 h at room temperature in the dark. By adding 10 µL 200 mM DTT at 37 °C, the alkylation reaction was then quenched for 1 h. Subsequently, the proteins were digested with 10 µg trypsin for 16 h at 37 °C. We stopped the digestion by adding 5 µL 50% formic acid and the generated peptides were purified using ZipTip C18 (Millipore, Billerica, MA, USA), and dried using a concentrator (Concentrator Plus, Eppendorf, Hamburg, Germany). Nano LC-Q exactive orbitrap mass spectrometry was performed with the settings described previously [12].
2.4. Data Analysis
Xcalibur (version 2.5.5), Mascot generic format (*.mgf) and ProteoWizard (version 3.0.331, Palo Alto, CA, USA) were used in data analysis, as described before [12]. Peptide and protein identifications were validated using Scaffold (Proteome Software Inc., Portland, OR, USA) with the minimum probability thresholds for peptide and protein at 95.0% and 99.0%, respectively, and at least two validated peptides as specified by the Prophet peptide [13] and protein algorithms [14]. Spectral counts after normalization were used for label-free quantification as described elsewhere [15]. A two-sampled t-test in Microsoft® Excel® for Mac (ver. 15.19.1) was used to compare the quantity of each protein in the sericin-containing group versus the non-sericin culture group. p-values were considered significant if <0.05. Fold change (FC) was calculated as follows: FC = ((mean of protein expressions in RPE cultured in DMEM with 1% sericin)/(mean of protein expressions in RPE cultured in DMEM without sericin)).
Similar to other proteomic studies [16,17,18], we used QIAGEN’s Ingenuity® Pathway Analysis (IPA®, QIAGEN Redwood City, CA, USA) to define functional patterns and analyze molecular pathways identified from the IPA library that were most significantly over-represented in the dataset by the right-tailed Fisher’s exact test. p-values were considered significant if <0.05.
2.5. Immunoblotting
Whole-cell lysates harvested with RIPA buffer and supplemented with protease inhibitor cocktail were used for Western blot experiments. Briefly, 40 µg total protein was loaded onto a 4–20% gradient gel (Criterion TGX; Bio-Rad, Hercules, CA, USA) and run at 120 V for 1 h. Protein was transferred to a PVDF membrane by wet transfer. The blots were probed with antibodies for cysteine-rich angiogenic inducer 61 protein (CYR61; 1:100; Santa Cruz sc-374129, Santa Cruz Biotechnology, Dallas, TX, USA), laminin subunit β2 (1:500; Santa Cruz sc-133241), superoxide dismutase 1 (SOD1; 1:200; Santa Cruz sc-17767), cellular retinaldehyde-binding protein (CRALBP; 1:1000; Abcam ab-154898, Abcam, Cambridge, UK), premelanosome protein (PMEL; 1:250; Santa Cruz sc-33590), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1000; Santa Cruz sc-47724). GAPDH was used as loading control. The blots were visualized with either Clarity™ Western ECL substrate (Bio-Rad) or SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo Scientific, Waltham, MA, USA). Blots were quantified in ImageJ (National Institutes of Health, Bethesda, MD, USA).
2.6. Light Microscopy
Three representative photomicrographs from each culture group were captured using a Leica DM microscope (Leica Microsystem, Wetzlar, Germany) and Canon EOS 5D mark II camera (Canon, Tokyo, Japan) at 200× magnification.
2.7. Scanning Electron Microscopy
Cells cultured on Thermanox sterile plastic coverslips (lot number 1153033, Nunc, Rochester, NY, USA), were fixed with glutaraldehyde and subsequently dehydrated in increasing ethanol concentrations and then dried according to the critical point method (Polaron E3100 Critical Point Drier, Polaron Equipment Ltd., Watford, UK) with CO2 as the transitional fluid. The specimens were attached to carbon stubs and coated with a 30 nm thick layer of platinum in a Polaron E5100 sputter coater before being photographed with an XL30 ESEM electron microscope (Philips, Amsterdam, The Netherlands).
2.8. Immunofluorescence
Cells were fixed in 100% ice-cold methanol for 15 min and subsequently washed thrice with fresh phosphate-buffered saline (PBS). Fixed cells were incubated for 45 min at room temperature in a blocking buffer consisting of 10% goat serum, 1% bovine serum albumin (BSA), 0.1% Triton X-100, 0.05% Tween-20 and 0.05% sodium azide in PBS. Cells were then incubated overnight at 4 °C with primary antibodies diluted (1:100) in blocking buffer. FITC-conjugated and Cy3-conjugated secondary antibodies were diluted (1:3000 and 1:250 respectively) in PBS with 1% BSA and incubated for one hour at room temperature. The cultures were thereafter rinsed three times in PBS and incubated with 1 μg/mL DAPI in PBS to stain cell nuclei before a final wash with PBS. Photomicrographs were captured at 200× magnification at predetermined locations in the culture wells using a Nikon Eclipse Ti fluorescence microscope with a DS-Qi1 black and white camera and a motorized stage. The exposure length and gain were maintained at a constant level for all samples and the fluorescence intensities of the FITC or Cy3 fluorochromes, which were conjugated to the secondary antibodies, were within the dynamic range of the camera. Immunofluorescence per cell was quantified by custom-made macros for ImageJ, as described previously [7,19].
