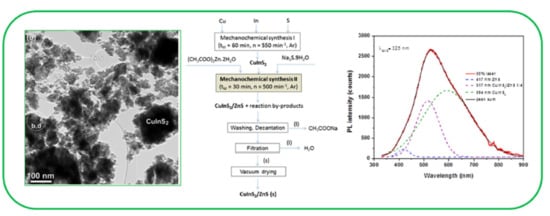
Mechanochemical Synthesis and Characterization of CuInS2/ZnS Nanocrystals
Abstract
1. Introduction
2. Results and Discussion
2.1. Why Mechanochemistry?
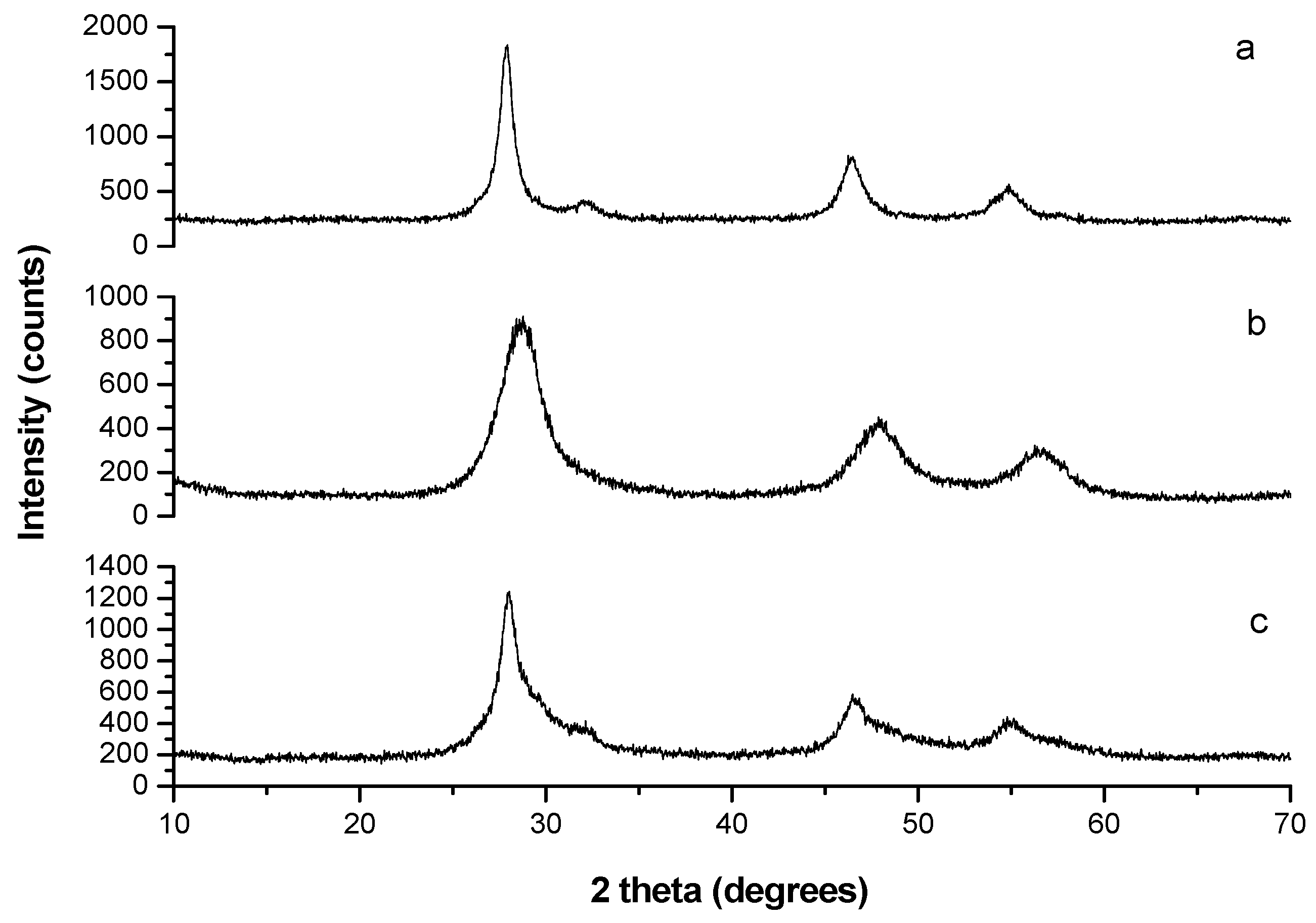
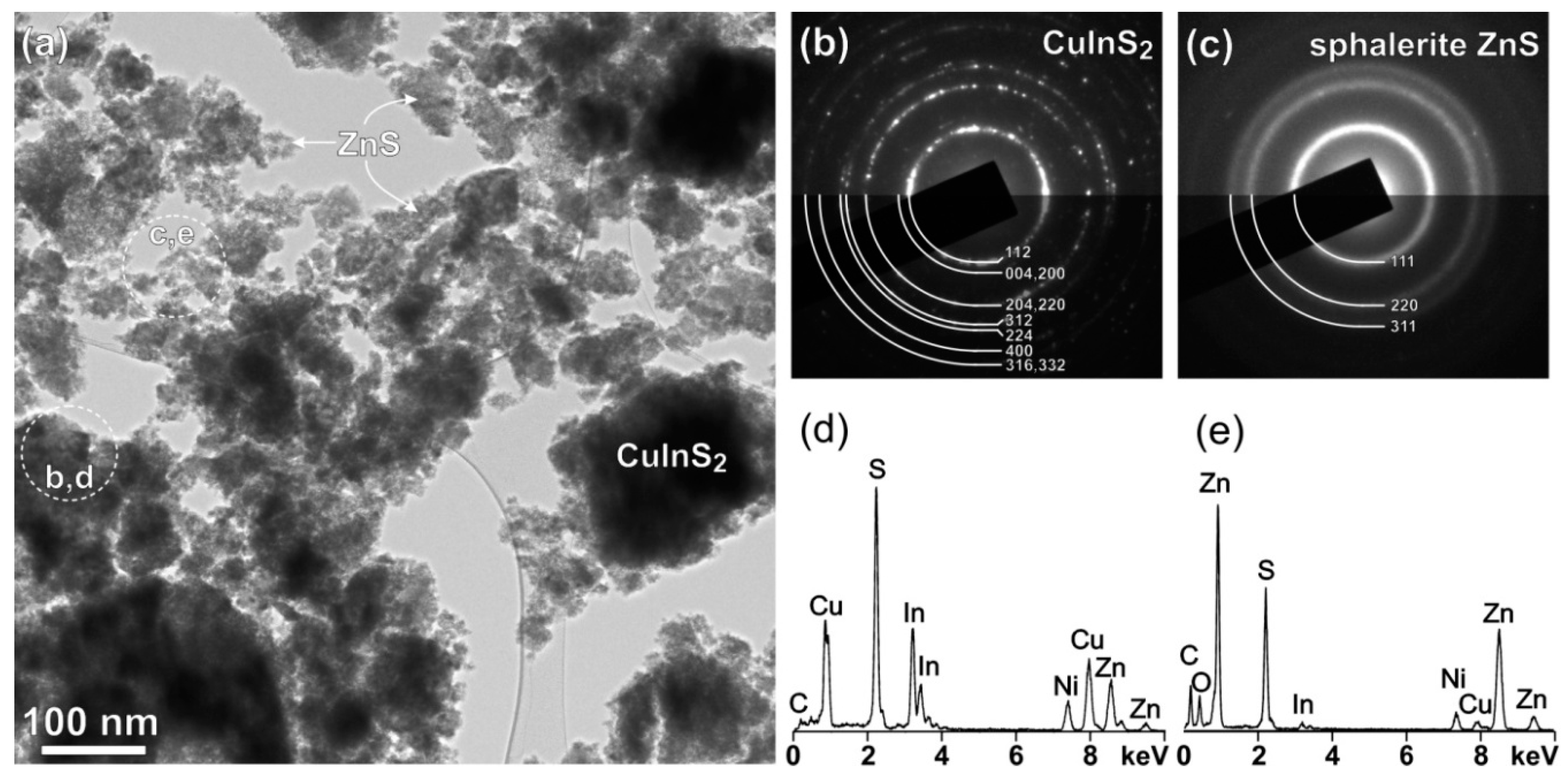
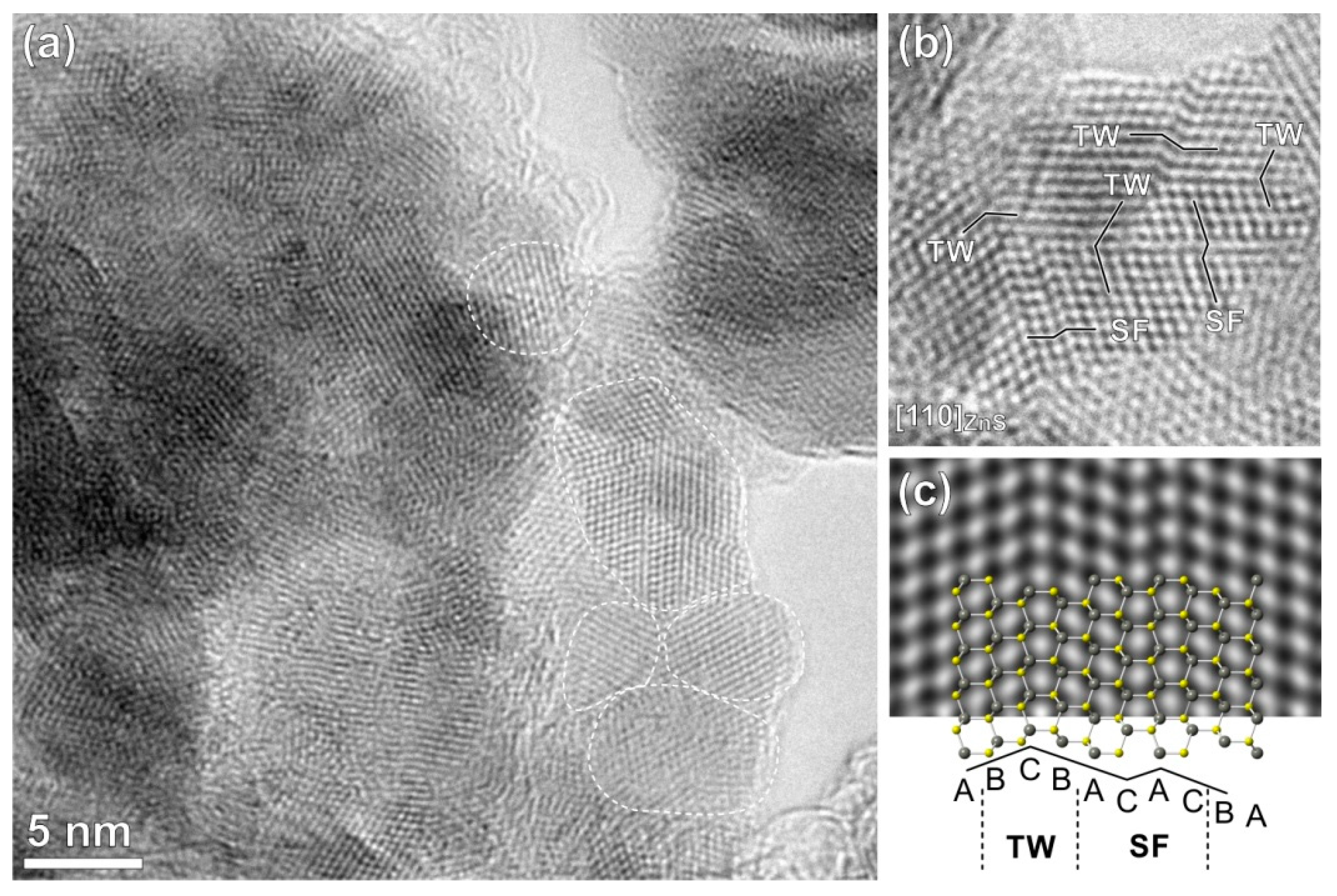
2.2. Structural Characterization
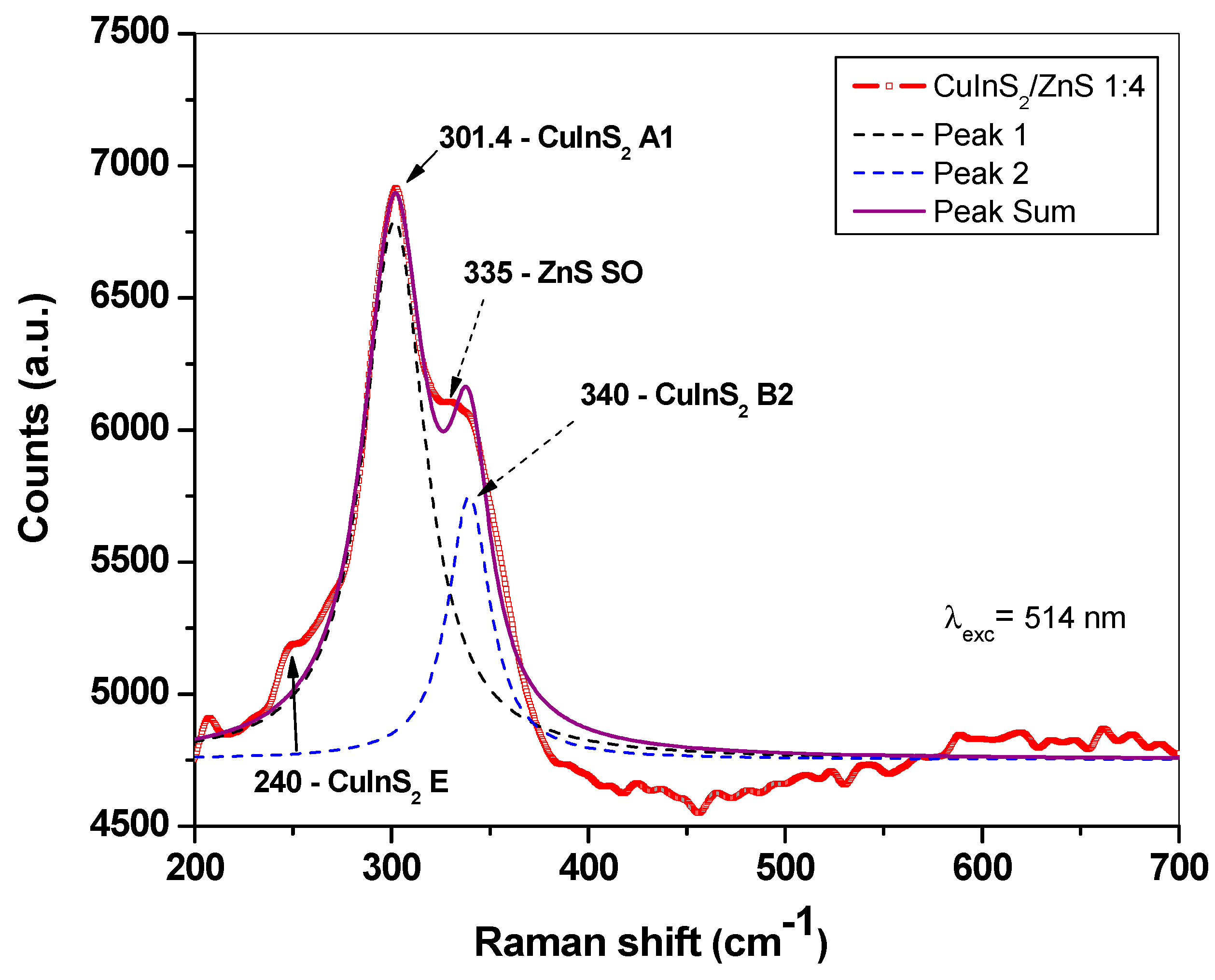
2.3. Optical Properties
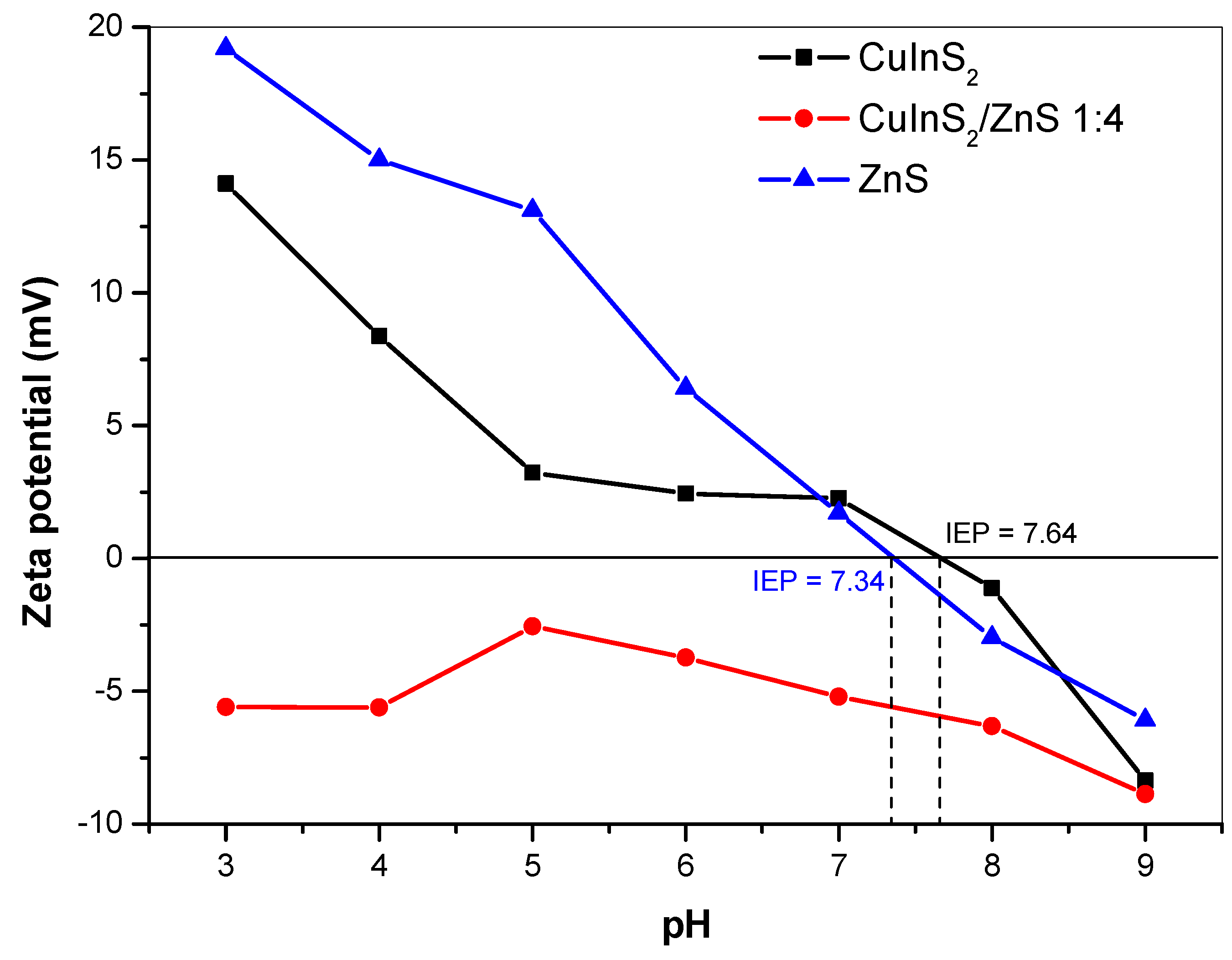
2.4. Surface Properties
3. Materials and Methods
3.1. Mechanochemical Synthesis of CuInS2/ZnS Nanocrystals
3.2. Characterization Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aldakov, D.; Lefrancois, A.; Reiss, P. Ternary and quaternary metal chalcogenide nanocrystals: Synthesis, properties and applications. J.Mater. Chem. C 2013, 1, 3756–3776. [Google Scholar] [CrossRef]
- Ye, E.Y.; Zhang, S.Y.; Lim, S.H.; Bosman, M.; Zhang, Z.H.; Win, K.Y.; Han, M.Y. Ternary Cobalt-Iron Phosphide Nanocrystals with Controlled Compositions, Properties, and Morphologies from Nanorods and Nanorice to Split Nanostructures. Chem. A Eur. J. 2011, 17, 5982–5988. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.J.; Wu, L.; Yu, S.H. Energetic I-III-VI2 and I-2-II-IV-VI4 nanocrystals: Synthesis, photovoltaic and thermoelectric applications. Energy Environ. Sci. 2014, 7, 190–208. [Google Scholar] [CrossRef]
- Regulacio, M.D.; Han, M.Y. Multinary I-III-VI2 and I-2-II-IV-VI4 Semiconductor Nanostructures for Photocatalytic Applications. Acc. Chem. Res. 2016, 49, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Regulacio, M.D.; Win, K.Y.; Lo, S.L.; Zhang, S.Y.; Zhang, X.H.; Wang, S.; Han, M.Y.; Zheng, Y.G. Aqueous synthesis of highly luminescent AgInS2-ZnS quantum dots and their biological applications. Nanoscale 2013, 5, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.P.; Xie, Y.; Tang, R.; Qian, Y.T. Synthesis and characterization of ternary CuInS2 nanorods via a hydrothermal route. J. Solid State Chem. 2001, 161, 179–183. [Google Scholar] [CrossRef]
