Abstract
It is well known that terrestrial environments host an immense microbial biodiversity. Exposed to different types of stress, such as UV radiation, temperature fluctuations, water availability and the inter- / intra-specific competition for resources, terrestrial microorganisms have been evolved to produce a large spectrum of bioactive molecules. Bacteria, archaea, protists, fungi and algae have shown a high potential of producing biomolecules for pharmaceutical or other industrial purposes as they combine a sustainable, relatively low-cost and fast-production process. Herein, we provide an overview of the different bioactive molecules produced by terrestrial microorganisms with skin protecting applications. The high content in polyphenolic and carotenoid compounds produced by several strains, as well as the presence of exopolysaccharides, melanins, indole and pyrrole derivatives, mycosporines, carboxylic acids and other molecules, are discussed in the context of their antioxidant, photo-protective and skin-whitening activity. Relevant biotechnological tools developed for the enhanced production of high added value natural products, as well as the protecting effect of some antioxidant, hydrolytic and degrading enzymes are also discussed. Furthermore, we describe classes of microbial compounds that are used or have the potential to be used as antimicrobials, moisturizers, biosurfactants, pigments, flavorings and fragrances.
1. Introduction
Microorganisms are extremely diverse organisms, including bacteria, archaea, protists, fungi and algae. In recent decades, there has been great progress on exploiting the immense chemical diversity available from the abundant microbial world [1,2]. After the discovery of the fungal metabolite penicillin in 1928, which was the beginning of the golden age of microbial-derived natural products and pharmaceuticals, treatments for fungal and parasitic infections as well as for several types of cancers followed [3]. In the forties and early fifties, almost all groups of important antibacterial antibiotics (tetracyclines, cephalosporins, aminoglycosides, macrolides) were discovered, while in the fifties and sixties, antitumor, antiviral and non-antibiotic-enzyme-inhibitory-metabolites were isolated, mainly from Streptomyces species [4].
The successful and wide utilization of microbial metabolites in various therapeutic areas (e.g., cyclosporine as immunosuppressant, doxorubicin as anticancer, and statins as cardio protective agents), as well as the wide application in livestock and agriculture (e.g., the antiparasitic avermectin, the feed additive monensin and the herbicide glufosinate) [5] were important features for broadening the research of bioactive microbial products in other sectors. In fact, in the last decade, microorganisms have attracted a great deal of attention as potential leading producers of promising compounds for cosmetic and/or cosmeceutical purposes [6]. Among these compounds, polyphenols, quinones and aldehydes have been reported in several studies as functional active ingredients for the maintenance of skin homeostasis (e.g., antioxidants, UV protecting, skin whitening) as well as coloring, flavoring, stabilizing and antibacterial agents [2].
Among the various environmental factors affecting skin homeostasis, ultraviolet (UV) irradiation is the most dangerous component, as it can cross the epidermis and reach the upper dermis. Additional parameters that affect all skin layers and thus contribute to skin aging are dietary (e.g., high fat diet) and lifestyle habits (e.g., smoking), various air pollutants, as well as internal factors such as metabolism, hormones, inflammatory processes, etc. [7,8,9] (Figure 1). Damaging agents modulate numerous molecular events and signaling pathways that (among others) lead to mitochondrial dysfunction, increased genome and proteome damage; increased synthesis and activity of matrix metalloproteases, decreased collagen production, triggering of stress-induced premature senescence (SIPS) and accumulation of the inflammatory senescence-associated secretory phenotype (SASP) [8,9,10,11]. The maintenance of a highly effective intra- or extracellular defense system capable of protecting against the adverse effects of irradiation and other stressors is crucial for safeguarding skin homeostasis. Adverse effects are macroscopically characterized by the loss of skin tone and an increase of wrinkles, dehydration (due to increased epidermal thickness), hyperpigmentation and sallowness (yellowing or pale tinted skin) (Figure 1).
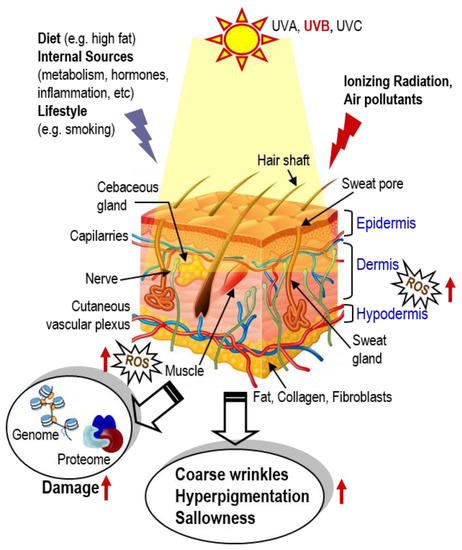
Figure 1.
During lifetime, skin is exposed to numerous environmental stressors such as UVA and UVB, ionizing radiation and air pollutants, as well as to stressors that may originate from diet (e.g., high fat diets), internal sources (e.g., metabolism or tissue inflammation) and/or lifestyle (e.g., smoking). While young, these stressors are effectively neutralized by cell protective mechanisms (e.g., the antioxidant transcription factor Nrf2). During aging, defenses are compromised, resulting in accumulating ROS, genome and proteome damage; damage of biomolecules then disrupts normal cell signaling and homeodynamics, resulting in (among others) coarse wrinkles, hyperpigmentation and skin shallowness.
Despite the large number of individual studies and evidences for the potential use of terrestrial microorganisms in the fast-growing cosmetic sector, so far no systematic review has addressed their applications. It is worth mentioning that the global market for cosmetic and cosmeceutical products was valued at USD 532.4 billion in 2017, and is expected to reach a market value of USD 805.6 billion by 2023, registering a CAGR (Compound Annual Growth Rate) of 7.14% during 2018–2023 [12].
In the current study, we provide an overview of the different bioactive compounds with skin protecting effect (and thus of cosmetic and cosmeceutical interest) isolated, from a broad range of terrestrial microorganisms including bacteria, algae, fungi and protists. Examples of biomolecules with skin protecting interest that are heterologously produced and/or biotransformed are included. The term “terrestrial” encompasses microorganisms from soil and freshwater, plant endophytes, and lichens. Marine microorganisms and mushrooms (all Basidiomycota and Ascomycota) are excluded, as they have been recently reviewed [2,13]. Representative bioactive compounds from terrestrial microorganisms with antioxidant, photo-protective, and skin-whitening activity, along with antimicrobial and moisturizing agents, pigments, fragrances and flavors are discussed. A detailed table including bioactive molecules, the source organisms and their habitat, the biological activity, as well as their presence in the list of cosmetic substances and ingredients of the European Union (CosIng inventory [14]) is provided.
2. Antioxidants
Oxidative stress is one of the prevailing causes of skin aging due to increased production and/or accumulation of Reactive Oxygen (ROS) and Nitrogen Species (RNS). Imbalance between their production and the endogenous antioxidant defense mechanisms may result in cellular oxidative stress, causing wrinkling, drying, photo-aging, pigmentation and elastosis of the skin. In addition, accumulation of free radicals may be responsible for cutaneous inflammation and skin cancer [15].
Reactive oxygen species (ROS) are formed as either by-products of normal metabolism (e.g., mitochondrial oxidative phosphorylation), as well by NAD(P)H oxidases, or by exogenous sources such as atmospheric pollutants, UV light, X- or gamma-rays [16]; if their concentration exceeds the cellular antioxidant capacity, ROS cause oxidative stress and damage to all cellular biomolecules [10].
Topical antioxidant products could act as scavengers of reactive species, inhibiting the initiation of chain reactions, responsible for cellular oxidative stress [17]. Many reports have demonstrated the ability of marine microorganisms to biosynthesize antioxidant compounds [2]. Concerning terrestrial microbes, compounds with a significant inhibition of oxidation reactions, like polyphenols, carotenoids or exopolysaccharides, are extensively discussed in the following sections.
Bioassays involving the neutralization of different radicals such as the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH), the cation radical 2,2′-azino-bis-3-ethylbenzotiazolin-6-sulfonic acid (ABTS), as well as the hydroxyl and nitric oxide radicals are widely applied for determining the in vitro antioxidant potential. Even if the relation to the in vivo antioxidant efficacy was not clearly described, the measured antioxidant activity can give an estimation of the amount of the compounds that can be oxidized under conditions of the assays [18].
2.1. Phenolic Compounds
Phenolic compounds are well known for their strong antioxidant and radical scavenging activity, as well as for their interaction with different pharmacological targets. The strong correlation between the microbial phenolic content and the antioxidant activity has been shown by several authors using different microorganisms. Huang et al. confirmed this positive correlation during their investigation of fungal endophytes isolated from medicinal Chinese plants [19]. The strong contribution to the antioxidant activity was also confirmed in Aspergillus austroafricanus, an endophytic fungus isolated from Zingiber officinale rhizome. HPLC analysis of the crude extract showed mainly the presence of hydroxycinnamic acids such as ferulic acid (1), p-coumaric acid (2) and cinnamic acid (3) [20] (Figure 2). Those molecules are well known in the plant kingdom and have been extensively studied for their antioxidant capacity (Table 1).
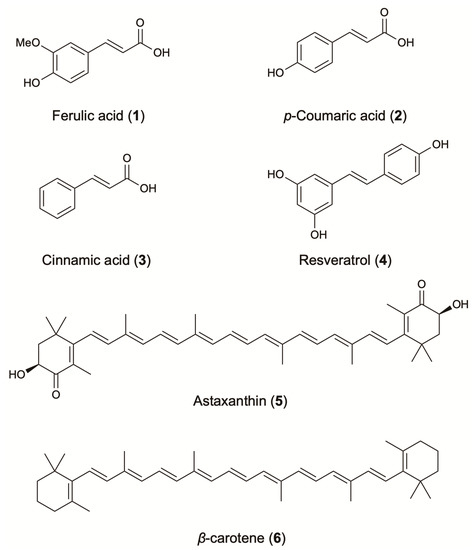
Figure 2.
Antioxidant agents from terrestrial microorganisms.

Table 1.