2.9. Viability Assay
The calcein-acetoxymethyl ester (CAM) viability assay permitted quantification of calcein-stained viable cells using custom-made macros with ImageJ. CAM is a non-fluorescent compound that is hydrolyzed to calcein (strongly fluorescent) by intracellular esterases in live cells. CAM can thus be used to detect live cells. At the end of the culture period, cell medium was aspirated and cells were incubated at 37 °C for 30 min with PBS containing 1.0 μM CAM. After washing the cultures thrice with PBS, photomicrographs were captured at 200× magnification and subsequently analyzed using custom-made macros with ImageJ, as reported elsewhere [7]. In brief, unevenly transmitted light (rolling = 50) was subtracted from all 16-bit photomicrographs using the “Subtract Background”-command in ImageJ before they were converted to binary photos, Then, the culture well area covered by calcein-stained cells was automatically measured using the “Area Fraction”-command.
2.10. Measurement of Superoxide and Hydrogen Peroxide Reactive Oxygen Species
The probe dihydroethidium (DHE) detects intracellular mitochondrial superoxide. The cationic probe dihydrorhodamine (DHR) is readily oxidized by mitochondrial peroxyl moieties, such as hydrogen peroxide (H2O2). DHR or DHE were added at a final concentration of 3 µM to separate wells (n = 6 for each probe). After incubation for 90 min at 24 °C ambient CO2 (DHR), or for 20 min at 32 °C ambient CO2 (DHE), cells were detached with 3 min trypsin incubation, washed and resuspended in ice-cold HBSS + 4% FBS. Samples were kept on ice and analyzed using a BD Accuri C6 flow cytometer. The probes were transformed from non-fluorescent to fluorescent compounds by oxidative burst intermediates within the cell. Excitation of the DHR dye at 488 nm produced green fluorescence with a peak at 530 nm detected using the standard filter 530/30 (FL1). Excitation of the DHE dye at 488 nm produced red fluorescence with a peak at 600 nm detected using the standard filter 585/40 nm (FL2). The mean fluorescence of each sample was recorded. Samples without addition of probe (probe negative control +/− 1% sericin) were included. The Mann-Whitney U-test was used to compare two groups. A two-tailed p-value < 0.05 was considered significant.
2.11. Quantification of Pigmentation
Cellular pigment was measured using ImageJ in light microscopy images of RPE cultured with or without sericin. In brief, 200× photomicrographs were converted to 8-bit images before employing the “Subtract Background”-command in ImageJ. Based on pixel whiteness, a threshold was then applied to quantify the dark area fraction (%) of the images that represented cellular pigment. The same macro was used on all photomicrographs. A two-sample two-tailed t-test was used to compare the sericin-supplemented and control groups. A p-value < 0.05 was considered significant.
2.12. Quantification of Microvilli
Three investigators independently counted the number of microvilli in 10 µm × 10 µm sections of SEM micrographs from each culture group (n = 3) which were obtained at predetermined locations. All three investigators were blinded to the origins of the micrographs. Means were calculated based on the counts from the investigators. A two-sample, two-tailed t-test was used to compare the sericin-supplemented and control groups. A p-value < 0.05 was considered significant.
3. Results
3.1. Effects of Sericin on Protein Expression
To investigate how exposure to sericin affects RPE in vitro, we analyzed the proteomic profile of RPE cultured in DMEM with 1% sericin in comparison to RPE cultured in DMEM alone. The quantitative MS/MS analysis identified 1337 proteins with two peptides or more at 95.0% minimum peptide threshold and 99.0% minimum protein threshold. Of these, 77 proteins were significantly (p < 0.05) upregulated (Table 1), while 69 proteins were significantly (p < 0.05) downregulated (Table 2) in RPE cultured in DMEM with 1% sericin compared to cells cultured in DMEM alone (Figure 1). To explore the functions of these up- and downregulated proteins, we searched each protein in the UniProt [20] and GeneCards [21] protein databases. The majority of the differentially expressed proteins were related to inflammation, RPE differentiation, cell survival, cell adhesion, Bruch’s membrane and cytoskeleton. Within these functions, a clear separation of protein expression direction was observed between the two culture groups (Figure 2). The quantitative MS/MS analysis thus indicated that in vitro sericin exposure significantly alters protein expression in RPE.

Table 1.
Significantly upregulated proteins in human fetal retinal pigment epithelial cells (RPE) cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% sericin compared to cells cultured in DMEM without sericin, sorted by decreasing fold change (FC). FC was calculated as follows: FC = ((mean of protein expressions in RPE cultured in DMEM with 1% sericin)/(mean of protein expressions in RPE cultured in DMEM without sericin)). N = 3. FC: Fold change.

