Microfluidic Technologies for High Throughput Screening Through Sorting and On-Chip Culture of C. elegans
Abstract
:1. Introduction
2. Traditional Methods for C. elegans Screening
3. Microfluidic Components for High-Throughput Screening
4. Microfluidic Sorting of Sizes and Developmental Stages
5. Neurobiology Studies
5.1. Neuronal Imaging Platforms
5.2. Platforms for Electrophysiological Readings
6. Platforms for Larval and Embryonic Development Studies
7. Platforms for Lifespan and Aging Studies
8. Platforms for Toxicity and Pathogenesis Screens
9. Platforms for Behavioral Screens
10. Drug Screening Platforms
11. Cellular Ablation Screening Platforms
12. Screening Platforms with Miscellaneous Applications
13. Robotics for Automated Chemical Screening
14. Non-Microfluidic Screening Methods
15. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Corsi, A.K. A Transparent window into biology: A primer on Caenorhabditis elegans. WormBook 2015, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Kimble, J.; Hirsh, D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 1979, 70, 396–417. [Google Scholar] [CrossRef]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 314, 1–340. [Google Scholar] [CrossRef]
- Hall, D.H.; Russell, R.L. The posterior nervous system of the nematode Caenorhabditis elegans: Serial reconstruction of identified neurons and complete pattern of synaptic interactions. J. Neurosci. 1991, 11, 1–22. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green fluorescent protein as a marker for gene expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. SOFT LITHOGRAPHY. Annu. Rev. Mater. Sci 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- San-Miguel, A.; Lu, H. Microfluidics as a tool for C. elegans research. WormBook 2013, 1–19. [Google Scholar] [CrossRef]
- Bakhtina, N.A.; Korvink, J.G. Microfluidic laboratories for C. elegans enhance fundamental studies in biology. RSC Adv. 2014, 4, 4691–4709. [Google Scholar] [CrossRef]
- Gupta, B.; Rezai, P. Microfluidic Approaches for Manipulating, Imaging, and Screening C. elegans. Micromachines 2016, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.E.; Shevkoplyas, S.S.; McGuigan, A.P.; Apfeld, J.; Fontana, W.; Whitesides, G.M. Lifespan-on-a-chip: Microfluidic chambers for performing lifelong observation of C. elegans. Lab. Chip 2010, 10, 589–597. [Google Scholar] [CrossRef]
- Chokshi, T.V.; Bazopoulou, D.; Chronis, N.; Mrsic-Flogel, T.D.; Hofer, S.B.; Stein, V.; Hendel, T.; Reiff, D.F.; Levelt, C.; Borst, A.; et al. An automated microfluidic platform for calcium imaging of chemosensory neurons in Caenorhabditis elegans. Lab. Chip 2010, 10, 2758. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; Bourgeois, F.; Chokshi, T.; Durr, N.J.; Hilliard, M.A.; Chronis, N.; Ben-Yakar, A. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat. Methods 2008, 5, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Cornaglia, M.; Lehnert, T.; Gijs, M.A.M. Microfluidic systems for high-throughput and high-content screening using the nematode Caenorhabditis elegans. Lab. Chip 2017, 17, 3736–3759. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Hill, J.J.; Bargmann, C.I. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. PNAS 2005, 90, 2227–2231. [Google Scholar] [CrossRef]
- Avery, L.; Horvitz, H.R. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 1990, 253, 263–270. [Google Scholar] [CrossRef]
- Kwok, T.C.Y.; Ricker, N.; Fraser, R.; Chan, A.W.; Burns, A.; Stanley, E.F.; McCourt, P.; Cutler, S.R.; Roy, P.J. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature 2006, 441, 91–95. [Google Scholar] [CrossRef]
- Petrascheck, M.; Ye, X.; Buck, L.B. A High-Throughput Screen for Chemicals that Increase the Lifespan of Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 2009, 1170, 698–701. [Google Scholar] [CrossRef]
- Doh, J.H.; Moore, A.B.; Celen, I.; Moore, M.T.; Sabanayagam, C.R. ChIP and Chips: Introducing the WormPharm for correlative studies employing pharmacology and genome-wide analyses in C. elegans. J. Biol. Methods 2016, 3, 44. [Google Scholar] [CrossRef]