- Batabyal, S.K.; Tian, L.; Venkatram, N.; Ji, W.; Vittal, J.J. Phase-Selective Synthesis of CuInS2 Nanocrystals. J. Phys. Chem. C 2009, 113, 15037–15042. [Google Scholar] [CrossRef]
- Li, D.S.; Zou, Y.; Yang, D.R. Controlled synthesis of luminescent CuInS2 nanocrystals and their optical properties. J. Lumin. 2012, 132, 313–317. [Google Scholar] [CrossRef]
- Klenk, R.; Klaer, J.; Scheer, R.; Lux-Steiner, M.C.; Luck, I.; Meyer, N.; Ruhle, U. Solar cells based on CuInS2-an overview. Thin Solid Films 2005, 480, 509–514. [Google Scholar] [CrossRef]
- Xie, R.G.; Rutherford, M.; Peng, X.G. Formation of High-Quality I-III-VI Semiconductor Nanocrystals by Tuning Relative Reactivity of Cationic Precursors. J. Am. Chem. Soc. 2009, 131, 5691–5697. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.L.; Bailey, S.G.; Raffaelle, R.P.; Banger, K.K.; Hepp, A.F. Nanocrystalline chalcopyrite materials (CuInS2 and CuInSe2) via low-temperature pyrolysis of molecular single-source precursors. Chem. Mater. 2003, 15, 3142–3147. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.W. CuInS2/ZnS core/shell quantum dots by cation exchange and their blue-shifted photoluminescence. J. Mater. Chem. 2011, 21, 3745–3750. [Google Scholar] [CrossRef]
- Nakamura, H.; Kato, W.; Uehara, M.; Nose, K.; Omata, T.; Otsuka-Yao-Matsuo, S.; Miyazaki, M.; Maeda, H. Tunable photoluminescence wavelength of chalcopyrite CuInS2-based semiconductor nanocrystals synthesized in a colloidal system. Chem. Mater. 2006, 18, 3330–3335. [Google Scholar] [CrossRef]
- Yue, W.J.; Han, S.K.; Peng, R.X.; Shen, W.; Geng, H.W.; Wu, F.; Tao, S.W.; Wang, M.T. CuInS2 quantum dots synthesized by a solvothermal route and their application as effective electron acceptors for hybrid solar cells. J. Mater. Chem. 2010, 20, 7570–7578. [Google Scholar] [CrossRef]
