Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals
Abstract
1. Introduction
2. Results and Discussion
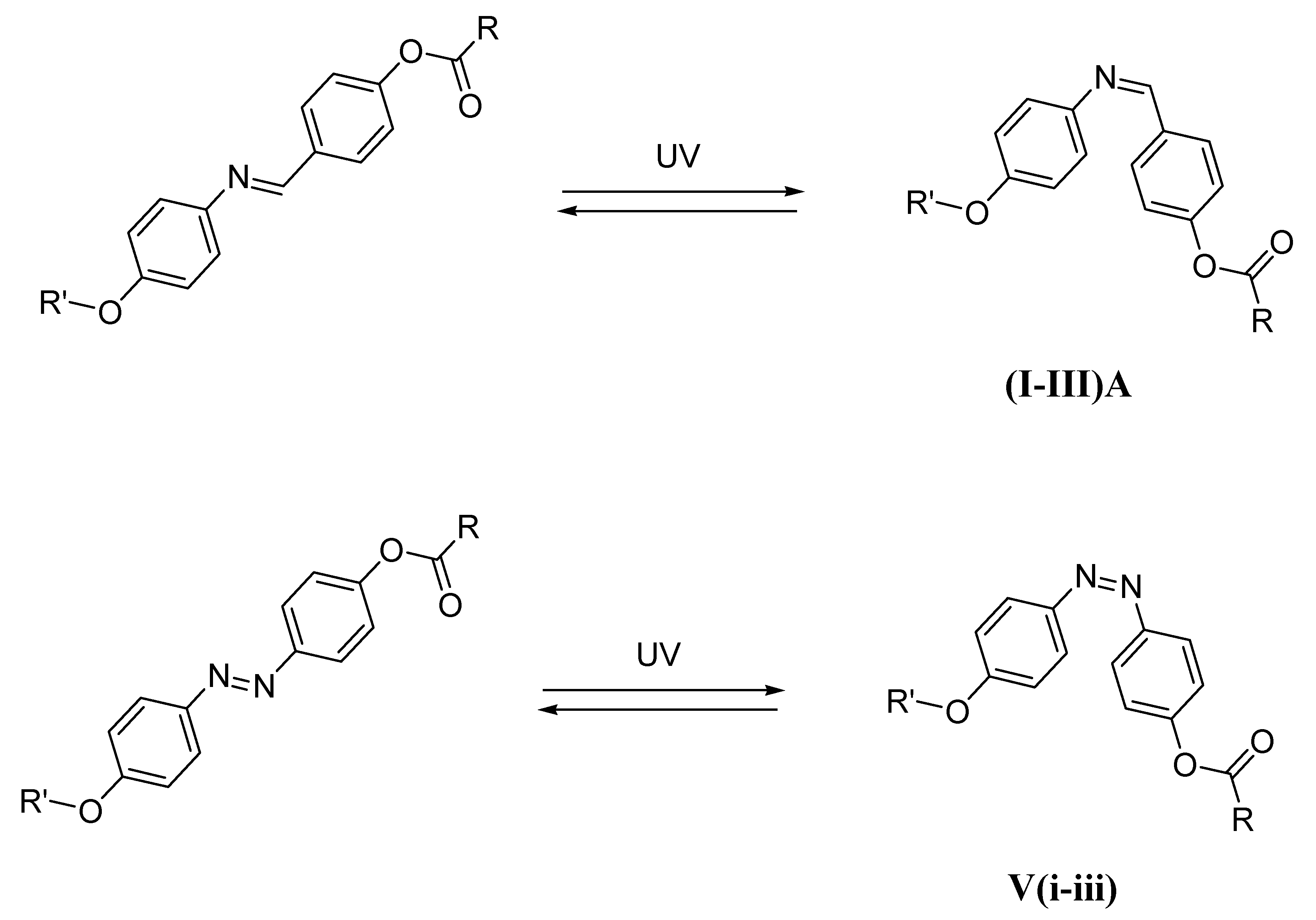
2.1. Characterization of Compounds (I–III)A
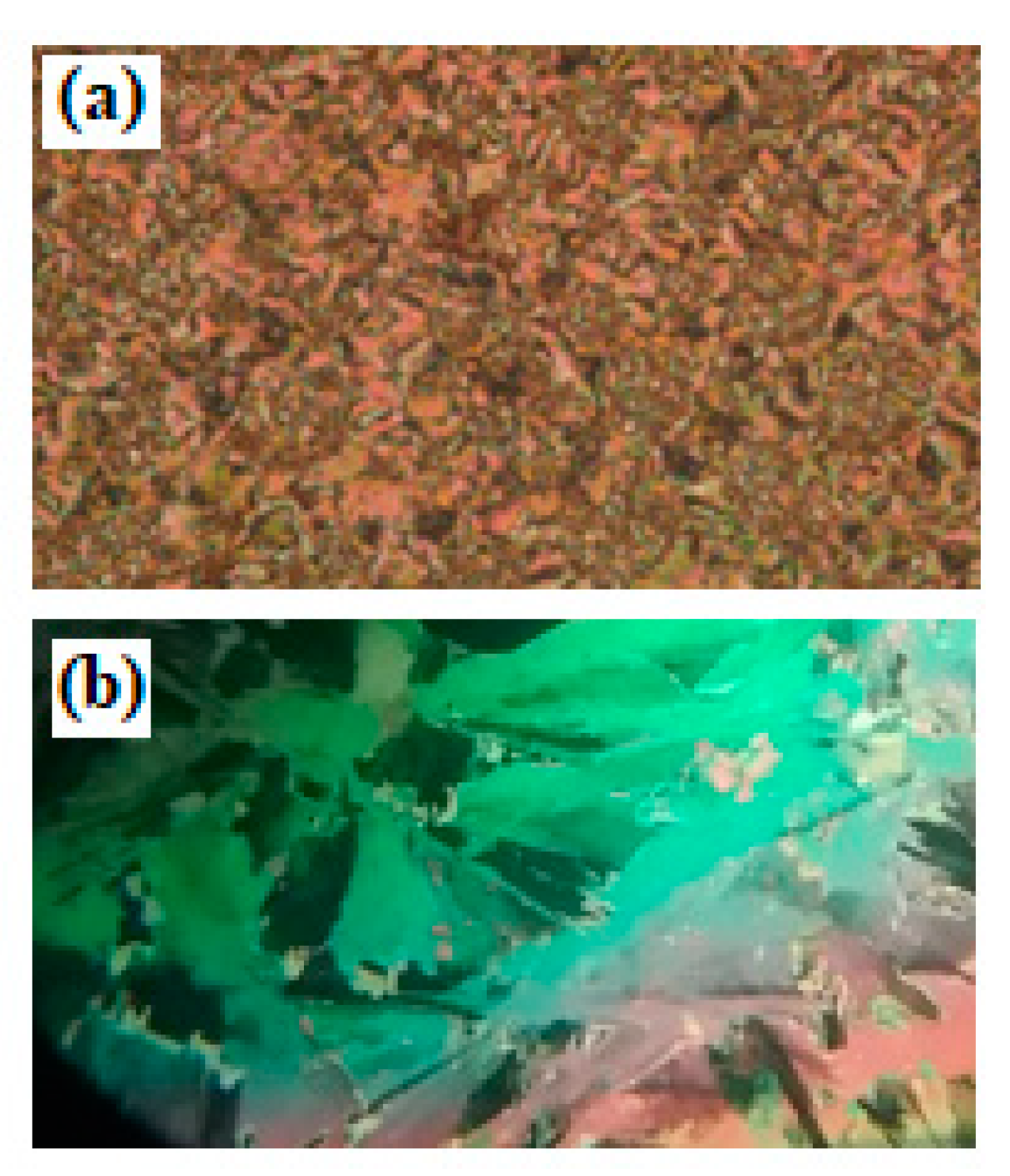
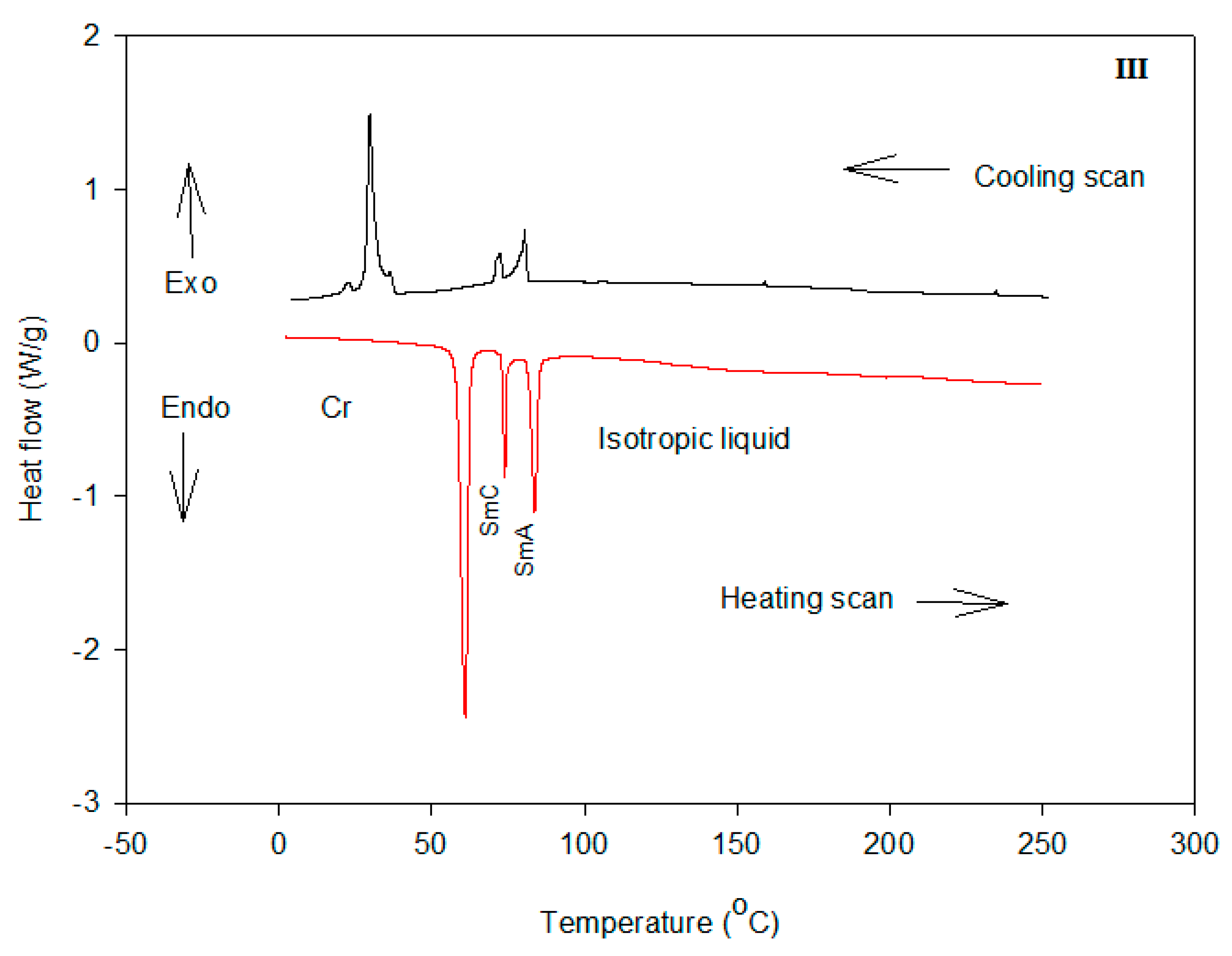
2.2. Mesomorphic Investigations
2.3. Effect of Different Mesogens and Terminal Groups on the Mesophase Behavior
2.4. DFT Calculations
2.4.1. Optimized Geometrical Structures
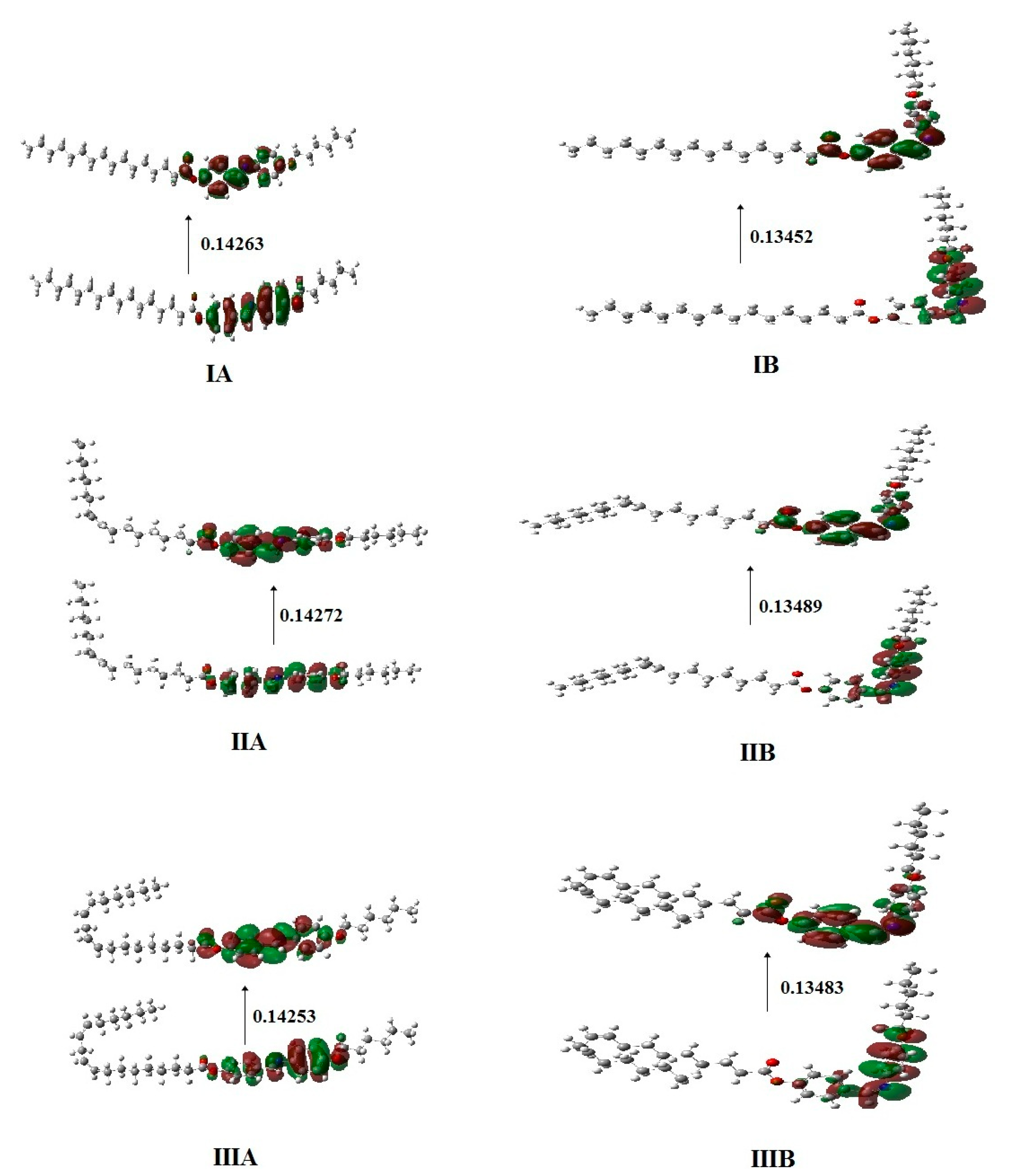
2.4.2. Frontier Molecular Orbitals and Molecular Electrostatic Potential
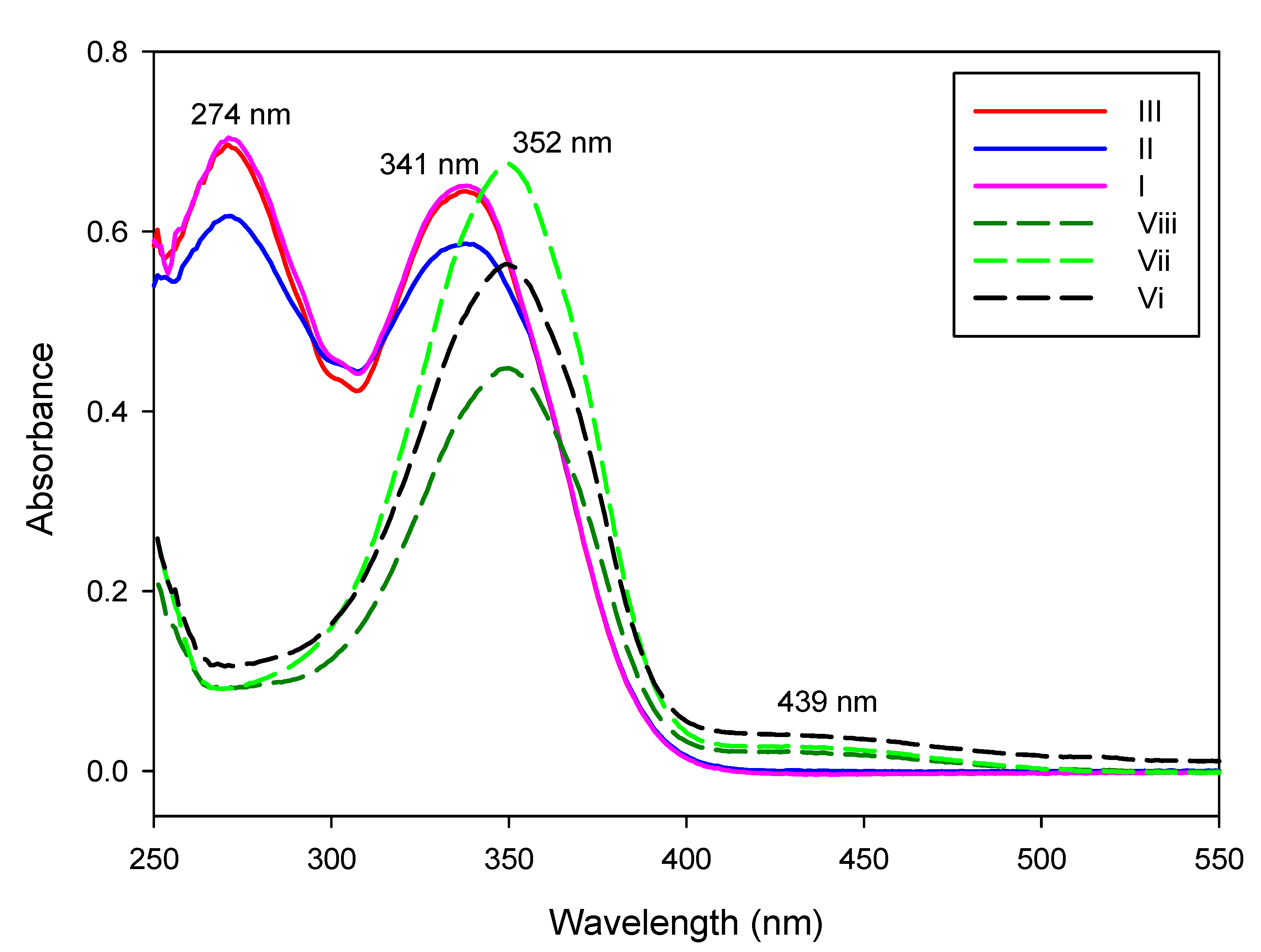
2.4.3. Photophysical Behavior of the Investigated Compounds and Their Corresponding Azo Analogues
3. Experimental
3.1. Synthesis
3.1.1. Synthesis of 4-(Hexyloxy)phenylimino)phenol A
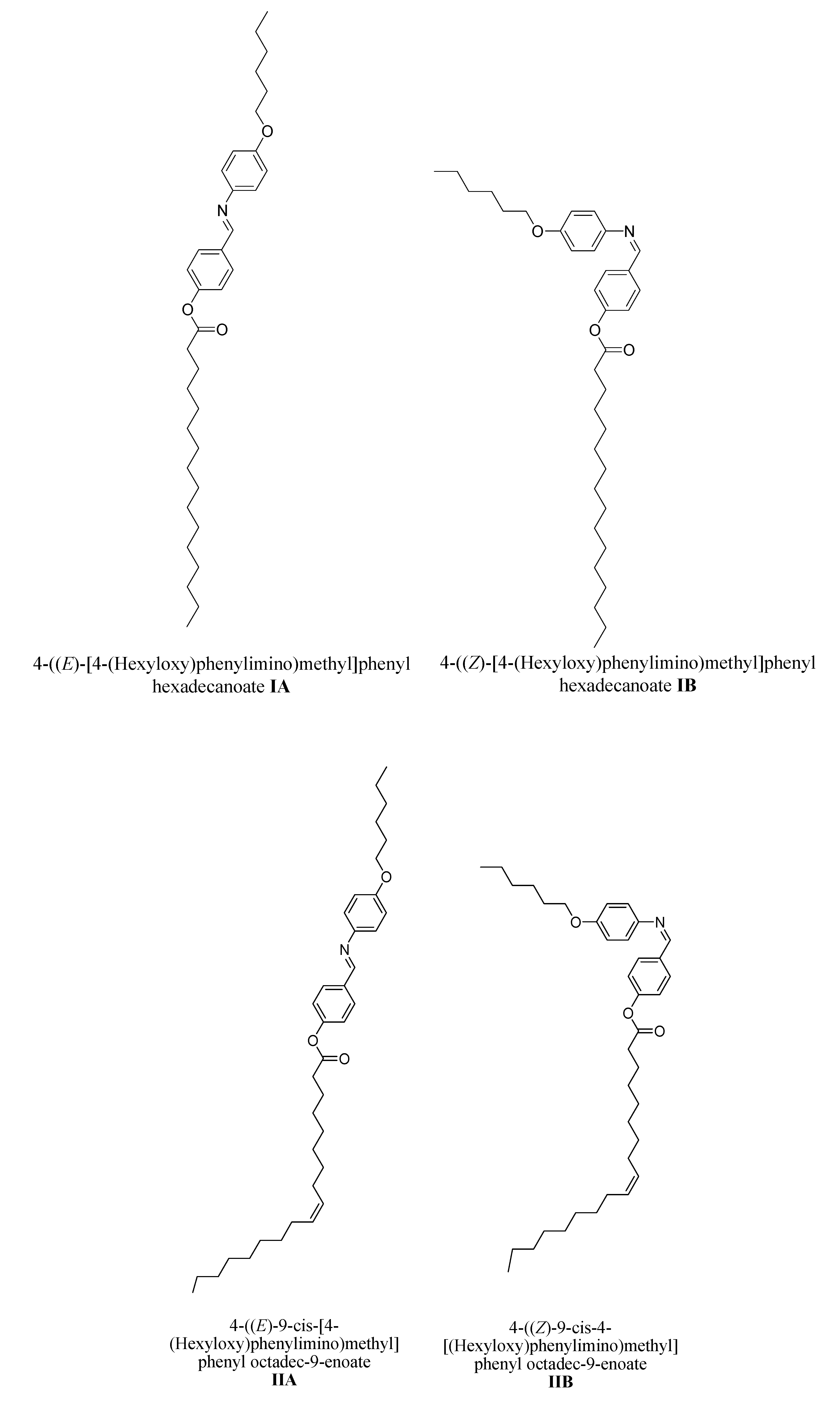
3.1.2. Synthesis of [4-(Hexyloxy)phenylimino)methyl]phenyl carboxylate (I–III)A
4-((E)-9-cis-[4-(Hexyloxy)phenylimino)methyl]phenyl hexadec-9-enoate IA
4-((E)-9-cis-[4-(Hexyloxy)phenylimino)methyl]phenyl octadec-9-enoate IIA
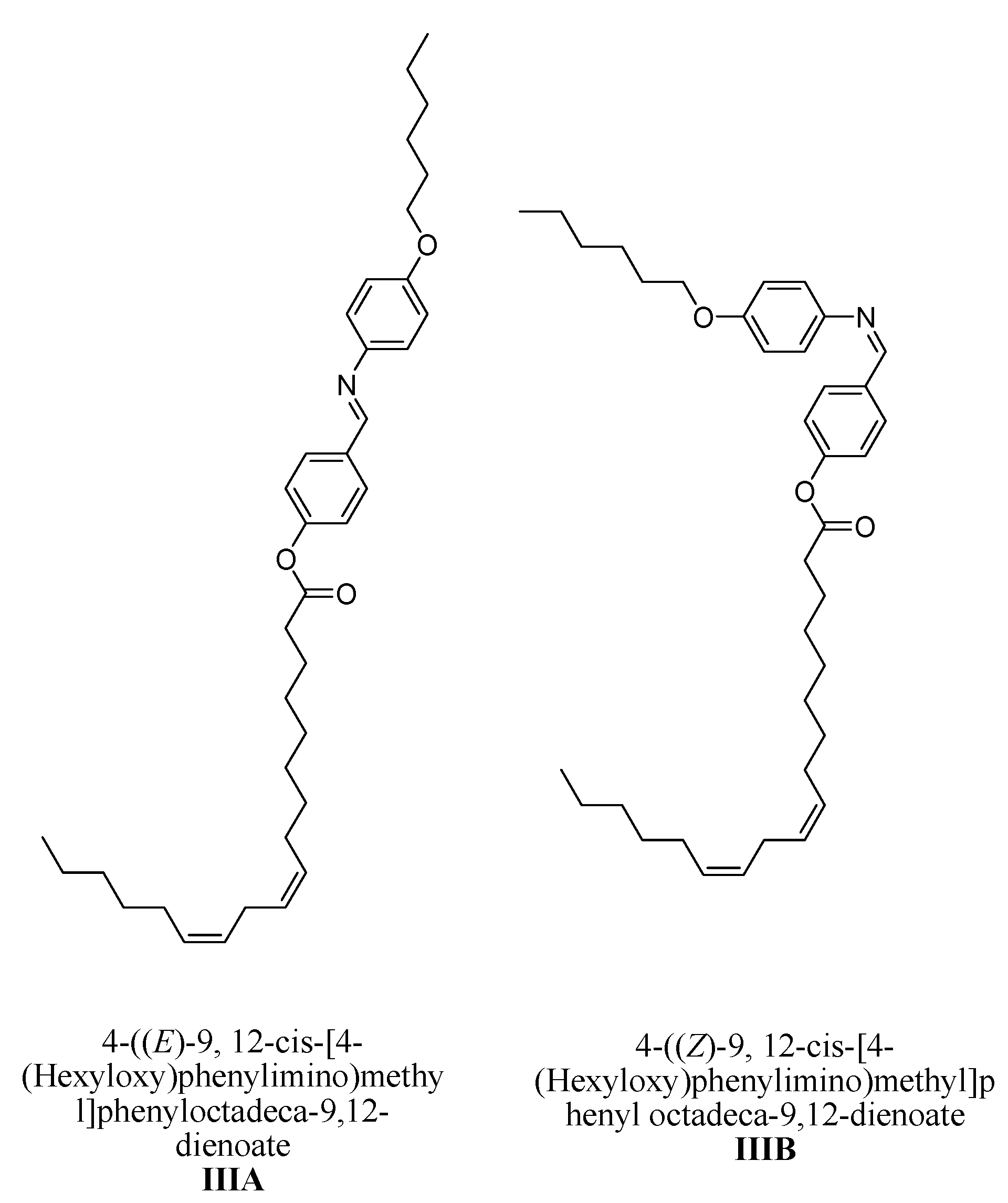
4-((E)-9, 12-cis-[4-(Hexyloxy)phenylimino)methyl]phenyloctadeca-9,12-dienoate IIIA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pramanik, A.; Das, M.K.; Das, B.; Żurowska, M.; Dąbrowski, R. Electro-optical properties of a new series of fluorinated antiferroelectric orthoconic liquid crystalline esters. Liq. Cryst. 2015, 42, 412–421. [Google Scholar] [CrossRef]
- Heilmeier, G.H.; Zanoni, L. Guest-host interactions in nematic liquid crystals. A new electro-optic effect. Appl. Phys. Lett. 1968, 13, 91–92. [Google Scholar] [CrossRef]
- Heilmeier, G.H.; Zanoni, L.A.; Barton, L.A. Dynamic scattering: A new electro-optic effect in certain classes of nematic liquid crystals. Proc. IEEE 1968, 56, 1162–1171. [Google Scholar] [CrossRef]
- Goodby, J. Ferroelectric liquid crystals: Principles properties and applications. Ferroelectr. Relat. Phenom. 1992, 7. [Google Scholar] [CrossRef]
- Carlton, R.J.; Hunter, J.T.; Miller, D.S.; Abbasi, R.; Mushenheim, P.C.; Tan, L.N.; Abbott, N.L. Chemical and biological sensing using liquid crystals. Liq. Cryst. Rev. 2013, 1, 29–51. [Google Scholar] [CrossRef]