Bioactive molecules produced by terrestrial microorganisms.
Similar conclusions were drawn using cultures of the microalgae Arthrospira platensis [21] and of other Arthrospira sp. [22,23]. Simple phenolics and hydroxycinnamic acids, such as gallic acid, chlorogenic acid, ferulic acid, and caffeic acid have been isolated from different species of microalgae e.g Chlorella vulgaris, Haematococcus pluvialis, Diacronema lutheri, Phaeodactylum tricornutum, Tetraselmis suecica, Ankistrodesmus sp., Spirogyra sp., Euglena cantabrica, Caespitella pascheri, and Porphyridium purpureum [24,25,26].
Studies on terrestrial cyanobacterial species from the genera Anabaena, Nostoc, Nodularia, Microcheate, Oscillatoria, Synechocystis, Hapalosiphon, Mastigocladus, Scytonema, Westiellopsis, Cylindrospermum, Aulosira, Chroococcus, Lyngbya, Calothrix, Dichothrix, Phormidiochaete, Limnothrix and Phormidium have also reported the correlation of their antioxidant activity with their total phenolic content. Chlorogenic and gallic acid were identified as main phenolics in several cyanobacterial species, with Dichothrix sp. being one the most efficient producer of those compounds (77.9 µg/g and 24.4 µg/g fresh weight, respectively) [26].
Resveratrol (4), another well-known natural compound produced by plants, has recently been reported from endophytes isolated from grapevine varieties [27,28]. It is considered one of the most famous compounds for its unique anti-aging properties (Figure 2). It has been widely reported to be a strong inhibitor of ROS production and protein oxidation and a more effective agent than vitamins E and C against lipid peroxidation [29]. Microorganisms have been successfully considered for the production of resveratrol, since its synthesis and/or its extraction from plants is considered inefficient due to high requirements of organic solvents, biomass and low final yield. Resveratrol was first industrially produced in 2009, using Saccharomyces cerevisiae. Since this development, different methods such as bioconversion and genetic engineering have been used in order to obtain higher yields. For instance, resveratrol has been produced by Alternaria sp. (1.4 μg/L), and by genetically modified S. cerevisiae (531.4 mg/L), and E. coli (2370 mg/L) [30]. The molecules discussed, as well as additional microbial phenolic compounds that have antioxidant or other related biological activities, are presented in Table 1.
2.2. Carotenoids
Carotenoids are the most common natural pigments; they are well known for their powerful antioxidant activity, as they are very efficient physical quenchers of singlet oxygen and scavengers of other ROS. Carotenoids are also well known for their capacity to act as quenchers of photosensitization products, giving them photo-protective properties [109].
In the last decade, the interest in microbial fermentation for the production of natural carotenoids has increased. Carotenoid production by bacteria, asporogenous yeasts, filamentous fungi [110] and microalgae [111] has been extensively reported, with cyanobacteria to be the most prominent source [112]. Accordingly, carotenogenic microbes Xanthophyllomyces dendrorhous, Blakeslea trispora, and Haematococcus pluvialis have been widely used in large-scale processes. Furthermore, the transformation of the non-carotenogenic microbes E. coli, S. cerevisiae, Candida utilis, and Zymomonas mobilis, with carotenoid genes from selected microbes has been successfully applied for the production of carotenoids [113]. In fact, E. coli in fed-batch fermentation produced 72.6 mg/g cdw (cell dry weight) of β-carotene [1] and 1.44 g/L of lycopene [49], while astaxanthin production was enhanced 1.4-fold compared to the X. dendrorhous parental strain, reaching 1.25 mg/L (Table 1) [46]. Astaxanthin (5), β-carotene (6) and lutein are the carotenoids with the highest added value (Figure 2) [114]. The oxycarotenoid lutein is mainly produced by microalgae of the genus Chlorella, Dunaliella, and Haematococcus [114]. Its profound effect on the antioxidant defense system is attributed to its chemical structure. In in vitro systems, it significantly scavenged the superoxide (IC50: 21 μg/mL), the hydroxyl (IC50: 1.75 μg/mL), the nitric oxide (IC50: 3.8 μg/mL), and the DPPH (IC50: 35 μg/mL) radical and inhibited lipid peroxidation (2.2 μg/mL). In in vivo systems, it has been proved to be an effective scavenger of superoxide radical (IC50: 21 μg/mL) [51].
2.3. Exopolysaccharides (EPSs)
EPSs are high-molecular-weight carbohydrate polymers demonstrating strong scavenging activities, metal chelating ability and lipid peroxidation inhibition. These compounds are among the most exploited bioactive substances for their anti-aging capacity [115].
EPSs are mainly biosynthesized by bacteria and fungi. The ability of a microorganism to produce antioxidant EPSs was first introduced with the study of Paenibacillus polymyxa. This endophytic bacterium, isolated from the root of Stemona japonica, produces different EPSs with strong scavenging activity against the superoxide and the hydroxyl radical [116,117] (Table 1). When tested at a concentration of 1 mg/mL, the scavenging effect of the crude EPS against the superoxide radical was 74.38%, while the activity of the purified EPS-1 and EPS-2 was higher than ascorbic acid. At the same concentration, EPS, EPS-1, EPS-2 were also very effective against the hydroxyl radical [56]. EPS-1 and EPS-2 were composed of mannose, fructose and glucose in molar ratios of 2.6:29.8:1 and 4.2:36.6:1, respectively. Since this discovery, many endophytes were found to produce antioxidant EPSs. A characteristic case is the purified rhamno-galactan fraction of Fusarium solani and Bacillus cereus isolated from Alstonia scholaris and Artemisia annua L., respectively. This EPS fraction showed a significant scavenging activity against the DPPH, (IC50:0.6 mg/mL), the superoxide (IC50: 2.6 mg/mL) and the hydroxyl radical (IC50: 3.1 mg/mL) [54,55].
Optimization of cultivation parameters of P. polymyxa using sucrose, yeast extract and CaCl2 showed an EPSs yield of 35.26 g/L (18.74%), which was 1.55-fold higher compared to the original medium [57]. EPSs structures are in a great variety. EPSs isolated from the culture medium of the endophytic fungus Aspergillus sp. were mainly composed of mannose and galactose (89.4:10.6) [59], while EPSs isolated from the endophytic bacteria Burkholderia tropica were mainly composed of rhamnose, glucose and glucuronic acid (2:2:1) [60]. Antioxidant EPSs have also been isolated from the terrestrial microalgae Rhodella reticulata. Its extracellular polysaccharides showed strong antioxidant activity, significantly higher than α-tocopherol. The radical scavenging ability against the superoxide radical of the deproteinized extracellular polysaccharide reached 328.48 U/L, compared to 174.03 U/L of α-tocopherol [118].
2.4. Enzymes
Enzymes are produced by microorganisms as a primary cell protective detoxification mechanism (e.g., from ROS) as they catalyze the removal of ROS through the formation of less reactive molecules such as oxygen or water. Superoxide dismutases, catalases, and peroxidases are involved in these mechanisms.
Superoxide dismutases (SODs) catalyze the neutralization of two superoxide radicals by the addition of two hydrogen ions to form hydrogen peroxide and oxygen. Belonging to the family of metalloisozymes, SODs are differentiated in their metal cofactor: Ni-SOD, CuZn-SOD, Fe-SOD and Mn-SOD; the last three are commonly found in microalgae. SOD biosynthesis is directly correlated with level of cellular ROS. In fact, a study carried out on microalgae Scenedesmus vacuolatus and Pinnularia viridis showed that the concentration and SOD activity is correlated with ROS related stress [119,120]. Similarly, the elimination of ROS in most Streptococcus and Lactococcus bacterial spp., conforms to this general antioxidant defense system since both genera express MnSOD. However, these bacteria possess only one type of SOD, namely the Mn-containing enzyme (MnSOD), rendering this enzyme an essential part of the antioxidant cell machinery [121].
Catalases contain porphyrin heme active sites that degrade hydrogen peroxide into water and oxygen [119]. One molecule of catalase is able to convert six billion molecules of hydrogen peroxide each minute [122]. In the yeast S. cerevisiae, the overexpression of catalase reduces lactic acid-induced oxidative stress [123]. Furthermore, a study involving the single-cell green alga Chlamydomonas reinhardtii showed that hydrogen peroxide from the media was faster degraded when the catalase inhibitor aminotriazole was absent; thus, catalase is one of the major enzymes involved in ROS detoxification [124].
Finally, peroxidases catalyze the oxidation of several substrates by hydrogen peroxide. Ascorbate, cytochrome C, pyrogallol, and glutathione are examples of these substrates. As for the other antioxidant enzymes, the induction of peroxidases activity upon ROS accumulation seem to be concentration- and time-dependent [119].
3. Photo–Protective Agents
Ultraviolet A (UVA, 315–400 nm) and ultraviolet B (UVB, 280–315 nm) play a major role in skin cell damage. UVA is mainly involved in the creation of ROS while UVB heavily affects DNA and proteins integrity. To protect themselves against UV radiation, terrestrial microorganisms have developed several strategies, one of which is the accumulation of photo-protective compounds [2].
Despite the evidence that several compounds from microorganisms have photo-protective activities, there has been surprisingly little work carried out involving in vivo skin models. This might be partially explained by the fact that the EU has banned the in vivo testing of cosmetics since 2013. Thus, potential skin protecting effects have been established based on existing in vitro studies [125].
3.1. Melanins
Bacteria, fungi and protists are able to produce a diverse group of pigments. Melanized fungi are mostly black yeasts, and melanized bacteria belong mainly to Actinobacteria [126].
The basic role of melanins in microorganisms are still a matter of controversy and speculation. The fact that these compounds are interceptors of UV photons leads to a lower vulnerability of micro-ecosystems to UV radiation. Melanins are also involved in energy production due to their ability to accept electrons. Finally, in some pathogenic microorganisms, these compounds act like virulence factors, lowering the defense mechanisms of the host [127].
