Designing Efficient Processes for Sustainable Bioethanol and Bio-Hydrogen Production from Grass Lawn Waste
Abstract
1. Introduction
2. Results and Discussion
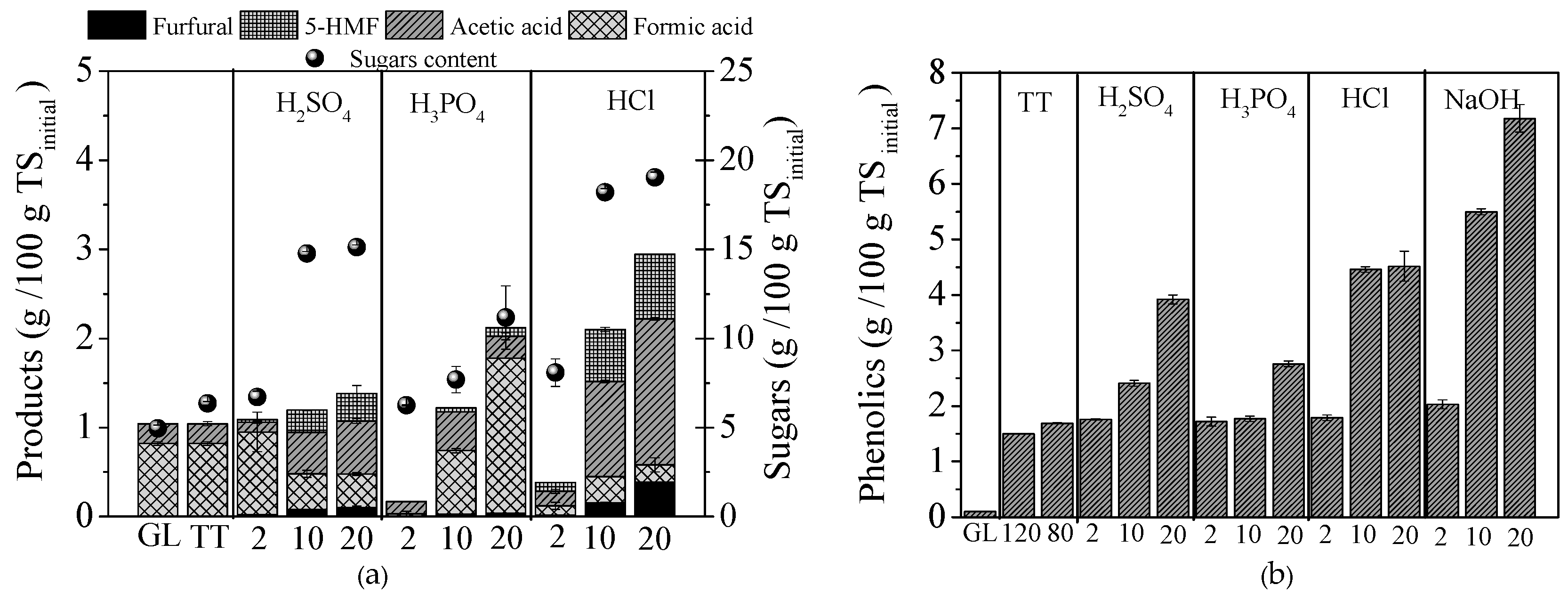
2.1. Composition of GL before and after Pretreatments
2.2. BHP Experiments
2.2.1. BHP under Different Enzymatic Loadings
2.2.2. BHP of the Whole Pretreated Slurry, at SSF
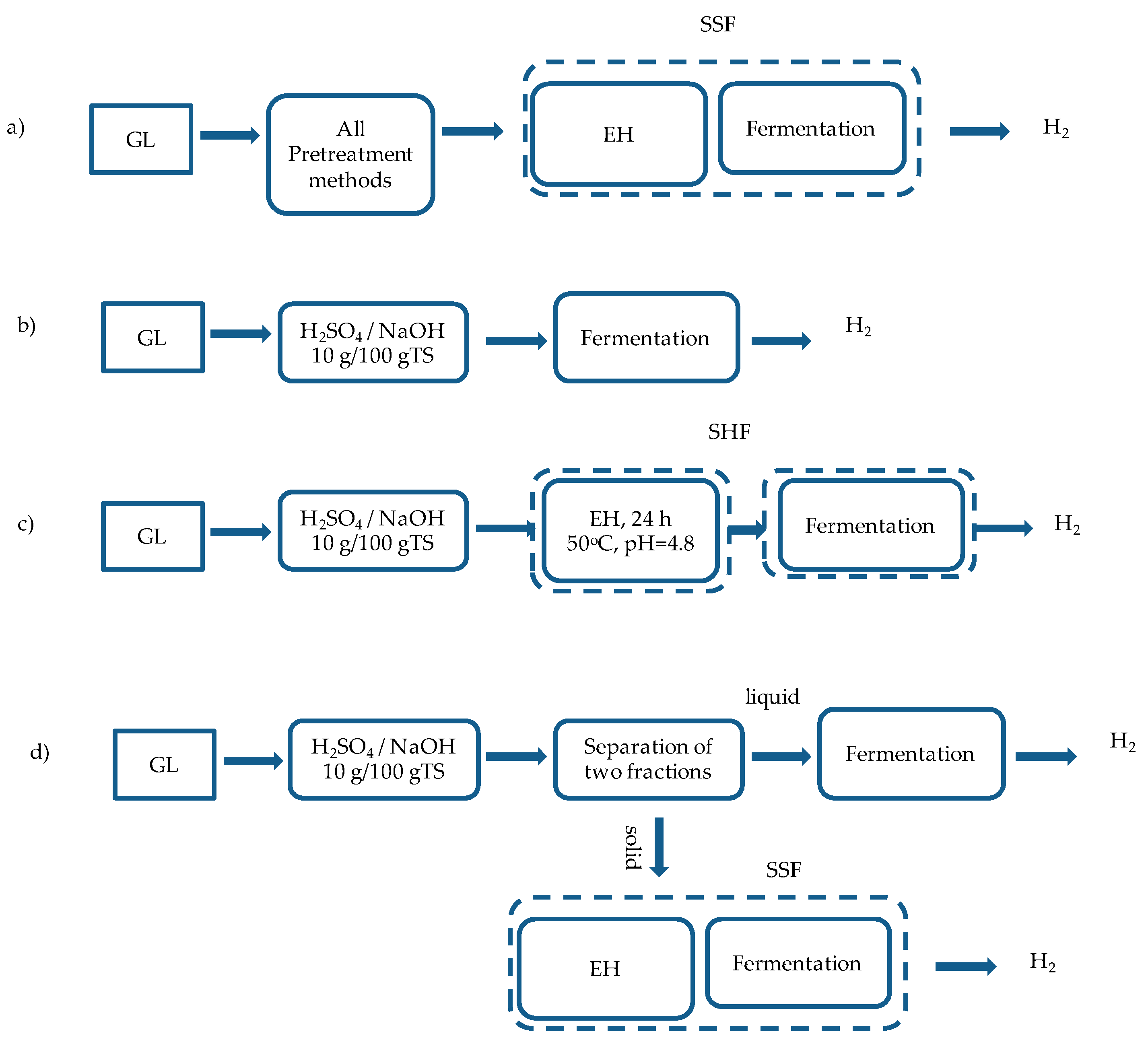
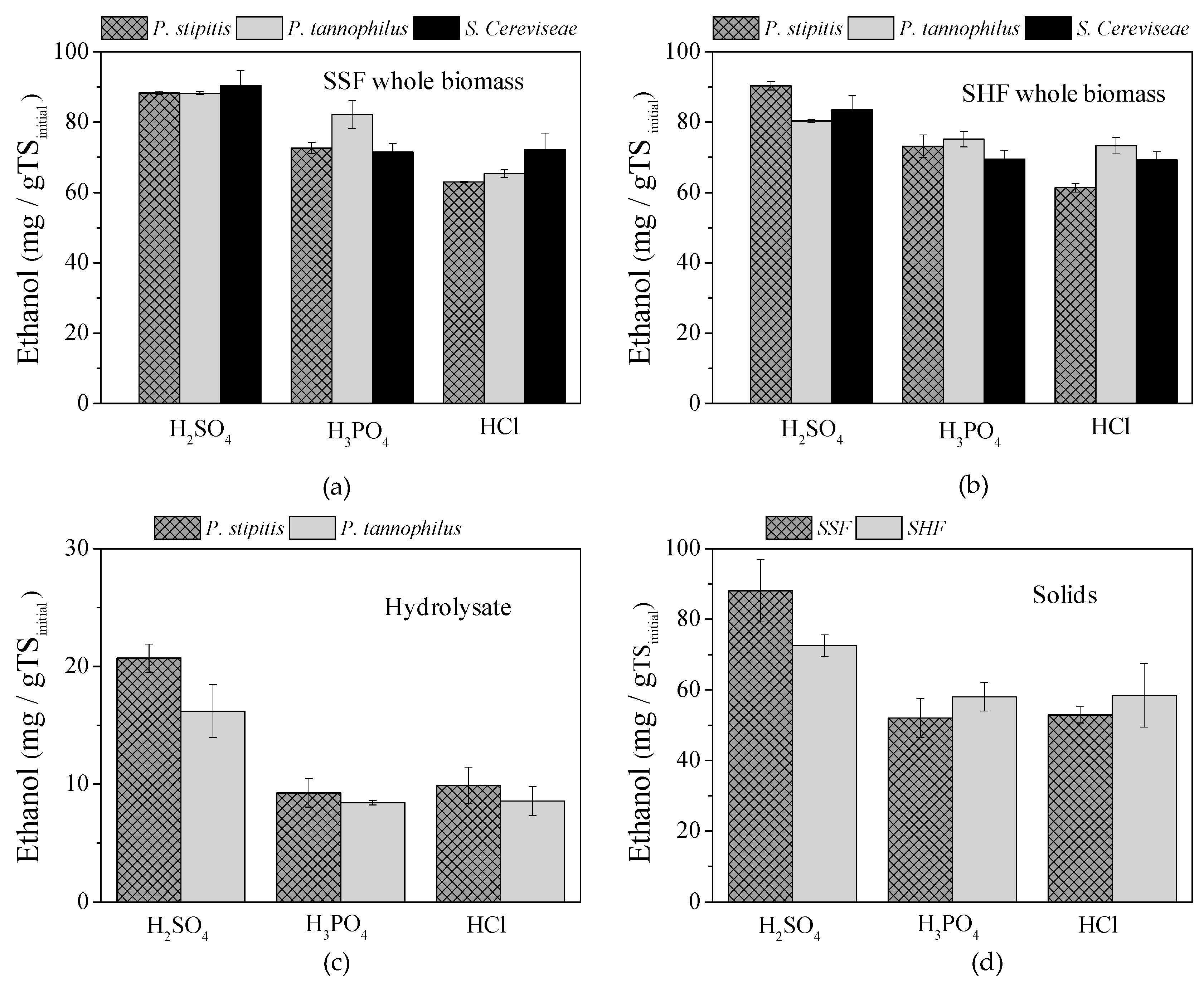
2.2.3. BHP for Different Hydrolysis and Fermentation Concepts
2.3. BP Experiments
2.3.1. BP of the Whole Pretreatment Slurry, at SSF
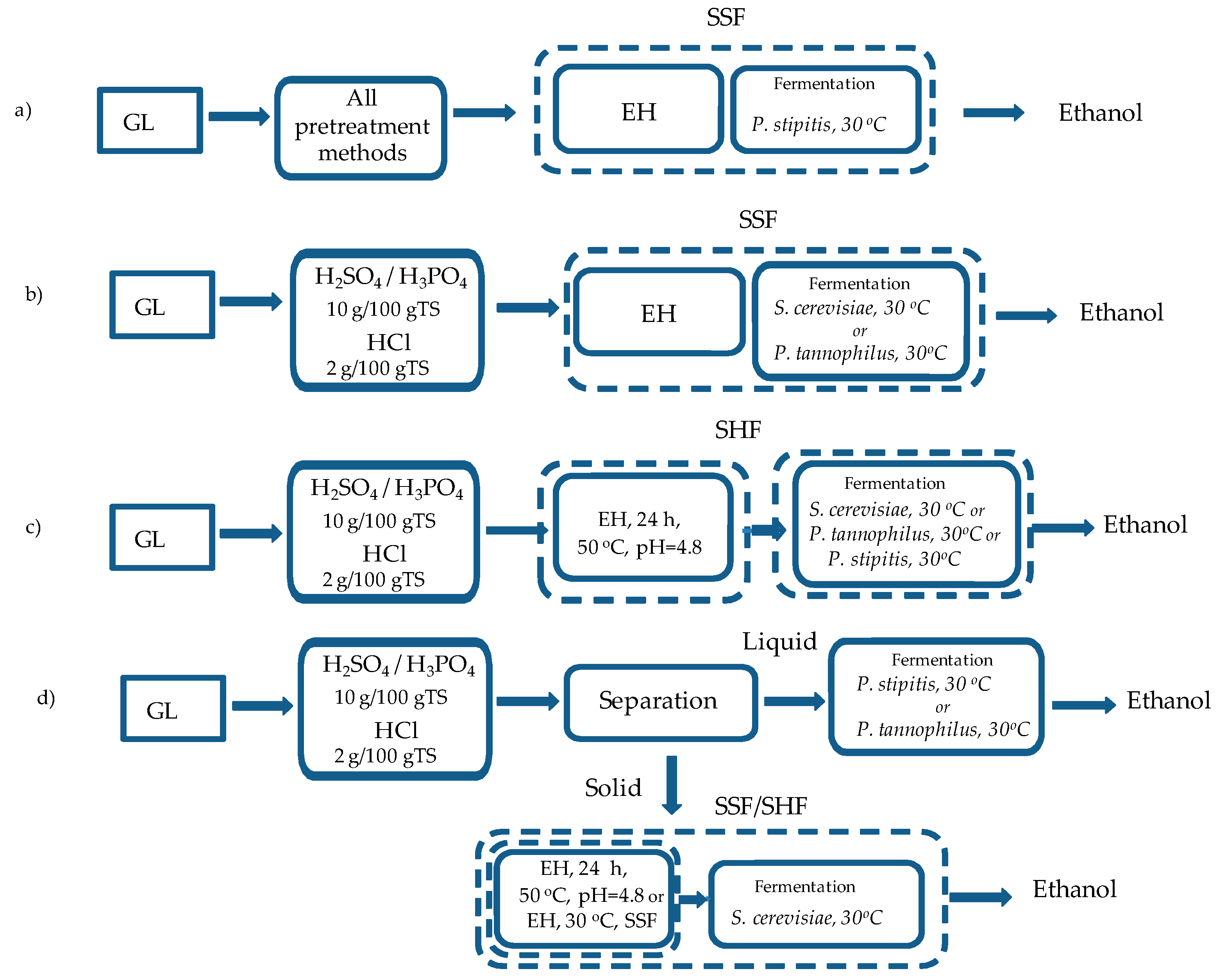
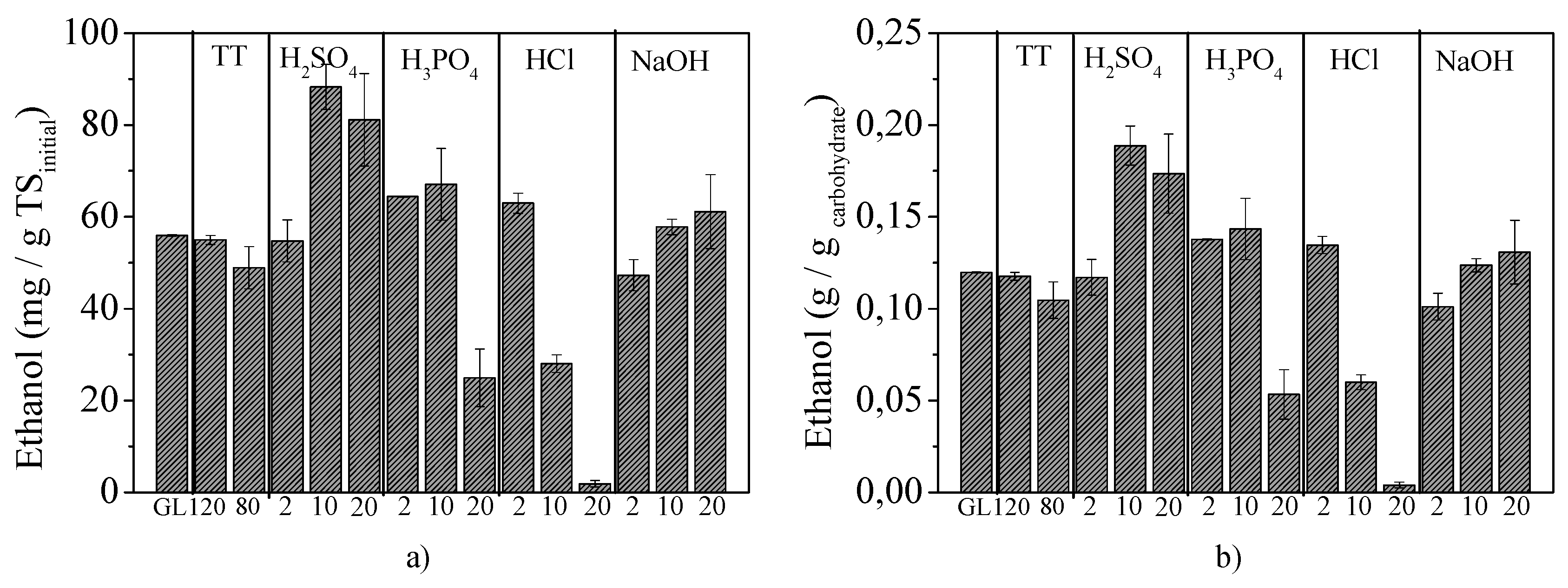
2.3.2. BP under Different Fermentation Concepts
2.4. Energy Recovery from Different Process Schemes
3. Materials and Methods
3.1. Biomass Used
3.2. Pretreatment Methods Used
3.3. BHP Experiments
3.3.1. Microbial Cultures
3.3.2. BHP Experiments
3.4. BP Experiments
3.4.1. Cultures and Media
3.4.2. Fermentation/Hydrolysis Experiments
3.5. Analytical Methods
3.6. Statistical Analysis
4. Conclusions
Funding
Conflicts of Interest
References
- Smullen, E.; Finnan, J.; Dowling, D.; Mulcahy, P. Bioconversion of switchgrass: Identification of a leading pretreatment option based on yield, cost and environmental impact. Renew. Energy 2017, 111, 638–645. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Vayenas, D.; Lyberatos, G. Ethanol and hydrogen production from sunflower straw: The effect of pretreatment on the whole slurry fermentation. Biochem. Eng. J. 2016, 116, 65–74. [Google Scholar] [CrossRef]