- Chuang, P.H.; Lin, C.C.; Liu, R.S. Emission-Tunable CuInS2/ZnS Quantum Dots: Structure, Optical Properties, and Application in White Light-Emitting Diodes with High Color Rendering Index. ACS Appl. Mater. Interfaces 2014, 6, 15379–15387. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Han, J.Y.; Kang, D.S.; Kim, S.W.; Jang, D.S.; Suh, M.; Kirakosyan, A.; Jeon, D.Y. Characteristics of CuInS2/ZnS quantum dots and its application on LED. J. Cryst. Growth 2011, 326, 90–93. [Google Scholar] [CrossRef]
- Lee, J.; Han, C.S. Large-scale synthesis of highly emissive and photostable CuInS2/ZnS nanocrystals through hybrid flow reactor. Nanoscale Res. Lett. 2014, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.S.; Chen, N.; Tu, Y.; Dong, C.H.; Zhang, B.B.; Hu, C.H.; Chang, J. Synthesis of Zn-Cu-In-S/ZnS Core/Shell Quantum Dots with Inhibited Blue-Shift Photoluminescence and Applications for Tumor Targeted Bioimaging. Theranostics 2013, 3, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.N.; Chen, Z.; Cao, C.B. Preparation of magnetic CuInS2-ZnS nanocomposites for bioimaging. Mater. Lett. 2014, 120, 50–53. [Google Scholar] [CrossRef]
- Tang, X.S.; Cheng, W.L.; Choo, E.S.G.; Xue, J.M. Synthesis of CuInS2-ZnS alloyed nanocubes with high luminescence. Chem. Commun. 2011, 47, 5217–5219. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.E.; Song, W.S.; Yang, H. Noninjection, one-pot synthesis of Cu-deficient CuInS2/ZnS core/shell quantum dots and their fluorescent properties. J. Colloid Interface Sci. 2011, 361, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.T.; Chen, S.Y.; Cheng, B.M.; Lin, C.C. Synthesis and characterization of highly luminescent CuInS2 and CuInS2/ZnS (core/shell) nanocrystals. Thin Solid Films 2008, 517, 1257–1261. [Google Scholar] [CrossRef]
