Abstract
We disclose herein the first synthetic method that is capable of offering heteroaryl[b]quinolines (HA[b]Qs) with structural diversity, which include tricyclic and tetracyclic structures with (benzo)thienyl, (benzo)furanyl, and indolyl rings. The target HA[b]Q is addressed by the annulation of o-acylanilines and MeO–heteroarenes with the aid of an indium Lewis acid that effectively works to make two different types of the N–C and C–C bonds in one batch. A series of indolo[3,2-b]quinolines prepared here can be subsequently transformed to structurally unprecedented cryptolepine derivatives. Mechanistic studies showed that the N–C bond formation is followed by the C–C bond formation. The indium-catalyzed annulation reaction thus starts with the nucleophilic attack of the NH2 group of o-acylanilines to the MeO-connected carbon atom of the heteroaryl ring in an SNAr fashion, and thereby the N–C bond is formed. The resulting intermediate then cyclizes to make the C–C bond through the nucleophilic attack of the heteroaryl-ring-based carbon atom to the carbonyl carbon atom, providing the HA[b]Q after aromatizing dehydration.
1. Introduction
Heteroaryl[b]quinolines (HA[b]Qs), wherein electron-rich heteroaryl rings are fused to the [b] site of quinoline, are important frameworks found in natural products [1,2,3] and biologically active molecules [1,4,5,6] as well as functional organic materials [7,8,9]. Due to their significance, numerous synthetic approaches have been developed for the construction of such structural motifs. These approaches could be categorized simply into three strategies on the basis of the ring-constructing method (Figure 1), which are the heteroaryl ring formation (strategy a) [10,11,12,13,14,15,16,17,18,19,20,21,22], the central pyridyl ring formation (strategy b) [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39], and the formation of both rings (strategy c) [4,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Although there are advantages and disadvantages to each strategy from various aspects, the strategy b seems to be the most user-friendly in terms of the accessibility of the starting substrates.
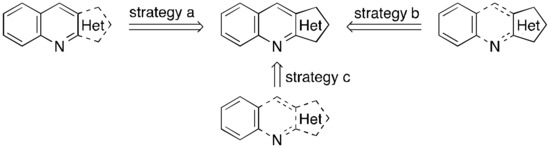
Figure 1.
Synthetic strategies for the construction of the heteroaryl[b]quinoline (HA[b]Q) structure.
On the other hand, we have recently reported a new C(heteroaryl)–N bond-forming reaction by reacting electron-rich methoxyheteroarenes with amines via a nucleophilic aromatic substitution (SNAr) reaction [54]. In addition to this, we have also developed several new C(heteroaryl)–C bond-forming reactions by reacting alkynes [55,56,57] or carbonyl compounds [58,59,60] with heteroarenes. All of these reactions are effectively catalyzed by a salt of an indium(III) Lewis acid, which has been also employed for various organic transformations by other research groups [61,62,63,64,65,66,67,68,69,70,71]. We therefore envisaged that conducting the two different types of reactions in a tandem fashion would be a new methodology of the strategy b to offer the HA[b]Q in an easy way, thereby also leading to the further expansion of our indium-based technology. Our working hypothesis is illustrated more intelligibly in Scheme 1. We thus expected that the synthesis of HA[b]Qs 4 could be achieved by mixing o-alkynylanilines 1 or o-acylanilines 2 with methoxyheteroarenes 3 in the presence of a catalytic indium Lewis acid (InX3 = In). The first stage is the SNAr-based N–C bond-forming reaction through the nucleophilic attack of the amino group of 1 or 2 to electrophilic complex A to afford 5 or 6, respectively. Intermediate 5 or 6 successively cyclizes by forming the C–C bond in an intramolecular fashion, thus giving 7 or 8, respectively, via the activation mode of B or C. The isomerization or dehydration as the final stage results in the formation of desired HA[b]Q 4. We also expected that combining the two indium transformations, both of which are compatible with a broad range of substrates, should lead to the development of the HA[b]Q synthesis with good substrate generality. As stated above, a lot of studies that synthesize the HA[b]Q have appeared so far in literature, but these studies have been limited to preparing HA[b]Qs with one to three types of heteroaryl rings, to the best of our knowledge [72,73,74]. We report herein that an indium salt effectively catalyzes the N–C and C–C bond-forming sequence to afford a range of HA[b]Qs including tricyclic and tetracyclic [2,3-b] and [3,2-b] structures with sulfur-, oxygen-, and nitrogen-based five-membered heteroaryl rings. Among the products, indolo[3,2-b]quinolines, which can be easily converted to cryptolepine derivatives that have been known to exhibit anti-malarial and anti-cancer activities, are included [75].