- Appiah-Nkansah, N.B.; Li, J.; Rooney, W.; Wang, W. A review of sweet sorghum as a viable renewable bioenergy crop and its techno-economic analysis. Renew. Energy 2019, 143, 1121–1132. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Angelopoulos, K.; Lyberatos, G. Biofuels generation from sweet sorghum: Fermentative hydrogen production and anaerobic digestion of the remaining biomass. Bioresour. Technol. 2008, 99, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, K.; Singh, A.; Singh, S.; Saxena, A.K.; Nain, L. Technological interventions for utilization of crop residues and weedy biomass for 2 s generation bio-ethanol production. Renew. Energy 2019, 132, 723–741. [Google Scholar] [CrossRef]
- Ben Atitallah, I.; Antonopoulou, G.; Ntaikou, I.; Alexandropoulou, M.; Narsi, M.; Mechichi, T.; Lyberatos, G. On the evaluation of different saccharification schemes for enhanced bioethanol production from potato peels waste via a newly isolated yeast strain of Wickerhamomyces anomalus. Bioresour. Technol. 2019, 289, 121614. [Google Scholar] [CrossRef]
- Ntaikou, I.; Menis, N.; Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. Valorization of kitchen biowaste for ethanol production via simultaneous saccharification and fermentation using co-cultures of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Bioresour. Technol. 2019, 263, 75–83. [Google Scholar] [CrossRef]
- Eisentraut, A. Sustainable Production of Second-Generation Biofuels: Potential and Perspectives in Major Economics and Developing Countries; International Energy Agency: Paris, France, 2010. [Google Scholar]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. The effect of aqueous ammonia soaking pretreatment on methane generation using different lignocellulosic biomasses. Waste Biomass Valor. 2015, 6, 281–291. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zou, Y.; Ying, H.; Chen, Y. Feasibility of ethanol production from expired rice by surface immobilization technology in a new type of packed bed pilot reactor. Renew. Energy 2020, 149, 321–328. [Google Scholar] [CrossRef]