Table 2.
Significantly downregulated proteins in human fetal retinal pigment epithelial cells (RPE) cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% sericin compared to cells cultured in DMEM without sericin. Fold change (FC) was calculated as follows: FC = ((mean of protein expressions in RPE cultured in DMEM with 1% sericin)/(mean of protein expressions in RPE cultured in DMEM without sericin)). N = 3. FC: Fold change.
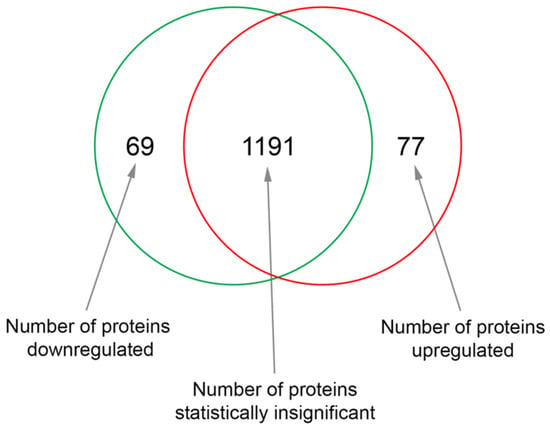
Figure 1.
The Venn diagram shows the number of proteins that were significantly upregulated or downregulated in human fetal retinal pigment epithelial cells (RPE) cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% sericin compared to cells cultured in DMEM without sericin.
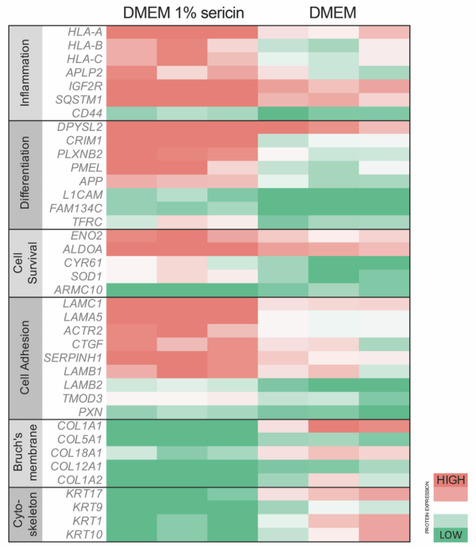
Figure 2.
Primary human fetal retinal pigment epithelial cells (RPE) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with or without 1% sericin. All cultures were subjected to proteomic analysis to evaluate differences in protein expression. Relative protein expression of key proteins related to inflammation, differentiation, cell survival and cell adhesion was higher in cells cultured in DMEM with 1% sericin, whereas cells cultured in DMEM without sericin showed higher expression levels of proteins associated with Bruch’s membrane and cytoskeleton. N = 3.
3.2. Immunofluorescence and Immunoblotting
To validate the MS/MS proteomic results, the expression levels of CYR61, laminin subunit β-2 and superoxide dismutase were assessed by immunofluorescence and immunoblotting in both culture groups. The rationale behind selecting targets to validate by Western blot was to verify relevant biological aspects of the proteomic dataset, as recommended elsewhere [22], instead of just validating the most abundant proteins. Because the proteomic dataset indicated that proteins related to inflammation, differentiation, cell adhesion and superoxide dismutase were upregulated in the sericin-treated group, targets to be validated by immunoblotting were chosen to represent some of these biological functions. Immunofluorescence and Western blot experiments confirmed the presence of the selected targets (Table 3 & Figure 3).

Table 3.
The table shows average fold change (FC) in protein levels upon incubation of primary human fetal retinal pigment epithelial cells (RPE) in Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% sericin for seven days in comparison to control (cultured in DMEM alone). FC was calculated as follows: FC = ((mean of protein expressions in RPE cultured in DMEM with 1% sericin)/(mean of protein expressions in RPE cultured in DMEM without sericin)). Data are averages from triplicates. CYR61 = Cysteine-rich angiogenic inducer 61 protein.
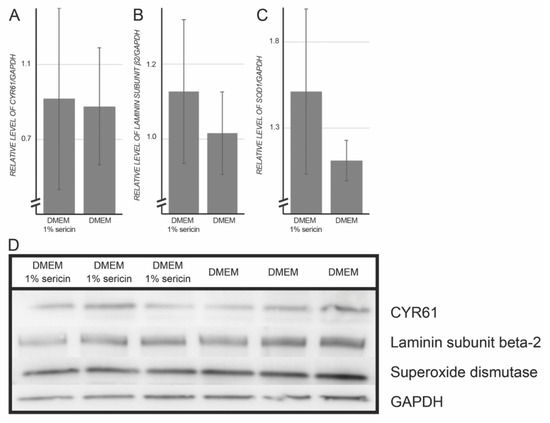
Figure 3.
