Insights into Triterpene Acids in Fermented Mycelia of Edible Fungus Poria cocos by a Comparative Study
Abstract
:1. Introduction
2. Results
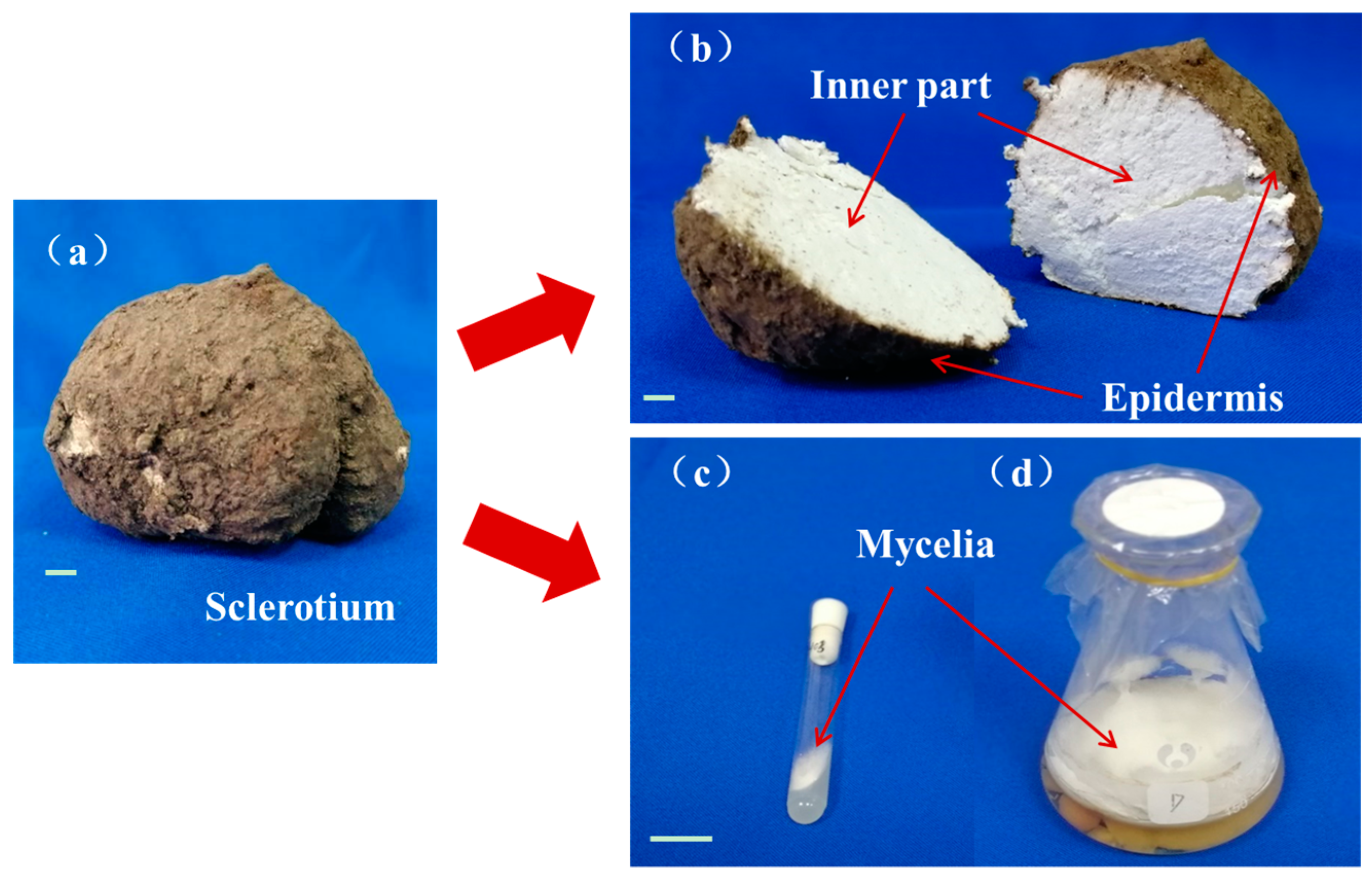
2.1. The Natural and Fermented Parts of Poria Cocos
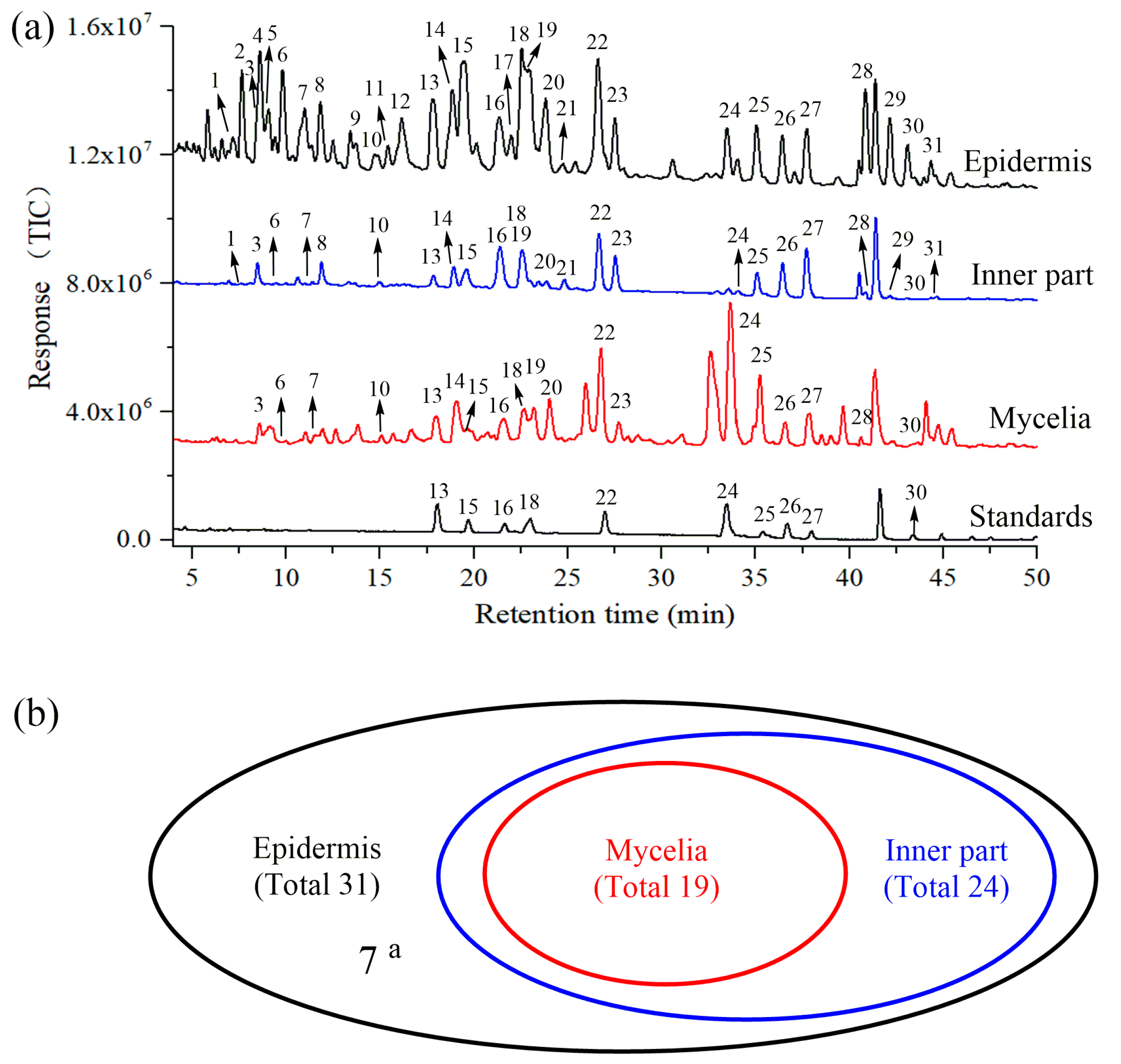
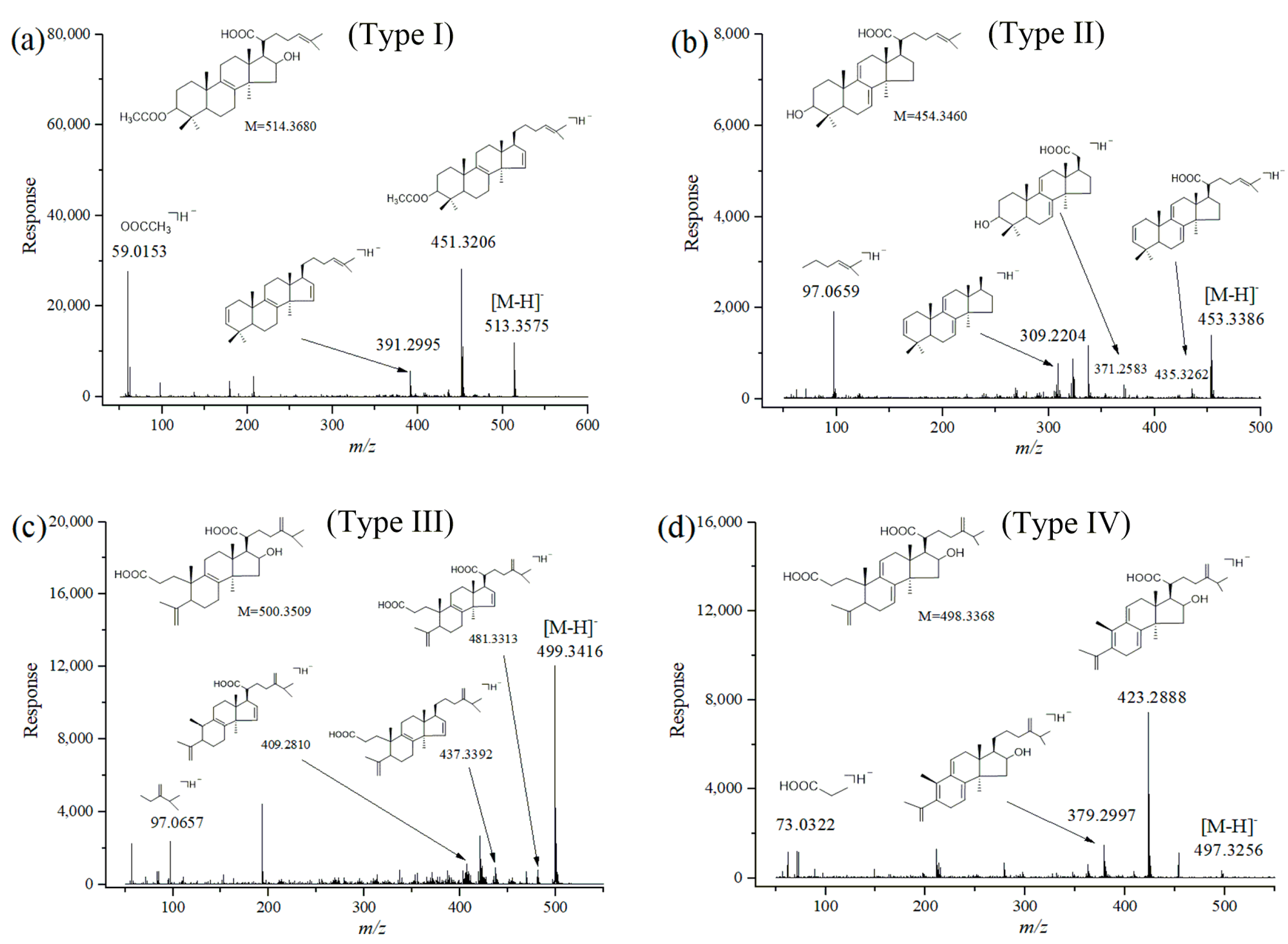
2.2. Identification of Triterpene Acids in Mycelia, Epidermis and the Inner Part
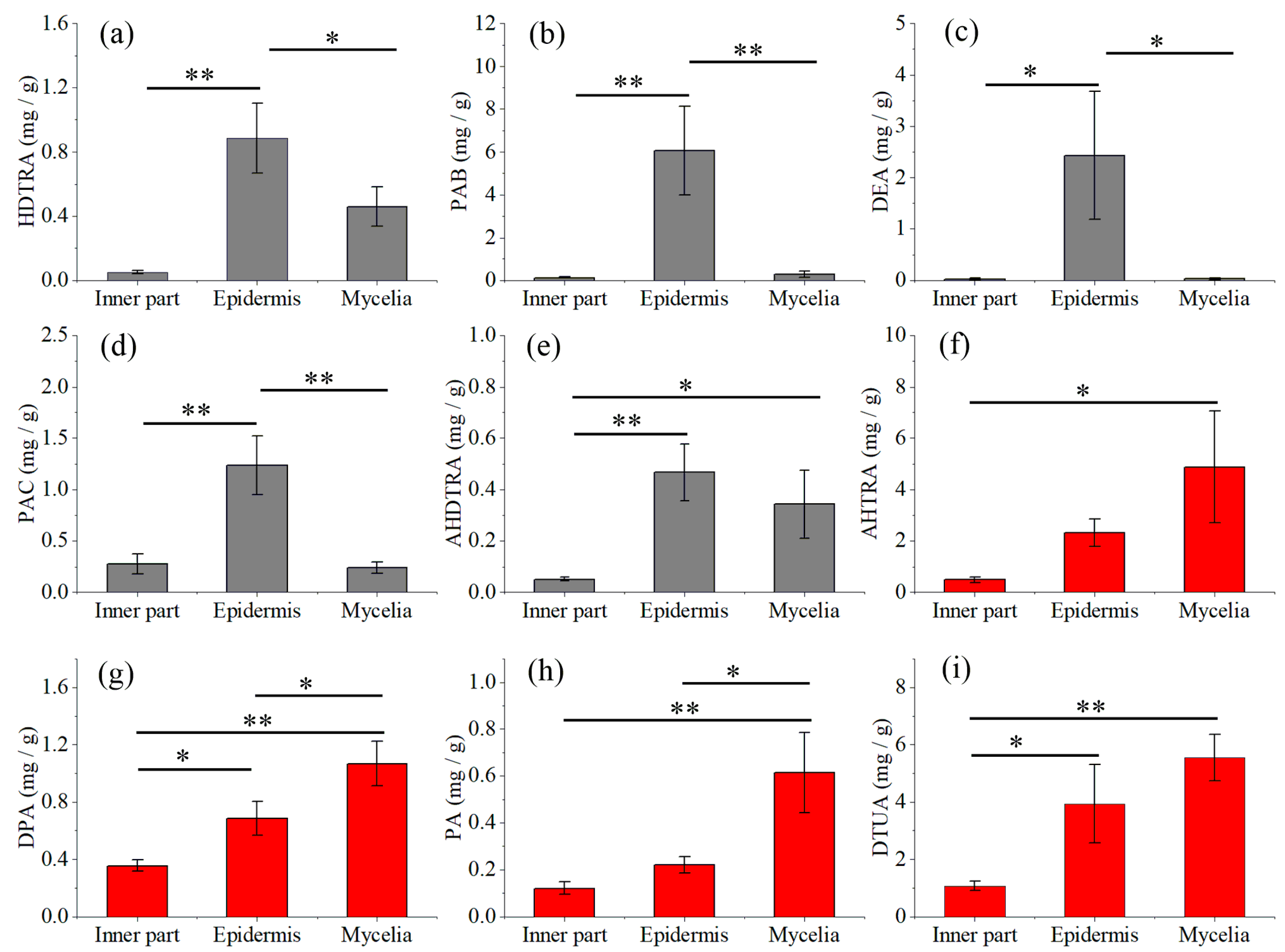
2.3. Quantitative Analysis of Nine Triterpene Acids in Mycelia, Epidermis and the Inner Part
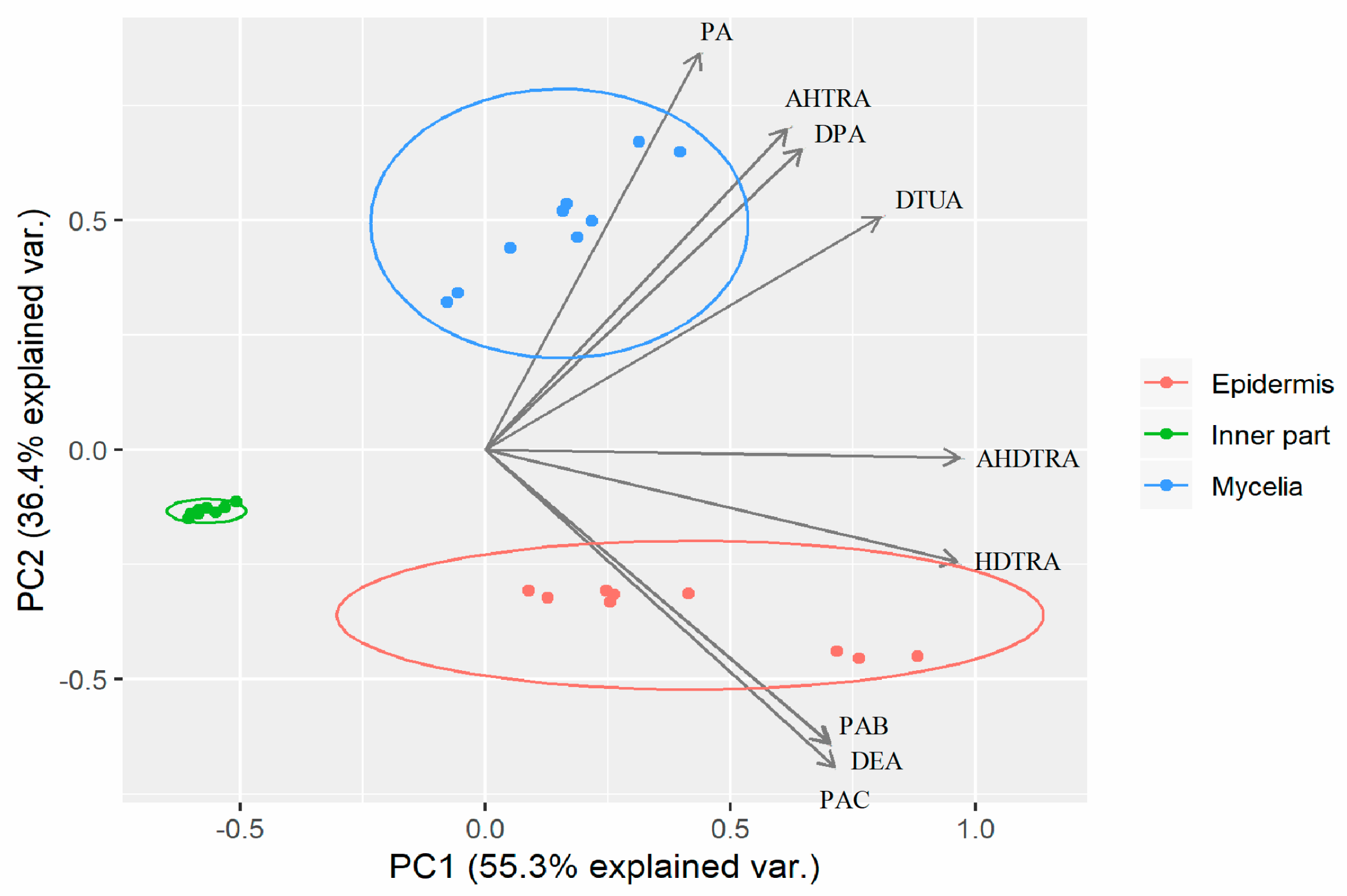
2.4. Principal Component Analysis of Mycelia, Epidermis and the Inner Part
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection of Sclerotia of Poria cocos
4.3. Strain Isolation
4.4. Mycelia Fermentation
4.5. Extraction of Triterpene Acids
4.6. HPLC-QTOF-MS/MS Analysis
4.7. Quantitative Characterization of Triterpene Acids
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.Z.; Zhang, J.; Zhao, Y.L.; Li, T.; Shen, T.; Li, J.Q.; Li, W.Y.; Liu, H.G. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb: A review. J. Ethnopharmacol. 2013, 147, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, E.; Yang, S.-M.; Ryoo, R.; Moon, E.; Kim, S.