- Cui, M.; Shen, J. Effects of acid and alkaline pretreatments on the biohydrogen production from grass by anaerobic dark fermentation. Int. J. Hydrogen Energy 2012, 37, 1120–1124. [Google Scholar] [CrossRef]
- Yana, X.; Cheng, J.-R.; Wang, Y.-T.; Zhu, M.-J. Enhanced lignin removal and enzymolysis efficiency of grass waste by hydrogen peroxide synergized dilute alkali pretreatment. Bioresour. Technol. 2020, 301, 122756. [Google Scholar]
- Antonopoulou, G.; Vayenas, D.; Lyberatos, G. Biogas production from physicochemically pretreated grass lawn waste: Comparison of different process schemes. Molecules 2020, 25, 296. [Google Scholar] [CrossRef] [PubMed]
- Inghels, D.; Dullaert, W.; Aghezzaf, E.H.; Heijungs, R. Towards optimal trade-offs between material and energy recovery for green waste. Waste Manag. 2019, 93, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Mishra, C.; Behera, S.S.; Thatoi, H. Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from grass biomass –a review. Renew. Sustain. Energy Rev. 2017, 78, 1007–1032. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Passos, F.; Affes, R.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of pretreatment strategies for the most common anaerobic digestion feedstocks: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Dimitrellos, G.; Beobide, A.S.; Vayenas, D.; Lyberatos, G. Chemical pretreatment of sunflower straw biomass: The effect on chemical composition and structural changes. Waste Biomass Valor. 2015, 6, 733–746. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Ntaikou, I.; Fragkou, E.; Lyberatos, G. Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J. Environ. Manag. 2017, 203, 704–713. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jin, Y.S.; Cha, Y.L.; Seo, J.H. Bioethanol production from cellulosic hydrolysates by engineered industrial Saccharomyces cerevisiae. Bioresour. Technol. 2017, 228, 355–361. [Google Scholar] [CrossRef]