- Wicks, S.R.; de Vries, C.J.; van Luenen, H.G.A.M.; Plasterk, R.H.A. CHE-3, a Cytosolic Dynein Heavy Chain, Is Required for Sensory Cilia Structure and Function in Caenorhabditis elegans. Dev. Biol. 2000, 221, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gabel, C.V.; Ha, H.-I.; Zhang, Y.; Samuel, A.D.T. Olfactory Behavior of Swimming C. elegans Analyzed by Measuring Motile Responses to Temporal Variations of Odorants. J. Neurophysiol. 2008, 99, 2617–2625. [Google Scholar] [CrossRef]
- Kamath, R.S.; Fraser, A.G.; Dong, Y.; Poulin, G.; Durbin, R.; Gotta, M.; Kanapin, A.; Le Bot, N.; Moreno, S.; Sohrmann, M.; et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003, 421, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, E.M.; Mango, S.E. The Art and Design of Genetic Screens: Caenorhabditis Elegans. Nat. Rev. Genet. 2002, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.E.; Shevkoplyas, S.S.; Apfeld, J.; Fontana, W.; Whitesides, G.M. A microfabricated array of clamps for immobilizing and imaging C. elegans. Lab. Chip 2007, 7, 1515. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Crane, M.M.; Lu, H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 2008, 5, 637–643. [Google Scholar] [CrossRef]
- de Cáceres, I.C.; Valmas, N.; Hilliard, M.A.; Lu, H. Laterally Orienting C. elegans Using Geometry at Microscale for High-Throughput Visual Screens in Neurodegeneration and Neuronal Development Studies. PLoS ONE 2012, 7, e35037. [Google Scholar] [CrossRef]
- Lee, H.; Crane, M.M.; Zhang, Y.; Lu, H. Quantitative screening of genes regulating tryptophan hydroxylase transcription in Caenorhabditis elegans using microfluidics and an adaptive algorithm. Integr. Biol. 2013, 5, 372–380. [Google Scholar] [CrossRef]
- Chokshi, T.V.; Ben-Yakar, A.; Chronis, N. CO2 and compressive immobilization of C. elegans on-chip. Lab. Chip 2009, 9, 151–157. [Google Scholar] [CrossRef]
- Keil, W.; Kutscher, L.M.; Shaham, S.; Siggia, E.D. Long-Term High-Resolution Imaging of Developing, C. elegans Larvae with Microfluidics. Dev. Cell 2017, 40, 202–214. [Google Scholar] [CrossRef]
- Rohde, C.B.; Zeng, F.; Gonzalez-Rubio, R.; Angel, M.; Fatih Yanik, M. Microfluidic System for On-Chip High-Throughput Whole-Animal Sorting and Screening at Subcellular Resolution. Proc. Natl. Acad. Sci. USA 2007, 104, 13891–13895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.; Zhan, M.; Srinivasan, J.; Sternberg, P.W.; Gong, E.; Schroeder, F.C.; Lu, H. Microfluidic chamber arrays for whole-organism behavior-based chemical screening. Lab. Chip 2011, 11, 3689–3697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solvas, X.C.I.; Geier, F.M.; Leroi, A.M.; Bundy, J.G.; Edel, J.B.; deMello, A.J. High-throughput age synchronisation of Caenorhabditis elegans. Chem. Commun. 2011, 47, 9801. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Zhuo, W.; Liang, Q.; McGrath, P.T.; Lu, H. A high-throughput device for size based separation of C. elegans developmental stages. Lab. Chip 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Cornaglia, M.; Lehnert, T.; Gijs, M.A.M. Versatile size-dependent sorting of C. elegans nematodes and embryos using a tunable microfluidic filter structure. Lab. Chip 2016, 16, 574–585. [Google Scholar] [CrossRef]
- Han, B.; Kim, D.; Hyun Ko, U.; Shin, J.H. A sorting strategy for C. elegans based on size-dependent motility and electrotaxis in a micro-structured channel. Lab. Chip 2012, 12, 4128. [Google Scholar] [CrossRef]
- Rezai, P.; Salam, S.; Selvaganapathy, P.R.; Gupta, B.P. Electrical sorting of Caenorhabditis elegans. Lab. Chip 2012, 12, 1831. [Google Scholar] [CrossRef]
- Wang, X.; Hu, R.; Ge, A.; Hu, L.; Wang, S.; Feng, X.; Du, W.; Liu, B.-F. Highly efficient microfluidic sorting device for synchronizing developmental stages of C. elegans based on deflecting electrotaxis. Lab. Chip 2015, 15, 2513–2521. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, W.; Tian, B.; Luo, Y.; Lan, J.; Wu, D.; Chen, D.; Wang, Z.; Pan, D. Using microfluidic impedance cytometry to measure C. elegans worms and identify their developmental stages. Sens. Actuators B Chem. 2018, 275, 470–482. [Google Scholar] [CrossRef]