-H.; Kim, K.H. Bioactive compounds from sclerotia extract of Poria cocos that control adipocyte and osteoblast differentiation. Bioorg. Chem. 2018, 81, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhou, Y.; Tan, H.S.; Ding, L.S.; Xu, H.X. Advanced ultra-performance liquid chromatography-photodiode array-quadrupole time-of-flight mass spectrometric methods for simultaneous screening and quantification of triterpenoids in Poria cocos. Food Chem. 2014, 152, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT Food Sci. Technol. 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Feng, G.F.; Zheng, Y.; Sun, Y.; Liu, S.; Pi, Z.F.; Song, F.R.; Liu, Z.Q. A targeted strategy for analyzing untargeted mass spectral data to identify lanostane–type triterpene acids in Poria cocos by integrating a scientific information system and liquid chromatography–tandem mass spectrometry combined with ion mobility spectrometry. Anal. Chim. Acta 2018, 1033, 87–99. [Google Scholar] [PubMed]
- Feng, Y.-L.; Lei, P.; Tian, T.; Yin, L.; Chen, D.-Q.; Chen, H.; Mei, Q.; Zhao, Y.-Y.; Lin, R.-C. Diuretic activity of some fractions of the epidermis of Poria cocos. J. Ethnopharmacol. 2013, 150, 1114–1118. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Li, H.-T.; Feng, Y.-L.; Bai, X.; Lin, R.-C. Urinary metabonomic study of the surface layer of Poria cocos as an effective treatment for chronic renal injury in rats. J. Ethnopharmacol. 