The term melanin encompasses three polymeric substances; eumelanins, pheomelanins and allomelanins. Bacteria contain mostly eumelanins and allomelanins, whereas fungi mostly express allomelanins [126]. Fungal melanins have been isolated from Cryptococcus neoformans, Candida albicans, Aspergillus sp., Sporothrix schenckii, Fonsecaea pedrosoi, Paracoccidioides brasiliensis, Coccidioides sp., and Histoplasma capsulatum [128]. Melanins are also widespread in a variety of bacteria, like E. coli, B. cereus, Klebsiella sp., Pseudomonas aeruginosa, Pseudomonas stutzeri, Bacillus thuringiensis, Vibrio cholera and Streptomyces kathirae [129]; the last has been selected as an ideal microorganism for melanin production. Under optimal conditions, the yield was maximized at 13.7 g/L. In that study S. kathirae was identified as an excellent candidate for industrial-scale production of melanins [67].
3.2. Indole and Pyrrole Derivatives
Scytonemin (7) is a yellow to brown alkaloid pigment composed of an indolic and a phenolic subunit. Until now, only four different derivatives have been reported: dimethoxyscytonemin (8), scytonin (9), scytonemin-3a-imine (10) and tetramethoxyscytonemin (11) (Figure 3). Known for their strong UV-absorbing function and free radical scavenging capacity, scytonemin and its derivatives are excellent candidates for skin protecting purposes. Scytonemin prevents up to 90% of solar UV radiations from entering the cell. The strong radical scavenging activity of this compound (IC50: 36 µM against the ABTS radical), combined with its localization in the bacterial cell wall explains its protective role and the inability of UV-A radiation to cross the cellular envelope [130,131].
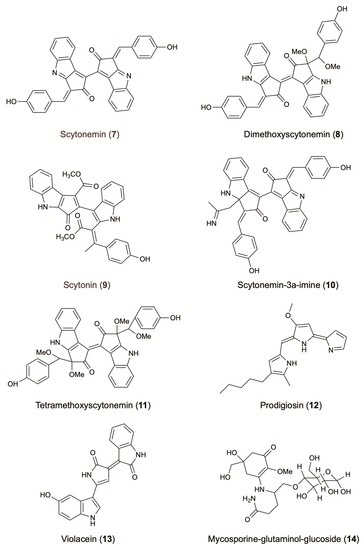
Figure 3.
Photo-protective agents from terrestrial microorganisms.
Almost exclusively synthesized by cyanobacteria from extreme environments, scytonemin (7) has been described in more than 300 cyanobacterial species, many of them terrestrial; e.g., Nostoc commune, Nostoc microscopicum, Phormidium sp. and Pleurocapsa sp. Scytonemin is also found in Scytonema hoffmani together with dimethoxyscytonemin (8), tetramethoxyscytonemin (11) and scytonin (9) [132]. To induce scytonemin (7) biosynthesis, modulation of temperature or photo-oxidative stress has to be combined with osmotic stress and periodic desiccation [126]. For industrial applications, the production of the UV-protecting scytonemin has been optimized in N. commune to yield 758 µg/g [73] (Table 1).
Prodigiosin (12) is characterized by a common pyrrolyl dipyrromethene skeleton containing a 4-methoxy-2,2′-bipyrrole ring system (Figure 3). This red pigment is mainly produced by strains belonging to the bacterial genus Serratia [75]. Well known for its antimalarial, antibacterial, and anticancer activity, prodigiosin has also demonstrated UV protective activity. When used as an additive in commercial sunscreens (4% w/w prodigiosin), the sunscreen protection factors (SPF) increased by 20–65%. In the same study, addition of 4% (w/w) of prodigiosin in photo-protective leaf extracts of Aloe vera and Cucumis sativus fruits showed an increasing of SPFs up to 3.5 orders of magnitude [133]. Bacteria Pseudomonas magneslorubra, Vibrio psychroerythrous, Vibrio gazogenes, Alteromonas rubra, and Rugamonas rubra, along with actinomycetes, such as Streptomyces rubrireticuli and S. longisporus ruber, have been studied for their capacity to produce prodigiosin or its derivatives [133]. Improvement in the production of prodigiosin (277 mg/L) was reported by the addition of a ram horn peptone (RHP, 0.4% w/v) in the culture media of S. marcescens MO-1 [75] (Table 1).
Violacein (13) is a purple pigment that presents an unusual structure consisting of a 2-pyrollidone and an oxindole ring system connected by a double bond, and a 5-hydroxyindole unit (Figure 3) [134]. Known to possess antibacterial effects against Staphylococcus aureus and other Gram-positive pathogens, violacein can also act as a photo-protective agent against UV irradiation. This compound absorbs at visible wavelengths and presents a broad absorption band extended out to 700 nm [69]. When used as an additive in commercial sunscreens (4% w/w violacein), the SPFs increased by 10–22%. Furthermore, the addition at 4% (w/w) of violacein in photo-protective extracts of A. vera leaves and C. sativus fruits, showed an increasing of SPFs up to 3.5 orders of magnitude [133]. Violacein is mainly produced by the bacterial strains Janthinobacterium lividum, Pseudoalteromonas sp. and Chromobacterium violaceum (Table 1). It is worth mentioning that the medium pH, culture volume, concentration of potassium nitrate, and L-tryptophan, affect significantly violacein production. In fact, the cultivation of C. violaceum, isolated from various plant waste sources, in a medium supplemented with sugar bagasse and L-tryptophan 10% (v/v), increased the final yield production of violacein to 0.82 g/L [70]. Similarly, optimized cultivation parameters of Duganella sp. increased by 4.8 folds the final yield of crude violacein (1.62 g/L) [71].
3.3. Mycosporines and Mycosporine-Like Amino Acids (MAAs)
Originally detected in the mycelia of terrestrial basidiomycetes, mycosporines present a central cyclohexenone or cyclohexenimine ring and a wide variety of substitutions. Mycosporine-like amino acids are imine derivate of mycosporines. The ring absorbs UV light and dissipates energy as heat, without generating ROS. Cyanobacteria and microalgae can synthesize mycosporines and MAAs, while fungi produce only mycosporines [126] (Table 1).
Mainly known for their photo-protective activity, MAAs are also efficient antioxidants and scavengers of ROS. These activities have led to several patents in the research of natural UV filters [135].
As in other cases, the production of microbial MAAs can be optimized following modification of culturing parameters. Khosravi et al. showed that the combination of UV irradiation and elevated salinity significantly increase the bioaccumulation of MAAs [136]. Indeed, the exposure of terrestrial fungi to UV radiation, desiccation and nutrient scarcity significantly increased the production of the UV-absorbing compound mycosporine-glutaminol-glucoside (14) (Figure 3) [137].
4. Skin-Whitening Agents
Skin-whitening agents are commercially available for cosmetic and clinical purposes, to obtain lighter skin complexion and treat hyperpigmentary disorders [138]. Uneven pigmentation of the skin may lead to blotches, patches of brown to grey discoloration or freckling which may require cosmetic interventions [13]. Whitening agents act at various levels of melanin production of the skin, either by inhibiting the activity of tyrosinase, the key enzyme in melanogenesis in plants and animals, or by inhibiting the transport of melanosomes from melanocytes to surrounding keratinocytes [139,140,141].
4.1. Pyrones
Kojic acid (15) is an inexpensive water-soluble fungal secondary metabolite (Figure 4). It has two OH- groups, the primary at C-7 and the secondary at C-5, which is essential to the radical scavenging and tyrosinase interference activity (IC50: 14 µM) [142,143]. The skin depigmenting activity of kojic acid results from the inhibition of the creolase and catecholase activities of tyrosinase. It prevents the conversion of the O-quinone to DL-DOPA and dopamine to its corresponding melanin. Decreased melanin content is demonstrated in melanocytes after their treatment with kojic acid [143]. This compound has been extensively used for skin depigmentation (and consequently as a cosmetic agent) with an excellent whitening effect, due to its ability to inhibit tyrosinase activity.
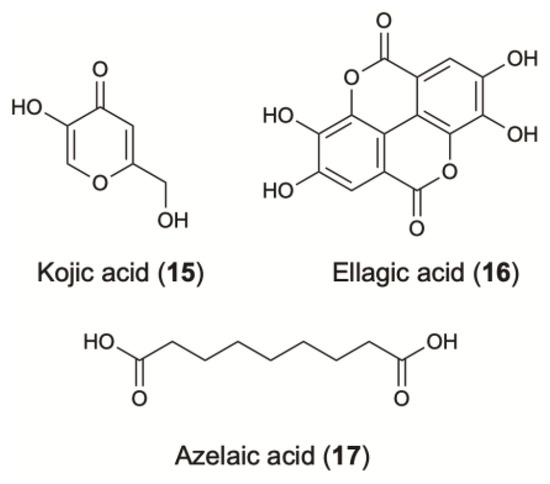
Figure 4.
Skin-whitening agents from terrestrial microorganisms.
Mainly produced by Penicillium sp. and Acetobacter sp., kojic acid has also been isolated from other terrestrial microorganisms, such as Aspergillus flavus, an endophytic fungus of Vigna unguiculata [81]. To produce this compound, fermentation of Aspergillus sp., is widely used. Other strains are also commonly employed, such as A. oryzae (0.26 g kojic acid/g glucose), A. parasiticus (0.089 g/g glucose) and A. candidus (0.3 g/g sucrose). A high yield of 0.453 g/g glucose was obtained with the culture of A. flavus [82,83,144] (Table 1).
4.2. Phenolic Lactones
Ellagic acid (16) is an antioxidant polyphenol that has generated commercial interest due to recommendations for topical use as a skin-whitening agent (Figure 4). This compound inhibits melanogenesis via the chemical reduction of O-quinones (O-dopaquinone) and semiquinones [145].
Ellagic acid can be produced from plant tannins via fermentation using different A. niger strains [146,147]. A yield of 6.3 and 4.6 mg of ellagic acid/g of dried pomegranate husk were obtained by converting of pomegranate ellagitannins into ellagic acid in a solid state fermentation [85] (Table 1).