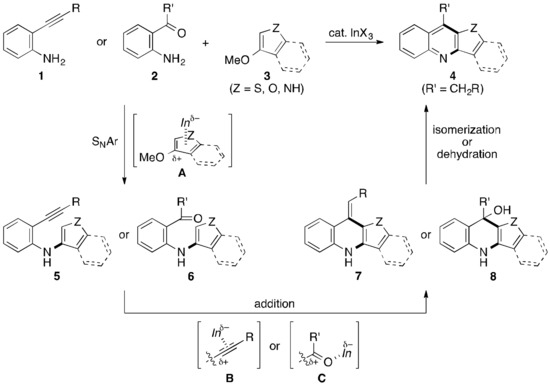
Scheme 1.
A working hypothesis for the synthesis of HA[b]Qs 4. In = InX3.
2. Results and Discussion
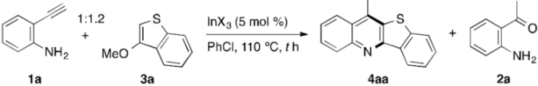
In order to verify the working hypothesis, we first investigated the possibility of whether o-ethynylaniline (1a) works as a substrate for the synthesis of the HA[b]Q under indium catalysis, and selected 3-methoxybenzothiophene (3a) as the substrate partner (Table 1). Upon treatment of 1a and 3a with 5 mol % of In(NTf2)3 (Tf = SO2CF3) in PhCl at 110 °C for 24 h, we were pleased to observe that the desired annulation proceeded to give 11-methyl[1]benzothieno[3,2-b]quinoline (4aa), albeit in low yield (entry 1). While the screening of other indium salts provided no significant improvements in the yield of 4aa, a small amount of o-acetylaniline (2a) was formed along with 4aa when using In(ONf)3 (Nf = SO2C4F9) as a catalyst (entries 2–6). In this context, a wide variety of Lewis acids, including indium salts, have been known to act as catalysts for the hydration of a C≡C bond to create a carbonyl functionality [76,77]. A possible explanation for the formation of 2a is thus the indium-catalyzed hydration of 1a with H2O, which could have been present in a small quantity in the reaction mixture. Accordingly, we presumed that, as routes for the formation of 4aa, there would be two possibilities: one is directly from 1a, and the other is indirectly from 2a formed in situ after the hydration of 1a. In order to get an insight into which routes operate here, the following experiments were conducted. Thus, the annulation carried out under the conditions of entry 3, additionally including five molar equivalents of H2O, resulted in higher yields of both 4aa and 2a (entry 7). Moreover, the prolonged reaction time from 24 h to 36 h raised the yield of 4aa to 61% with the complete consumption of 2a (entry 8). These results suggest that 4aa is likely to be formed through the generation of 2a by the hydration of 1a, whereas the contribution of the direct route from 1a cannot be completely excluded.

Table 1.
Indium-catalyzed annulation of o-ethynylaniline with 3-methoxybenzothiophene a.
