Sensory Metabolite Profiling in a Date Pit Based Coffee Substitute and in Response to Roasting as Analyzed via Mass Spectrometry Based Metabolomics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Components and Their Contribution to the Aroma of Roasted Pits and Coffee via SPME Analysis
2.1.1. Furans and Furanones
2.1.2. Pyrans
2.1.3. Pyrroles
2.1.4. Mono/Sesquiterpenoids
2.1.5. Oxygenated Monoterpenoids
2.1.6. Phenolics
2.1.7. Sulfur Containing Volatiles
2.1.8. Aldehydes/Ketones/Lactones
2.2. Multivariate Data Analysis of Headspace SPME-GCMS Volatiles Dataset
2.3. GCMS Analysis of Non-Volatile Primary Metabolites Post Silylation
2.3.1. Alcohols and Organic Acids
2.3.2. Amino Acids/Nitrogenous Compounds
2.3.3. Fatty Acids
2.3.4. Sugars
2.3.5. Sterols and Triterpenes
2.4. Multivariate Data Analyses of Silylated Primary Metabolites
2.5. Acute Toxicity Study
2.6. Neuropharmacological Tests
2.6.1. Phenobarbital Sodium Induced Sleeping Time
2.6.2. Open Field Test
3. Materials and Methods
3.1. Plant Material, Animals and Chemicals
3.2. The Preparations of Roasted Date Pits, Commercial Pit Product and Coffee Product Extracts for Chemical and Biological Analyses
3.3. SPME Volatiles Analysis
3.4. GCMS Headspace Volatile Analysis
3.5. GCMS Analysis of Silylated Primary Metabolites
3.6. Multivariate Data Analyses
3.7. Acute Toxicity Study
3.8. Neuropharmacological Tests
3.8.1. Treatment Schedule
3.8.2. Phenobarbital Sodium Induced Sleeping Time
3.8.3. Open Field Test
3.8.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scott-Thomas, C. Food Waste ‘One of the Great Paradoxes of Our Times’. Available online: http://www.foodnavigator.com (accessed on 17 October 2013).
- Devshony, S.; Eteshola, E.; Shani, A. Characteristics and some potential applications of date palm (Phoenix dactylifera L.) seeds and seed oil. J. Am. Oil Chem. Soc. 1992, 69, 595–597. [Google Scholar] [CrossRef]
- Hamada, J.S.; Hashim, I.B.; Sharif, F.A. Preliminary analysis and potential uses of date pits in foods. Food Chem. 2002, 76, 135–137. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Waly, M.I.; Singh, V.; Sequeira, V.; Rahman, M.S. Chemical composition of date-pits and its potential for developing value-added product—A review. Pol. J. Food Nutr. Sci. 2014, 64, 215–226. [Google Scholar] [CrossRef]
- Saafi-Ben Salah, E.B.; El Arem, A.; Louedi, M.; Saoudi, M.; Elfeki, A.; Zakhama, A.; Najjar, M.F.; Hammami, M.; Achour, L. Antioxidant-rich date palm fruit extract inhibits oxidative stress and nephrotoxicity induced by dimethoate in rat. J. Physiol. Biochem. 2012, 68, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.N.A.; Fekry, M.I.; Farag, M.A. Metabolome based volatiles profiling in 13 date palm fruit varieties from Egypt via SPME GC–MS and chemometrics. Food Chem. 2017, 217, 171–181. [Google Scholar] [CrossRef]
- Grosch, W. Flavour of coffee. A review. Nahrung 1998, 42, 344–350. [Google Scholar] [CrossRef]