- Bhat, S.G.; Ramachandra, G.S.; Bhagavath, P.; Subrao, M.; Potukuchi, D.; Maddasani, S. Self-assembled liquid crystalline materials with fatty acids. J. Therm. Anal. Calorim. 2018, 132, 989–1000. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A.; Yeap, G.-Y. Liquid crystal oligomers: going beyond dimers. Liq. Cryst. 2009, 36, 755–777. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Lee, H.-C.; Mahmood, W.A.K.; Imrie, C.T.; Takeuchi, D.; Osakada, K. Synthesis, thermal and optical behavior of non-symmetric liquid crystal dimers α-(4-benzylidene-substituted-aniline-4′-oxy)-ω-[pentyl-4-(4′-phenyl) benzoateoxy] hexane. Phase Transit. 2011, 84, 29–37. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Osman, F.; Imrie, C.T. Non-symmetric dimers: effects of varying the mesogenic linking unit and terminal substituent. Liq. Cryst. 2015, 42, 543–554. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Hng, T.-C.; Yeap, S.-Y.; Gorecka, E.; Ito, M.M.; Ueno, K.; Okamoto, M.; Mahmood, W.A.K.; Imrie, C.T. Why do non-symmetric dimers intercalate? The synthesis and characterisation of the α-(4-benzylidene-substituted-aniline-4′-oxy)-ω-(2-methylbutyl-4′-(4 ″-phenyl) benzoateoxy) alkanes. Liq. Cryst. 2009, 36, 1431–1441. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. Experimental and theoretical approaches of molecular geometry and mesophase behavior relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1440–1451. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. Mesophase stability of new Schiff base ester liquid crystals with different polar substituents. Liq. Cryst. 2018, 45, 1324–1332. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Saad, G. Impact of the proportionation of dialkoxy chain length on the mesophase behavior of Schiff base/ester liquid crystals; experimental and theoretical study. Liq. Cryst. 2019, 46, 1611–1620. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. Synthesis and mesophase behavior of Schiff base/ester 4-(arylideneamino) phenyl-4 ″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; El-Sayed TH, B.; Alnoman, R. Schiff base/ester liquid crystals with different lateral substituents: Mesophase behavior and DFT calculations. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alhaddad, O. New chair shaped supramolecular complexes-based aryl nicotinate derivative; mesomorphic properties and DFT molecular geometry. Rsc Adv. 2019, 9, 16366–16374. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. New azobenzene-based natural fatty acid liquid crystals with low melting point: synthesis, DFT calculations and binary mixtures. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; El-Sayed, T.; Alnoman, R. Mesophase behavior and DFT conformational analysis of new symmetrical diester chalcone liquid crystals. J. Mol. Liq. 2019, 285, 96–105. [Google Scholar] [CrossRef]
- Veerabhadraswamy, B.N.; Rao, D.S.S.; Yelamaggad, C.V. Ferroelectric Liquid Crystals: Synthesis and Thermal Behavior of Optically Active, Three-Ring Schiff Bases and Salicylaldimines. Chem. Asian J. 2018, 13, 1012–1023. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hsu, C.-C.; Chen, L.-W.; Cheng, Y.-L. The effect of position of (S)-2-octyloxy tail on the formation of frustrated blue phase and antiferroelectric phase in Schiff base liquid crystals. Soft Matter 2014, 10, 9343–9351. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Gowda, A.; Jacob, L.; Joy, N.; Philip, R.; Pratibha, R.; Kumar, S. Thermal and nonlinear optical studies of newly synthesized EDOT based bent-core and hockey-stick like liquid crystals. New J. Chem. 2018, 42, 2047–2057. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O. Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Kelker, H.; Scheurle, B. A Liquid-crystalline (Nematic) Phase with a Particularly Low Solidification Point. Angew. Chem. Int. Ed. 1969, 8, 884–885. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A. Liquid crystal dimers and oligomers. Curr. Opin. Colloid Interface Sci. 2002, 7, 298–311. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A. Liquid crystal dimers and higher oligomers: between monomers and polymers. Chem. Soc. Rev. 2007, 36, 2096–2124. [Google Scholar] [CrossRef]