2013, 148, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Nakamura, Y.; Tokuda, H.; Uchiyama, E.; Suzuki, T.; Kimura, Y.; Uchikura, K.; Nishino, H. Triterpene acids from Poria cocos and their anti-tumor-promoting effects. J. Nat. Prod. 2007, 70, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.G.; Cai, Y. Triterpenes from the fungus Poria cocos and their inhibitory activity on nitric oxide production in mouse macrophages via blockade of activating protein-1 pathway. Chem. Biodivers. 2011, 8, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.; Lee, S.O.; Ryu, J.Y.; Choi, S.Z.; Kang, K.S.; Kim, K.H. Anti-inflammatory activity of the sclerotia of edible fungus, Poria cocos Wolf and their active lanostane triterpenoids. J. Funct Foods 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhang, W.; Liu, R.-S.; Zhu, L.-W.; Zhong, J.-J. Scale-up study on the fed-batch fermentation of Ganoderma lucidum for the hyperproduction of ganoderic acid and Ganoderma polysaccharides. Process. Biochem. 2011, 46, 404–408. [Google Scholar] [CrossRef]
- Zheng, P.; Xia, Y.; Xiao, G.; Xiong, C.; Hu, X.; Zhang, S.; Zheng, H.; Huang, Y.; Zhou, Y.; Wang, S.; Zhao, G.-P.; Liu, X.; St Leger, R.J.; Wang, C. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional chinese medicine. Genome Biol. 2011, 12, R116. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-K.; Cheng, J.