- Li, J.; Kempken, B.; Dzhagan, V.; Zahn, D.R.T.; Grzelak, J.; Mackowski, S.; Parisi, J.; Kolny-Olesiak, J. Alloyed CuInS2-ZnS nanorods: Synthesis, structure and optical properties. Crystengcomm 2015, 17, 5634–5643. [Google Scholar] [CrossRef]
- Balaz, P.; Achimovičová, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Balaz, P.; Balaz, M.; Achimovicova, M.; Bujinakova, Z.; Dutkova, E. Chalcogenide mechanochemistry in materials science: Insight into synthesis and applications (a review). J.Mater. Sci. 2017, 52, 11851–11890. [Google Scholar] [CrossRef]
- Sepelak, V.; Duvel, A.; Wilkening, M.; Becker, K.D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef] [PubMed]
- Balaz, P.; Sayagues, M.J.; Balaz, M.; Zorkovska, A.; Hronec, P.; Kovac, J.; Kovac, J.; Dutkova, E.; Mojzisova, G.; Mojzis, J. CdSe@ZnS nanocomposites prepared by a mechanochemical route: No release of Cd2+ ions and negligible in vitro cytotoxicity. Mater. Res. Bull. 2014, 49, 302–309. [Google Scholar] [CrossRef]
- Bujnakova, Z.; Balaz, M.; Dutkova, E.; Balaz, P.; Kello, M.; Mojzisova, G.; Mojzis, J.; Vilkova, M.; Imrich, J.; Psotka, M. Mechanochemical approach for the capping of mixed core CdS/ZnS nanocrystals: Elimination of cadmium toxicity. J. Colloid Interface Sci. 2017, 486, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Bujnakova, Z.; Dutkova, E.; Zorkovska, A.; Balaz, M.; Kovac, J.; Kello, M.; Mojzis, J.; Briancin, J.; Balaz, P. Mechanochemical synthesis and in vitro studies of chitosan-coated InAs/ZnS mixed nanocrystals. J. Mater. Sci. 2017, 52, 721–735. [Google Scholar] [CrossRef]