Whole-cell lysates of human retinal pigment epithelial cells (RPE) cultured for seven days in Dulbecco’s Modified Eagle’s Medium (DMEM) with or without 1% sericin were subjected to Western blot experiments. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. Fold change (FC) was calculated as follows: FC = ((mean of protein expressions in RPE cultured in DMEM with 1% sericin)/(mean of protein expressions in RPE cultured in DMEM without sericin)). (A) Cysteine-rich angiogenic inducer 61 (CYR61); FC: 1.05; p-value: 0.90. (B); Laminin subunit β-2; FC: 1.11; p-value: 0.43. (C) Superoxide dismutase; FC: 1.36; p-value: 0.23. (D) Western blot. Error bars represent standard deviation of the mean. N = 3.
3.3. Sericin Increases Viability Despite Inducing an Inflammatory Response
The proteomic data revealed that RPE cultured for seven days in DMEM with 1% sericin expressed significantly higher levels of inflammation-related proteins in comparison to RPE cultured in DMEM only (Figure 2). IPA predicted that the NF-κB pathway, important in pro-inflammatory signaling [23], was upregulated in RPE cultured in DMEM with 1% sericin (p = 0.001). The prediction was based on upregulation of several of its promoters and the concurrent downregulation of regulators that are known to be inhibited by NF-κB (Figure 4). Despite upregulation of inflammatory proteins, IPA suggested a downstream effect compatible with increased cell viability and survival in the sericin-supplemented group (Figure 5A). To confirm this finding, we performed a CAM-based viability assay. CAM is a non-fluorescent compound that is hydrolyzed to calcein (strongly fluorescent) by intracellular esterases in live cells. CAM can thus be used to detect live cells. We measured the culture well area covered by CAM-stained live RPE cells in both culture groups. RPE cultured in DMEM with 1% sericin yielded significantly higher cell viability compared to cells cultured without sericin (p = 0.04) (Figure 5B). These results show that sericin increases viability despite initiating an inflammatory response in RPE in vitro.
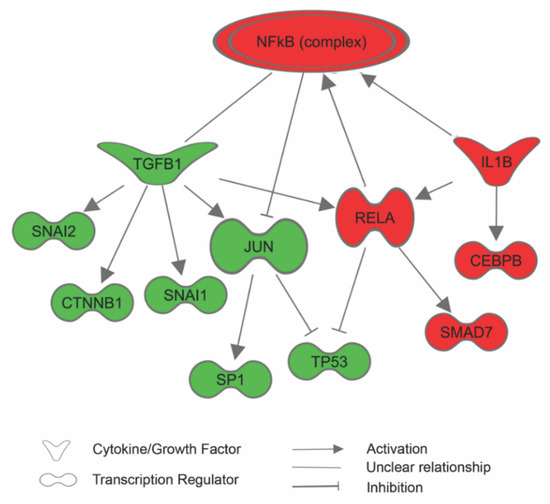
Figure 4.
Based on the differentially expressed proteins, Ingenuity Pathway Analysis (IPA) predicted that the NF-κB pathway was activated in human fetal retinal pigment epithelial cells (RPE) cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% sericin. The prediction was based on upregulation (red symbols) of several of its promoters and the concurrent downregulation (green symbols) of regulators that are known to be inhibited by the NF-κB pathway. For the sake of clarity, some extraneous relationships have been omitted, but are included in the Supplementary Material.
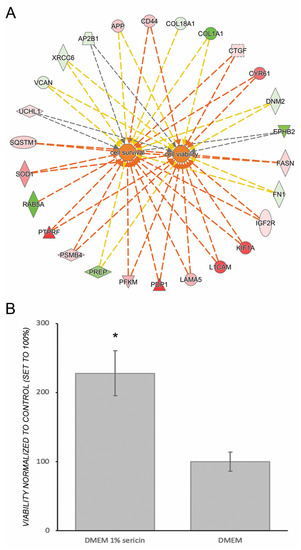
Figure 5.
Ingenuity Pathway Analysis (IPA) predicted a downstream effect compatible with increased viability and survival of human fetal retinal pigment epithelial cells (RPE) cultured in in Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% sericin compared to cells cultured without sericin (A). Red and pink symbols indicate increased protein levels upon culturing in presence of sericin, while green symbols indicate reduced protein levels. The dotted lines indicate indirect relationships leading to activation (orange). Yellow and grey dotted lines indicate inconsistent relationships and no predicted effects, respectively. To verify the effects on cell survival and viability predicted by IPA, the culture well area of CAM-stained live cells was measured in confluent layers of RPE cultured in DMEM with 1% sericin or DMEM alone. The bar chart (B) shows culture well area covered by live cells in RPE cultured in DMEM with 1% sericin normalized to the control cultures, i.e., RPE cultured in DMEM alone (100%). RPE cultured in DMEM with 1% sericin yielded significantly higher cell viability compared to cells cultured without sericin (p = 0.04). Error bars represent standard deviation of the mean. * p < 0.05. N = 3.