-J.; Lin, C.-Y.; Chang, C.-C. Purification, structural elucidation, and anti-inflammatory effect of a water-soluble 1,6-branched 1,3-α-d-galactan from cultured mycelia of Poria cocos. Food Chem. 2010, 118, 349–356. [Google Scholar] [CrossRef]
- Hu, G.-S.; Huang, C.-G.; Zhang, Y.; Xiao, W.; Jia, J.-M. Accumulation of biomass and four triterpenoids in two-stage cultured Poria cocos mycelia and diuretic activity in rats. Chinese J. Nat. Med. 2017, 15, 265–270. [Google Scholar] [CrossRef]
- Zhong, J.J.; Xiao, J.H. Secondary metabolites from higher fungi: discovery, bioactivity, and bioproduction. Adv. Biochem. Eng. Biotechnol. 2009, 113, 79–150. [Google Scholar] [PubMed]
- Wang, W.; Dong, H.; Yan, R.; Li, H.; Li, P.; Chen, P.; Yang, B.; Wang, Z. Comparative study of lanostane-type triterpene acids in different parts of Poria cocos (Schw.) Wolf by UHPLC–Fourier transform MS and UHPLC-triple quadruple MS. J. Pharm. Biomed. Anal. 2015, 102, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-X.; Xu, J.; Wang, R.-J.; Li, H.-X.; Tan, Y.-Z.; Chen, H.-B.; Dong, X.-P.; Zhao, Z.-Z. Correlation between quality and geographical origins of Poria cocos revealed by qualitative fingerprint profiling and quantitative determination of triterpenoid acids. Molecules 2018, 23, 