- López-Abelairas, M.; Lu-Chau, T.A.; Lema, J.M. Fermentation of biologically pretreated wheat straw for ethanol production: Comparison of fermentative microorganisms and process configurations. Appl. Biochem. Biotechnol. 2013, 170, 1838–1852. [Google Scholar]
- Sanchez, G.; Pilcher, L.; Roslander, C.; Modig, T.; Galbe, M.; Liden, G. Dilute-acid hydrolysis for fermentation of the Bolivian straw material Paja Brava. Bioresour. Technol. 2004, 93, 249–256. [Google Scholar] [CrossRef]
- Jin, M.; Lau, M.W.; Balan, V.; Dale, B.E. Two-step SSCF to convert AFEX-treated switchgrass to ethanol using commercial enzymes and Saccharomyces cerevisiae 424A (LNH-ST). Bioresour. Technol. 2010, 101, 8171–8178. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Effectiveness of dilute oxalic acid pretreatment of Miscanthus× giganteus biomass for ethanol production. Biomass Bioenergy 2013, 59, 540–548. [Google Scholar] [CrossRef]
- Agbodike, T.C.; Ado, S.A.; Abdullahi, I.O. Bio-ethanol production from elephant grass using co-cultures of Aspergillus niger and Saccharomyces cerevisiae in simultaneous saccharification and fermentation. South Asian J. Exp. Biol. 2013, 3, 152–157. [Google Scholar]
- Mutreja, R.; Das, D.; Goyal, D.; Goyal, A. Bioconversion of agricultural waste to ethanol by SSF using recombinant cellulase from Clostridium thermocellum. Enzym. Res. 2011, 6, 340279. [Google Scholar]
- Yasuda, M.; Miura, A.; Shiragami, T.; Matsumoto, J.; Kamei, I.; Ishii, Y.; Ohta, K. Ethanol production from non-pretreated napier grass through a simultaneous saccharification and fermentation process followed by a pentose fermentation with Escherichia coli KO11. J. Biosci. Bioeng. 2012, 114, 188–192. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Ultrasound combined with dilute acid pretreatment of grass for improvement of fermentative hydrogen production. Bioresour. Technol. 2019, 275, 10–18. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Pretreatment of grass waste using combined ionizing radiation- acid treatment for enhancing fermentative hydrogen production. Bioresour. Technol. 2018, 255, 7–15. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Mudhoo, A.; Rene, E.R.; Saratale, G.D.; Kobayashi, T.; Xu, K.; Kim, S.H.; Kim, D.H. Fermentative hydrogen production using lignocellulose biomass: An overview of pre-treatment. Renew. Sustain. Energy Rev. 2017, 77, 28–42. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, S.C.; Antonopoulou, G.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. A Leptolyngbya-based microbial consortium for agro-industrial wastewaters treatment and biodiesel production. Environ. Sci. Pollut. Res. 2018, 25, 17957–17966. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Aemig, Q.; Trably, E.; Steyer, J.-P.; Carrère, H. Specific inhibition of biohydrogen producing Clostridium sp. after dilute-acid pretreatment of sunflower stalks. Int. J. Hydrogen Energy. 2013, 38, 12273–12282. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. Influence of pH on fermentative hydrogen production from sweet sorghum extract. Int. J. Hydrogen Energy. 2010, 35, 1921–1928. [Google Scholar] [CrossRef]
- Quemeneur, M.; Bittel, M.; Trably, E.; Dumas, C.; Fourage, L.; Ravot, G.; Steyer, J.-P.; Carrere, H. Effect of enzyme addition on fermentative hydrogen production from wheat straw. Int. J. Hydrogen Energy 2012, 37, 10639–10647. [Google Scholar] [CrossRef]
- Delgenes, J.P.; Moletta, R.; Navarro, J.M. Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis and Candida shehatae. Enzym. Microb. Technol. 1996, 19, 220–225. [Google Scholar] [CrossRef]