3.4. Sericin Promotes Pigmentation of Cultured Human Retinal Pigment Epithelial Cells
Light microscopy and scanning electron microscopy (SEM) were employed to assess sericin’s effect on the morphology of cultured RPE. Light microscopy showed pigmented RPE in sericin-supplemented cultures (Figure 6A), whereas cells cultured in only DMEM showed markedly less pigmentation (Figure 6B). Quantification of the dark area fraction in light microscopy images revealed significantly higher pigmented culture well area in the sericin-supplemented group (314% ± 68%; p < 0.001) in comparison to cultures not supplemented with sericin (set to 100%) (Figure 6C). SEM showed tightly adjoined hexagonal cells with typical cobblestone morphology in both culture groups (Figure 6J,K). However, apical microvilli appeared to be more abundantly present in cells cultured in DMEM with 1% sericin (Figure 6D) compared to DMEM only (Figure 6E). Quantification of microvilli, albeit not significant, supported this observation (microvilli count in DMEM with 1% sericin: 142 ± 64; microvilli count in DMEM only: 38 ± 14; p = 0.051) (Figure 6F). To assess the quantity of RPE-specific maturation markers CRALBP and PMEL, immunoblotting was employed (Figure 6G). CRALBP and PMEL were both most abundant in RPE cultured in DMEM with 1% sericin (344% ± 14%; p = 0.01; Figure 6H, and 290% ± 47%; p < 0.001; Figure 6I, respectively) compared to cells cultured in DMEM only (set to 100%). Collectively, these findings suggest that sericin-supplementation promotes the pigmentation and maintains the epithelial morphology of RPE in vitro.
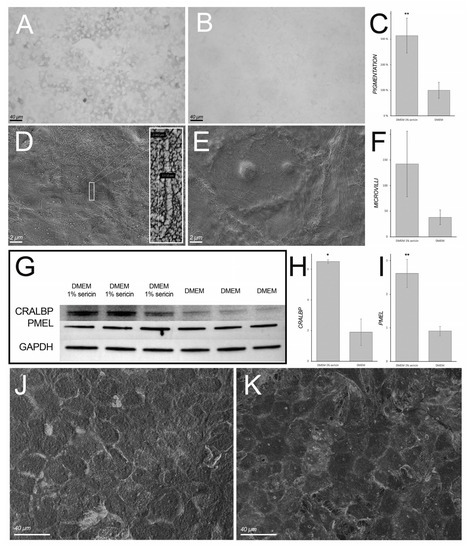
Figure 6.
Light microscopy images (grayscale) of RPE cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with (A) or without (B) 1% sericin in which the former group contained significantly more pigmented cells. The bar chart (C) shows culture well area covered by pigmented cells normalized to control cultures (RPE cultured in DMEM only; set to 100%). In RPE cultured in DMEM with 1% sericin (D), apical microvilli appeared to be more abundant in comparison to cells cultured in DMEM only (E). However, the difference was not statistically significant (F). The bar chart shows mean number of microvilli in 10 µm × 10 µm sections of SEM micrographs counted independently by three blinded investigators (F). To assess RPE-specific maturation markers, immunoblotting (G) was used to quantify cellular retinaldehyde-binding protein (CRALBP) and premelanosome protein (PMEL), which were both most abundant in the sericin-supplemented cultures. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. The bar charts show relative expression of CRALBP/GAPDH (H) and relative expression of PMEL/GAPDH (I). Tightly adjoined, hexagonal cells with typical cobblestone morphology were observed in both groups (J: DMEM with 1% sericin; K: DMEM only). All bar charts (C,F,H,I) show mean values ± standard deviation error bars. * p < 0.05. ** p < 0.001.
3.5. Effects of Sericin on Superoxide Dismutase Substrate and Product
IPA revealed higher levels of superoxide dismutase, an enzyme that catalyzes the dismutation of superoxide into oxygen, water and hydrogen peroxide [24], in RPE cultured with sericin in comparison to RPE cultured without sericin. Quantification of superoxide (one of the substrates of superoxide dismutase) with a dihydroethidium (DHE) assay revealed no significant increase in superoxide in the sericin-containing RPE cultures compared to control cultures (p = 0.052; Figure 7B). A dihydrorhodamine (DHR) assay showed significantly higher levels of hydrogen peroxide (one of the products of the superoxide dismutase enzyme) in RPE cultured with sericin (10.5-fold increase, compared to the control where no sericin was added; p = 0.002; Figure 7C).
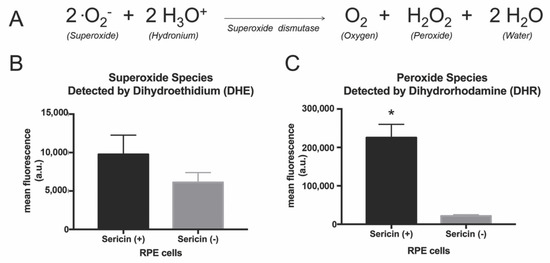
Figure 7.