- Gulbas, H.; Coskun, D.; Gursel, Y.; Bilgin-Eran, B. Synthesis, characterization and mesomorphic properties of side chain liquid crystalline oligomer having schiff base type mesogenic group. Adv. Mater. 2014, 5, 333–338. [Google Scholar] [CrossRef]
- Tschierske, C. Mirror symmetry breaking in liquids and liquid crystals. Liq. Cryst. 2018, 45, 2221–2252. [Google Scholar] [CrossRef]
- Goodby, J.W. Free volume, molecular grains, self-organisation, and anisotropic entropy: Machining materials. Liq. Cryst. 2017, 44, 1755–1763. [Google Scholar] [CrossRef]
- Zannoni, C. From idealised to predictive models of liquid crystals. Liq. Cryst. 2018, 45, 1880–1893. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Nafee, S.S.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar]
- Prasad, V.; Nagendrappa Gowdru, N.; Manjunath, M. Thermally stable azo-substituted bent-core nematogens: Observation of chiral domains in the nematic mesophases composed of smectic nano clusters. Liq. Cryst. 2018, 45, 666–679. [Google Scholar] [CrossRef]
- Paterson, D.A.; Walker, R.; Abberley, J.P.; Forestier, J.; Harrison, W.T.; Storey, J.M.; Pociecha, D.; Gorecka, E.; Imrie, C.T. Azobenzene-based liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2017, 44, 2060–2078. [Google Scholar] [CrossRef]
- Nesrullajev, A.; Bilgin-Eran, B. Mesomorphic, morphologic and thermotropic properties of 4-hexyl-N-(4-hexadecyloxysalicylidene) aniline. Mater. Chem. Phys. 2005, 93, 21–25. [Google Scholar] [CrossRef]
- Yasa-Sahin, O.; Yazici, O.; Karaagac, B.; Sakar, D.; Cankurtaran, O.; Bilgin-Eran, B.; Karaman, F. A new liquid crystal of considerable value for the separation of closely related solvents by gas chromatography. Liq. Cryst. 2010, 37, 1111–1118. [Google Scholar] [CrossRef]
- Canli, N.Y.; Günes, S.; Pivrikas, A.; Fuchsbauer, A.; Sinwel, D.; Sariciftci, N.; Yasa, Ö.; Bilgin-Eran, B. Chiral (S)-5-octyloxy-2-[{4-(2-methylbuthoxy)-phenylimino}-methyl]-phenol liquid crystalline compound as additive into polymer solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1089–1099. [Google Scholar] [CrossRef]