- Nijssen, L.; Visscher, C.; Maarse, H.; Willemsens, L.; Boelens, M. Volatile Compounds in Food: Qualitative and Quantitative Data, 7th ed.; Central Institute for Nutrition and Food Research, TNO: Zeist, The Netherlands, 1996. [Google Scholar]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H. Analysis of the headspace volatiles of freshly brewed Arabica coffee using solid-phase microextraction. J. Food Sci. 2007, 72, C388–C396. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Augusto, F.; Salva, T.J.G.; Thomaziello, R.A.; Ferreira, M.M.C. Prediction of sensory properties of Brazilian Arabica roasted coffees by headspace solid phase microextraction-gas chromatography and partial least squares. Anal. Chim. Acta 2009, 634, 172–179. [Google Scholar] [CrossRef]
- Blank, I.; Sen, A.; Grosch, W. Potent odorants of the roasted powder and brew of Arabica coffee. Z. Lebensm. Unters. Forsch. 1992, 195, 239–245. [Google Scholar] [CrossRef]
- Shahidi, F.; Ho, C.T. Flavor Chemistry of Ethnic Foods, 3rd ed.; Springer: Boston, MA, USA, 2012. [Google Scholar]
- Yang, N.; Liu, C.; Liu, X.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016, 211, 206–214. [Google Scholar] [CrossRef]
- Flament, I.; Bessière-Thomas, Y. Coffee Flavor Chemistry; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Silwar, R.; Lullmann, C. Investigation of aroma formation in Robusta coffee during roasting. Café Cacao 1993, 37, 145–152. [Google Scholar]
- Rocha, S.; Maeztu, L.; Barros, A.; Cid, C.; Coimbra, M.A. Screening and distinction of coffee brews based on headspace solid phase microextraction/gas chromatography/principal component analysis. J. Sci. Food Agric. 2004, 84, 43–51. [Google Scholar] [CrossRef]
- Del Terra, L.; Lonzarich, V.; Asquini, E.; Navarini, L.; Graziosi, G.; Suggi Liverani, F.; Pallavicini, A. Functional characterization of three Coffea arabica L. monoterpene synthases: Insights into the enzymatic machinery of coffee aroma. Phytochemistry 2013, 89, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.; Blank, I.; Dunkel, A.; Hofmann, T. Chapter 12—The chemistry of roasting—Decoding flavor formation. In The Craft and Science of Coffee; Academic Press: London, UK, 2017; pp. 273–309. [Google Scholar]
- Cannon, R.J.; Trinnaman, L.; Grainger, B.; Trail, A. The key odorants of coffee from various geographical locations. In Flavors in Noncarbonated Beverages; American Chemical Society: Washington, DC, USA, 2010; Volume 1036, pp. 77–90. [Google Scholar]
- Merritt, C., Jr.; Robertson, D.; McAdoo, D. The relationship of volatile compounds in roasted coffee beans to their precursors. In Proceedings of the COLLOQUE International sur la Chimie des Cafés, 4, Amsterdam, The Netherlands, 2–6 June 1969; pp. 144–148. [Google Scholar]
- Sugie, S.; Okumura, A.; Tanaka, T.; Mori, H. Inhibitory effects of benzyl isothiocyanate and benzyl thiocyanate on diethylnitrosamine-induced hepatocarcinogenesis in rats. Jpn. J. Cancer Res. 1993, 84, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Rana, M.K. Salad Crops|Leaf-types. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: San Diego, CA, USA, 2003; pp. 5046–5055. [Google Scholar]
- Harrak, H.; Reynes, M.; Lebrun, M.; Hamouda, A.; Brat, P. Identification and comparison of volatile components of fruits of eight Moroccan date varieties. Fruits Paris 2005, 60, 267–268. [Google Scholar] [CrossRef]
- Lyman, D.J.; Benck, R.; Dell, S.; Merle, S.; Murray-Wijelath, J. FTIR-ATR analysis of brewed coffee: Effect of roasting conditions. J. Agric. Food Chem. 2003, 51, 3268–3272. [Google Scholar] [CrossRef]