Superoxide dismutase is an antioxidant enzyme that catalyzes the dismutation of superoxide into oxygen, water and hydrogen peroxide (A). Proteomic analysis revealed that this enzyme was increased 5.4-fold in cells cultured with sericin compared to cells cultured without sericin. We performed dihydroethidium (DHE) and dihydrorhodamine (DHR) assays to study the levels of the key substrate and product of this enzyme. The DHE assay measures superoxide, while the DHR assay quantifies hydrogen peroxide. The DHE assay revealed no significant increase in superoxide in the sericin-containing RPE cultures compared to control cultures (B). The DHR assay showed significantly higher levels of hydrogen peroxide in RPE cultured with sericin (C). All bar charts show mean values ± standard deviation error bars. * p < 0.05.
4. Discussion
In the current study, we investigated how sericin affects the proteome of primary human fetal RPE cells. Following a seven-day culture period, we found that cultures supplemented with sericin showed higher levels of proteins associated with inflammation, differentiation, cell survival and cell adhesion. The sericin-treated cultures in the present experiments showed better pigmentation at the end of the culture period. Furthermore, IPA analysis of the proteomic dataset predicted that the NF-κB pathway, important in pro-inflammatory signaling, was upregulated in RPE cultured in DMEM with 1% sericin. This is in agreement with a previous transcription analysis report by our group in which we demonstrated that sericin conduces pigmentation of RPE by activating the NF-κB pathway [8]. Despite the higher levels of inflammation-associated proteins, and the predicted activation of the NF-κB pathway in sericin-supplemented RPE cultures, significantly higher cell viability was observed in these cultures compared to cells cultured without sericin.
Analysis of the proteome of cultured RPE revealed higher levels of inflammatory proteins and superoxide dismutase in the sericin-supplemented cultures. These findings are supported by reports proposing sericin-associated superoxide dismutase activity [25,26]. Moreover, through Affymetrix microarray analysis, we recently reported that sericin upregulates superoxide dismutase genes in RPE cultured with sericin [8]. Superoxide dismutase is an enzyme that catalyzes the dismutation of superoxide into oxygen, water and hydrogen peroxide. Since NF-κB can be activated by hydrogen peroxide [27], we wished to investigate whether sericin promotes activation of the NF-κB pathway through regulating the level of this reactive oxygen species. A DHR assay showed a significantly higher level of hydrogen peroxide (one of the products of the superoxide dismutase enzyme) in RPE cultured with sericin. However, because superoxide dismutase is an enzyme, its elevated levels in sericin-supplemented cultures (as shown by the proteomic and western blot experiments), and the elevated levels of its product (as shown by the DHR assay), could also be caused by an increased amount of its substrates (superoxide and hydronium). This would fit with the proteomic data indicating a sericin-induced inflammatory reaction, and also the fact that sericin is a xenobiotic that, in theory, could cause ROS-generation through activation of inflammatory processes. However, quantification of superoxide (one of the substrates of superoxide dismutase) with a DHE assay revealed no significant increase in superoxide in the sericin-containing RPE cultures compared to control cultures. Even though the DHE assay did not show significantly elevated levels of superoxide, we cannot rule out that an increased catalytic activity of superoxide dismutase is responsible for this finding. Hence, the data presented herein cannot be used to conclude whether the addition of sericin causes higher levels of superoxide, which in turn increase the activity of the superoxide dismutase enzyme, or if the increased levels of superoxide dismutase are directly induced by sericin.
The DHR assay showed a significantly higher level of hydrogen peroxide in RPE cultured with sericin. Interestingly, hydrogen peroxide has been reported to activate NF-κB [27], and we have shown that increased NF-κB is associated with increased pigmentation in cultured RPE [8]. In epidermal cells, generation of hydrogen peroxide through ultraviolet radiation is known to cause melanogenesis [28]. Furthermore, it has been shown that hydrogen peroxide increases melanin synthesis and pigmentation in primary human melanocytes and keratinocytes [29]. Recently, Kim et al. reported that temporary exposure of melanoma cells to hydrogen peroxide can induce melanogenesis by increasing ATP, intracellular cAMP and melanogenesis-related genes [30]. Thus, we hypothesize that the pro-pigmentation effects of sericin in RPE in vitro are associated with inflammation and increased levels of hydrogen peroxide. However, significantly higher RPE cell viability with sericin addition suggests that hydrogen peroxide is maintained at a manageable level. Indeed, Tang et al. showed that a low level of hydrogen peroxide resulted in melanin synthesis in melanocytes, while high levels decreased melanin synthesis and increased cell death [29]. Our proteomic results indicate that hydroxyacylglutathione hydrolase (HAGH) was significantly increased (Table 1) in sericin-supplemented cells. HAGH is a thiolesterase enzyme that produces reduced glutathione and D-lactate, which is used in the antioxidant reaction converting hydrogen peroxide to water and oxygen, combatting oxidative stress. Thus, increased antioxidant activity by HAGH may be implicated in maintenance of hydrogen peroxide at a balanced level that promotes RPE pigmentation and maintains viability.