- Dong, X.; Song, P.; Liu, X. An Automated Microfluidic System for Morphological Measurement and Size-Based Sorting of C. elegans. IEEE Trans. Nanobioscience 2019, 18, 373–380. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, L.; Shi, W.; Qin, J.; Lin, B. A programmable microvalve-based microfluidic array for characterization of neurotoxin-induced responses of individual C. elegans. Biomicrofluidics 2009, 3, 044114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsch, J.; Ventimiglia, D.; Bargmann, C.I.; Albrecht, D.R. High-throughput imaging of neuronal activity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2013, 110, E4266–E4273. [Google Scholar] [CrossRef] [Green Version]
- Lockery, S.R.; Hulme, S.E.; Roberts, W.M.; Robinson, K.J.; Laromaine, A.; Lindsay, T.H.; Whitesides, G.M.; Weeks, J.C. A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans. Lab. Chip 2012, 12, 2211. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Dillon, J.; Kearn, J.; Murray, C.; O’Connor, V.; Holden-Dye, L.; Morgan, H. NeuroChip: A Microfluidic Electrophysiological Device for Genetic and Chemical Biology Screening of Caenorhabditis elegans Adult and Larvae. PLoS ONE 2013, 8, e64297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uppaluri, S.; Brangwynne, C.P. A size threshold governs Caenorhabditis elegans developmental progression. Proc. Biol. Sci. 2015, 282, 20151283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornaglia, M.; Mouchiroud, L.; Marette, A.; Narasimhan, S.; Lehnert, T.; Jovaisaite, V.; Auwerx, J.; Gijs, M.A.M. An automated microfluidic platform for C. elegans embryo arraying, phenotyping, and long-term live imaging. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Letizia, M.C.; Cornaglia, M.; Trouillon, R.; Sorrentino, V.; Mouchiroud, L.; Bou Sleiman, M.S.; Auwerx, J.; Gijs, M.A.M. Microfluidics-enabled phenotyping of a whole population of C. elegans worms over their embryonic and post-embryonic development at single-organism resolution. Microsyst. Nanoeng. 2018, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Atakan, H.B.; Cornaglia, M.; Mouchiroud, L.; Auwerx, J.; Gijs, M.A.M. Automated high-content phenotyping from the first larval stage till the onset of adulthood of the nematode Caenorhabditis elegans. Lab. Chip 2019, 19, 120–135. [Google Scholar] [CrossRef]
- Xian, B.; Shen, J.; Chen, W.; Sun, N.; Qiao, N.; Jiang, D.; Yu, T.; Men, Y.; Han, Z.; Pang, Y.; et al. WormFarm: A quantitative control and measurement device toward automated Caenorhabditis elegans aging analysis. Aging Cell 2013, 12. [Google Scholar] [CrossRef]
- Wen, H.; Shi, W.; Qin, J. Multiparameter evaluation of the longevity in C. elegans under stress using an integrated microfluidic device. Biomed. Microdevices 2012, 14, 721–728. [Google Scholar] [CrossRef]
- Li, S.; Stone, H.A.; Murphy, C.T. A microfluidic device and automatic counting system for the study of C. elegans reproductive aging. Lab. Chip 2015, 15, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Banse, S.A.; Blue, B.W.; Robinson, K.J.; Jarrett, C.M.; Phillips, P.C. The Stress-Chip: A microfluidic platform for stress analysis in Caenorhabditis elegans. PLoS ONE 2019, 14, e0216283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Li, Y.; He, Q.; Qin, J.; Yu, Y.; Li, X.; Zhang, L.; Yao, M.; Liu, J.; Chen, Z. Microfluidic platform integrated with worm-counting setup for assessing manganese toxicity. Biomicrofluidics 2014, 8, 054110. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.H.; Cha, Y.J.; Hong, S.J.; Chung, S.K.; Park, T.H.; Choi, S.S.C. elegans-on-a-chip for in situ and in vivo Ag nanoparticles’ uptake and toxicity assay. Sci. Rep. 2017, 7, 40225. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Ching, P.; Shi, Q.; Li, X. An integrated microfluidic platform for evaluating in vivo antimicrobial activity of natural compounds using a whole-animal infection model. Lab. Chip 2013, 13, 3373. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ge, A.; Wang, X.; Wang, S.; Yue, X.; Wang, J.; Feng, X.; Du, W.; Liu, B.-F. Real-time monitoring of immune responses under pathogen invasion and drug interference by integrated microfluidic device coupled with worm-based biosensor. Biosens. Bioelectron. 