- Farag, M.A.; Labib, R.M.; Noleto, C.; Porzel, A.; Wessjohann, L.A. NMR approach for the authentication of 10 cinnamon spice accessions analyzed via chemometric tools. LWT 2018, 90, 491–498. [Google Scholar] [CrossRef]
- Farag, M.A.; Ali, S.E.; Hodaya, R.H.; El-Seedi, H.R.; Sultani, H.N.; Laub, A.; Eissa, T.F.; Abou-Zaid, F.O.F.; Wessjohann, L.A. Phytochemical Profiles and antimicrobial activities of Allium cepa Red cv. and A. sativum subjected to different drying methods: A comparative MS-based metabolomics. Molecules 2017, 22, 761. [Google Scholar] [CrossRef]
- Buffo, R.A.; Cardelli-Freire, C. Coffee flavour: An overview. Flavour Fragr. J. 2004, 19, 99–104. [Google Scholar] [CrossRef]
- Tressl, R. Bildung von aromastoffen curch Maillard reaktion (Formation of aroma compounds by the Maillard reaction). In Proceedings of the COLLOQUE Scientifique International sur le Café, 9, London, UK, 16–20 June 1980. [Google Scholar]
- Casal, S.; Oliveira, M.B.; Alves, M.R.; Ferreira, M.A. Discriminate analysis of roasted coffee varieties for trigonelline, nicotinic acid, and caffeine content. J. Agric. Food Chem. 2000, 48, 3420–3424. [Google Scholar] [CrossRef]
- Liang, T.; Liao, S. Inhibition of steroid 5α-reductase by specific aliphatic unsaturated fatty acids. Biochem. J. 1992, 285, 557–562. [Google Scholar] [CrossRef]
- O’Brien, R.D. Fats and Oils: Formulating and Processing for Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Antonelli, T.; Carla, V.; Lambertini, L.; Moroni, F.; Bianchi, C. Pyroglutamic acid administration modifies the electrocorticogram and increases the release of acetylcholine and GABA from the guinea-pig cerebral cortex. Pharmacol. Res. Commun. 1984, 16, 189–197. [Google Scholar] [CrossRef]
- Buck, W.B.; Osweiler, G.D.; Van Gelder, G.A. Clinical and Diagnostic Veterinary Toxicology, 2nd ed.; Kendall/Hunt Publishing Co.: Lowa, IA, USA, 1976. [Google Scholar]
- Tripathi, K.D. Essentials of Medical Pharmacology, 4th ed.; Jaypee Brothers, Medical Publishers: New Delhi, India, 1999. [Google Scholar]
- Kulkarni, S.K. Handbook of Experimental Pharmacology, 3rd ed.; Vallabh Prakashan Publication: New Delhi, India, 1999. [Google Scholar]
- Singh, B.R.; Dwivedi, S.; Al-Khedhairy, A.A.; Musarrat, J. Synthesis of stable cadmium sulfide nanoparticles using surfactin produced by Bacillus amyloliquifaciens strain KSU-109. Colloids Surf. B Biointerfaces 2011, 85, 207–213. [Google Scholar] [CrossRef]
- Farag, M.A.; Wessjohann, L.A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J. Food Sci. 2012, 77, C1179–C1184. [Google Scholar] [CrossRef]
- Farag, M.A.; Rasheed, D.M.; Kamal, I.M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle) cultivars via headspace SPME-GC-MS and chemometrics. Food Res. Int. 2015, 78, 327–335. [Google Scholar] [CrossRef]
- Litchfield, J.T., Jr.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Williamson, E.M.; Okpako, D.T.; Evans, F.J. Selection, Preparation and Pharmacological Evaluation of Plant Material; John Wiley & Sons: New York, NY, USA, 1996; Volume 1. [Google Scholar]