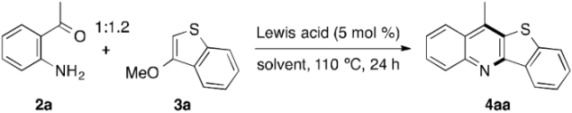
On the basis of the above results, we turned our attention to the annulation with 2a instead of 1a (Table 2). As expected, under the same reaction conditions as those for entry 3 of Table 1, 4aa was produced in significantly higher yield of 62% (entry 1). Inspired by this result, we continuously examined the effect of various indium salts other than In(ONf)3 for the same annulation reaction of 2a with 3a. Thus, In(OTf)3 and In(NTf2)3 with the strong electron-withdrawing ligands as In(ONf)3 also catalyzed the annulation, and the yield of 4aa increased to 74% in the use of In(NTf2)3 (entries 2 and 3). Among the indium halides examined, InBr3 and InI3 were found to be highly effective, giving 4aa in 92% yield in both the cases, in sharp contrast to the inactivity of the fluoride salt (entries 4–7). However, the corresponding hydroxide and acetate salts were totally inactive (entries 8 and 9). Due to the remarkable catalytic activity of InBr3, metal bromides of, for instance, Sc, Fe, Co, Pd, Cu, Ag, Zn, Pb, and Bi were tested, but proved to be less effective (entries 10–18). No 4aa was formed in the absence of a catalyst, which is thus indispensable for the progress of the annulation (entry 19). With InBr3 as the promising catalyst, a continuous survey of the solvent effect indicated that PhCl would be the most suitable solvent of choice for the annulation, and that the reaction rate greatly decreases in H2O (entries 20–27). While the lowering of the catalyst loading to 1 mol % accompanies the decrease of the reaction rate, the good yield of 4aa can be secured by extending the reaction time to 96 h (entry 28). Favorably, the annulation can be also carried out under an atmosphere of air instead of argon to afford 4aa in 88% yield (entry 29).

Table 2.
Lewis acid-catalyzed annulation of o-acetylaniline with 3-methoxybenzothiophene a.
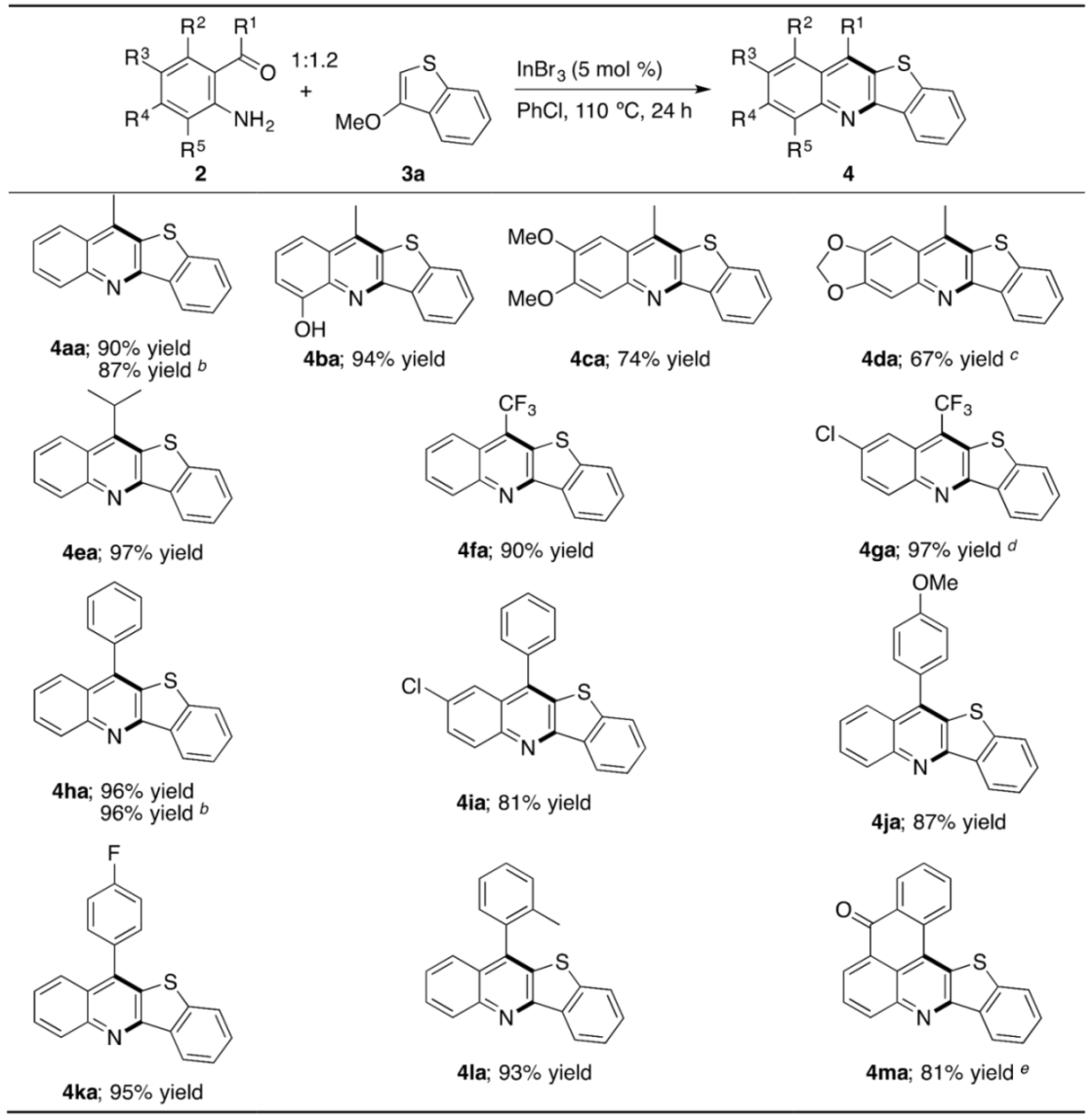