- Soares, L.B.; Bonan, C.I.D.G.; Biazi, L.E.; Dionísio, S.R.; Bonatelli, M.L.; Andrade, A.L.D.; Renzano, E.C.; Costa, A.C.; Ienczak, J.L. Investigation of hemicellulosic hydrolysate inhibitor resistance and fermentation strategies to overcome inhibition in non-saccharomyces species. Biomass Bioenergy 2020, 137, 105549. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Dien, B.S.; Berhow, M.A.; Kurtzman, C.P.; Gorsich, S.W. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J. Ind. Microbiol. Biotechnol. 2004, 31, 345–352. [Google Scholar] [CrossRef]
- Demiray, E.; Karatay, S.E.; Donmez, G. Evaluation of pomegranate peel in ethanol production by Saccharomyces cerevisiae and Pichia stipitis. Energy 2018, 159, 988–994. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, T.-S.; Guo, G.-L.; Huang, W.-S. Ethanol production from rice straw hydrolysates by Pichia stipitis. Energy Procedia 2012, 14, 1261–1266. [Google Scholar] [CrossRef][Green Version]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Second generation bioethanol production from Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hack. Bioresour. Technol. 2010, 101, 5358–5365. [Google Scholar] [CrossRef]
- Nandal, P.; Sharma, S.; Arora, A. Bioprospecting non-conventional yeasts for ethanol production from rice straw hydrolysate and their inhibitor tolerance. Renew. Energy 2020, 147, 1694–1703. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Alexandropoulou, M.; Ntaikou, I.; Lyberatos, G. From waste to fuel: Energy recovery from household food waste via its bioconversion to energy carriers based on microbiological processes. Sci. Total Environ. 2020, 732, 139230. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater; Franson, M.A., Ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Characteristic | Value |
|---|---|
| TS (%) | 92.2 ± 0.1 |
| VS (g/100 g TS) | 83.4 ± 0.1 |