- Dey, P.; Chandra, S.; Chatterjee, P.; Bhattacharya, S. Neuropharmacological properties of Mikania scandens (L.) Willd. (Asteraceae). J. Adv. Pharm. Technol. Res. 2011, 2, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, B.S.; Mishra, B.; Veerapur, V.P.; Gupta, G. Anxiolytic activity of Nymphaea alba Linn. in mice as experimental models of anxiety. Indian J. Pharmacol. 2011, 43, 50–55. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
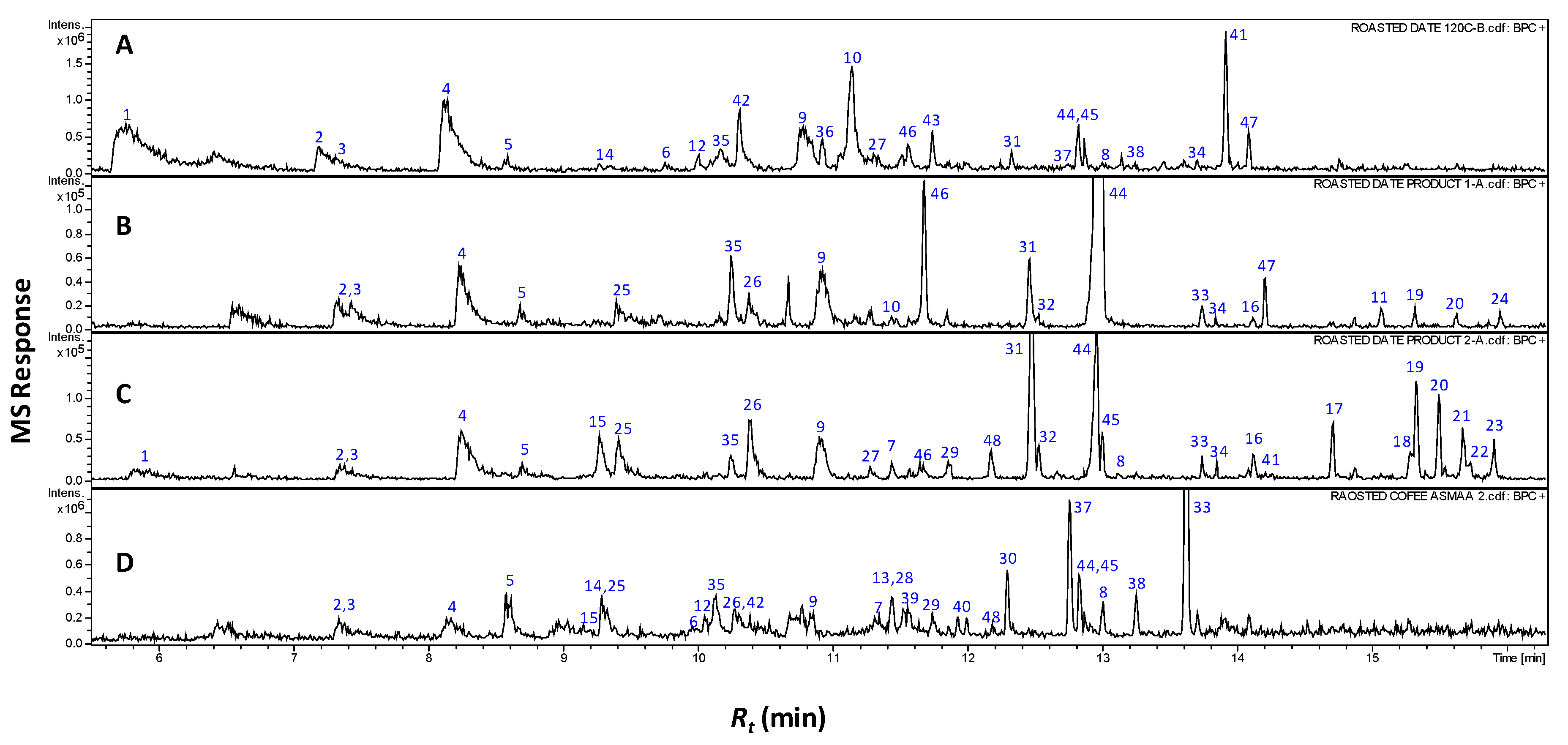
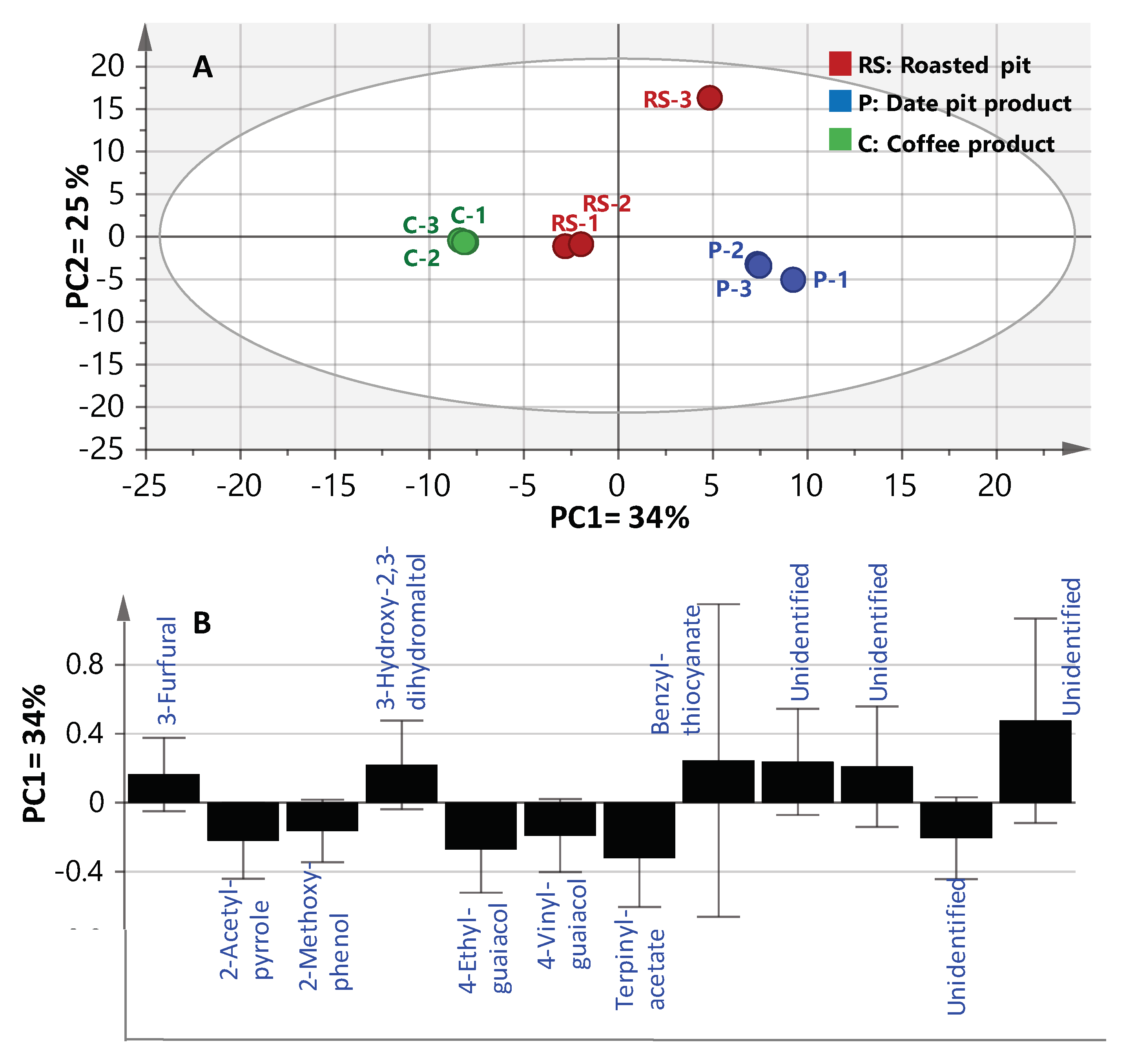
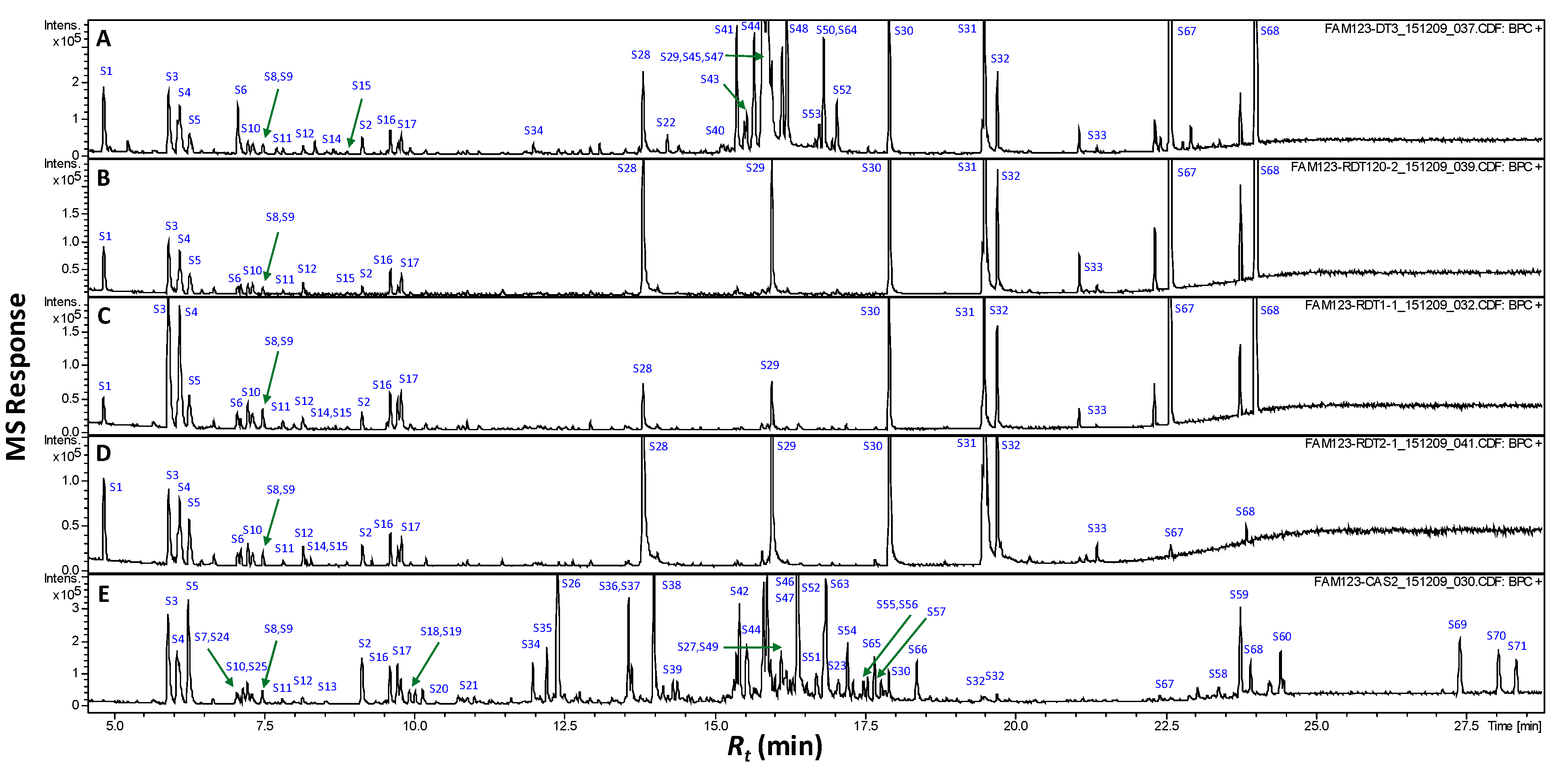
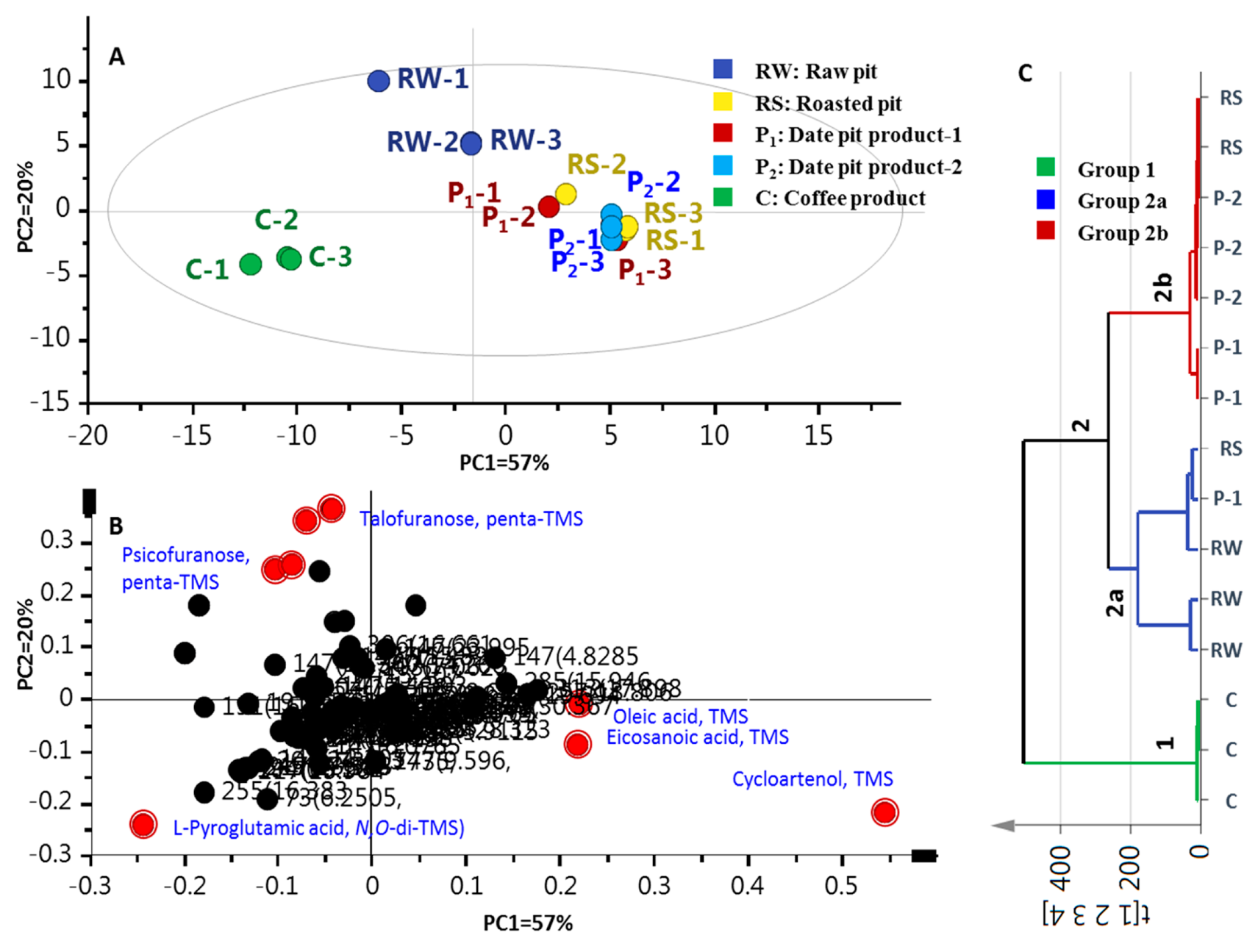
| Peak No. | Rt (min) | RI | Compound | RS | P 2 | C |
|---|---|---|---|---|---|---|
| Furans | ||||||
| 1 | 5.74 | 840.5 | 3-Furfural | 7.58 ± 5.84 | 0.05 ± 0.02 | 0.02 ± 0.02 |
| 2 | 7.18 | 912.0 | 2-Acetylfuran | 0.45 ± 0.02 | 0.13 ± 0.09 | 0.02 ± 0.02 |
| 3 | 7.31 | 919.2 | Dihydro-4-methyl-2(3H)-furanone | 0.14 ± 2.46 | 0.12 ± 0.03 | 0.14 ± 0.10 |
| 4 | 8.11 | 965.2 | 5-Methyl-2-furaldehyde | 15.92 ± 1.20 | 4.88 ± 5.82 | 3.73 ± 0.96 |
| 5 | 8.57 | 991.0 | Furfuryl acetate | 1.19 ± 0.10 | 0.27 ± 0.36 | 4.21 ± 0.84 |
| 6 | 9.96 | 1079.5 | 2-Furfurylfuran | 1.00 ± 0.05 | 0.06 ± 0.08 | 2.02 ± 0.18 |
| 7 | 11.34 | 1174.2 | 5-Methyl-2-furfurylfuran | - | 0.33 ± 0.44 | 1.16 ± 0.35 |
| 8 | 13.00 | 1298.2 | Difurfuryl ether | 0.21 ± 0.15 | 0.02 ± 0.00 | 1.33 ± 0.19 |
| Total furans | 26.50 | 5.87 | 12.71 | |||
| Pyrans | ||||||
| 9 | 10.39 | 1128.1 | Maltol | 9.86 ± 0.04 | 8.60 ± 10.92 | 0.86 ± 0.02 |
| 10 | 11.14 | 1160.2 | 3-Hydroxy-2,3-dihydromaltol | 2.78 ± 1.15 | - | - |
| 11 | 15.07 | 1467.2 | Benzo-α-pyrone | - | 0.05 ± 0.00 | - |
| Total pyrans | 12.64 | 8.65 | 0.86 | |||
| Pyrroles | ||||||
| 12 | 10.05 | 1085.2 | 2-Acetyl-pyrrole | 1.00 ± 0.44 | - | 4.10 ± 1.46 |
| 13 | 11.43 | 1180.3 | 1-(2-Furanyl-methyl)-1H-pyrrole | - | - | 1.60 ± 0.18 |
| Total pyrroles | 1.00 | - | 5.70 | |||
| Lactones | ||||||
| 14 | 9.34 | 1039.7 | Cyclotene (Maple lactone) | 1.47 ± 0.12 | - | 1.58 ± 0.27 |
| Total lactones | 1.47 | - | 1.58 | |||
| Monoterpenoid hydrocarbons | ||||||
| 15 a | 9.17 | 1029.1 | α-Phellandrene | - | 2.22 ± 1.80 | 1.13 ± 3.03 |
| Total monoterpenoid hydrocarbons | - | 2.22 | 1.13 | |||
| Sesquiterpenoid hydrocarbons | ||||||
| 16 | 14.01 | 1380.5 | α-Copaene | - | 0.30 ± 0.37 | - |
| 17 | 14.58 | 1427.4 | (E,β)-Farnesene | - | 1.11 ± 0.96 | - |
| 18 a | 15.15 | 1473.9 | α-Humulene | - | 0.57 ± 0.57 | - |
| 19 | 15.20 | 1478.0 | Curcumene | - | 3.65 ± 3.01 | - |
| 20 a | 15.36 | 1491.1 | α-Zingiberene | - | 2.61 ± 2.50 | - |
| 21 a | 15.53 | 1504.5 | β-Bisabolene | - | 2.63 ± 2.99 | - |
| 22 | 15.58 | 1508.0 | δ-Selinene | - | 0.40 ± 0.07 | - |
| 23 | 15.77 | 1520.7 | β-Sesquiphellandrene | - | 1.76 ± 1.68 | - |
| 24 | 15.95 | 1533.6 | Calamenene | - | - | - |
| Total sesquiterpenoid hydrocarbons | - | 13.04 | - | |||
| Oxygenated monoterpenoids | ||||||
| 25 a | 9.25 | 1034.0 | Cineole | - | 1.09 ± 1.13 | 8.22 ± 0.81 |
| 26 a | 10.25 | 1097.8 | Linalool | - | 2.45 ± 2.84 | 1.94 ± 0.39 |
| 27 a | 11.14 | 1160.3 | p-Menthone | 0.42 ± 0.01 | 0.04 ± 0.02 | - |
| 28 | 11.44 | 1181.4 | Isopulegone | - | 0.03 ± 0.05 | 1.60 ± 0.15 |
| 29 | 11.72 | 1200.6 | α-Terpineol | - | 0.85 ± 0.85 | 1.10 ± 0.08 |