- Bujnakova, Z.; Balaz, M.; Zduriencikova, M.; Sedlak, J.; Caplovicova, M.; Caplovic, L.; Dutkova, E.; Zorkovska, A.; Turianicova, E.; Balaz, P.; et al. Preparation, properties and anticancer effects of mixed As4S4/ZnS nanoparticles capped by Poloxamer 407. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 71, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Bujnakova, Z.; Dutkova, E.; Kello, M.; Mojzis, J.; Balaz, M.; Balaz, P.; Shpotyuk, O. Mechanochemistry of Chitosan-Coated Zinc Sulfide (ZnS) Nanocrystals for Bio-imaging Applications. Nanoscale Res. Lett. 2017, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Balaz, P.; Balaz, M.; Dutkova, E.; Zorkovska, A.; Kovac, J.; Hronec, P.; Kovac, J.; Caplovicova, M.; Mojzis, J.; Mojzisova, G.; et al. CdS/ZnS nanocomposites: From mechanochemical synthesis to cytotoxicity issues. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 58, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Zhong, H.Z.; Zhang, W.Q.; Tan, Z.A.; Li, Y.F.; Yu, C.R.; Zhai, T.Y.; Bando, Y.S.; Yang, S.Y.; Zou, B.S. Highly Emissive and Color-Tunable CuInS2-Based Colloidal Semiconductor Nanocrystals: Off-Stoichiometry Effects and Improved Electroluminescence Performance. Adv. Funct. Mater. 2012, 22, 2081–2088. [Google Scholar] [CrossRef]
- Zhang, A.D.; Dong, C.Q.; Li, L.; Yin, J.J.; Liu, H.; Huang, X.Y.; Ren, J.C. Non-blinking (Zn)CuInS/ZnS Quantum Dots Prepared by In Situ Interfacial Alloying Approach. Sci. Rep. 2015, 5, 15227. [Google Scholar] [CrossRef] [PubMed]
- Balaz, P.; Hegedus, M.; Achimovicova, M.; Balaz, M.; Tesinsky, M.; Dutkova, E.; Kanuchova, M.; Briancin, J. Semi-industrial Green Mechanochemical Syntheses of Solar Cell Absorbers Based on Quaternary Sulfides. ACS Sustain. Chem. Eng. 2018, 6, 2132–2141. [Google Scholar] [CrossRef]
- Hegedus, M.; Balaz, M.; Tesinsky, M.; Sayagues, M.J.; Siffalovic, P.; Krulakova, M.; Kanuchova, M.; Briancin, J.; Fabian, M.; Balaz, P. Scalable synthesis of potential solar cell absorber Cu2SnS3 (CTS) from nanoprecursors. J. Alloys Compd. 2018, 768, 1006–1015. [Google Scholar] [CrossRef]