2200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, J.; Zhang, S.; Wang, R.; Huang, Q.; Chen, H.; Dong, X.; Zhao, Z. Qualitatively and quantitatively comparing secondary metabolites in three medicinal parts derived from Poria cocos (Schw.) Wolf using UHPLC-QTOF-MS/MS-based chemical profiling. J. Pharm. Biomed. Anal. 2018, 150, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-J.; Xue, Z.-Z.; Geng, Y.-L.; Wang, X.; Yang, B. Lanostane triterpenes isolated from epidermis of Poria cocos. Phytochem. Lett. 2017, 22, 102–106. [Google Scholar] [CrossRef]
- Yun, L.; Mingcang, C.; Ke, W.; Zhaolin, S.; Zhixiong, L.; Bin, W.; Chenggang, H. Systematic screening and characterization of the major bioactive components of Poria cocos and their metabolites in rats by LC-ESI-MS(n). Biomed. Chromatogr. 2012, 26, 1109–1117. [Google Scholar]
- Shah, V.K.; Choi, J.J.; Han, J.-Y.; Lee, M.K.; Hong, J.T.; Oh, K.-W. Pachymic acid enhances pentobarbital-induced sleeping behaviors via GABAA-ergic systems in mice. Biomol. Ther. (Seoul) 2014, 22, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xu, X.; Jiang, N.; Wei, W.; Li, F.; He, L.; Luo, X. Dehydropachymic acid decreases bafilomycin A1 induced β-Amyloid accumulation in PC12 cells. J. Ethnopharmacol. 