| 30 a | 12.29 | 1243.9 | Linalyl acetate | - | - | 6.58 ± 0.01 |
| 31 | 12.34 | 1247.9 | Pulegone | 0.68 ± 0.19 | 11.67 ± 11.18 | - |
| 32 a | 12.39 | 1251.8 | Carvone | - | 1.33 ± 1.01 | - |
| 33 | 13.62 | 1348.2 | Terpinyl acetate | 0.04 ± 0.05 | 0.29 ± 0.25 | 26.21 ± 2.86 |
| 34 a | 13.73 | 1357.8 | Eugenol | 0.11 ± 0.06 | 0.36 ± 0.21 | - |
| Total oxygenated monoterpenoids | 1.25 | 18.09 | 45.65 | |||
| Phenolics | ||||||
| 35 | 10.13 | 1089.9 | 2-Methoxy phenol | 0.22 ± 0.04 | 0.24 ± 0.18 | 8.44 ± 2.07 |
| 36 | 10.92 | 1144.9 | Benzyonitrile | 3.45 ± 1.22 | - | - |
| 37 | 12.75 | 1279.2 | 4-Ethylguaiacol | 0.11 ± 0.07 | 0.00 ± 0.01 | 9.96 ± 1.62 |
| 38 | 13.25 | 1318.4 | 4-Vinylguaiacol | 0.10 ± 0.07 | - | 3.92 ± 1.61 |
| Total phenolics | 3.87 | 0.25 | 22.32 | |||
| Sulfur compounds | ||||||
| 39 | 11.63 | 1194.2 | 2-Propionylthiophene | - | - | 0.29 ± 0.15 |
| 40 | 11.93 | 1216.7 | 2-Furfuryl methyl disulfide | - | - | 0.09 ± 0.02 |
| 41 | 13.92 | 1372.7 | Benzyl-thiocyanate | 6.42 ± 3.79 | 42.34 ± 59.82 | - |
| Total sulfur compounds | 6.42 | 42.34 | 0.37 | |||
| Aldehydes/ketones | ||||||
| 42 a | 10.31 | 1101.7 | Nonanal | 2.20 ± 1.01 | 0.01 ± 0.01 | 0.37 ± 0.15 |
| 43 a | 11.73 | 1201.9 | n-Decanal | 1.13 ± 0.76 | - | - |
| 44 a | 12.83 | 1284.4 | (E)-Cinnamaldehyde | 1.90 ± 0.40 | 7.86 ± 10.28 | 5.68 ± 2.07 |
| 45 | 12.87 | 1287.9 | 2-Undecanone | 1.03 ± 0.47 | 1.03 ± 0.75 | 1.70 ± 0.46 |
| Total aldehydes/ketones | 6.25 | 8.89 | 7.75 | |||
| Hydrocarbons | ||||||
| 46 | 11.56 | 1189.5 | 2-Methylundecane | 0.87 ± 0.04 | 0.15 ± 0.05 | - |
| 47 | 14.20 | 1396.3 | 4-Methyltridecane | 1.10 ± 0.04 | 0.03 ± 0.05 | - |
| Total hydrocarbons | 1.97 | 0.18 | - | |||
| Unidentified volatiles | ||||||
| 48 | 12.17 | 1235.4 | Unknown | - | 0.62 ± 0.61 | - |
| 49 | 20.06 | 1766.7 | Unknown | 5.96 ± 3.80 | - | - |
| 50 | 20.62 | 1795.7 | Unknown | 17.04 ± 7.90 | - | - |
| 51 | 20.85 | 1807.9 | Unknown | 0.33 ± 0.10 | - | 3.04 ± 1.28 |
| 52 | 21.25 | 1828.6 | Unknown | 15.30 ± 11.24 | - | - |
| Total unidentified volatiles | 38.63 | 0.62 | 3.04 | |||
| Peak No. | Rt (min) | RI | Compound | RW | RS | P1 | P2 | C |
|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||
| S1 | 4.3 | 971.6 | Ethylene glycol, di-TMS | 3.22 ± 0.63 | 5.19 ± 1.83 | 5.97 ± 2.36 | 4.07 ± 2.25 | 0.24 ± 1.02 |
| S2 | 9.12 | 1281.9 | Glycerol, tri-TMS | 0.21 ± 0.04 | 0.12 ± 0.12 | 0.19 ± 0.04 | 0.32 ± 0.07 | 0.50 ± 0.15 |
| Total alcohols | 3.43 | 5.30 | 6.16 | 4.39 | 0.74 | |||
| Acids and lactones | ||||||||
| S3 | 5.91 | 1051.2 | Glycolic acid acetate, TMS | 2.38 ± 0.95 | 3.03 ± 0.01 | 7.96 ± 1.18 | 3.14 ± 3.96 | 2.65 ± 1.43 |
| S4 | 6.08 | 1062.0 | Lactic acid, di-TMS | 1.41 ± 0.64 | 1.45 ± 0.55 | 3.50 ± 0.50 | 2.08 ± 1.72 | 2.54 ± 0.83 |
| S5 | 6.25 | 1076.1 | Glycolic acid, di-TMS | 1.27 ± 0.51 | 1.95 ± 0.54 | 2.81 ± 0.82 | 4.26 ± 1.24 | 5.15 ± 1.88 |
| S6 | 7.06 | 1134.6 | Oxalic acid, di-TMS | 0.34 ± 0.32 | 0.04 ± 0.01 | 0.04 ± 0.17 | 0.03 ± 0.08 | 0.01 ± 0.07 |
| S7 | 7.07 | 1135.1 | 2-Furancarboxylic acid, TMS | 0.03 ± 0.02 | 0.04 ± 0.01 | 0.11 ± 0.02 | 0.22 ± 0.05 | 0.44 ± 0.11 |
| S8 | 7.23 | 1146.4 | 3-Hydroxypropionic acid, di-TMS | 0.60 ± 0.27 | 0.94 ± 0.24 | 1.34 ± 0.39 | 0.93 ± 0.59 | 0.46 ± 0.27 |