2018, 110, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Stirman, J.N.; Brauner, M.; Gottschalk, A.; Lu, H. High-throughput study of synaptic transmission at the neuromuscular junction enabled by optogenetics and microfluidics. J. Neurosci. Methods 2010, 191, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, D.R.; Bargmann, C.I. High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments. Nat. Methods 2011, 8, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Salam, S.; Ansari, A.; Amon, S.; Rezai, P.; Selvaganapathy, P.R.; Mishra, R.K.; Gupta, B.P. A microfluidic phenotype analysis system reveals function of sensory and dopaminergic neuron signaling in C. elegans electrotactic swimming behavior. Worm 2013. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Gupta, B.; Selvaganapathy, P.R. An automated microfluidic system for screening Caenorhabditis elegans behaviors using electrotaxis. Biomicrofluidics 2016, 10, 014117. [Google Scholar] [CrossRef] [Green Version]
- Johari, S.; Nock, V.; Alkaisi, M.M.; Wang, W. High-Throughput Microfluidic Sorting of C. elegans for Automated Force Pattern Measurement. Mater. Sci. Forum 2011, 700, 182–187. [Google Scholar] [CrossRef]
- Carr, J.A.; Parashar, A.; Gibson, R.; Robertson, A.P.; Martin, R.J.; Pandey, S. A microfluidic platform for high-sensitivity, real-time drug screening on C. elegans and parasitic nematodes. Lab. Chip 2011, 11, 2385. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Hegarty, E.; Martin, C.; Gökçe, S.K.; Ghorashian, N.; Ben-Yakar, A. Large-scale microfluidics providing high-resolution and high-throughput screening of Caenorhabditis elegans poly-glutamine aggregation model. Nat. Commun. 2016, 7, 13023. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Njus, Z.; Kong, T.; Su, W.; Ho, C.-M.; Pandey, S. Effective drug combination for Caenorhabditis elegans nematodes discovered by output-driven feedback system control technique. Sci. Adv. 2017, 3, eaao1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Jankele, R.; Cornaglia, M.; Lehnert, T.; Gönczy, P.; Gijs, M.A.M. Integrated Microfluidic Device for Drug Studies of Early C. elegans Embryogenesis. Adv. Sci. 2018, 5, 1700751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliozzi, D.; Cornaglia, M.; Mouchiroud, L.; Uhlmann, V.; Unser, M.A.; Auwerx, J.; Gijs, M.A.M. Multimodal imaging and high-throughput image-processing for drug screening on living organisms on-chip. J. Biomed. Opt. 2018, 24, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, P.B.; Sgro, A.E.; Chao, D.L.; Doepker, B.E.; Scott Edgar, J.; Shen, K.; Chiu, D.T. Single-synapse ablation and long-term imaging in live C. elegans. J. Neurosci. Methods 2008, 173, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.; Lu, H. Automated high-throughput cell microsurgery on-chip. Lab. Chip 2009, 9, 2764. [Google Scholar] [CrossRef]
- Samara, C.; Rohde, C.B.; Gilleland, C.L.; Norton, S.; Haggarty, S.J.; Yanik, M.F. Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 18342–18347. [Google Scholar] [CrossRef] [Green Version]
- Gokce, S.K.; Guo, S.X.; Ghorashian, N.; Everett, W.N.; Jarrell, T.; Kottek, A.; Bovik, A.C.; Ben-Yakar, A. A Fully Automated Microfluidic Femtosecond Laser Axotomy Platform for Nerve Regeneration Studies in C. elegans. PLoS ONE 2014, 9, e113917. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, S.A.; Coakley, S.; Mugno, P.; Hammarlund, M.; Hilliard, M.A.; Lu, H. A multi-channel device for high-density target-selective stimulation and long-term monitoring of cells and subcellular features in C. elegans. Lab. Chip 2014, 14, 4513–4522. [Google Scholar] [CrossRef] [Green Version]
- Ghorashian, N.; Gökçe, S.K.; Guo, S.X.; Everett, W.N.; Ben-Yakar, A. An Automated Microfluidic Multiplexer for Fast Delivery of C. elegans Populations from Multiwells. PLoS ONE 2013, 8, e74480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubry, G.; Zhan, M.; Lu, H. Hydrogel-droplet microfluidic platform for high-resolution imaging and sorting of early larval Caenorhabditis elegans. Lab. Chip 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Desta, I.T.; Al-Sharif, A.; AlGharibeh, N.; Mustafa, N.; Orozaliev, A.; Giakoumidis, N.; Gunsalus, K.C.; Song, Y.-A. Detecting and Trapping of a Single C. elegans Worm in a Microfluidic Chip for Automated Microplate Dispensing. SLAS Technol. Transl. Life Sci. Innov. 2017, 22, 431–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagoy, R.C.; Albrecht, D.R. Automated fluid delivery from multiwell plates to microfluidic devices for high-throughput experiments and microscopy. Sci. Rep. 2018, 8, 6217. [Google Scholar] [CrossRef] [PubMed]