2017, 198, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Yue, K.; Ye, M.; Lin, X.; Zhou, Z. The artificial cultivation of medicinal caterpillar fungus, Ophiocordyceps sinensis (Ascomycetes): A review. Int. J. Med. Mushrooms 2013, 15, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kan, L.; Nie, S.; Chen, H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured Cordyceps sinensis. LWT Food Sci. Technol. 2015, 63, 2–7. [Google Scholar] [CrossRef]
- Cui, J.D. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit. Rev. Biotechnol. 2015, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Gaosheng, H.U. Dynamic accumulation of three main triterpenic acids in submerged cultivation mycelium of Poria cocos. China J. Chin. Mater. Med. 2013, 38, 1355–1359. [Google Scholar]
- Jin, J.; Kang, W.; Zhong, C.; Qin, Y.; Zhou, R.; Liu, H.; Xie, J.; Chen, L.; Qin, Y.; Zhang, S. The pharmacological properties of Ophiocordyceps xuefengensis revealed by transcriptome analysis. J. Ethnopharmacol. 2018, 219, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lao, J.; Zhou, R.; He, W.; Qin, Y.; Zhong, C.; Xie, J.; Liu, H.; Wan, D.; Zhang, S.; Qin, Y. Simultaneous identification and dynamic analysis of saccharides during steam processing of rhizomes of Polygonatum cyrtonema by HPLC–QTOF–MS/MS. Molecules 2018, 23, 2855. [Google Scholar] [CrossRef]