| S9 | 7.30 | 1151.9 | Pantolactone, TMS | 0.25 ± 0.13 | 0.35 ± 0.09 | 0.52 ± 0.14 | 0.37 ± 0.24 | 0.19 ± 0.12 |
| S10 | 7.47 | 1163.5 | 3-Hydroxyisobutyric acid, di-TMS | 0.74 ± 0.35 | 0.96 ± 0.28 | 1.81 ± 0.38 | 1.08 ± 0.86 | 0.68 ± 0.36 |
| S11 | 7.81 | 1187.9 | Cyclohexane-carboxylic acid, TMS | 0.13 ± 0.06 | 0.19 ± 0.05 | 0.31 ± 0.07 | 0.25 ± 0.14 | 0.12 ± 0.09 |
| S12 | 8.14 | 1211.3 | Acetoacetic acid, di-TMS | 0.14 ± 0.05 | 0.37 ± 0.07 | 0.37 ± 0.18 | 0.49 ± 0.16 | 0.13 ± 0.19 |
| S13 | 8.53 | 1239.3 | 4-Hydroxybutyric acid, di-TMS | 0.05 ± 0.04 | 0.03 ± 0.01 | 0.07 ± 0.02 | 0.06 ± 0.04 | 0.10 ± 0.02 |
| S14 | 8.69 | 1250.9 | Benzoic acid, TMS | 0.19 ± 0.11 | 0.23 ± 0.06 | 0.32 ± 0.09 | 0.33 ± 0.14 | 0.05 ± 0.13 |
| S15 | 8.88 | 1264.5 | Octanoic acid, TMS | 0.11 ± 0.04 | 0.17 ± 0.05 | 0.21 ± 0.07 | 0.18 ± 0.09 | 0.04 ± 0.06 |
| S16 | 9.60 | 1316.9 | Succinic acid, di-TMS | 0.99 ± 0.38 | 1.78 ± 0.43 | 2.72 ± 0.79 | 3.39 ± 1.23 | 1.88 ± 1.39 |
| S17 | 9.77 | 1330.2 | 2-Methyl succinic acid, di- TMS | 0.56 ± 0.18 | 1.10 ± 0.27 | 1.78 ± 0.51 | 1.47 ± 0.81 | 1.86 ± 0.49 |
| S18 | 9.92 | 1340.9 | Glyceric acid, tri-TMS | 0.05 ± 0.00 | 0.03 ± 0.03 | 0.02 ± 0.02 | 0.05 ± 0.01 | 0.14 ± 0.02 |
| S19 | 10.02 | 1348.5 | Fumaric acid, di-TMS | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.20 ± 0.01 |
| S20 | 10.14 | 1357.5 | Methyl maleic acid, di-TMS | 0.09 ± 0.03 | 0.15 ± 0.04 | 0.28 ± 0.06 | 0.23 ± 0.13 | 1.04 ± 0.09 |
| S21 | 11.00 | 1423.1 | 3-Deoxytetronic acid, di TMS | 0.01 ± 0.00 | 0.14 ± 0.01 | 0.08 ± 0.08 | 0.05 ± 0.04 | 0.42 ± 0.02 |
| S22 | 14.21 | 1688.7 | 3-Oxo-glutaric acid, tri-TMS | 0.20 ± 0.16 | 0.01 ± 0.03 | 0.00 ± 0.09 | 0.00 ± 0.04 | 0.00 ± 0.04 |
| Total acids and lactones | 9.56 | 12.94 | 24.25 | 18.64 | 18.10 | |||
| Phenolic acid | ||||||||
| S23 | 17.07 | 1961.3 | Hydrocaffeic acid, tri-TMS | - | - | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.12 ± 0.00 |
| Total phenolic acid | - | - | 0.01 | 0.01 | 0.12 | |||
| Amino acids and other nitrogenous compounds | ||||||||
| S24 | 7.10 | 1137.2 | Sarcosine, N,O-di-TMS | 0.20 ± 0.06 | 0.49 ± 0.09 | 0.57 ± 0.24 | 0.55 ± 0.24 | 0.19 ± 0.18 |
| S25 | 7.15 | 1140.5 | 3-Pyridinol, TMS | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.06 ± 0.03 | 0.93 ± 0.03 |
| S26 | 12.38 | 1533.6 | L-Pyroglutamic acid, N,O-di-TMS | 0.26 ± 0.04 | 0.11 ± 0.16 | 0.16 ± 0.06 | 0.41 ± 0.06 | 10.82 ± 0.21 |
| S27 | 16.10 | 1865.2 | Caffeine | 0.03 ± 0.02 | - | 0.00 ± 0.01 | 0.00 ± 0.01 | 3.61 ± 0.00 |
| Total amino acids | 0.46 | 0.60 | 0.73 | 0.96 | 11.01 | |||
| Fatty acids | ||||||||
| S28 | 13.81 | 1653.8 | Lauric acid, TMS | 2.37 ± 0.65 | 7.58 ± 1.21 | 4.53 ± 3.85 | 7.62 ± 1.75 | 0.02 ± 2.97 |
| S29 | 15.95 | 1850.2 | Myristic acid, TMS | 2.23 ± 0.59 | 5.39 ± 1.16 | 3.50 ± 2.62 | 5.89 ± 1.18 | 0.04 ± 2.41 |
| S30 | 17.90 | 2045.1 | Palmitic acid, TMS | 3.45 ± 0.89 | 7.70 ± 1.81 | 6.14 ± 3.70 | 9.49 ± 2.17 | 0.60 ± 3.86 |
| S31 | 19.49 | 2205.5 | Oleic acid, TMS | 3.74 ± 1.17 | 11.51 ± 1.82 | 7.09 ± 5.79 | 14.28 ± 2.75 | 0.08 ± 5.98 |
| S32 | 19.69 | 2226.5 | Stearic acid, TMS | 1.28 ± 0.43 | 3.32 ± 0.60 | 2.14 ± 1.62 | 4.20 ± 0.79 | 0.19 ± 1.78 |