In a previous study on preservation of cultured primary human fetal RPE, we showed that the fraction of pigmented cells was inversely related to storage temperature [7]. The precise mechanisms behind these results were not fully understood. However, in light of the current investigation suggesting a role of superoxide dismutase and hydrogen peroxide in melanogenesis, the reported findings can be explained based on the temperature dependence of superoxide dismutase activity. Contrary to classic temperature-dependent enzyme activity, superoxide dismutase activity increases with decreasing temperature [31]. Hence, if superoxide dismutase plays a central role in melanogenesis in RPE, an increase in temperature would decrease superoxide dismutase activity, lower hydrogen peroxide-levels and thus decrease melanogenesis.
Accompanying the upregulation of differentiation-related proteins in sericin-supplemented RPE cultures (in comparison to cells cultured in DMEM only) is an increase of proteins associated with cell adhesion (Figure 2). This observation is particularly interesting in the light of a report that showed that cell adhesion was important for RPE melanogenesis, suggesting an interrelationship between RPE cell-to-cell adhesion and pigmentation [32]. The same study noted that the process of melanogenesis was not evenly distributed in the RPE cultures. Rather, it apparently started in scattered cells, subsequently spreading to neighboring cells. This phenomenon was also observed by us during the present experiments and is evident when studying the photomicrograph in Figure 6A. Aside from underscoring the importance of cell-to-cell adhesion, this interesting phenomenon suggests that RPE cells may stimulate adjacent cells through intercellular junctions and/or paracrine mediators. It also serves to establish a relationship between the upregulated protein groups presented in this study.
In conclusion, we have demonstrated that sericin-induced melanogenesis in cultured fetal RPE is associated with elevated levels of superoxide dismutase, hydrogen peroxide and inflammatory proteins. In the future, it could be interesting to study whether the effects of sericin reported herein can be replicated in other cells such as melanocytes, keratinocytes, iris pigment epithelial cells or induced pluripotent stem cell-derived RPE. If so, new avenues for treating pigmentation disorders may unfold, and the time-consuming differentiation of induced pluripotent stem cell-derived RPE from pluripotent stem cells could be shortened, thereby reducing a significant hurdle in regenerative medicine strategies for blinding diseases (e.g., AMD).
Supplementary Materials
Supplementary Materials are available online. Based on the differentially expressed proteins, Ingenuity Pathway Analysis (IPA) predicted that the NF-κB pathway was activated in primary human fetal retinal pigment epithelial cells (RPE) cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% sericin. Activation of the NF-κB pathway was indicated by upregulation of several of its regulator proteins and the concurrent downregulation of proteins that are known to be inhibited by the NF-κB pathway. The entries in the table show how a target (row) supports the prediction of a regulator (column).
Author Contributions
Conceptualization, A.Z.K. and J.R.E.; formal analysis, A.Z.K., C.J.J., S.R., D.S., O.K.O. and B.T.; writing—original draft preparation, A.Z.K.; writing—review and editing, C.J.J., T.P.U., S.R., D.S., A.Z.K. and B.T.; visualization, A.Z.K.; supervision, T.P.U. and J.R.E.; funding acquisition, T.P.U., J.R.E. and A.Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Norwegian Research Council [Forskerlinje-grant to A.Z.K., 2014–2019]; Futura Fond til vitenskapelig medisinsk forskning [grant to A.Z.K., 2019]; and Fylkesmann H.B. Guldahl og hustru Lucy Guldahls legat til bekjempelse av kreft og andre alvorlige sykdommer [grant to A.Z.K., 2019].
Acknowledgments
The authors thank Muhammad Saeed and Evan Michael Vallenari at Institute of Oral Biology, Faculty of Dentistry, University of Oslo for assistance with SEM and immunoblotting, respectively. We would also like to express our gratitude to Ingunn Rode Grorud, Senior Executive Officer at the Division of Laboratory Medicine, Faculty of Medicine, University of Oslo for her kind, flexible and timely logistical support during the months the experiments were undertaken.
Conflicts of Interest
A patent application has been filed by the research group on the use of sericin in culture media (European Patent Application No. 16733583.5).