4.3. Carboxylic Acids
Azelaic acid (17) is a saturated dicarboxylic acid which is produced by Malassezia furfur (also known as Pityrosporum ovale), a yeast that lives on normal skin [91] (Figure 4) (Table 1). It is effective in treating several skin conditions, such as acne, inflammation and hyperpigmentation. As a competitive inhibitor of tyrosinase in vitro, it has been used to treat melasma, Lentigo maligna and post-inflammatory hyperpigmentation. The minimum concentration at which azelaic acid demonstrates its anti-enzymatic activity is 10−3 mol/L and it is approximately equal to the 20% content of azelaic acid in a cream applied topically [148,149]. Furthermore, the efficacy of 20% azelaic acid cream is superior than a 2% hydroquinone (HQ) cream while severe side effects were not reported [90,150]. Clinical trials demonstrated that this cream was also effective against melasma when used in parallel with a broad-spectrum sunscreen. Thus, the ability of azelaic acid to reduce the amount of melanin in a specific region of skin tissue as well as the lack of side effects makes it widely used in cosmetic formulations.
Lactic acid is also used as skin whitener (Table 1). At a dose of 500 µg/mL it inhibits melanin formation in a dose-dependent manner without affecting cell growth [151]. Recent studies have shown that species of Rhizopus could offer a valuable alternative source for lactic acid production [152]. The filamentous fungus R. oryzae converts both glucose and xylose under aerobic conditions into l(+)-lactic acid with yields varying between 0.55 and 0.8 g/g [87].
Poly γ-glutamic acid (γ-PGA) is a natural polymer produced by different species of Bacillus (yields vary from 10 to 50 g/L depending on the species) [88] (Table 1). Studies related to the inhibitory effect against mushroom tyrosinase and tyrosinase in B16 melanoma cells reported a dose dependent activity. γ-PGAs, and especially the low molecular weight polymers, has attracted much attention owing to its great potential in cosmetics as skin-whitening agents [153].
4.4. Enzymes and Derived Products
The possibility of using melanolytic enzymes in skin lightening was examined by screening the potential melanolytic activity of wild fungal isolates. Among them, Sporotrichum pruinosum was the most promising from the very limited number of fungi that decolorize synthetic melanin [154]. As described in the US 20030077236 Patent, compositions containing melanin-degrading enzymes derived from Aspergillus fumigatus or S. cerevisiae were twice as effective as kojic acid in producing a whitening effect on the skin.
A large variety of compounds with potential skin protecting applications can be obtained through biotechnological processes by using enzymes isolated from terrestrial microorganisms. This is the case of retinol, the most active form of Vitamin A, a skin-whitening agent that has been synthesized by the esterification of palmitic acid using a modified lipase B from Candida antarctica (CALB) and a modified lipase from Pseudomonas fluorescens, in order to maximize its solubility in water and minimize skin irritation. Other Vitamin A modifications include the esterification with oleic, lactic, succinic or methylsuccinic, catalyzed by CALB or by Rhizomucor miehei lipase [155].
A better dermal absorption and a 10% higher skin-whitening activity, as compared to the well-known tyrosinase inhibitor arbutin, was demonstrated by its derivative arbutin undecylenic acid ester which has been enzymatically synthesized using an alkaline protease from Bacillus subtilis [94,155]. In addition, α-arbutin glycosides were synthesized by the trans glycosylation reaction of cyclomaltodextrin glucanotransferase from Bacillus macerans. Synthesized glucosides exhibited higher inhibition on human tyrosinase than α-arbutin [156].
5. Additives and Other Active Ingredients
Additive products provide long-term physical stability, inhibit germination and influence the sensory perception. Recently, the cosmetic industry has been strongly criticized for the addition of chemicals such as formaldehyde, dioxane, parabens, and phthalates. Controversies regarding the human health impact of those synthetic molecules and their analogues has encouraged the research of new additives from natural sources.
5.1. Antimicrobial Agents
One of the most widely used antimicrobial agents against bacteria, viruses and fungi contamination in cosmetics is chitosan (18) (Figure 5). This polysaccharide is composed mostly of glucosamine and a variable number of N-acetylglucosamine residues. Although chitosan is present in large amounts in the exoskeleton of crustaceans, insects, crabs, and shrimps, its production is limited due to factors such as seasonality, production sustainability and processing cost. To face these difficulties, chitosan can be produced by an alternative and more effective sources of microbial origin since 22–44% of the cell wall of fungi is composed of chitosan [2]. An optimal production was found in Rhizopus oryzae (0.5 g/L), R. japonicus (0.6 g/L) and Mucor indicus (0.75 g/L) (Table 1) [63], while A. niger, isolated from the lichen Roccella montagnei, showed a higher yield of 1.3 g/L, which was further increased to 1.93 g/L when glucose was added [65]. In addition to the antimicrobial activity, chitosan is known for its emulsifying and delivering properties. This compound has a better water-binding capacity than methyl-cellulose, which is commonly used in cosmetics [2]. Consequently, chitosan and its derivatives, like the copolymer chitin-glucan, can present potential candidates for cosmetic and cosmeceutical formulations. Other examples with anti-aging activity that also combine antimicrobial activity are presented in Table 1.
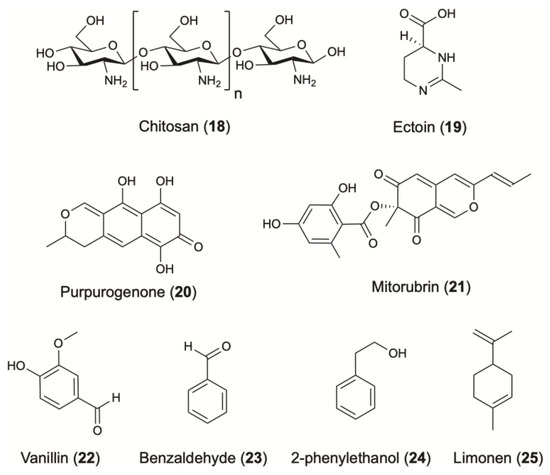
Figure 5.
Additives and other active ingredients from terrestrial microorganisms.
5.2. Moisturizers and Biosurfactants
Concerning moisturizing care, ectoine (19) is commonly used for its strong hydration properties (Figure 5). This cyclic amino acid is produced by several bacterial species in response to osmotic stress. Corynebacterium glutamicum is widely studied as a microbial cell factory for the biotechnological production of ectoine. The optimization of some cultivation parameters led to the production of 6.7 g/L/day of ectoine [98] (Table 1).
Glycolipids represent an important class of biosurfactants. Among them, sophorolipids and trehalolipids are efficient biosurfactants. Sophorolipids are mainly produced by yeasts belonging to the genus Candida (formerly called Torulopsis), like C. bombicola, C. petrophilum and C. apicola, while trehalolipids by Rhodococcus sp., Mycobacterium sp., Nocardia sp., and Corynebacterium sp. Trehalolipids represent structures with a variation in the number of carbon atoms and the degree of unsaturation.
Rhamnolipids are commonly used in cosmetics as moisturizers and biosurfactants [108]. Rhamnolipids, primarily crystalline acids, are composed of a β-hydroxy fatty acid attached by the carboxyl end to a rhamnose sugar molecule and are classified as mono and di-rhamnolipids [157]. Compared to chemical surfactants, biosurfactants have several advantages, because of their better compatibility, lower toxicity and higher biodegradability [158]. Rhamnolipids are mainly produced by Pseudomonas aeruginosa as well as by other Pseudomonas sp. They are also used in the pharmaceutical industry for their antiviral and antimicrobial properties [159,160] and for others targets related to skin regeneration such as wound healing with reduced fibrosis, cure of burn shock and treatment of wrinkles [161].
5.3. Pigments
Microorganisms produce several compounds that can be used as natural pigments. A lot of synthetic dyes have been commercialized, but few of them are eligible in cosmetics. Natural pigments are more stable and less allergenic compared to synthetics [162]. Pigments commonly biosynthesized by fungi include aromatic polyketides such as quinones, anthraquinones, naphthoquinones, melanins, flavins and ankaflavins. Purpurogenone (20) and mitorubrin (21) are two characteristic examples, produced by the fungus Penicillium purpurogenum [95] (Figure 5) (Table 1). Recently, the potential use of terrestrial fungi as a source of natural pigments has been considerably investigated [163,164,165].
Cyanobacteria are an interesting source of pigments, that have the ability to produce phycobiliproteins, which are brilliantly colored fluorescent proteins. Among phycobiliproteins, phycocyanins are already used in diagnostic assays such as flow cytometry, fluorescence activated cell sorting, histochemistry, etc. Their intense blue color allows their use in cosmetics as natural dyes [166]. Phycocyanins are mainly produced by the photoautotrophic cyanobacterium Arthrospira platensis (3.2 g/L) [167]. However, the unicellular rhodophyte Galdieria sulphuraria showed excellent results; this red alga, growing usually in acidic springs, produced c-phycocyanin with a yield of 2.9 g/L [168] (Table 1).
5.4. Flavoring and Fragrances
Many flavoring and fragrance compounds on the market are still produced through plant and animal sources. However, a rapid and sustainable alternative is given as such high value compounds can be also produced by microorganisms [169]. Numerous yeasts and terrestrial fungal and bacterial strains are able to synthesize potentially valuable fragrance compounds, including alcohols, aldehydes, esters, fatty acids, ketones, lactones, aromatic compounds and pyrazines [170]. In support, several articles and reviews have been published and offer sufficient information regarding the use of microbial cultures or enzyme preparations for the production of flavor compounds valuable for the cosmetic industry [171,172,173,174]. Vanillin (22) is a very good example of a natural fragrance where the increasing demand and value have led to the development of alternative strategies for its production [175] (Figure 5). Strains including Pseudomonas putida, Aspergillus niger, Corynebacterium glutamicum, Corynebacterium sp., Arthrobacter globiformis and Serratia marcescens were successfully introduced for its production by converting eugenol or isoeugenol to vanillin [170].