- Bhola, G.; Bhoya, U. Molecular structural flexibility dependence of mesomorphism through ortho-substituted bromo group. Mol. Cryst. Liq. Cryst. 2016, 630, 188–196. [Google Scholar] [CrossRef]
- Galewski, Z.; Coles, H. Liquid crystalline properties and phase situations in 4-chlorobenzylidene-4′-alkylanilines. J. Mol. Liq. 1999, 79, 77–87. [Google Scholar] [CrossRef]
- Galewski, Z. Liquid crystalline properties of 4-alkoxy-aniline-4′-alkoxy-anilines. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1994, 249, 43–49. [Google Scholar] [CrossRef]
- Dave, J.S.; Menon, M. Azomesogens with a heterocyclic moiety. Bull. Mater. Sci. 2000, 23, 237–238. [Google Scholar] [CrossRef]
- Dietrich, D. Calamitic liquid crystals. In Handbook of Liquid Crystals: Low Molecular Weight Liquid Crystals I; Goodby, J.W., Gray, G.W., Spiess, H.W., Vill, V., Eds.; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2011; Volume 2A. [Google Scholar]
- Hagar, M.; Ahmed, H.; Alhaddadd, O. DFT Calculations and mesophase study of coumarin esters and its azoesters. Crystals 2018, 8, 359. [Google Scholar] [CrossRef]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Quinazolin-4-yl-sulfanylacetyl-hydrazone derivatives; Synthesis, molecular structure and electronic properties. J. Mol. Struct. 2013, 1049, 177–188. [Google Scholar] [CrossRef]
- Soliman, S.M.; Hagar, M.; Ibid, F.; El Sayed, H. Experimental and theoretical spectroscopic studies, HOMO–LUMO, NBO analyses and thione–thiol tautomerism of a new hybrid of 1, 3, 4-oxadiazole-thione with quinazolin-4-one. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015, 145, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Synthesis, molecular structure and spectroscopic studies of some new quinazolin-4 (3H)-one derivatives; an account on the N-versus S-Alkylation. J. Mol. Struct. 2016, 1108, 667–679. [Google Scholar] [CrossRef]
- Aboelnaga, A.; Hagar, M.; Soliman, S.M. Ultrasonic synthesis, molecular structure and mechanistic study of 1, 3-dipolar cycloaddition reaction of 1-alkynylpyridinium-3-olate and acetylene derivatives. Molecules 2016, 21, 848. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alhaddad, O.A. Phase behavior and DFT calculations of laterally methyl supramolecular hydrogen-bonding complexes. Crystals 2019, 9, 133. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2019, 46, 550–559. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Saad, G.R. New calamitic thermotropic liquid crystals of 2-hydroxypyridine ester mesogenic core: mesophase behaviour and DFT calculations. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Naoum, M.; Ahmed, H. Effect of dipole moment and conformation on the mesophase behavior of di-laterally substituted phenylazophenyl benzoate liquid crystals. Thermochim. Acta 2011, 521, 202–210. [Google Scholar] [CrossRef]
- Imrie, C.T. Non-symmetric liquid crystal dimers: how to make molecules intercalate. Liq. Cryst. 2006, 33, 1449–1485. [Google Scholar] [CrossRef]
- Date, R.; Imrie, C.; Luckhurst, G.; Seddon, J. Smectogenic dimeric liquid crystals. The preparation and properties of the α, ω-bis (4-n-alkylanilinebenzylidine-4′-oxy) alkanes. Liq. Cryst. 1992, 12, 203–238. [Google Scholar] [CrossRef]
- Naoum, M.M.; Metwally, N.H.; Abd Eltawab, M.M.; Ahmed, H.A. Polarity and steric effect of the lateral substituent on the mesophase behavior of some newly prepared liquid crystals. Liq. Cryst. 2015, 42, 1351–1369. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Ha, S.-T.; Lim, P.-L.; Boey, P.-L.; Mahmood, W.A.K.; Ito, M.M.; Sanehisa, S. Synthesis and mesomorphic properties of schiff base esters ortho-hydroxy-para-alkyloxybenzylidene-para-substituted anilines. Mol. Cryst. Liq. Cryst. 2004, 423, 73–84. [Google Scholar] [CrossRef]
- McCaffrey, M.T.; Castellano, J.A. Liquid Crystals VII. The mesomorphic behavior of homologous: p-alkoxy-p’-acyloxyazoxybenzenes. Mol. Cryst. Liq. Cryst. 1972, 18, 209–225. [Google Scholar] [CrossRef]
- Alam, M.Z.; Yoshioka, T.; Ogata, T.; Nonaka, T.; Kurihara, S. Influence of helical twisting power on the photoswitching behavior of chiral azobenzene compounds: applications to high-performance switching devices. Chem. (Weinh. Der Bergstr. Ger.) 2007, 13, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Ruslim, C.; Komitov, L.; Ichimura, K. Formation and behavior of monolayers of 3, 3′-and 4, 4′-disubstituted azobenzenes on a water surface. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 2001, 367, 125–132. [Google Scholar] [CrossRef]