With the proper reaction conditions in hand, we next examined the scope of the o-acylaniline substrate to 3a (Table 3). Similar to o-acetylaniline (2a), its derivatives with the OH, OMe, or methylenedioxy group successfully participated in the annulation (4aa–4da). The formation of 4ba in such high yield shows that the OH group does not interfere with the progress of the desired annulation by acting as the nucleophilic site, as the NH2 group does. No undesired ring fragmentation of the acetal moiety in 4da was observed, even under the Lewis acidic conditions [78]. The bulkier isopropyl group on the carbonyl carbon atom does not affect the efficiency of the annulation, giving 4ea in 97% yield. A CF3 group, the C–F bond of which is known to increase metabolic stability and membrane permeability, thus leading to improvement in bioavailability [79], can be also installed onto the C11-position of the benzothieno[3,2-b]quinoline structure (4fa and 4ga). A commercially available hydrochloride–hydrate adduct of o-acylaniline 2g can be used as a substrate without neutralizing and drying. Our protocol is applicable as well to o-acylanilines with a series of aryl groups with different electronic and steric natures, in which the simple phenyl group for 4ha and 4ia, p-MeOC6H4 for 4ja, p-FC6H4 for 4ka, o-MeC6H4 for 4la, and o-fused-aroylC6H4 for 4ma are included. The atmosphere of air was again confirmed to be available on the synthesis of 4ha. In the reaction of aminoanthraquinone 2m with two carbonyl moieties, only the one adjacent to the NH2 group worked as a reaction site to provide hexacyclic-fused ring system 4ma in one shot. Of importance to note is that the MeO, Cl, and F groups on the aryl ring are known to behave as leaving groups in the general SNAr reaction, but were found to be compatible with the reaction conditions, thus contributing to the high-yield formation of the target molecules (4ca, 4ga, 4ia, 4ja, and 4ka) [80].

Table 3.
Indium-catalyzed annulation of o-acylanilines with 3-methoxybenzothiophene a.