| S33 | 21.36 | 2394.5 | Eicosanoic acid, TMS | 6.59 ± 2.05 | 15.26 ± 3.21 | 16.62 ± 7.32 | 17.06 ± 6.87 | 4.31 ± 5.76 |
| Total fatty acids | 19.64 | 50.77 | 40.01 | 58.54 | 5.24 | |||
| Sugars | ||||||||
| S34 | 11.97 | 1499.6 | L-Threitol, tetra-TMS | 0.38 ± 0.15 | 0.14 ± 0.16 | 0.29 ± 0.01 | 0.25 ± 0.14 | 1.26 ± 0.12 |
| S35 | 12.21 | 1518.9 | 2-Deoxy-D-ribose, tri-TMS | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 | 2.50 ± 0.02 |
| S36 | 13.56 | 1632.7 | Ribofuranose, tetra-TMS | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 3.24 ± 0.01 |
| S37 | 13.62 | 1637.3 | Arabinopyranose, tetra-TMS | - | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.59 ± 0.01 |
| S38 | 13.98 | 1669.2 | Ribopyranose, tetra-TMS | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 3.61 ± 0.01 |
| S39 | 14.30 | 1696.9 | Ribofuranose, tetra-TMS isomer | 0.36 ± 0.21 | 0.11 ± 0.10 | 0.09 ± 0.06 | 0.10 ± 0.02 | 0.43 ± 0.04 |
| S40 | 15.21 | 1779.9 | Sorbofuranose, penta-TMS | 0.53 ± 0.43 | 0.01 ± 0.07 | 0.01 ± 0.23 | 0.01 ± 0.11 | 0.57 ± 0.11 |
| S41 | 15.36 | 1794.1 | Fructofuranose, penta-TMS | 1.55 ± 0.75 | 0.28 ± 0.57 | 0.10 ± 0.24 | 0.21 ± 0.24 | 1.54 ± 0.02 |
| S42 | 15.41 | 1798.6 | Psicopyranose, penta-TMS | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 1.11 ± 0.01 |
| S43 | 15.49 | 1806.1 | Tagatofuranose, penta-TMS | 0.46 ± 0.28 | 0.02 ± 0.12 | 0.02 ± 0.13 | 0.02 ± 0.06 | 0.13 ± 0.06 |
| S44 | 15.65 | 1821.7 | Talofuranose, penta-TMS | 6.73 ± 2.96 | 0.18 ± 2.66 | 0.04 ± 1.53 | 0.09 ± 1.31 | 0.28 ± 0.78 |
| S45 | 15.79 | 1835.4 | Psicofuranose, penta-TMS | 5.68 ± 2.39 | 0.21 ± 2.33 | 0.30 ± 1.24 | 0.27 ± 1.02 | 1.77 ± 0.51 |
| S46 | 15.81 | 1837.3 | Talopyranose, penta-TMS | 1.30 ± 0.30 | 0.01 ± 0.71 | 0.01 ± 0.35 | 0.02 ± 0.35 | 2.40 ± 0.19 |
| S47 | 15.87 | 1843.2 | Talofuranose, penta-TMS isomer | 5.01 ± 3.29 | 0.03 ± 1.21 | 0.01 ± 1.65 | 0.03 ± 0.85 | 1.02 ± 0.81 |
| S48 | 16.12 | 1867.1 | Galactopyranose, penta-TMS | 3.53 ± 1.25 | 0.05 ± 1.61 | 0.01 ± 0.82 | 0.02 ± 0.80 | 0.28 ± 0.46 |
| S49 | 16.30 | 1884.0 | 3-Deoxy-arabino-hexonic acid, penta-TMS | 4.90 ± 1.60 | 0.26 ± 2.33 | 0.26 ± 1.05 | 0.24 ± 1.04 | 5.49 ± 0.47 |
| S50 | 16.66 | 1920.1 | Psicose, penta-TMS, TMS-oxy oxime | 0.57 ± 0.56 | 0.00 ± 0.01 | 0.00 ± 0.32 | 0.00 ± 0.18 | 0.04 ± 0.16 |
| S51 | 16.69 | 1922.0 | Glucuronic acid γ-lactone TMS, trimelthyoxyoxime | 0.06 ± 0.03 | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.27 ± 0.00 |
| S52 | 16.81 | 1934.9 | Glucose, penta-TMS | 1.23 ± 0.60 | 0.01 ± 0.45 | 0.00 ± 0.31 | 0.00 ± 0.23 | 0.02 ± 0.16 |
| S53 | 17.03 | 1957.5 | Gulonic acid, 1,4-lactone, tetra-TMS | 1.32 ± 0.82 | 0.10 ± 0.35 | 0.01 ± 0.37 | 0.05 ± 0.20 | 0.21 ± 0.16 |
| S54 | 17.23 | 1977.1 | Mannitol, hexa-TMS | 0.32 ± 0.25 | 0.08 ± 0.05 | 0.13 ± 0.10 | 0.13 ± 0.04 | 1.59 ± 0.05 |
| S55 | 17.47 | 2002.1 | Mannonic acid, 1,4-lactone, tetra-TMS | 0.14 ± 0.08 | 0.15 ± 0.05 | 0.07 ± 0.05 | 0.17 ± 0.01 | 0.27 ± 0.08 |
| S56 | 17.54 | 2008.7 | Glucopyranose, penta-TMS | 0.55 ± 0.37 | 0.03 ± 0.13 | 0.02 ± 0.18 | 0.03 ± 0.08 | 0.72 ± 0.08 |
| S57 | 17.77 | 2031.7 | Glucuronic acid, penta-TMS | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.70 ± 0.01 |