- Imrie, C. Laterally substituted dimeric liquid crystals. Liq. Cryst. 1989, 6, 391–396. [Google Scholar] [CrossRef]
- Foo, K.-L.; Ha, S.-T.; Yeap, G.; Lee, S. Mesomorphic behaviors of a series of heterocyclic thiophene-imine-ester-based liquid crystals. Phase Transit. 2018, 91, 509–520. [Google Scholar] [CrossRef]
- Gaussian 09; revision a. 02; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- GaussView, version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009.
- Ahmed, H.A.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC. Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef]
- Lyons, C.; Rathi, N.; Dev, P.; Byrne, O.; Surolia, P.K.; Maji, P.; MacElroy, J.; Yella, A.; Grätzel, M.; Magner, E. Organic dyes containing coplanar dihexyl-substituted dithienosilole groups for efficient dye-sensitised solar cells. Int. J. Photoenergy 2017, 2017, 7594869. [Google Scholar] [CrossRef]
- Sui, M.Y.; Pan, Q.Q.; Yin, H.; Sun, G.Y.; Geng, Y.; Su, Z.M. Design of hexabenzocoronene derivatives as non-fullerene acceptors in organic photovoltaics by bridging dimers and modulating structural twists. Sol. Rrl. 2017, 1, 1700060. [Google Scholar] [CrossRef]
- Yamamoto, R.; Yamamoto, T.; Ohara, K.; Naito, T. Dye-sensitized molecular charge transfer complexes: magnetic and conduction properties in the photoexcited states of ni (dmit) 2 salts containing photosensitive dyes. Magnetochemistry 2017, 3, 20. [Google Scholar] [CrossRef]
Sample Availability: A(I–III) are available from the authors. |


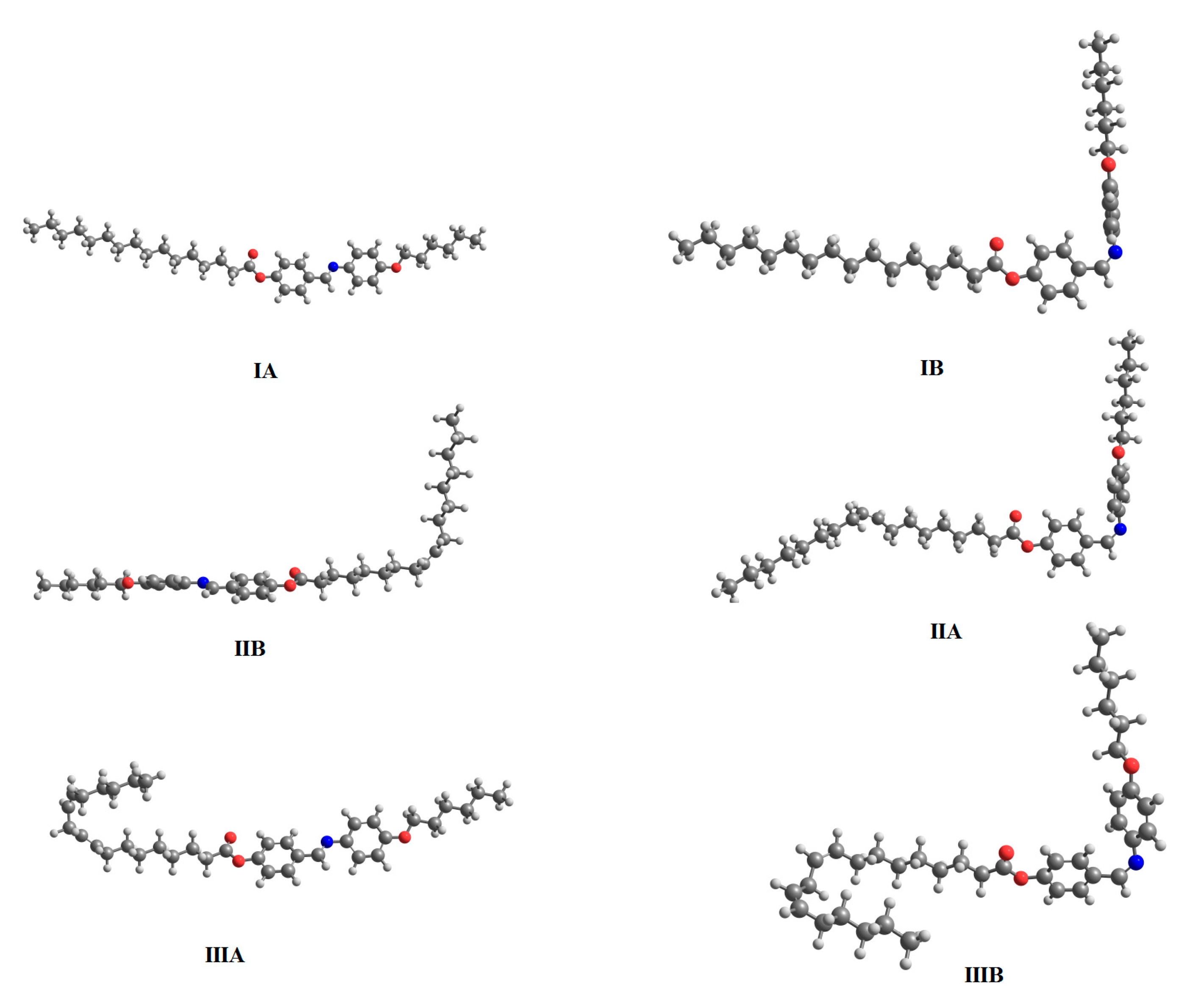
| Compound | TCr-SmC | ∆HCr-SmC | TSmC-SmA | ∆HSmC-SmA | TSmC-I | ∆HSmC-I | TSmA-I | ∆HSmA-I | ∆S/R |
|---|---|---|---|---|---|---|---|---|---|
| IA | 93.5 | 10.98 | - | - | 97.7 | 0.65 | - | - | 0.89 |
| IIA | 67.9 | 8.39 | - | - | 77.4 | 0.80 | - | - | 1.14 |
| IIIA | 61.0 | 8.05 | 74.0 | 0.73 | - | - | 83.8 | 0.88 | 1.24 |
| Compound | Compound Structure | Phase Transitions |
|---|---|---|
| IV [54] | 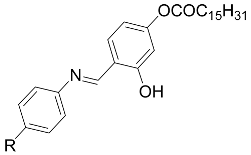 R = -OCH3 | Cr 98.0 SmA 111.0 I |