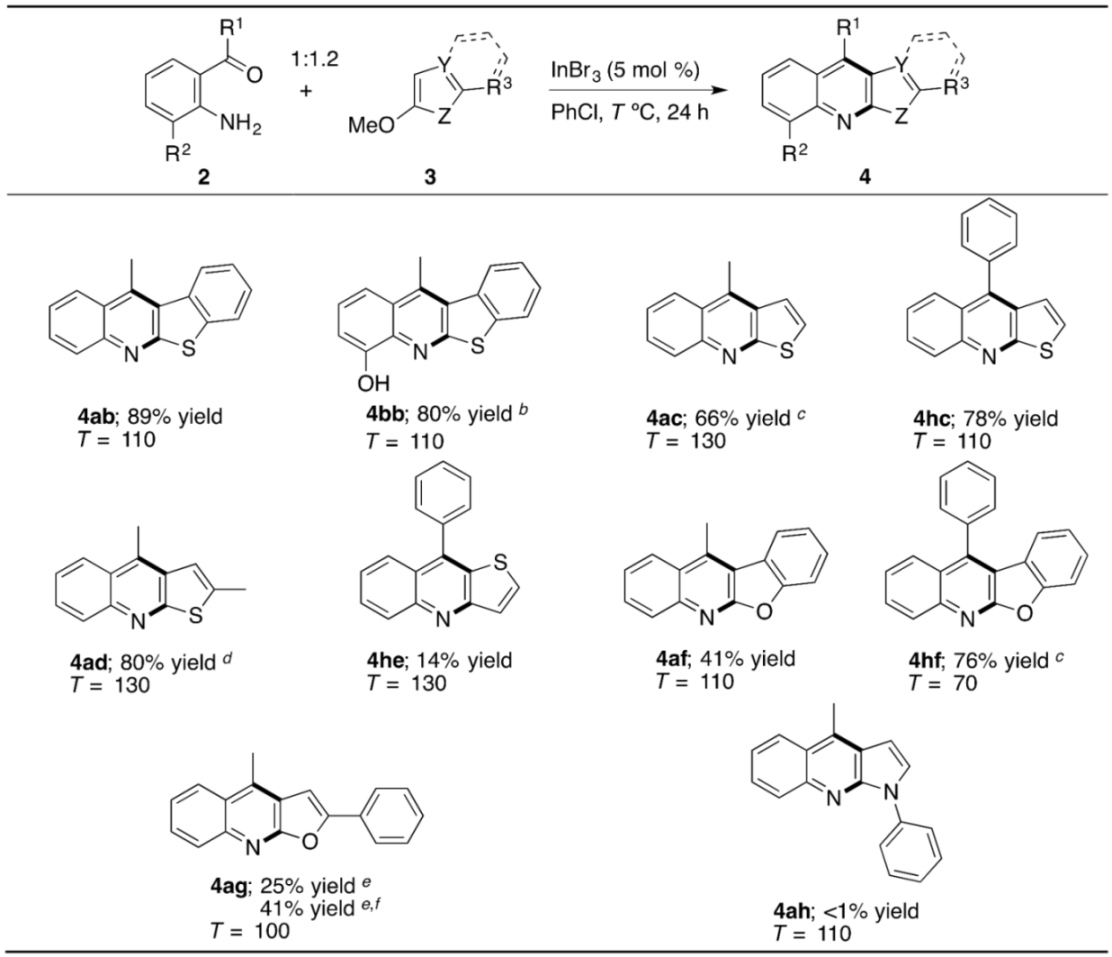
Besides the benzothieno[3,2-b]quinoline, our method is applicable to preparing a range of HA[b]Qs by using other sulfur- and oxygen-based methoxyheteroarenes (Table 4). The replacement of 3a with 2-methoxybenzothiophene (3b) enables the switch of the fused-ring orientation from the [3,2-b] to the [2,3-b], and products 4ab and 4bb were obtained in high yields. However, in contrast to the successful construction of thieno[2,3-b]quinoline 4ac, 4hc, and 4ad, changing the fused-ring orientation to the [3,2-b] in this case resulted in low yield of 4he. In the reaction of 3-methoxythiophene (3e), a self-condensation reaction, in which two molecules of 2h react with each other to form cyclic diimine 9, occurred as a major side reaction (Figure 2). This result is likely to be related, at least in part, to the relatively low reaction rate of the desired SNAr process between 2h and 3e, and, in fact, 70% of 3e loaded for the reaction remained unconsumed. In this context, we have previously confirmed that the SNAr amination reaction of 3-methoxythiophene (3e) requires a higher loading of an indium catalyst as well as higher temperature compared to those for the reaction of 2-methoxythiophene (3c) [54]. In addition to the sulfur-containing HA[b]Qs, the tetracyclic and tricyclic oxygen-containing analogues can be addressed by our method in moderate to good yields (4af, 4hf, and 4ag). When preparing 4ag, InI3 worked as a catalyst more efficiently than InBr3. Unfortunately, no annulation reaction of 2a with 2-methoxy-1-phenylpyrrole (3h) for the synthesis of pyrrolo[2,3-b]quinoline 4ah proceeded, due to some undesired side reactions, including N-methylation of 2a by the MeO group of 3h acting as a source of a methyl group.