- Pulak, R. Techniques for Analysis, Sorting, and Dispensing of C. elegans on the COPASTM Flow-Sorting System. In C. elegans; Humana Press: Totowa, NJ, USA, 2006; pp. 275–286. [Google Scholar] [CrossRef]
- Gomez-Amaro, R.L.; Valentine, E.R.; Carretero, M.; LeBoeuf, S.E.; Rangaraju, S.; Broaddus, C.D.; Solis, G.M.; Williamson, J.R.; Petrascheck, M. Measuring Food Intake and Nutrient Absorption in Caenorhabditis elegans. Genetics 2015, 200, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churgin, M.A.; Jung, S.-K.; Yu, C.-C.; Chen, X.; Raizen, D.M.; Fang-Yen, C. Longitudinal imaging of Caenorhabditis elegans in a microfabricated device reveals variation in behavioral decline during aging. Elife 2017, 6. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006. [Google Scholar] [CrossRef] [Green Version]
- Rezai, P.; Siddiqui, A.; Selvaganapathy, P.R.; Gupta, B.P. Electrotaxis of Caenorhabditis elegans in a microfluidic environment. Lab. Chip 2010, 10, 220–226. [Google Scholar] [CrossRef]
- Gabel, C.V.; Gabel, H.; Pavlichin, D.; Kao, A.; Clark, D.A.; Samuel, A.D.T. Neural circuits mediate electrosensory behavior in Caenorhabditis elegans. J. Neurosci. 2007, 27, 7586–7596. [Google Scholar] [CrossRef]
- Crane, M.M.; Stirman, J.N.; Ou, C.-Y.; Kurshan, P.T.; Rehg, J.M.; Shen, K.; Lu, H. Autonomous screening of C. elegans identifies genes implicated in synaptogenesis. Nat. Methods 2012, 9, 977–980. [Google Scholar] [CrossRef] [Green Version]
- San-Miguel, A.; Kurshan, P.T.; Crane, M.M.; Zhao, Y.; McGrath, P.T.; Shen, K.; Lu, H. Deep phenotyping unveils hidden traits and genetic relations in subtle mutants. Nat. Commun. 2016, 7, 12990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeli, S.; Klang, I.; Sivapatham, R.; Mark, K.; Zucker, D.; Bhaumik, D.; Lithgow, G.J.; Andersen, J.K. A DNA synthesis inhibitor is protective against proteotoxic stressors via modulation of fertility pathways in Caenorhabditis elegans. Aging (Albany. NY) 2013, 5, 759–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, H.; Gao, X.; Qin, J. Probing the anti-aging role of polydatin in Caenorhabditis elegans on a chip. Integr. Biol. 2014, 6, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yin, F.; Wang, L.; Wei, W.; Jiang, L.; Qin, J. Modeling type 2 diabetes-like hyperglycemia in C. elegans on a microdevice. Integr. Biol. 2016, 8, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Barnes, D.E.; Matsunaga, Y.; Benian, G.M.; Ono, S.; Lu, H. Muscle contraction phenotypic analysis enabled by optogenetics reveals functional relationships of sarcomere components in Caenorhabditis elegans. Sci. Rep. 2016, 6, 19900. [Google Scholar] [CrossRef]
- Mondal, S.; Hegarty, E.; Sahn, J.J.; Scott, L.L.; Gökçe, S.K.; Martin, C.; Ghorashian, N.; Satarasinghe, P.N.; Iyer, S.; Sae-Lee, W.; et al. High-Content Microfluidic Screening Platform Used To Identify σ2R/Tmem97 Binding Ligands that Reduce Age-Dependent Neurodegeneration in C. elegans SC_APP Model. ACS Chem. Neurosci. 2018, 9, 1014–1026. [Google Scholar] [CrossRef] [Green Version]
- Fang-Yen, C.; Gabel, C.V.; Samuel, A.D.T.; Bargmann, C.I.; Avery, L. Laser microsurgery in Caenorhabditis elegans. Methods Cell Biol. 2012, 107, 177–206. [Google Scholar] [CrossRef] [Green Version]
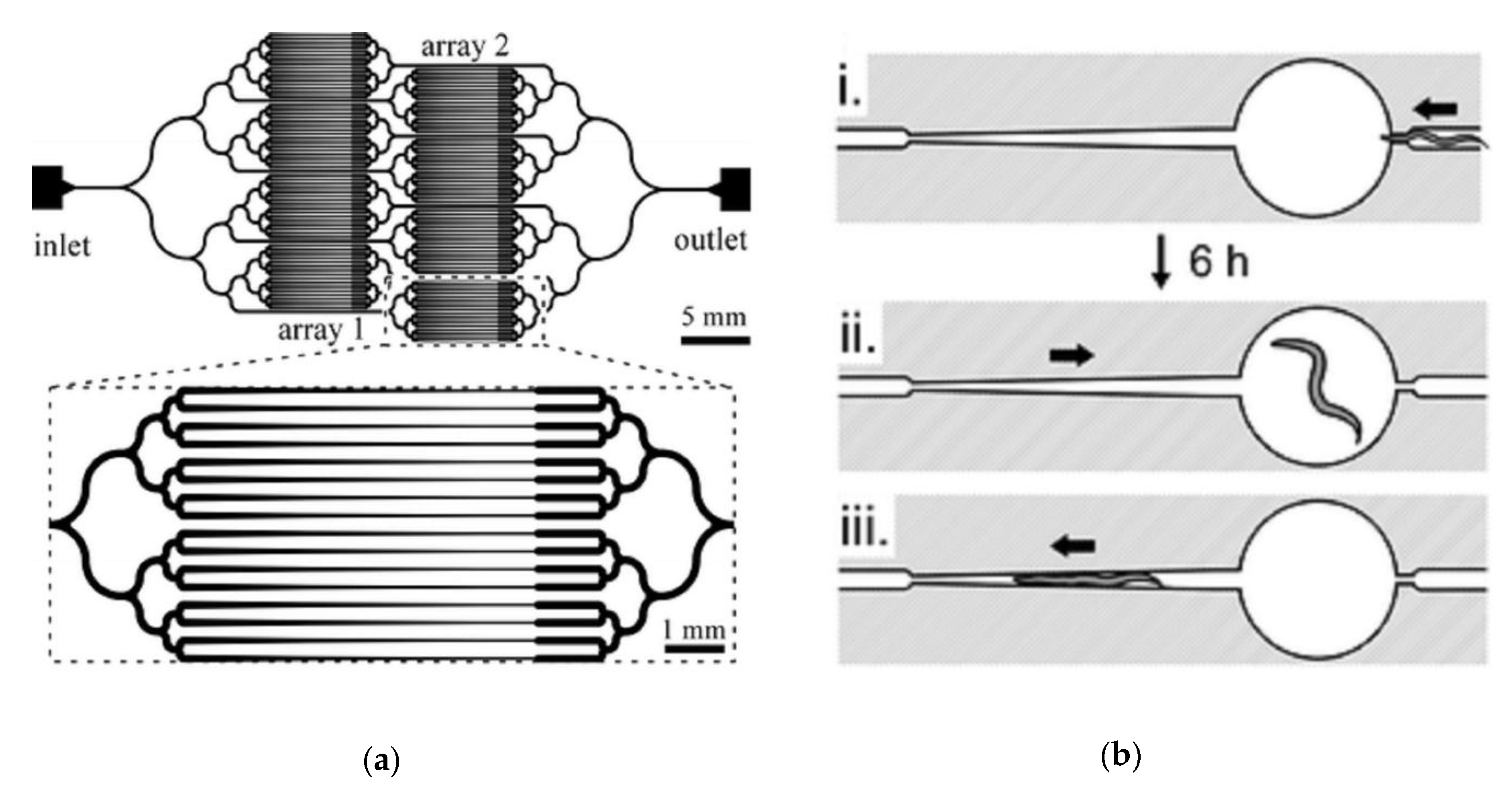
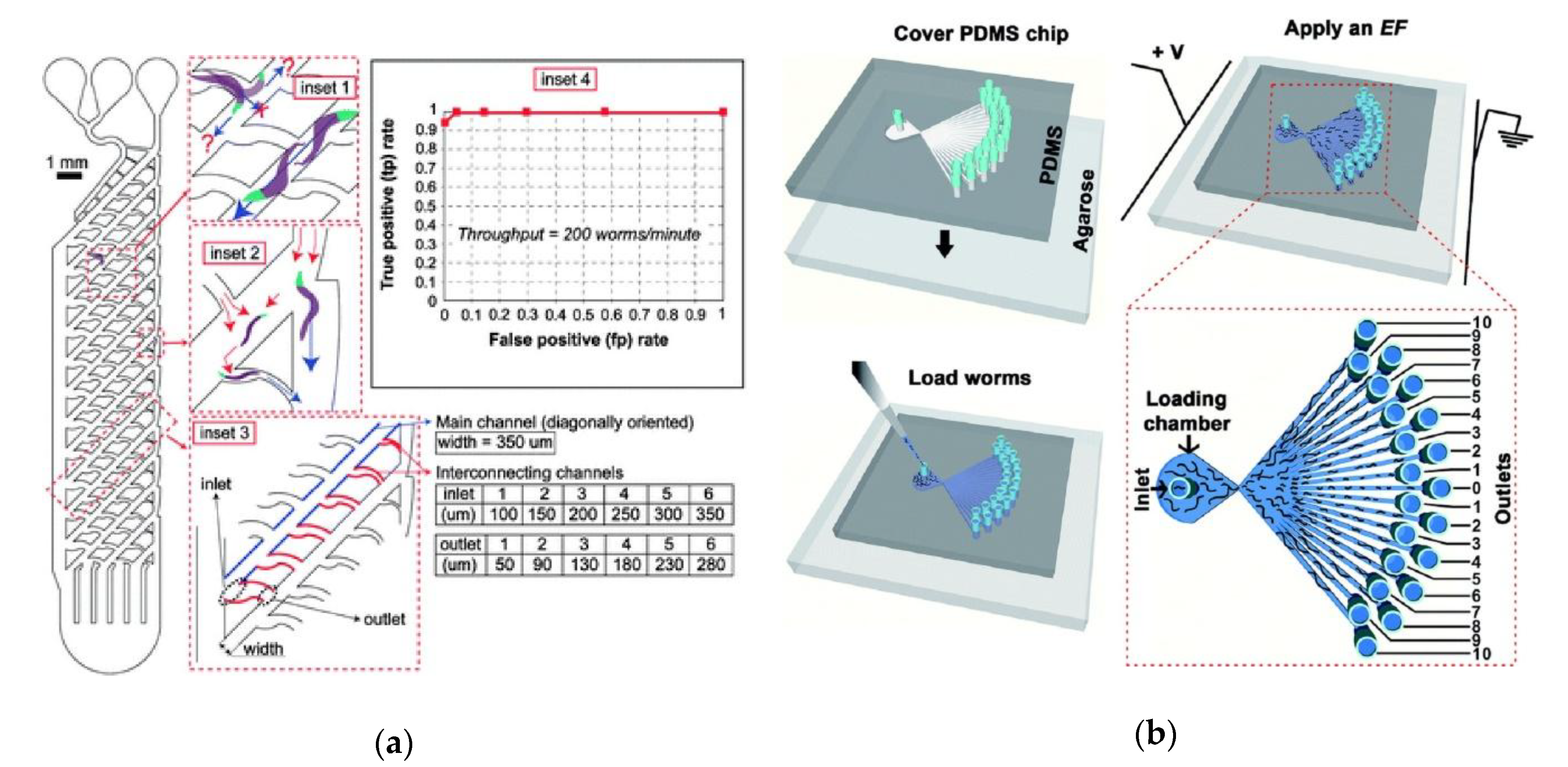
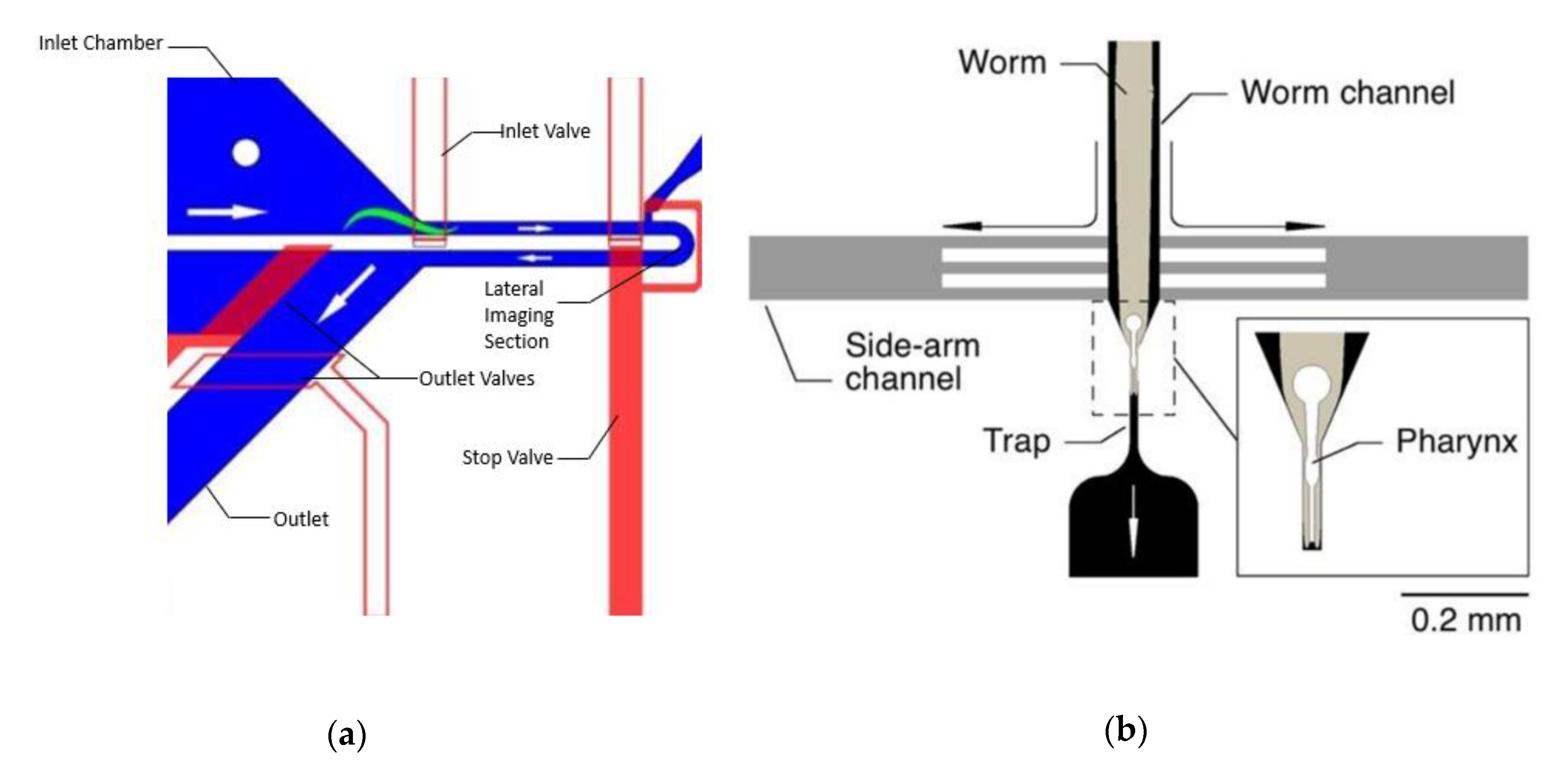
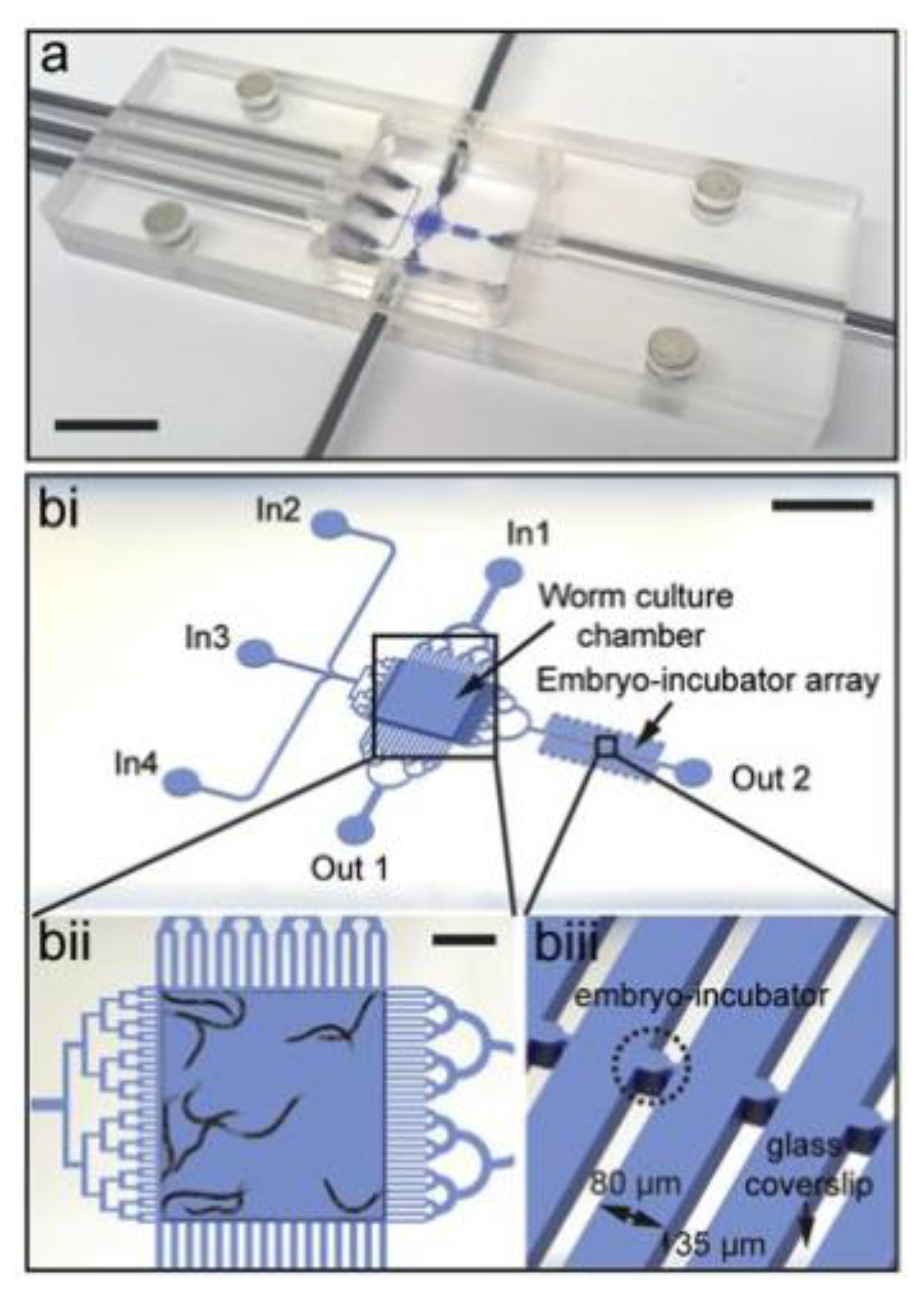
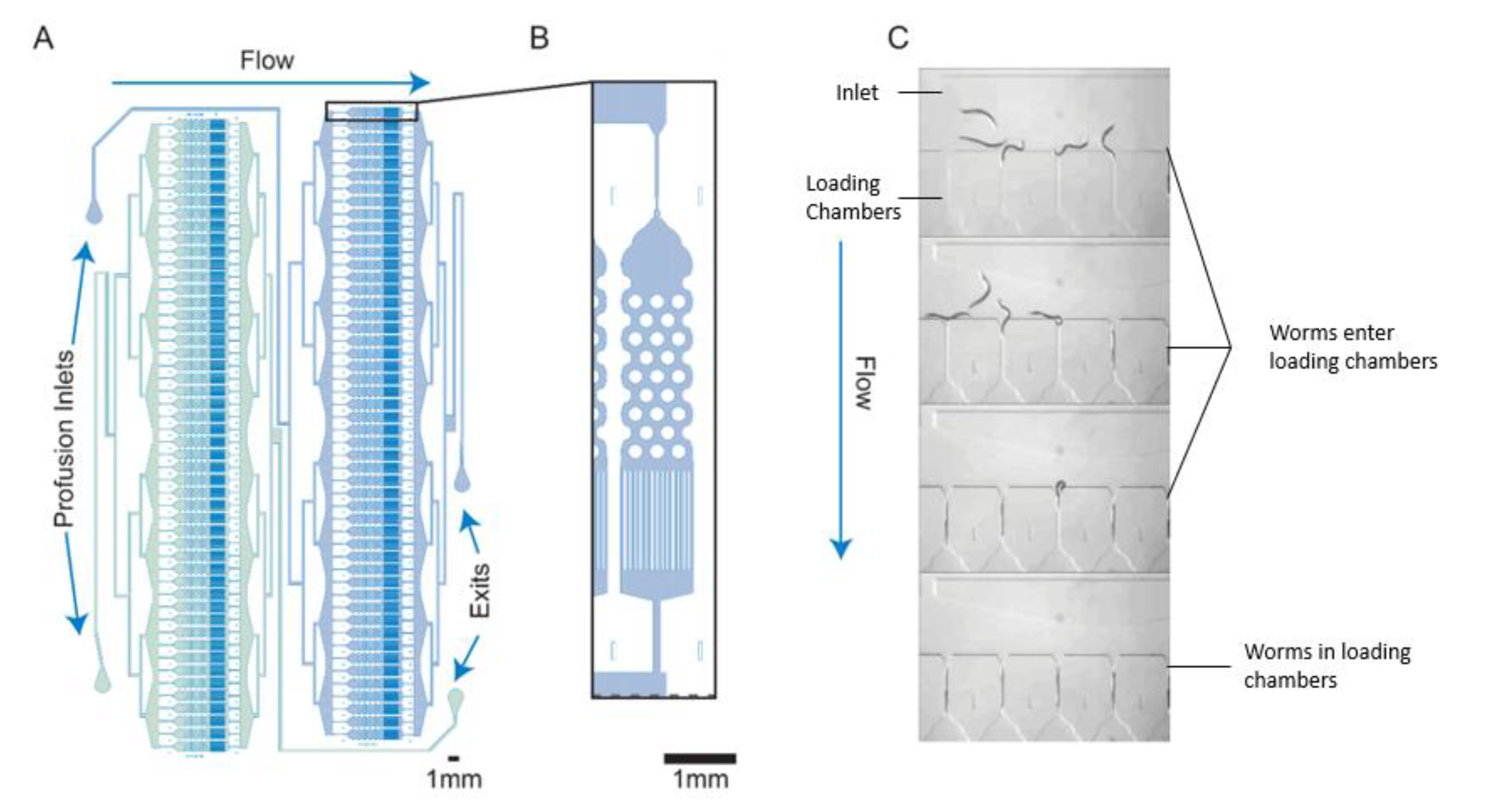
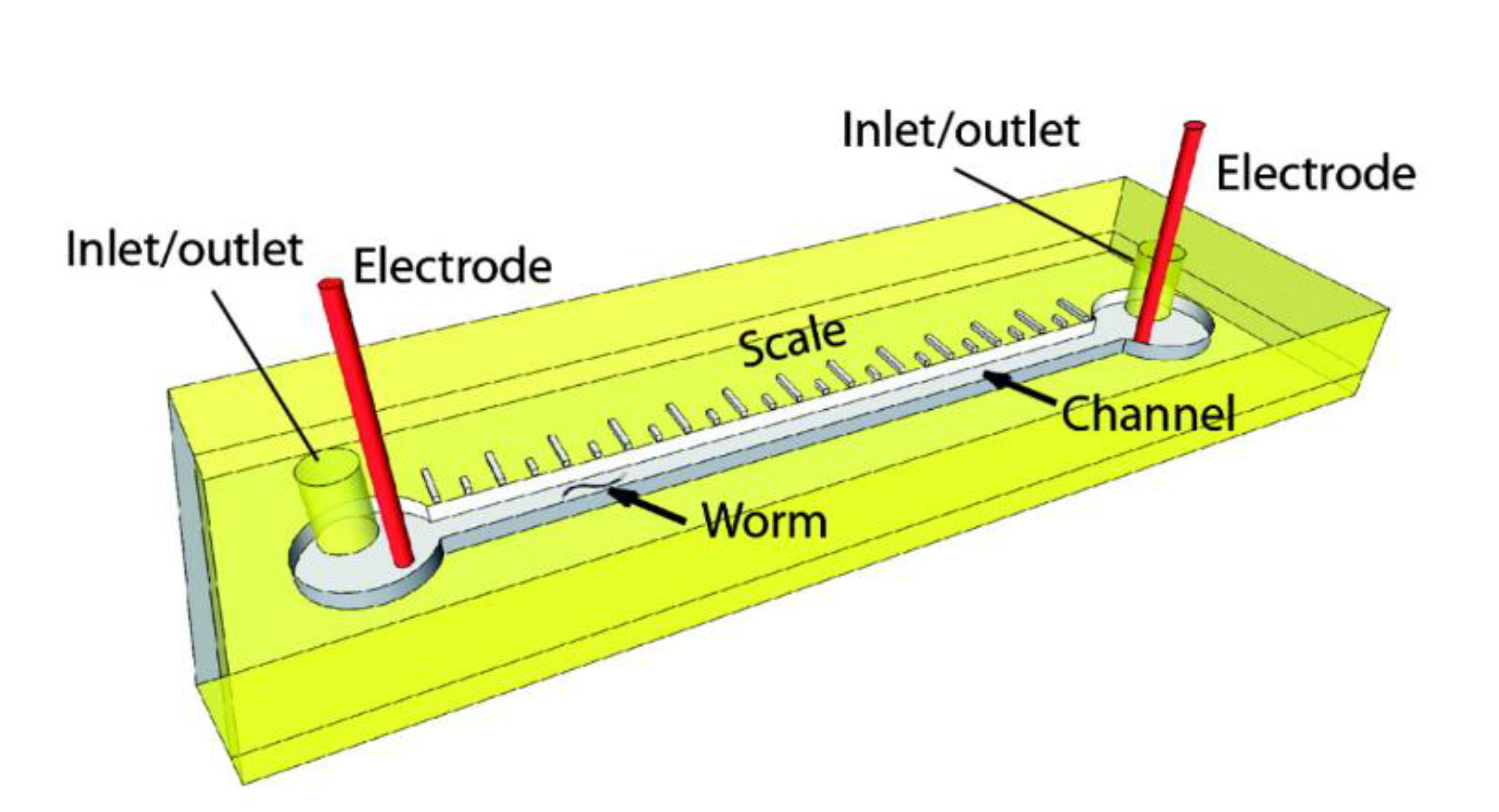
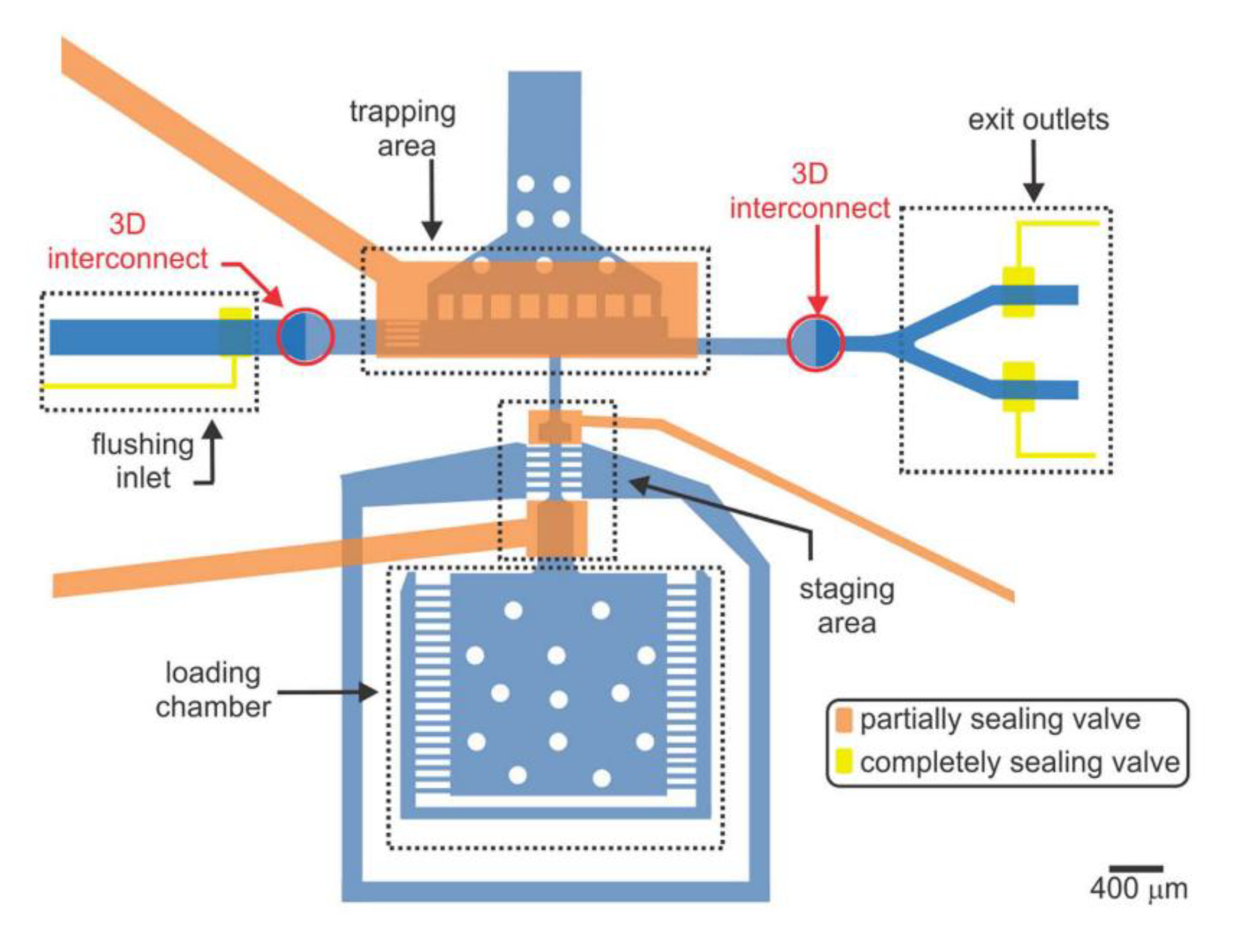
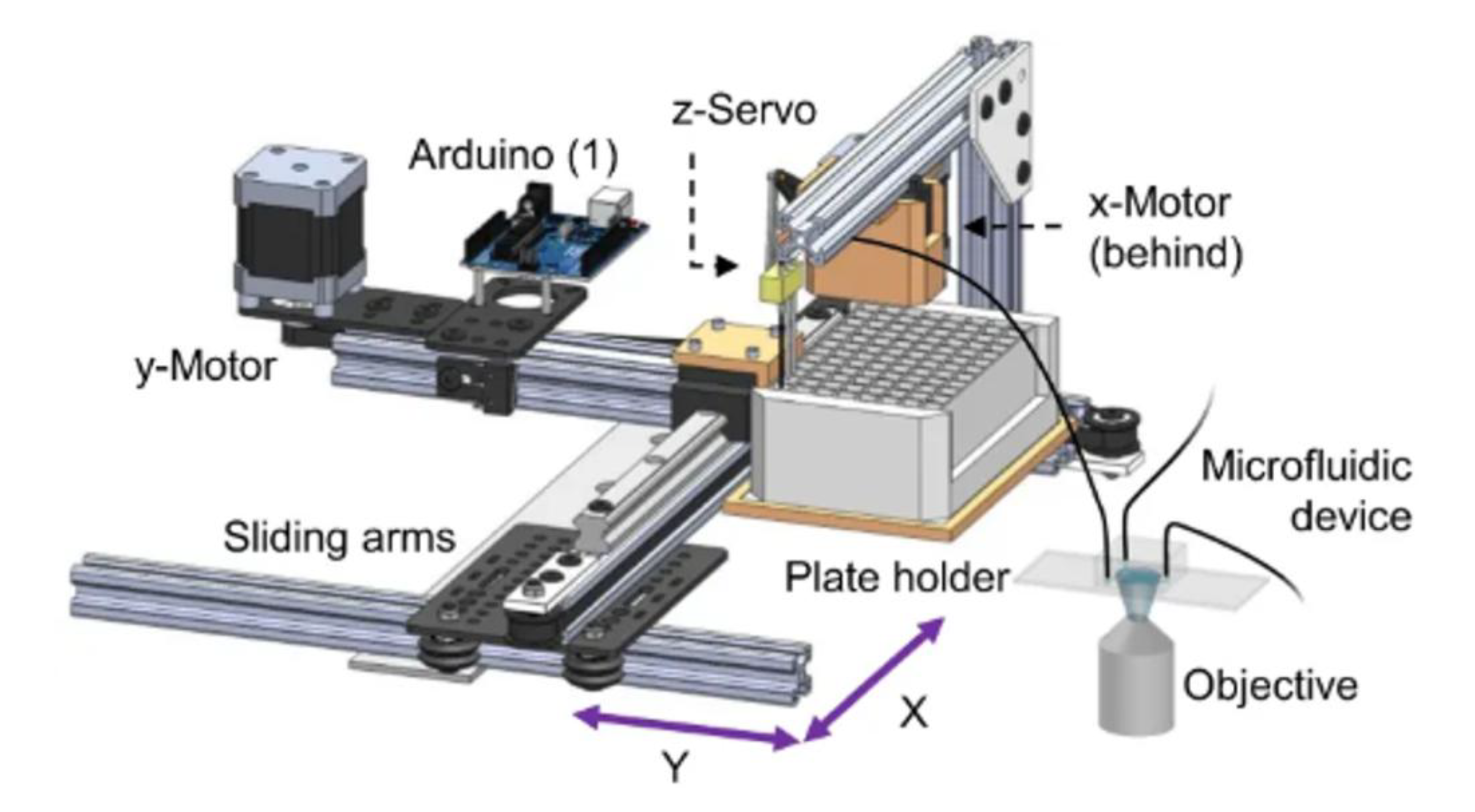
| Authors, Year | Reference | Purpose | Advantage(s) | Disadvantage(s) |
|---|---|---|---|---|
| Platforms for Size-Based Sorting | ||||
| Solvas et al. (2011) | [33] | Size-Based Sorting | Can separate larvae from adults with high accuracy and efficiency | Does not separate between differing larval stages |