| V [13,55] |  R = i; C15H31, ii; C17H33, iii; C17H31 | (i) Cr 93.0 SmA 88.6I (ii) Cr 51.5 SmA 56.5I (iii)Cr 43.3 SmA 51.0I |
| VI [56] | 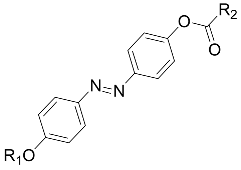 R1 = C6H13, R2 = C7H15 | Cr 78.0 N 107.0I |
| Parameter | IA | IB | IIA | IIB | IIIA | IIIB |
|---|---|---|---|---|---|---|
| Ecorr | 0.83 | 0.83 | 0.86 | 0.86 | 0.84 | 0.84 |
| ZPVE | −1644.86 | −1644.85 | −1722.20 | −1722.19 | −1720.99 | −1720.98 |
| Etot | −1644.81 | −1644.80 | −1722.15 | −1722.14 | −1720.95 | −1720.93 |
| H | −1644.81 | −1644.80 | −1722.15 | −1722.14 | −1720.94 | −1720.93 |
| G | −1644.95 | −1644.94 | −1722.30 | −1722.28 | −1721.09 | −1721.07 |
| ΔH(A:B and A:C) | 0.000 | 0.012 | 0.000 | 0.012 | 0.000 | 0.012 |
| Total Dipole Moment | 2.6402 | 2.1197 | 2.8932 | 2.1722 | 2.8946 | 1.9983 |
| Polarizability α | 441.42 | 409.27 | 463.25 | 433.73 | 459.39 | 426.10 |
| Compound | EHOMO (a.u) | ELUMO (a.u) | ΔE(ELUMO−EHOMO) (a.u) | S = 1/ΔE |
|---|---|---|---|---|
| IA | −0.20031 | −0.05768 | 0.14263 | 7.011148 |
| IB | −0.19102 | −0.05650 | 0.13452 | 7.433839 |
| IIA | −0.20040 | −0.05768 | 0.14272 | 7.006726 |
| IIB | −0.19144 | −0.05655 | 0.13489 | 7.413448 |
| IIIA | −0.20041 | −0.05788 | 0.14253 | 7.016067 |
| IIIB | −0.19180 | −0.05697 | 0.13483 | 7.416747 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnoman, R.; Al-Nazawi, F.k.; Ahmed, H.A.; Hagar, M. Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals. Molecules 2019, 24, 4293. https://doi.org/10.3390/molecules24234293
Alnoman R, Al-Nazawi Fk, Ahmed HA, Hagar M. Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals. Molecules. 2019; 24(23):4293. https://doi.org/10.3390/molecules24234293
Chicago/Turabian StyleAlnoman, Rua, Fares khalid Al-Nazawi, Hoda A. Ahmed, and Mohamed Hagar. 2019. "Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals" Molecules 24, no. 23: 4293. https://doi.org/10.3390/molecules24234293
APA StyleAlnoman, R., Al-Nazawi, F. k., Ahmed, H. A., & Hagar, M. (2019). Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals. Molecules, 24(23), 4293. https://doi.org/10.3390/molecules24234293