- Godocikova, E.; Balaz, P.; Gock, E.; Choi, W.S.; Kim, B.S. Mechanochemical synthesis of the nanocrystalline semiconductors in an industrial mill. Powder Technol. 2006, 164, 147–152. [Google Scholar] [CrossRef]
- Li, L.; Daou, T.J.; Texier, I.; Tran, T.K.C.; Nguyen, Q.L.; Reiss, P. Highly Luminescent CuInS2/ZnS Core/Shell Nanocrystals: Cadmium-Free Quantum Dots for In Vivo Imaging. Chem. Mater. 2009, 21, 2422–2429. [Google Scholar] [CrossRef]
- Benchikhi, M.; El Ouatib, R.; Guillemet-Fritsch, S.; Chane-Ching, J.Y.; Demai, J.J.; Er-Rakho, L.; Durand, B. Synthesis of CuInS2 nanometric powder by reaction in molten KSCN. Mater. Lett. 2014, 136, 431–434. [Google Scholar] [CrossRef]
- Dutková, E.; Sayagues, M.J.; Briancin, J.; Zorkovska, A.; Bujnakova, Z.; Kovac, J.; Kovac, J.J.; Balaz, P.; Ficeriova, J. Synthesis and characterization of CuInS2 nanocrystalline semiconductor prepared by high-energy milling. J. Mater. Sci. 2016, 51, 1978–1984. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Jin, C.Q.; Gao, F.; Wu, X.L.; Zhong, W.; Li, S.H.; Chu, P.K. Raman scattering study of zinc blende and wurtzite ZnS. J. Appl. Phys. 2009, 106, 123505. [Google Scholar] [CrossRef]
- Kim, J.H.; Rho, H.; Kim, J.; Choi, Y.J.; Park, J.G. Raman spectroscopy of ZnS nanostructures. J. Raman Spectrosc. 2012, 43, 906–910. [Google Scholar] [CrossRef]
- Srot, V.; Recnik, A.; Scheu, C.; Sturm, S.; Mirtic, B. Stacking faults and twin boundaries in sphalerite crystals from the Trepca mines in Kosovo. Am. Mineral. 2003, 88, 1809–1816. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Mange, Y.J.; Dewi, M.; McFadden, A.; Skinner, W.M.; Nann, T. Cation Exchange of green chalcopyrite CuInS2 quantum dots. Cryst. Eng. Com. 2014, 16, 9455–9460. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Cho, S.; Kim, S.; Park, I.Y.; Choi, Y.D. Role of growth parameters on structural and optical properties of ZnS nanocluster thin films grown by solution growth technique. Mater. Chem. Phys. 2003, 77, 254–260. [Google Scholar] [CrossRef]