Benzaldehyde (23) is among the most commonly used flavoring agent, with a strong cherry and almond-like aroma. An E. coli strain was successfully engineered to produce this aromatic [100,176], while the fungus Ashbya gossypii has been tested for its ability to synthetize the rose flavour 2-phenylethanol (24) [104]. Among terpenes, limonene (25) is one of the most widely used terpene due to its unique citrus scent [169]. Optimization of the expression pathway in E. coli led to a yield of 435 mg/L with 1% of glucose as carbon source [177]. When the impact of a different carbon source have been explored, the fermentation using glycerol led to the titers of 2.7 g/L [106] (Figure 5) (Table 1).
6. Other Targets of Skin Protecting Interest
Elastase and collagenase inhibitors of microbial origin are promising cosmeceutical agents that worth to be further explored. Elastase, a member of the chymotrypsin family of serine proteases, is responsible primarily for the breakdown of elastin, which is an important protein found within the extracellular matrix of the skin, whose damage has a significant impact in skin ageing. Nostopeptins A and B isolated from the freshwater cyanobacterium Nostoc minutum are the only reported inhibitors of elastase (IC50: 1.3 and 11.0 µg/mL) [178]. On the other hand, collagen, the major constituent of the skin (80% of skin dry weight), is responsible for the tensile strength. The metalloproteinases named collagenases are capable of cleaving collagen and elastin. To the best of our knowledge, terrestrial microorganisms, apart from the aforementioned example of nostopeptins A and B, have not been investigated thoroughly yet for their ability to produce metabolites with elastase and collagenase inhibitory effects although that large screening programs on terrestrial microorganism and endophytes have been recently presented [179,180].
7. Targets for Future Developments
Beyond the above applications of microbial-derived natural products, it is worth mentioning some new cosmeceutical targets with great potential for future development.
It is well known that skin retains its young-looking appearance for many years due to numerous cell genome and proteome protective mechanisms; these are mostly driven by protein machines that execute both DNA and proteome damage responses. Proteome quality control is carried out through the curating activity of the proteostasis network (PN) and is critical for cellular functionality [7,10,11]. Key components of the PN are the two main degradation machineries, namely the autophagy-lysosome and the ubiquitin-proteasome pathways; several short-lived transcription factors are also considered to be part of the PN as they mobilize genomic cytoprotective responses [11]. These, among many others, include Nrf2, which responds to oxidative, electrophilic, and/or proteotoxic stress [11,181,182]. Deregulation of the PN functionality is associated with ageing and it is considered a major risk factor for a wide spectrum of age-related protein conformational diseases such as immunological and metabolic disorders, cardiovascular and neurodegenerative diseases and cancer [11,183]. On the other hand, several studies have shown that the activation of proteostatic modules by genetic, dietary and/or pharmacological interventions increases organismal health- and/or life-span and delays cellular senescence [7,182].
Concomitantly, natural compounds that activate the PN have also been reported to possess anti-aging properties at either cell-based or in vivo models [7,11,182]; likewise, natural products significantly delay the appearance of the aged skin hallmarks. To the best of our knowledge, only few molecules of microbial origin were reported to activate proteostatic modules. Betulinic acid was recently isolated from the endophytic fungi Phomopsis sp. and its preferentially activating the chymotrypsin-like proteasomal activity with no or minimal effects on trypsin-like and caspase-like activities [184,185]. The second case of a microbial natural product, that is well known for its anti-aging proprieties is rapamycin. This molecule isolated from Streptomyces hygroscopicus, delay cellular senescence through (among others) the inhibition of the TOR pathway and the downstream induced alterations to both autophagy and the rate of protein synthesis [7].
8. Conclusions
Naturally derived molecules are traditionally used in skin protection products (Table 1, CosIng inventory). Consequently, natural compounds isolated and/or produced using biotechnological tools from microorganisms are already used for dermatologic purposes in topical cosmetic formulations. These products can aesthetically improve the skin’s appearance but can also prevent and/or treat age-related skin disorders. Beyond the “established” molecules, there are several small molecules and/or enzymes derived from microorganisms that have great potential to be used in cosmetics or cosmeceutical formulations (Table 1).
Interestingly, several biomolecules that are already included in the European Inventory of accepted cosmetic ingredients (CosIng inventory) [14] are registered for one of their biological activities, but are used differently in cosmetic applications. A characteristic example is kojic acid, which is registered as “antioxidant”, while the main application in cosmetics is its strong anti-tyrosinase activity, and thus its application as a skin whitening agent (Table 1).
Considering the immense microbial biodiversity and microbial adaptation to virtually any environment on earth, it is to be expected that microbes represent an extraordinary inventory of highly diverse structural scaffolds of biomolecules with potential skin protective activities. Although research on marine environment has started match later that the terrestrial environment, we have several cases where cosmetic applications and patents are in favor of marine-derived microorganisms. As mentioned in the case of MAAs known for their photo-protective activity, they are included in several patents for natural UV filters. However most of them were developed with microorganisms from marine environments (72.2%), while patents developed on terrestrial and fresh water microorganisms have not exceeded 21.4% and 2.4%, respectively [135]. This study reflects that to date, in some cases the terrestrial environment has been neglected.
Overall, taking into consideration that most of the world’s microbial terrestrial biodiversity remains largely uninvestigated and that microorganisms offer a sustainable, relatively low-cost and fast production process, we remain confident that in the near future, systematic research will reveal additional microorganisms that can be used as cell factories for producing high added value biomolecules with applications in the cosmetic industry as active ingredients.
Author Contributions
All authors have contributed to the preparation of this article.
Funding
This work has been financially supported by EU under the frame of MICROSMETICS project (FP7-PEOPLE- Industry-Academia Partnerships and Pathways), Grant agreement No. 612276.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABTS | 2,2′-Azino-bis-3-ethylbenzthiazolin-6-sulphonic acid |
| CALB | Candida antarctica lipase B |
| CAGR: | compound annual growth rate |
| CDW | cell dry weight |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EPSs | exopolysaccharides |
| HQ | hydroquinone |
| LTA | lipoteichoic acid |
| MAAs | mycosporine-like amino acids |
| MIC | minimum inhibitory concentration |
| γ-PGA | poly- γ-glutamic acid |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SPFs | sunscreen protection factors |
| SSR | solar-simulated radiation |
| SOD | superoxide dismutases |
| UVA | ultraviolet A |
| UVB | ultraviolet B |
References
- Ma, T.; Deng, Z.; Liu, T. Microbial production strategies and applications of lycopene and other terpenoids. World J. Microbiol. Biotechnol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Lobanovska, M.; Pilla, G. Penicillin’s discovery and antibiotic resistance: lessons for the future? J. Biol. Med. 2017, 90, 135–145. [Google Scholar]
- Raja, A.; Prabakarana, P. Actinomycetes and Drug-An Overview. Am. J. Drug Discov. Dev. 2011, 1, 75–84. [Google Scholar] [CrossRef]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [PubMed]
- Ferreira, A.; Vecino, X.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Novel cosmetic formulations containing a biosurfactant from Lactobacillus paracasei. Colloids Surf. B Biointerfaces 2017, 155, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, A.; Aligiannis, N.; Trougakos, I.P.; Skaltsounis, A.L. Natural compounds with anti-ageing activity. Nat. Prod. Rep. 2013, 30, 1412–1437. [Google Scholar] [CrossRef]
- Cavinato, M.; Jansen-Durr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar]
- Velarde, M.C.; Demaria, M. Targeting senescent cells: Possible implications for delaying skin aging: A mini-review. Gerontology 2016, 62, 513–518. [Google Scholar]
- Trougakos, I.P.; Sesti, F.; Tsakiri, E.; Gorgoulis, V.G. Non-enzymatic post-translational protein modifications and proteostasis network deregulation in carcinogenesis. J. Proteomics 2013, 92, 274–298. [Google Scholar]
- Sklirou, A.; Papanagnou, E.D.; Fokialakis, N.; Trougakos, I.P. Cancer chemoprevention via activation of proteostatic modules. Cancer Lett. 2018, 413, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Market Research Reports. Available online: https://www.reuters.com/brandfeatures/venture-capital/article?id=30351 (accessed on 22 August 2017).
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi—An unusual source for cosmetics. Fungal Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- CosIng Inventory. Available online: http://ec.europa.eu/growth/sectors/cosmetics/cosing_en (accessed on 22 March 2018).