| Ai et al. (2014) | [34] | Size-Based Sorting | Sequential separation of all larval stages at >85% efficiency | Multiple devices needed for separating all stages, leading to increased chance of operational error |
| Dong et al. (2016) | [35] | Size-Based Sorting | One device can separate any developmental stage based on pressure input | Throughput is 3.5 worms per second, lower than other platforms |
| Han et al. (2012) | [36] | Size-Based Sorting | Worms of all developmental stages can be sorted, first use of electrotaxis for size-based separation in C. elegans | Imperfect separation for L2–L4 stages, only 80% of worms undergo directed movement due to electrotaxis |
| Rezai et al. (2012) | [37] | Size-Based Sorting | Selectivity for a given developmental stage is at least 90% | Uncertain structural and molecular effects due to paralysis |
| Wang et al. (2015) | [38] | Size-Based Sorting | Separates all worm stages simultaneously in one device, can also isolate male worms and size mutants | Purity for certain separations as low as 82% |
| Zhu et al. (2018) | [39] | Size Measurement | Quantitatively measures worm size using impedance cytometry, individual worms can be sorted for forward genetic screening | Accuracy for identifying L3 worms is only 81% |
| Dong et al. (2019) | [40] | Size Measurement | Worm size determined using automated image analysis, individual worms can be sorted for forward genetic screens | Throughput limited to 10.4 worms per minute, clogging of channel can completely disrupt sorting |
| Neurobiological Studies | ||||
| Rohde et al. (2007) | [31] | High-Resolution Imaging and Sorting | Worms immobilized for high-resolution imaging and sorted, worms not imaged can be recycled, sorted worms can be fed to well plate | Multiple active steps required to load one worm for imaging |
| Chung et al. (2008) | [26] | High-Resolution Imaging and Sorting | Simplified loading step allows for fast sequential imaging | Additional microfluidic control layer must be interfaced with flow layer |
| Cacéres et al. (2012) | [27] | High-Resolution Imaging and Sorting | Modified channel orients worms in the lateral orientation for nerve cord imaging | Additional microfluidic control layer must be interfaced with flow layer |
| Lee et al. (2013) | [28] | High-Resolution Imaging and Sorting | Similar function to [26] but only consists of one microfluidic layer | Device clogging can lead to disruption in sorting |
| Ma et al. (2009) | [41] | Long-Term High-Resolution Imaging | Individual worms can be imaged at multiple time points throughout most of their lifespan | No straightforward method for recovery of individual worms after imaging |
| Larsch et al. (2013) | [42] | Calcium Transient Imaging | Calcium transients can be imaged in multiple worms | Limited to lower resolution phenotypes |
| Lockery et al. (2012) | [43] | Electrophysiological Measurements of Neurons | Electropharyngeograms of multiple worms can be measured simultaneously | Worms cannot be recovered after data acquisition |
| Hu et al. (2013) | [44] | Electrophysiological Sorting | Worms can be sorted sequentially based on electrophysiological data, three times faster than manual methods | Data acquisition on chip not fully automated |
| Larval and Embryonic Development Studies | ||||
| Uppaluri et al. (2015) | [45] | Environmental Effects on Larval Growth | Software quantitatively tracks size growth of individual worms | Only eight larvae can be tracked simultaneously on a chip |
| Keil et al. (2017) | [30] | High-Resolution Imaging of Larvae | Individual larvae can be imaged at high resolution | No worm outlet |
| Cornaglia et al. (2015) | [46] | High-Resolution Imaging of Embryos | Individual embryos maintained in incubation chambers for high-resolution imaging, operation is passive | Screening multiple chemicals would require operation of multiple platforms in parallel |
| Letizia et al. (2018) | [47] | High-Resolution Imaging of Embryos and Worms | Operates using same mechanisms as [46], but can image worms after each embryo hatches | Screening multiple chemicals would require operation of multiple platforms in parallel |