References
- Buchholz, D.E.; Hikita, S.T.; Rowland, T.J.; Friedrich, A.M.; Hinman, C.R.; Johnson, L.V.; Clegg, D.O. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells 2009, 27, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Minoura, N.; Aiba, S.I.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of cultured fibroblast cells on silk protein matrices. J. Biomed. Mater. Res. 1995, 29, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef]
- Nagai, N.; Murao, T.; Ito, Y.; Okamoto, N.; Sasaki, M. Enhancing effects of sericin on corneal wound healing in rat debrided corneal epithelium. Biol. Pharm. Bull. 2009, 32, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.I.; Brancalhao, R.M.C.; Ribeiro, L.D.C.; Natali, M.R.M. Silkworm sericin: Properties and biomedical applications. Biomed. Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef]
- Siritientong, T.; Angspatt, A.; Ratanavaraporn, J.; Aramwit, P. Clinical potential of a silk sericin-releasing bioactive wound dressing for the treatment of split-thickness skin graft donor sites. Pharm. Res. 2014, 31, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Utheim, T.P.; Reppe, S.; Sandvik, L.; Lyberg, T.; Roald, B.B.H.; Ibrahim, I.B.; Eidet, J.R. Cultured human retinal pigment epithelial (hRPE) sheets: A search for suitable storage conditions. Microsc. Microanal. 2018, 24, 147–155. [Google Scholar] [CrossRef]
- Eidet, J.R.; Reppe, S.; Pasovic, L.; Olstad, O.K.; Lyberg, T.; Khan, A.Z.; Fostad, I.G.; Chen, D.F.; Utheim, T.P. The silk-protein sericin induces rapid melanization of cultured primary human retinal pigment epithelial cells by activating the NF-κB pathway. Sci. Rep. 2016, 6, 22671. [Google Scholar] [CrossRef]
- Fan, J.B.; Wu, L.P.; Chen, L.S.; Mao, X.Y.; Ren, F.Z. Antioxidant activities of silk sericin from silkworm Bombyx mori. J. Food Biochem. 2009, 33, 74–88. [Google Scholar] [CrossRef]
- Schenk, H.; Klein, M.; Erdbrugger, W.; Droge, W.; Schulzeosthoff, K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-κB and AP-1. Proc. Natl. Acad. Sci. USA 1994, 91, 1672–1676. [Google Scholar] [CrossRef]
- Ma, Q.; Kinneer, K.; Ye, J.; Chen, B.J. Inhibition of nuclear factor kappaB by phenolic antioxidants: Interplay between antioxidant signaling and inflammatory cytokine expression. Mol. Pharmacol. 2003, 64, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Koehler, C.J.; Thiede, B. Predominant cleavage of proteins N-terminal to serines and threonines using scandium (III) triflate. J. Biol. Inorg. Chem. 2020, 25, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef]
- Tran, T.T.; Strozynski, M.; Thiede, B. Quantitative phosphoproteome analysis of cisplatin-induced apoptosis in Jurkat T cells. Proteomics 2017, 17, 1600470. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Luo, C.; Cai, J.; Powell, D.W. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4220–4233. [Google Scholar] [CrossRef]
- Luo, C.; Yang, X.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Tezel, G. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5697–5707. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Luo, C.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Kaplan, H.J. Oxidative stress and the regulation of complement activation in human glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5071–5082. [Google Scholar] [CrossRef]
- Khan, A.Z.; Utheim, T.P.; Jackson, C.J.; Reppe, S.; Lyberg, T.; Eidet, J.R. Nucleus morphometry in cultured epithelial cells correlates with phenotype. Microsc. Microanal. 2016, 22, 612–620. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Handler, D.C.L.; Pascovici, D.; Mirzaei, M.; Gupta, V.; Salekdeh, G.H.; Haynes, P.A. The art of validating quantitative proteomics data. Proteomics 2018, 18, 1800222. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Ersel, M.; Uyanikgil, Y.; Akarca, F.K.; Ozcete, E.; Altunci, Y.A.; Karabey, F.; Cavusoglu, T.; Meral, A.; Yigitturk, G.; Cetin, E.O. Effects of silk sericin on incision wound healing in a dorsal skin flap wound healing rat model. Med. Sci. Monit. 2016, 22, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, D.; Sikka, P.; Singh, P. Sericin supplementation improves semen freezability of buffalo bulls by minimizing oxidative stress during cryopreservation. Anim. Reprod. Sci. 2015, 152, 26–31. [Google Scholar] [CrossRef]
- Zhang, J.; Johnston, G.; Stebler, B.; Keller, E.T. Hydrogen peroxide activates NFκB and the interleukin-6 promoter through NFκB-inducing kinase. Antioxid. Redox Signal. 2001, 3, 493–504. [Google Scholar] [CrossRef]
- Pelle, E.; Mammone, T.; Maes, D.H.; Frenkel, K. Keratinocytes act as a source of reactive oxygen species by transferring hydrogen peroxide to melanocytes. J. Investig. Dermatol. 2005, 124, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, J.; Lin, X.; Wu, W.; Kang, K.; Fu, W. Oxidation levels differentially impact melanocytes: Low versus high concentration of hydrogen peroxide promotes melanin synthesis and melanosome transfer. Dermatology 2012, 224, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Lee, S.G. Induction of ATP synthase β by H2O2 induces melanogenesis by activating PAH and cAMP/CREB/MITF signaling in melanoma cells. Int. J. Biochem. Cell Biol. 2013, 45, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Perelman, A.; Dubinsky, Z.; Martínez, R. Temperature dependence of superoxide dismutase activity in plankton. J. Exp. Mar. Biol. Ecol. 2006, 334, 229–235. [Google Scholar]
- Rak, D.J.; Hardy, K.M.; Jaffe, G.J.; McKay, B.S. Ca++-switch induction of RPE differentiation. Exp. Eye Res. 2006, 82, 648–656. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).