| Cellulose (g/100 g TS) | 20.4 ± 0.1 |
| Hemicellulose (g/100 g TS) | 24.0 ± 2.0 |
| Lignin (g/100 g TS) | 12.3 ± 1.2 |
| Acid Insoluble lignin | 7.6 ± 0.4 |
| Soluble lignin | 4.7 ± 0.1 |
| Extractives (g/100 g TS) | 25.6 ± 3.1 |
| Proteins (g/100 g TS) | 10.5 ± 0.5 |
| Pretreatment Conditions | Reduction (%) | |||
|---|---|---|---|---|
| Lignin | Cellulose | Hemicellulose | ||
| Thermal | 80 °C | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.7 ± 0.2 |
| 120 °C | 0.0 ± 0.0 | 0.9 ± 0.1 | 0.8 ± 0.1 | |
| H2SO4 | 2 g/100 g TS | 0.0 ± 0.0 | 0.8 ± 0.0 | 4.7 ± 0.4 |
| 10 g/100 g TS | 0.0 ± 0.0 | 1.1 ± 0.2 | 54.1 ± 0.8 | |
| 20 g/100 g TS | 0.0 ± 0.0 | 3.4 ± 0.4 | 77.9 ± 1.4 | |
| H3PO4 | 2 g/100 g TS | 0.0 ± 0.0 | 3.5 ± 0.4 | 7.3 ± 0.3 |
| 10 g/100 g TS | 0.0 ± 0.0 | 4.5 ± 0.2 | 14.6 ± 0.5 | |
| 20 g/100 g TS | 0.0 ± 0.0 | 5.1 ± 0.7 | 33.8 ± 1.4 | |
| HCl | 2 g/100 g TS | 3.3 ± 0.3 | 0.0 ± 0.0 | 9.2 ± 0.5 |
| 10 g/100 g TS | 6.6 ± 0.4 | 0.0 ± 0.0 | 84.4 ± 1.2 | |
| 20 g/100 g TS | 6.8 ± 0.5 | 0.1 ± 0.0 | 93.4 ± 2.4 | |
| NaOH | 2 g/100 g TS | 16.7 ± 1.5 | 7.1 ± 0.4 | 10.3 ± 1.5 |
| 10 g/100 g TS | 61.7 ± 1.2 | 6.8 ± 0.2 | 23.5 ± 0.3 | |
| 20 g/100 g TS | 94.5 ± 1.4 | 5.4 ± 0.8 | 31.8 ± 0.5 | |
| Whole Biomass Slurry | Separated Fractions | |||||
|---|---|---|---|---|---|---|
| No Enzymes a | SSF b | SHF c | Liquid d | Solid-SSF d | Sum | |
| H2SO4 | 19.2 ± 0.3 | 230.7 ± 1.6 | 154.5 ± 18.8 c | 159.1 ± 12.8 | 111.1 ± 9.8 | 270.2 |
| NaOH | 11.2 ± 0.7 | 170.0 ± 10.7 | 100.6 ± 6.6 c | 85.7 ± 12.7 | 85.0 ± 6.7 | 170.7 |
| Untreated | 11.6 ± 1.2 | 81.8 ± 2.2 | 65.3 ± 5.4 c | n.t. e | n.t. e | |
| Whole Biomass Slurry | Separated Fractions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P. stipitis | P. tannophilus | S. cerevisiae | P. stipitis (Liquid) | P. tannophilus (Liquid) | ||||||