- Chai, T.T.; Law, Y.C.; Wong, F.C.; Kim, S.K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A Review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, T.E.S.; Kauppila, J.H.K.; Larsson, N.G. Mammalian mitochondria and aging: an update. Cell. Metab. 2017, 25, 57–71. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Natural antioxidants in cosmetics. Stud. Nat. Prod. Chem. 2013, 40, 485–505. [Google Scholar]
- Abramovič, H.; Grobin, B.; Ulrih, N.P.; Cigić, B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Xing, J. A potential antioxidant resource: endophytic fungi from medicinal plants. Econ. Bot. 2007, 61, 14–30. [Google Scholar] [CrossRef]
- Danagoudar, A.; Joshi, C.G.; Kumar, R.S.; Poyya, J.; Nivya, T.; Hulikere, M.M.; Appaiah, K.A.A. Molecular profiling and antioxidant as well as anti-bacterial potential of polyphenol producing endophytic fungus-Aspergillus austroafricanus CGJ-B3. Mycology 2017, 8, 28–38. [Google Scholar] [CrossRef]
- Colla, L.M.; Furlong, E.B.; Costa, J.A.V. Antioxidant properties of Spirulina (Arthospira) platensis cultivated under different temperatures and nitrogen regimes. Braz. Arch. Biol. Technol. 2007, 50, 161–167. [Google Scholar] [CrossRef]
- Miranda, M.S.; Sato, S.; Mancini-Filho, J. Antioxidant activity of the microalga Chlorella vulgaris cultered on special conditions. Boll. Chim. Farm. 2001, 140, 165–168. [Google Scholar]
- Shalaby, E.S.; Shanab, S.M.M. Antiradical and antioxidant activities of different Spirulina platensis extracts against DPPH and ABTS radical assays. Indian J. Geomarine Sci. 2013, 42, 556–564. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; GJ, B.; De Cooman, L. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Verma, S.; Meena, K.K.; Yandigeri, M. Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech. 2017. [Google Scholar] [CrossRef]
- Liu, Y.; Nan, L.; Liu, J.; Yan, H.; Zhang, D.; Han, X. Isolation and identification of resveratrol-producing endophytes from wine grape Cabernet Sauvignon. SpringerPlus 2016. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, Q.; Liu, Y.; Pan, Z. Alternaria sp. MG1, a resveratrol-producing fungus: isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 2012, 95, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.A. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, J.; Liu, Y. Bioconversion of resveratrol using resting cells of non-genetically modified Alternaria sp. Biotechnol. Appl. Biochem. 2013, 60, 236–243. [Google Scholar] [CrossRef]
- An, S.M.; Koh, J.S.; Boo, Y.C. P-coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother. Res. 2010, 24, 1175–1180. [Google Scholar]
- Lourith, N.; Kanlayavattanakul, M. Antioxidant activities and phenolics of Passiflora edulis seed recovered from juice production residue. J. Oleo. Sci. 2013, 62, 235–240. [Google Scholar] [CrossRef]
- Lopez-Burillo, S.; Tan, D.X.; Mayo, J.C.; Sainz, R.M.; Manchester, L.C.; Reiter, R.J. Melatonin, xanthurenic acid, resveratrol, EGCG, vitamin C and alpha-lipoic acid differentially reduce oxidative DNA damage induced by Fenton reagents: a study of their individual and synergistic actions. J. Pineal. Res. 2003, 34, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Kubo, I. Resveratrol as a kcat type inhibitor for tyrosinase: Potentiated melanogenesis inhibitor. Bioorg. Med. Chem. 2012, 20, 1090–1099. [Google Scholar] [CrossRef]
- Li, M.; Kildegaard, K.R.; Chen, Y.; Rodriguez, A.; Borodina, I.; Nielsen, J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.G.; Fowler, Z.L.; Hueller, T.; Schaffer, S.; Koffas, M.A. High-yield resveratrol production in engineered Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 3451–3460. [Google Scholar] [CrossRef]
- Sydor, T.; Schaffer, S.; Boles, E. Considerable increase in resveratrol production by recombinant industrial yeast strains with use of rich medium. Appl. Environ. Microbiol. 2010, 76, 3361–3363. [Google Scholar] [CrossRef]
- Balestrazzi, A.; Bonadei, M.; Calvio, C.; Mattivi, F.; Carbonera, D. Leaf-associated bacteria from transgenic white poplar producing resveratrol-like compounds: isolation, molecular characterization, and evaluation of oxidative stress tolerance. Can. J. Microbiol. 2009, 55, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, Q.; Gao, Y.-Q.; Tang, J.-J.; Zhang, A.-L.; Gao, J.-M. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Meng, J.; Fu, X.; Wang, X.; Tian, J.; Wang, M.; Peng, Y.; Zhou, L. Antimicrobial and antioxidant activities and effect of 1-hexadecene addition on palmarumycin C2 and C3 yields in liquid culture of endophytic fungus Berkleasmium sp. Dzf12. Molecules 2013, 18, 15587–15599. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.; Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef]
- Sonthalia, S.; Daulatabad, D.; Sarkar, R. Glutathione as a skin whitening agent: facts, myths, evidence and controversies. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 262–272. [Google Scholar] [CrossRef]
- Fei, L.; Wang, Y.; Chen, S. Improved glutathione production by gene expression in Pichia pastoris. Bioprocess. Biosyst. Eng. 2009, 32, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Wu, H.; Li, Z.; Ye, Q. Heterologous gshF gene expression in various vector systems in Escherichia coli for enhanced glutathione production. J. Biotechnol. 2015, 214, 63–68. [Google Scholar] [CrossRef]
- Palozza, P.; Krinsky, N.I. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch. Biochem. Biophys. 1992, 267, 291–295. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hara, K.Y.; Morita, T.; Nishimura, A.; Sasaki, D.; Ishii, J. Enhancement of astaxanthin production in Xanthophyllomyces dendrorhous by efficient method for the complete deletion of genes. Microb. Cell Fact. 2016, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, U.; Sarada, R.; Ravishankar, G.A. Effect of culture conditions on growth of green alga - Haematococcus pluvialis and astaxanthin production. Acta Physiol. Plant. 2002, 24, 323–329. [Google Scholar] [CrossRef]
- Mueller, L.; Boehm, V. Antioxidant activity of beta-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef]
- Zhu, F.; Lu, L.; Fu, S.; Zhong, X.; Hu, M.; Deng, Z. Targeted engineering and scale up of lycopene overproduction in Escherichia coli. Process. Biochem. 2015, 50, 341–346. [Google Scholar] [CrossRef]
- Nelis, H.J.; De Leenheer, A. P, Reinvestigation of Brevibacterium sp. strain KY-4313 as a source of canthaxanthin. Appl. Environ. Microbiol. 1989, 55, 2505–2510. [Google Scholar]
- Sindhu, E.R.; Preethi, K.C.; Kuttan, R. Antioxidant activity of carotenoid lutein in vitro and in vivo. Indian J. Exp. Biol. 2010, 843–848. [Google Scholar]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Angeles Vargas, M.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Venugopalan, V.; Tripathi, S.K.; Nahar, P.; Saradhi, P.P.; Das, R.H.; Gautam, H.K. Characterization of canthaxanthin isomers isolated from a new soil Dietzia sp. and their antioxidant activities. J. Microbiol. Biotechnol. 2013, 23, 237–245. [Google Scholar] [CrossRef]
- Mahapatra, S.; Banerjee, D. Evaluation of in vitro antioxidant potency of exopolysaccharide from endophytic Fusarium solani SD5. Int. J. Biol. Macromol. 2013, 53, 62–66. [Google Scholar] [CrossRef]
- Zheng, L.P.; Zou, T.; Ma, Y.J.; Wang, J.W.; Zhang, Y.Q. Antioxidant and DNA damage protecting activity of exopolysaccharides from the endophytic bacterium Bacillus cereus SZ1. Molecules 2016, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Medium optimization and structural characterization of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2010, 79, 206–213. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Tao, H.; Zhu, W.; Qi, X.; Chen, Y. Structural characterization and antioxidant properties of an exopolysaccharide produced by the mangrove endophytic fungus Aspergillus sp. Y16. Bioresour. Technol. 2011, 102, 8179–8184. [Google Scholar] [CrossRef] [PubMed]
- Serrato, R.V.; Sassaki, G.L.; Cruz, L.M.; Pedrosa, F.O.; Gorin, P.A.; Iacomini, M. Culture conditions for the production of an acidic exopolysaccharide by the nitrogen-fixing bacterium Burkholderia tropica. Can. J. Microbiol. 2006, 52, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, L.; Chaieb, O.; Mnari, A.; Abid-Essafi, S.; Aleya, L. Partial characterization and antioxidant and antiproliferative activities of the aqueous extracellular polysaccharides from the thermophilic microalgae Graesiella sp. BMC Complement. Altern. Med. 2016. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Tasar, O.C.; Erdal, S.; Taskin, M. Chitosan production by psychrotolerant Rhizopus oryzae in non-sterile open fermentation conditions. Int. J. Biol. Macromol. 2016, 89, 428–433. [Google Scholar] [CrossRef]
- Asachi, R.; Karimi, K. Enhanced ethanol and chitosan production from wheat straw by Mucor indicus with minimal nutrient consumption. Process. Biochem. 2013, 48, 1524–1531. [Google Scholar] [CrossRef]
- Logesh, A.R.; Thillaimaharani, K.A.; Sharmila, K.; Kalaiselvam, M.; Raffi, S.M. Production of chitosan from endolichenic fungi isolated from mangrove environment and its antagonistic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 140–143. [Google Scholar] [CrossRef]
- Ordonñez, L.; Garciía, J.; Bolanños, G. Producing chitin and chitin-glucan complexes from Aspergillus niger biomass using subcritical water. In Proceedings of the Ibero-american Conference on Supercritical Fluids, Cartagena, Colombia, 1–5 April 2013. [Google Scholar]
- Guo, J.; Rao, Z.; Yang, T.; Man, Z.; Xu, M.; Zhang, X. High-level production of melanin by a novel isolate of Streptomyces kathirae. FEMS Microbiol. Lett. 2014, 357, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tarangini, K.; Mishra, S. Production of melanin by soil microbial isolate on fruit waste extract: two step optimization of key parameters. Biotechnol. Rep. 2014, 4, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Beckstead, A.A.; Zhang, Y.; Hilmer, J.K.; Smith, H.J.; Bermel, E.; Foreman, C.M. Ultrafast excited-state deactivation of the bacterial pigment violacein. J. Phys. Chem. A 2017. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.A.; Yusof, N.Z.; Nordin, N.; Zakaria, Z.A.; Rezali, M.F. Production and characterization of violacein by locally isolated Chromobacterium violaceum grown in agricultural wastes. Appl. Biochem. Biotechnol. 2012, 167, 1220–1234. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, P.; Lu, Y.; Ruan, Z.; Jiang, R.; Xing, X.-H. Optimization of culture conditions for violacein production by a new strain of Duganella sp. B2. Biochem. Eng. J. 2009, 44, 119–124. [Google Scholar] [CrossRef]