- Fu, M.; Luan, W.; Tu, S.T.; Mleczko, L. Green Synthesis of CuInS2/ZnS Nanocrystals with High Photoluminescence and Stability. J. Nanomater. 2015, 16, 401. [Google Scholar] [CrossRef]
- Akaki, Y.; Ohno, Y.; Momiki, T. Synthesis of porous CuInS2 crystals using a stirrer. Phys. Status Solidi C Curr. Top. Solid State Phys. 2013, 10, 1009–1011. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Perez-Ramirez, J. Pore size determination in modified micro-and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Tomic, S.; Bernasconi, L.; Searle, B.G.; Harrison, N.M. Electronic and Optical Structure of Wurtzite CuInS2. J. Phys. Chem. C 2014, 118, 14478–14484. [Google Scholar] [CrossRef]
- Liu, J.C.; Huang, C.P. Electrokinetic Characteristics of Some Metal Sulfide Water Interfaces. Langmuir 1992, 8, 1851–1856. [Google Scholar] [CrossRef]
- Pugh, R.J.; Tjus, K. Electrokinetic studies on Cu(II) hydroxy coated zincsulfide particles. J. Colloid Interface Sci. 1987, 117, 231–241. [Google Scholar] [CrossRef]
- Williams, R.; Labib, M.E. Zinc-Sulfide Surface-Chemistry-an Electrokinetic Study. J. Colloid Interface Sci. 1985, 106, 251–254. [Google Scholar] [CrossRef]
- Dutkova, E.; Balaz, P.; Pourghahramani, P.; Balek, V.; Nguyen, A.V.; Satka, A.; Kovac, J.; Ficeriova, J. Mechanochemically synthesised ZnxCd1-xS nanoparticles for solar energy applications. J. Nano Res. 2012, 18–19, 247–256. [Google Scholar] [CrossRef]
- Wieczorek-Ciurowa, K.; Gamrat, K. Some aspects of mechanochemical reactions. Mater. Sci. Pol. 2007, 25, 219–232. [Google Scholar]