- Wu, T.; Taylor, C.; Nebl, T.; Ng, K.; Bennett, L.E. Effects of chemical composition and baking on in vitro digestibility of proteins in breads made from selected gluten-containing and gluten-free flours. Food Chem. 2017, 233, 514–524. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compounds | Range (μg/mL) | Regression Equation a | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| HDTRA | 7.2–143.3 | y = 0.0463x + 0.2607 | 0.999 | 0.018 | 0.061 |
| PAB | 17.3–145.6 | y = 0.0767x + 0.3884 | 0.999 | 0.031 | 0.103 |
| DTUA | 7.1–141.1 | y = 0.1353x − 0.0356 | 0.998 | 0.056 | 0.187 |
| PAC | 6.6–133.3 | y = 0.0529x + 0.1776 | 0.998 | 0.023 | 0.077 |
| AHDTRA | 5.1–102.2 | y = 0.0540x + 0.3526 | 0.992 | 0.350 | 1.166 |
| AHTRA | 7.0–140.0 | y = 0.2383x + 3.2067 | 0.999 | 0.020 | 0.067 |
| DPA | 6.7–135.6 | y = 0.0535x − 0.488 | 0.997 | 0.024 | 0.081 |
| PA | 4.8–97.7 | y = 0.0186x + 0.1617 | 0.999 | 0.016 | 0.054 |
| DEA | 6.4–128.9 | y = 0.0474x + 30.2911 | 0.999 | 0.016 | 0.055 |
| Compounds | Precision (n = 6) | Stability (48 h) (RSD, %) | Recovery (n = 3) | ||
|---|---|---|---|---|---|
| Intra-day (RSD, %) | Inter-day (RSD, %) | Mean (%) | RSD (%) | ||
| HDTRA | 1.53 | 2.53 | 3.02 | 92.14 | 8.91 |
| PAB | 1.69 | 3.46 | 4.81 | 95.03 | 6.45 |
| DTUA | 1.80 | 2.87 | 2.52 | 91.51 | 7.38 |
| PAC | 1.63 | 2.92 | 2.89 | 94.62 | 8.01 |
| AHDTRA | 3.21 | 4.15 | 4.11 | 92.78 | 7.49 |
| AHTRA | 1.45 | 2.38 | 2.91 | 96.16 | 5.71 |