| SSF | SHF | SSF | SHF | SSF | SHF | Solid SSF a | Solid SHF a | Solid SSF a | Solid SHF a | |
| H2SO4 | 88.3 ± 0.5 | 90.3 ± 1.2 | 88.2 ± 0.4 | 80.3 ± 0.4 | 90.5 ± 4.2 | 83.5 ± 4.1 | 108.8 ± 8.6 | 92.6 ± 4.3 | 104.3 ± 9.6 | 88.8 ± 7.2 |
| H3PO4 | 72.6 ± 1.6 | 73.1 ± 3.3 | 82.1 ± 3.9 | 75.2 ± 2.2 | 71.5 ± 2.5 | 69.5 ± 2.5 | 61.3 ± 4.2 | 67.3 ± 3.9 | 60.5 ± 2.5 | 66.5 ± 2.8 |
| HCl | 63.0 ± 0.2 | 61.3 ± 1.2 | 65.3 ± 1.1 | 73.3 ± 2.4 | 72.3 ± 4.6 | 69.3 ± 2.3 | 62.8 ± 3.9 | 68.4 ± 5.9 | 61.5 ± 3.7 | 67.0 ± 5.4 |
| Scheme | Pretreatment, Biofuel, Conditions | Energy (MJ/kg TS) | Source |
|---|---|---|---|
| Whole biomass | 10 g/100 g TS H2SO4, H2, SSF | 2.9 | This study |
| 10 g/100 g TS H2SO4, Ethanol, SSF (S. cerevisiae) | 2.4 | This study | |
| 20 g/100 g TS NaOH, CH4 | 13.7 | [13] | |
| Separation of fractions | 10 g/100 g TS H2SO4, H2 | 3.4 | This study |
| 10 g/100 g TS H2SO4, Ethanol (P. stipitis/solids—SSF) | 2.9 | This study | |
| 20 g/100 g TS NaOH, CH4 | 14.1 | [13] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonopoulou, G. Designing Efficient Processes for Sustainable Bioethanol and Bio-Hydrogen Production from Grass Lawn Waste. Molecules 2020, 25, 2889. https://doi.org/10.3390/molecules25122889
Antonopoulou G. Designing Efficient Processes for Sustainable Bioethanol and Bio-Hydrogen Production from Grass Lawn Waste. Molecules. 2020; 25(12):2889. https://doi.org/10.3390/molecules25122889
Chicago/Turabian StyleAntonopoulou, Georgia. 2020. "Designing Efficient Processes for Sustainable Bioethanol and Bio-Hydrogen Production from Grass Lawn Waste" Molecules 25, no. 12: 2889. https://doi.org/10.3390/molecules25122889
APA StyleAntonopoulou, G. (2020). Designing Efficient Processes for Sustainable Bioethanol and Bio-Hydrogen Production from Grass Lawn Waste. Molecules, 25(12), 2889. https://doi.org/10.3390/molecules25122889