- Balaz, P.; Pourghahramani, P.; Achimovicova, M.; Dutkova, E.; Kovac, J.; Satka, A.; Jiang, J.Z. Mechanochemical synthesis and reactivity of PbS nanocrystals. J. Cryst. Growth 2011, 332, 1–6. [Google Scholar] [CrossRef]
- Balaz, P.; Boldizarova, E.; Godocikova, E.; Briancin, J. Mechanochemical route for sulphide nanoparticles preparation. Mater. Lett. 2003, 57, 1585–1589. [Google Scholar] [CrossRef]
- Dutkova, E.; Balaz, P.; Pourghahramani, P.; Velumani, S.; Ascencio, J.A.; Kostova, N.G. Properties of Mechanochemically Synthesized ZnS Nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 6600–6605. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Radius (Å) |
|---|---|---|---|
| CuInS2 | 6 | 2.599 × 10−2 | 8.40782 × 101 |
| CuInS2:ZnS 1:4 | 86 | 1.32 × 10−1 | 3.06475 × 101 |
| ZnS | 125 | 2.365 × 10−1 | 3.79239 × 101 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutková, E.; Daneu, N.; Lukáčová Bujňáková, Z.; Baláž, M.; Kováč, J.; Kováč, J., Jr.; Baláž, P. Mechanochemical Synthesis and Characterization of CuInS2/ZnS Nanocrystals. Molecules 2019, 24, 1031. https://doi.org/10.3390/molecules24061031
Dutková E, Daneu N, Lukáčová Bujňáková Z, Baláž M, Kováč J, Kováč J Jr., Baláž P. Mechanochemical Synthesis and Characterization of CuInS2/ZnS Nanocrystals. Molecules. 2019; 24(6):1031. https://doi.org/10.3390/molecules24061031
Chicago/Turabian StyleDutková, Erika, Nina Daneu, Zdenka Lukáčová Bujňáková, Matej Baláž, Jaroslav Kováč, Jaroslav Kováč, Jr., and Peter Baláž. 2019. "Mechanochemical Synthesis and Characterization of CuInS2/ZnS Nanocrystals" Molecules 24, no. 6: 1031. https://doi.org/10.3390/molecules24061031
APA StyleDutková, E., Daneu, N., Lukáčová Bujňáková, Z., Baláž, M., Kováč, J., Kováč, J., Jr., & Baláž, P. (2019). Mechanochemical Synthesis and Characterization of CuInS2/ZnS Nanocrystals. Molecules, 24(6), 1031. https://doi.org/10.3390/molecules24061031