| DPA | 2.65 | 3.10 | 3.37 | 93.42 | 8.05 |
| PA | 1.42 | 2.46 | 3.64 | 90.25 | 9.42 |
| DEA | 2.28 | 3.98 | 4.25 | 91.06 | 8.76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Zhou, R.; Xie, J.; Ye, H.; Liang, X.; Zhong, C.; Shen, B.; Qin, Y.; Zhang, S.; Huang, L. Insights into Triterpene Acids in Fermented Mycelia of Edible Fungus Poria cocos by a Comparative Study. Molecules 2019, 24, 1331. https://doi.org/10.3390/molecules24071331
Jin J, Zhou R, Xie J, Ye H, Liang X, Zhong C, Shen B, Qin Y, Zhang S, Huang L. Insights into Triterpene Acids in Fermented Mycelia of Edible Fungus Poria cocos by a Comparative Study. Molecules. 2019; 24(7):1331. https://doi.org/10.3390/molecules24071331
Chicago/Turabian StyleJin, Jian, Rongrong Zhou, Jing Xie, Huixuan Ye, Xuejuan Liang, Can Zhong, Bingbing Shen, You Qin, Shuihan Zhang, and Luqi Huang. 2019. "Insights into Triterpene Acids in Fermented Mycelia of Edible Fungus Poria cocos by a Comparative Study" Molecules 24, no. 7: 1331. https://doi.org/10.3390/molecules24071331
APA StyleJin, J., Zhou, R., Xie, J., Ye, H., Liang, X., Zhong, C., Shen, B., Qin, Y., Zhang, S., & Huang, L. (2019). Insights into Triterpene Acids in Fermented Mycelia of Edible Fungus Poria cocos by a Comparative Study. Molecules, 24(7), 1331. https://doi.org/10.3390/molecules24071331