| Atakan et al. (2019) | [48] | Imaging and Behavioral Analysis of Embryonic Development | Can acquire both imaging data and locomotion rate for groups of worms | Immobilization not complete, so high-resolution imaging is not possible, only three worms can fit in each chamber for unrestricted motion |
| Lifespan and Aging Studies | ||||
| Xian et al. (2013) | [49] | Lifespan Analysis of Worm Populations with Age | Worm populations can be studied for their whole lifespan by automated analysis | Multiple chambers must be arranged in parallel for screening multiple chemicals |
| Doh et al. (2016) | [20] | Lifelong Behavioral Analysis Using Axenic Media | Can feed worms and determine worm size at defined intervals | Effects of axenic media on some aspects of worm biology are still unknown |
| Wen et al. (2012) | [50] | Lifelong Stress Studies | Worms can be imaged and stored in individual chambers over time | Multiple device layers make fabrication more challenging |
| Li et al. (2015) | [51] | Lifelong Reproductive Measurement | Chambers in device converge in parallel for real-time counting of progeny from each worm | Device contains many narrow regions which may increase the chance of clogging |
| Banse et al. (2019) | [52] | Measurement of Survival Under Stress | Large data sets (~600 worms per device) can be acquired, individual worms can be tracked over time | Chip cannot perform on-chip immobilization for high-resolution imaging |
| Toxicology and Pathogenesis Studies | ||||
| Zhang et al. (2014) | [53] | Toxic Effects on Neurons | Device inlets are mixed to create a gradient of concentrations across the device, counting mechanism loads the desired number of worms | Device must me interfaced with an electrode layer, making fabrication more complicated |
| Kim et al. (2017) | [54] | Toxic Effects of Nanoparticles | Tapered channels are used for immobilization, and the distance traveled along the channel is correlated with worm size | Small features could lead to device clogging |
| Yang et al. (2013) | [55] | Effect of Pathogens and Antimicrobials | Device inlets are mixed to create a gradient of concentrations across the device, counting mechanism loads the desired number of worms | Only four gradient mechanisms are present on each chip, limiting the number of drugs that can be screened at a time |
| Hu et al. (2018) | [56] | Effect of Pathogens and Antimicrobials | Worm survival can be studied for several days, device can perform high-resolution imaging | Multiple layers are required for fabrication and device assembly |
| Behavioral Studies | ||||
| Stirman et al. (2010) | [57] | Optogenetic Response | Data acquisition rate orders of magnitude higher than for data acquired on plate | Individual worms cannot be recovered |
| Albrecht et al. (2011) | [58] | Chemotaxis Response | Channels in device can create a variety of spatiotemporal odorant patterns | Different devices must be used for different types of spatial patterns |
| Chung et al. (2011) | [32] | Chemical Effects on Behavior | Worms are simultaneously loaded into chambers, making the loading process quick | Small features can lead to clogging |
| Salam et al. (2013) | [59] | Electrotaxis Response | Response to electrotaxis can be quantified | Needs to be scaled up to perform a large-scale screen |
| Liu et al. (2016) | [60] | Electrotaxis Response | Worms can be sorted based on electrotaxis response | Only 20 worms can be screened per hour |
| Johari et al. (2011) | [61] | Mechanical Strength Measurements | Mechanical strength can be detected by measuring the deflection of PDMS | More chambers with a separate inlet for each chamber could increase the chambers size for chemical screens |
| Drug Screening Platforms | ||||
| Carr et al. (2011) | [62] | Drug Behavioral Response | Device can be used to many behavioral parameters, individual worms are assessed for the entire period of drug application | Does not incorporate a feature for high-resolution imaging |
| Mondal et al. (2016) | [63] | High Resolution Imaging Drug Screens | High-resolution phenotypes can be acquired, ~4000 worms can be screened in 16 min | Imaging may be challenging with young adults or larvae |