- Pathak, J.; Sonker, A.S.; Richa, R.; Rajneesh, R.; Kannaujiya, V.K.; Singh, V.; Ahmed, H. Screening and partial purification of photoprotective pigment scytonemin from cyanobacterial crusts dwelling on the historical monuments in and around Varanasi, India. Microbiol. Res. 2017. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, L.; Xu, J.; Yang, R.; He, S.; Yan, X. Determination of oxidized scytonemin in Nostoc commune Vauch cultured on different conditions by high performance liquid chromatography coupled with triple quadrupole mass spectrometry. J. Appl. Phycol. 2013, 25, 1001–1007. [Google Scholar] [CrossRef]
- Nakashima, T.; Anzai, K.; Kuwahara, N.; Komaki, H.; Miyadoh, S.; Harayama, S. Physicochemical characters of a tyrosinase inhibitor produced by Streptomyces roseolilacinus NBRC 12815. Biol. Pharm. Bull. 2009, 32, 832–836. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Ozdal, M.; Ozdal, O.G.; Algur, O.F. Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz. J. Microbiol. 2015, 46, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Boric, M.; Danevcic, T.; Stopar, D. Prodigiosin from Vibrio sp. DSM 14379; a new UV-protective pigment. Microb. Ecol. 2011, 62, 528–536. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2017, 178, 1353–1363. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Libkind, D.; Moline, M.; Sommaruga, R.; Sampaio, J.P.; van Broock, M. Phylogenetic distribution of fungal mycosporines within the Pucciniomycotina (Basidiomycota). Yeast (Chichester, England) 2011, 28, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kogej, T.; Gostincar, C.; Volkmann, M.; Gorbushina, A.A.; Gunde-Cimerman, N.; Kogej, T.; Gorbushina, A.A. Mycosporines in extremophilic fungi - Novel complementary osmolytes? Environ. Chem. 2006, 3, 105–110. [Google Scholar] [CrossRef]
- Wei, S.; Xu, N.; Ji, Z. Identification of a kojic-acid producing Aspergillus flavus F52. Acta Microbiol. Sin. 2014, 1155–1160. [Google Scholar]
- El-Aasar, S.A. Cultural conditions studies on kojic acid production by Aspergillus parasiticus. Int. J. Agric. Biol. 2006, 8, 468–473. [Google Scholar]
- Mohamad, R.; Ariff, A.B. Biotransformation of various carbon sources to kojic acid by cell-bound enzyme system of A. flavus Link 44-1. Biochem. Eng. J. 2007, 35, 203–209. [Google Scholar] [CrossRef]
- Zhao, L.; Kim, J.C.; Paik, M.J.; Lee, W.; Hur, J.S. A multifunctional and possible skin UV protectant, (3R)-5-hydroxymellein, produced by an endolichenic fungus isolated from Parmotrema austrosinense. Molecules 2016, 22, 26. [Google Scholar] [CrossRef]
- Robledo, A.; Aguilera-Carbo, A.; Rodriguez, R.; Martinez, J.L.; Garza, Y.; Aguilar, C.N. Ellagic acid production by Aspergillus niger in solid state fermentation of pomegranate residues. J. Ind. Microbiol. Biotechnol. 2008, 35, 507–513. [Google Scholar] [CrossRef]
- Smith, W.P. The effects of topical L(+) lactic acid and ascorbic acid on skin whitening. Int. J. Cosmet. Sci. 1999, 21, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.H.; Springer, J.; Eggink, G.; Weusthuis, R.A. Xylose metabolism in the fungus Rhizopus oryzae: effect of growth and respiration on L+-lactic acid production. J. Ind. Microbiol. Biotechnol. 2008, 35, 569–578. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T. The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wood, J.W. A possible mechanism of action for azelaic acid in the human epidermis. Arch. Dermatol. Res. 1990, 282, 168–171. [Google Scholar] [CrossRef]
- Balina, L.M.; Graupe, K. The treatment of melasma, 20% azelaic acid versus 4% hydroquinone cream. Int. J. Dermatol. 1991, 30, 893–895. [Google Scholar] [CrossRef]
- Nazzaro-Porro, M.; Passi, S.; Morpurgo, G.; Breathnach, A.S. Identification of tyrosinase inhibitors in cultures of Pityrosporum and their melanocytotoxic effect. Pigment. Cell 1979, 4, 234–243. [Google Scholar]
- Yin, C.; Zhang, C.; Gao, M. Enzyme-catalyzed synthesis of vitamin E succinate using a chemically modified novozym-435. Chin. J. Chem. Eng. 2011, 19, 135–139. [Google Scholar] [CrossRef]
- Wicken, A.J.; Gibbens, J.W.; Knox, K.W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J. Bacteriol. 1973, 113, 365–372. [Google Scholar]
- Tokiwa, Y.; Kitagawa, M.; Raku, T. Enzymatic synthesis of arbutin undecylenic acid ester and its inhibitory effect on mushroom tyrosinase. Biotechnol. Lett. 2007, 29, 481–486. [Google Scholar] [CrossRef]
- Mapari, S.A.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009. [Google Scholar] [CrossRef]
- Dufossé, L. Encyclopedia of Microbiology, 3rd ed.; Academic Press: New York, NY, USA, 2009; Microbial Pigments; pp. 457–471. [Google Scholar]
- Buenger, J.; Driller, H. Ectoin: An effective natural substance to prevent UVA-induced premature photoaging. Skin Pharmacol. Physiol. 2004, 17, 232–237. [Google Scholar] [CrossRef]
- Becker, J.; Schafer, R.; Kohlstedt, M.; Harder, B.J.; Borchert, N.S.; Stoveken, N. Systems metabolic engineering of Corynebacterium glutamicum for production of the chemical chaperone ectoine. Microb. Cell Fact. 2013. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Di Giorgio, C.; Sabatier, A.S.; De Meo, M. Genotoxicity of visible light (400–800 nm) and photoprotection assessment of ectoin, L-ergothioneine and mannitol and four sunscreens. J. Photochem. Photobiol. B. 2008, 91, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.; McKenna, R.; Halloum, I.; Nielsen, D.R. Engineering Escherichia coli for renewable benzyl alcohol production. Metab. Eng. 2015, 2, 39–45. [Google Scholar] [CrossRef]
- Ni, J.; Tao, F.; Du, H.; Xu, P. Mimicking a natural pathway for de novo biosynthesis: natural vanillin production from accessible carbon sources. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Okada, Y.; Yoshida, T.; Nagasawa, T. Vanillin production using Escherichia coli cells over-expressing isoeugenol monooxygenase of Pseudomonas putida. Biotechnol. Lett. 2008, 30, 665–670. [Google Scholar] [CrossRef]
- Zhao, L.-Q.; Sun, Z.-H.; Zheng, P.; Zhu, L.-L. Biotransformation of isoeugenol to vanillin by a novel strain of Bacillus fusiformis. Biotechnol. Lett. 2005, 27, 1505–1509. [Google Scholar] [CrossRef]
- Ravasio, D.; Wendland, J.; Walther, A. Major contribution of the Ehrlich pathway for 2-phenylethanol/rose flavor production in Ashbya gossypii. FEMS Yeast Res. 2014, 14, 833–844. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Schrader, J. An aqueous–organic two-phase bioprocess for efficient production of the natural aroma chemicals 2-phenylethanol and 2-phenylethylacetate with yeast. Appl. Microbiol. Biotechnol. 2006, 71, 440–443. [Google Scholar] [CrossRef]
- Willrodt, C.; David, C.; Cornelissen, S.; Buhler, B.; Julsing, M.K.; Schmid, A. Engineering the productivity of recombinant Escherichia coli for limonene formation from glycerol in minimal media. Biotechnol. J. 2014, 9, 1000–1012. [Google Scholar] [CrossRef]
- Dobler, L.; de Carvalho, B.R.; Alves, S.; Neves, B.C.; Freire, G.; Almeida, R.V. Enhanced rhamnolipid production by Pseudomonas aeruginosa overexpressing estA in a simple medium. PloS ONE 2017, 12, e0183857. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 47–64. [Google Scholar]
- Castelblanco-Matiz, L.M.; Barbachano-Torres, A.; Ponce-Noyola, T.; Ramos-Valdivia, A.C.; Cerda García-Rojas, C.M.; Flores-Ortiz, C.M. Carotenoid production and gene expression in an astaxanthin-overproducing Xanthophyllomyces dendrorhous mutant strain. Arch. Microbiol. 2015, 197, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Zorrilla-Lopez, U.; Farre, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally important carotenoids as consumer products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Henríquez, V.; Escobar, C.; Galarza, J.; Gimpel, J. Carotenoids in microalgae. In Carotenoids in Nature; Strange, E., Ed.; Springer International Publishing: New York, NY, USA, 2016; pp. 219–237. [Google Scholar]
- Lau, N.-S.; Matsui, M.; Abdullah, A.A.-A. Cyanobacteria: photoautotrophic microbial factories for the sustainable synthesis of industrial products. J. Biomed. Biotechnol. 2015, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Yoon, S.H.; Lee, S.H.; Kim, J.Y.; Oh, D.K.; Kim, S.W. An update on microbial carotenoid production: application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 2007, 77, 505–512. [Google Scholar] [CrossRef]
- Mata-Gomez, L.C.; Montanez, J.C.; Mendez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Recent advances in endophytic exopolysaccharides: Production, structural characterization, physiological role and biological activity. Carbohydr. Polym. 2017, 157, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ooi, V.E.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Jin, M.; Cai, Y.X.; Li, J.R.; Zhao, H. 1,10-phenanthroline-Fe2+ oxidative assay of hydroxyl radical produced by H2O2/Fe. Prog. Biochem. Biophys. 1996, 23, 553–555. [Google Scholar]
- Chen, B.; You, W.; Huang, J.; Yu, Y.; Chen, W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2010, 26, 833–840. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Scott, J.A.; Ross, G.M. Management of oxidative stress by microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M. Cytotoxicity of oxyradicals and the evolution of superoxide dismutases. In Oxygen, Gene Expression and Cellular Function; Massaro, D., Clerch, L., Eds.; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Bruno-Barcena, J.M.; Azcarate-Peril, M.A.; Hassan, H.M. Role of antioxidant enzymes in bacterial resistance to organic acids. Appl. Environ. Microbiol. 2010, 76, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar]
- Abbott, D.A.; Suir, E.; Duong, G.H.; de Hulster, E.; Pronk, J.T.; van Maris, A.J. Catalase overexpression reduces lactic acid-induced oxidative stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 2320–2325. [Google Scholar] [CrossRef]
- Shao, N.; Beck, C.F.; Lemaire, S.D.; Krieger-Liszkay, A. Photosynthetic electron flow affects H2O2 signaling by inactivation of catalase in Chlamydomonas reinhardtii. Planta 2008, 228, 1055–1066. [Google Scholar] [CrossRef]
- European Commission. Ban on Animal Testing. Available online: https://ec.europa.eu/growth/sectors/cosmetics/animal-testing_en (accessed on 14 April 2019).