| S58 | 23.74 | 2635.0 | Lactose, octa-TMS (isomer 2) | 0.08 ± 0.06 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.01 ± 0.01 | 1.21 ± 0.01 |
| S59 | 23.91 | 2652.8 | 3-Mannobiose, octa-TMS | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.32 ± 0.00 |
| S60 | 24.41 | 2703.1 | 2-Mannobiose, octa-TMS | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.07 ± 0.01 | 0.01 ± 0.04 | 2.68 ± 0.02 |
| Total sugar | 34.81 | 1.79 | 1.54 | 1.81 | 34.23 | |||
| Sterols and triterpenes | ||||||||
| S61 | 30.37 | 3304.6 | β-Sitosterol, TMS | 0.71 ± 0.13 | 2.03 ± 0.41 | 2.65 ± 1.02 | 2.98 ± 1.16 | 0.05 ± 1.09 |
| S62 | 31.50 | 3419.0 | Cycloartenol, TMS | 2.37 ± 0.65 | 7.58 ± 1.21 | 4.53 ± 1.85 | 4.87 ± 1.75 | 0.00 ± 1.59 |
| Total sterols/triterpenes | 3.08 | 9.61 | 7.18 | 7.85 | 0.05 | |||
| Unidentified | ||||||||
| S63 | 16.38 | 1892.4 | Unknown | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.04 ± 0.00 | 0.00 ± 0.02 | 5.98 ± 0.01 |
| S64 | 16.73 | 1927.3 | Unknown | 2.14 ± 0.87 | 0.03 ± 0.90 | 0.01 ± 0.49 | 0.02 ± 0.44 | 4.34 ± 0.26 |
| S65 | 17.65 | 2020.4 | Unknown | - | - | - | 0.01 ± 0.00 | 0.36 ± 0.01 |
| S66 | 18.36 | 2091.4 | Unknown | 0.25 ± 0.37 | 0.00 ± 0.08 | 0.00 ± 0.19 | 0.00 ± 0.09 | 1.09 ± 0.09 |
| S67 | 22.58 | 2518.2 | Unknown | 7.22 ± 12.18 | 10.40 ± 3.50 | 11.28 ± 4.58 | 0.86 ± 4.21 | 0.08 ± 2.05 |
| S68 | 24.00 | 2661.0 | Unknown | 2.82 ± 4.22 | 4.08 ± 0.99 | 5.07 ± 1.83 | 0.46 ± 2.15 | 0.79 ± 0.90 |
| S69 | 27.39 | 3003.6 | Unknown | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 | 3.28 ± 0.01 |
| S70 | 28.03 | 3068.6 | Unknown | - | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.99 ± 0.00 |
| S71 | 28.32 | 3098.1 | Unknown | 0.66 ± 0.59 | 3.52 ± 0.05 | 0.63 ± 1.86 | 2.31 ± 0.93 | 1.06 ± 0.71 |
| Total unidentified | 13.11 | 18.06 | 17.07 | 3.68 | 17.97 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Otify, A.M.; El-Sayed, A.M.; Michel, C.G.; ElShebiney, S.A.; Ehrlich, A.; Wessjohann, L.A. Sensory Metabolite Profiling in a Date Pit Based Coffee Substitute and in Response to Roasting as Analyzed via Mass Spectrometry Based Metabolomics. Molecules 2019, 24, 3377. https://doi.org/10.3390/molecules24183377
Farag MA, Otify AM, El-Sayed AM, Michel CG, ElShebiney SA, Ehrlich A, Wessjohann LA. Sensory Metabolite Profiling in a Date Pit Based Coffee Substitute and in Response to Roasting as Analyzed via Mass Spectrometry Based Metabolomics. Molecules. 2019; 24(18):3377. https://doi.org/10.3390/molecules24183377
Chicago/Turabian StyleFarag, Mohamed A., Asmaa M. Otify, Aly M. El-Sayed, Camilia G. Michel, Shaimaa A. ElShebiney, Anja Ehrlich, and Ludger A. Wessjohann. 2019. "Sensory Metabolite Profiling in a Date Pit Based Coffee Substitute and in Response to Roasting as Analyzed via Mass Spectrometry Based Metabolomics" Molecules 24, no. 18: 3377. https://doi.org/10.3390/molecules24183377
APA StyleFarag, M. A., Otify, A. M., El-Sayed, A. M., Michel, C. G., ElShebiney, S. A., Ehrlich, A., & Wessjohann, L. A. (2019). Sensory Metabolite Profiling in a Date Pit Based Coffee Substitute and in Response to Roasting as Analyzed via Mass Spectrometry Based Metabolomics. Molecules, 24(18), 3377. https://doi.org/10.3390/molecules24183377