| Ding et al. (2017) | [64] | Anthelmintic Drug Screens | Feedback control system automatically optimizes concentration of drugs fed to device based on previous data | Only three chambers and three drug inlets per device |
| Dong et al. (2018) | [65] | Embryonic Drug Screens | Worms are compressed to extract embryos for drug screening | Larvae cannot be studied after embryos hatch |
| Migliozzi et al. (2018) | [66] | Multimodal Imaging for Drug Screening | Large data quantities extracted from both brightfield and fluorescent images | Only three drug inlets per device |
| Cellular Ablation Studies | ||||
| Allen et al. (2008) | [67] | Neuronal Laser Ablation | Worms are immobilized on-chip in parallel for laser ablation | Worms must leave device through inlet, requires more time than for devices with inlet and outlet |
| Guo et al. (2008) | [14] | Neuronal Laser Ablation | Complete immobilization is achieved for precise ablation | Sorting requires more steps than other platforms |
| Chung et al. (2009) | [68] | Neuronal Laser Ablation | Laser ablation is more high-throughput than for previous platforms | Multiple layers are required for device assembly |
| Samara et al. (2010) | [69] | Laser Ablation Chemical Screen | Worms transferred from multi-well plate to channel for ablation | Operation is not fully automated |
| Gokce et al. (2014) | [70] | Neuronal Laser Ablation | Device operation is fully automated | Multiple layers are required for device assembly |
| Lee et al. (2014) | [71] | Optogenetic KillerRed Ablation | Ablation can occur in many worms simultaneously | Separate strains must be generated to ablate different cells |
| Miscellaneous Applications | ||||
| Ghorashian et al. (2013) | [72] | Well-Plate Retrieval | Recover worms from a well plate within seconds for use in a microfluidic device | Only 16 wells are interfaced with the device |
| Aubry et al. (2015) | [73] | High Resolution L1 Larval Imaging | Hydrogel immobilization does not require tiny features that clog easily, worms can be recovered after imaging | Hydrogel and spacing fluid are new elements that are not commonly present in microfluidic labs |
| Robotics for Microfluidic Screening | ||||
| Desta et al. (2017) | [74] | Transfer worms from device to well plate | Robotic arm automatically transfers worms from a screening platform to a well plate | Robot setup may be challenging to replicate in a different lab environment |
| Lagoy et al. (2018) | [75] | Deliver chemicals from well-plate to device | Robotic arm transfers specified chemicals to device for screening | More time-consuming than delivery to devices with one inlet per well |
| Non-Microfluidic Screening Platforms | ||||
| Pulak (2006) and others | [76] | COPAS Flow Cytometry Sorting | Can sort ~100 worms per second | Platform is expensive, only large-scale phenotypes can be assessed |
| Gomez-Amaro et al. (2015) | [77] | Measuring Food Absorption | Techniques can either measure food consumed or protein accumulation in an organism | Assessing protein accumulation requires mass-spectrometry equipment |
| Churgin et al. (2017) | [78] | Behavioral Decline with Age | Worms can be maintained within individual chambers on solid media, without requiring regular bacterial perfusion | Worms must be manually placed in each chamber |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Midkiff, D.; San-Miguel, A. Microfluidic Technologies for High Throughput Screening Through Sorting and On-Chip Culture of C. elegans. Molecules 2019, 24, 4292. https://doi.org/10.3390/molecules24234292
Midkiff D, San-Miguel A. Microfluidic Technologies for High Throughput Screening Through Sorting and On-Chip Culture of C. elegans. Molecules. 2019; 24(23):4292. https://doi.org/10.3390/molecules24234292
Chicago/Turabian StyleMidkiff, Daniel, and Adriana San-Miguel. 2019. "Microfluidic Technologies for High Throughput Screening Through Sorting and On-Chip Culture of C. elegans" Molecules 24, no. 23: 4292. https://doi.org/10.3390/molecules24234292
APA StyleMidkiff, D., & San-Miguel, A. (2019). Microfluidic Technologies for High Throughput Screening Through Sorting and On-Chip Culture of C. elegans. Molecules, 24(23), 4292. https://doi.org/10.3390/molecules24234292