- Gao, Q.; Garcia-Pichel, F. Microbial ultraviolet sunscreens. Nat. Rev. Microbiol. 2011, 9, 791–802. [Google Scholar] [CrossRef]
- Plonka, P.M.; Grabacka, M. Melanin synthesis in microorganisms-biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar]
- Nosanchuk, J.D.; Stark, R.E.; Casadevall, A. Fungal melanin: What do we know about structure? Front. Microbiol. 2015. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Melanin: a photoprotection for Bacillus thuringiensis based biopesticides. Biotechnol. Lett. 2015, 37, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Nazifi, E.; Hirai, Y.; Wada, N.; Matsugo, S.; Sakamoto, T. The cyanobacterial UV-absorbing pigment scytonemin displays radical-scavenging activity. J. Gen. Appl. Microbiol. 2012, 58, 137–144. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Sherry, N.D.; Castenholz, R.W. Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem. Photobiol. 1992, 56, 17–23. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial sunscreen scytonemin: role in photoprotection and biomedical research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563. [Google Scholar] [CrossRef]
- Darshan, N.; Manonmani, H.K. Prodigiosin and its potential applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef]
- Duran, N.; Justo, G.Z.; Duran, M.; Brocchi, M.; Cordi, L.; Tasic, L. Advances in Chromobacterium violaceum and properties of violacein-Its main secondary metabolite: A review. Biotechnol. Adv. 2016, 34, 1030–1045. [Google Scholar] [CrossRef]
- Colabella, F.; Moline, M.; Libkind, D. UV sunscreens of microbial origin: mycosporines and mycosporine- like aminoacids. Recent Pat. Biotechnol. 2014, 8, 179–193. [Google Scholar] [CrossRef]
- Khosravi, S.; Khodabandeh, S.; Agh, N.; Bakhtiarian, M. Effects of salinity and ultraviolet radiation on the bioaccumulation of mycosporine-like amino acids in Artemia from Lake Urmia (Iran). Photochem. Photobiol. 2013, 89, 400–405. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Whitehead, K.; Dornieden, T.; Niesse, A.; Schulte, A.; Hedges, J.I. Black fungal colonies as units of survival: hyphal mycosporines synthesized by rock-dwelling microcolonial fungi. Can. J. Bot. 2003, 81, 131–138. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents-existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Yang, M.S.; Jang, D.S.; Choe, S.U.; Park, G.H. Inhibitory activities of flavanone derivatives isolated from Sophora flavecens for melanogenesis. Bull. Korean Chem. Soc. 2001, 22, 97–99. [Google Scholar]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Rosfarizan, M.; Mohd, S.M.; Nurashikin, S.; Madihah, M.S.; Arbakariya, B.A. Kojic acid: Applications and development of fermentation process for production. Biotech. Mol. Biol. 2010, 5, 24–37. [Google Scholar]
- Ortiz-Ruiz, C.V.; Berna, J.; Tudela, J.; Varon, R.; Garcia-Canovas, F. Action of ellagic acid on the melanin biosynthesis pathway. J. Dermatol. Sci. 2010, 5, 24–37. [Google Scholar] [CrossRef]
- Ventura, J.; Belmares, R.; Aguilera-Carbo, A.; Gutiérrez-Sanchez, G.; Rodríguez-Herrera, R. Fungal biodegradation of tannins from Creosote bush (Larrea tridentata) and Tar bush (Fluorensia cernua) for gallic and ellagic acid production. Food Technol. Biotechnol. 2008, 46, 213–217. [Google Scholar]
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carboó, A.; Aguilar, C.N. Ellagic acid: Biological properties and biotechnological development for production processes. Afr. J. Biotechnol. 2011, 10, 4518–4523. [Google Scholar]
- Breathnach, A.C.; Nazzaro-Porro, M.; Passi, S.; Zina, G. Azelaic acid therapy in disorders of pigmentation. Clin. Dermatol. 1989, 7, 106–119. [Google Scholar] [CrossRef]
- Sieber, M.A.; Hegel, J.K. Azelaic acid: Properties and mode of action. Skin Pharmacol. Physiol. 2014, 27, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.S. Melanin hyperpigmentation of skin: melasma, topical treatment with azelaic acid, and other therapies. Cutis 1989, 7, 106–119. [Google Scholar]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp. Dermatol. 2003, 12, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jin, B.; Kelly, J.M. Production of lactic acid from renewable materials by Rhizopus fungi. Biochem. Eng. J. 2007, 35, 251–263. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Liu, S.; Li, H.; Ling, P.; Zhu, X. Poly-gamma-glutamate from Bacillus subtilis inhibits tyrosinase activity and melanogenesis. Appl. Microbiol. Biotechnol. 2013, 97, 9801–9809. [Google Scholar] [CrossRef]
- Mohorčič, M.; Friedrich, J.; Renimel, I.; André, P.; Mandin, D.; Chaumont, J.-P. Production of melanin bleaching enzyme of fungal origin and its application in cosmetics. Biotechnol. Bioprocess. Eng. 2007, 12, 200–206. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulos, P.; Faraco, V. Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nomura, K.; Nishimura, T.; Kiso, T.; Sugimoto, K.; Kuriki, T. Syntheses of alpha-arbutin-alpha-glycosides and their inhibitory effects on human tyrosinase. J. Biosci. Bioeng. 2005, 99, 272–276. [Google Scholar] [CrossRef]
- Sekhon Randhawa, K.K.; Rahman, P.K. Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front. Microbiol. 2014. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: a sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Wullbrandt, D. Rhamnose lipids-biosynthesis, microbial production and application potential. Appl. Microbiol. Biotechnol. 1999, 51, 22–32. [Google Scholar] [CrossRef]
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322. [Google Scholar] [CrossRef]
- Piljac, G.; Piljac, T. Use of rhamnolipids in wound healing, treating burn shock, atherosclerosis, organ transplants, depression, schizophrenia and cosmetics. U.S. Patent No US7262171B1. 2000.
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: current trends and future perspectives. J. Food Sci. Tech. 2015, 52, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and colorants from filamentous fungi. Fungal Metab. 2017, 499–568. [Google Scholar] [CrossRef]
- Souza, P.N.; Grigoletto, T.L.; Abreu, L.M.; Guimarães, L.H.; Santos, C.; Galvão, L.R.; Cardoso, P.G. Production and chemical characterization of pigments in filamentous fungi. Microbiology 2016, 162, 12–22. [Google Scholar]
- Rao, N.; Prabhu, M.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017. [Google Scholar] [CrossRef]
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent developments in production and biotechnological applications of C-phycocyanin. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M. Microalgae Nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Graverholt, O.S.; Eriksen, N.T. Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin. Appl. Microbiol. Biotechnol. 2007, 77, 69–75. [Google Scholar] [CrossRef]
- Carroll, A.L.; Desai, S.H.; Atsumi, S. Microbial production of scent and flavor compounds. Curr. Opin. Chem. Biol. 2016, 37, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- Soccol, C.R.; Medeiros, A.B.; Vandenberghe, L.P.; Woiciechowski, A.L. Flavor compounds produced by fungi, yeasts, and bacteria. Handb. Food Prod. Manuf. 2007, 1, 179–191. [Google Scholar]
- Vandamme, E.J. Bioflavours and fragrances via fungi and their enzymes. Fungal Divers. 2003, 13, 153–166. [Google Scholar]
- Carrau, F.; Boido, E.; Dellacassa, E. Yeast Diversity and Flavor Compounds. In Fungal Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–29. [Google Scholar]
- Priefert, H.; Rabenhorst, J.; Steinbüchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef]
- Kunjapur, A.M.; Tarasova, Y.; Prather, K.L.J. Synthesis and accumulation of aromatic aldehydes in an engineered strain of Escherichia coli. J. Am. Chem. Soc. 2014, 136, 11644–11654. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gutierrez, J.; Chan, R.; Batth, T.S.; Adams, P.D.; Keasling, J.D.; Petzold, C.J. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41. [Google Scholar] [CrossRef]
- Okino, T.; Qi, S.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Nostopeptins A and B, elastase inhibitors from the cyanobacterium Nostoc minutum. J. Nat. Prod. 1997, 60, 158–161. [Google Scholar] [CrossRef]
- Georgousaki, K.; DePedro, N.; Chinchilla, A.; Aliagiannis, N.; Vicente, F.; de Castro, P.; Fotinos, S.; Genilloud, O.; Fokialakis, N. High throughput screening of microbial biodiversity for the discovery of novel cosmeceutical agents. Panta Med. 2015. [Google Scholar] [CrossRef]
- Georgousaki, K.; Tsafantakis, N.; Cheilari, A.; Gumeni, S.; González, I.; González, V.; José, R.T.; Genilloud, O.; Fotinos, S.; Trougakos, I.; et al. Discovery of novel cosmeuceutical agents from endophytic microorganisms of Spanish biodiversity. Planta Med. 2017. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010. [Google Scholar] [CrossRef] [PubMed]
- Wedel, S.; Manola, M.; Cavinato, M.; Trougakos, I.P.; Jansen-Durr, P. Targeting protein quality control mechanisms by natural products to promote healthy ageing. Molecules 2018, 23, 1219. [Google Scholar] [CrossRef]
- Morimoto, R.I.; Cuervo, A.M. Proteostasis and the aging proteome in health and disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, C.H. Proteasome regulators: activators and inhibitors. Curr. Med. Chem. 2009, 16, 931–939. [Google Scholar] [CrossRef]
- Peyrat, L.A.; Eparvier, V.; Eydoux, C.; Guillemot, J.C.; Litaudon, M.; Stien, D. Betulinic acid, the first lupane-type triterpenoid isolated from both a Phomopsis sp. and its host plant Diospyros carbonaria Benoist. Chem. Biodivers. 2017. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).