Table 4.
Indium-catalyzed annulation of o-acylanilines with methoxyheteroarenes a.
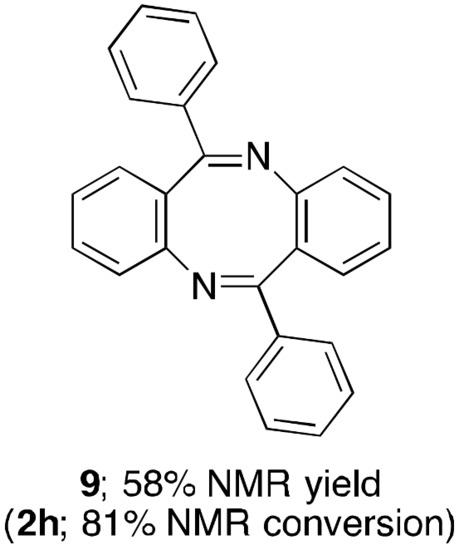
Figure 2.
A major byproduct formed in the reaction of 2h with 3e.
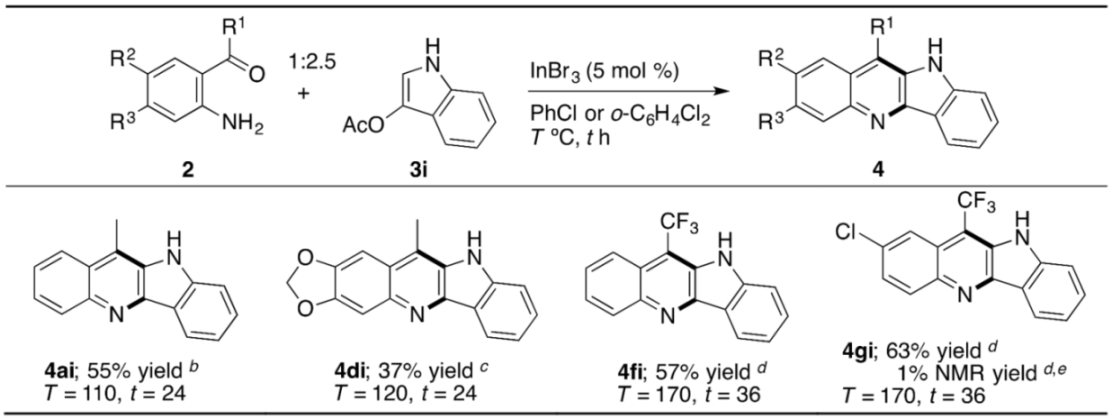
As collected separately in Table 5, we successively present the result of constructing the framework of the indolo[3,2-b]quinoline, which is alternatively named quindoline, having been known to show cytotoxic activity against human cancer cell lines [81]. As in our preceding SNAr amination [54], commercially unavailable 3-methoxyindole was not required, but rather commercially available 3-acetyloxyindole (3i) can be used here again as a substrate. Thus, mixing 2a, 3i, and InBr3 (5 mol %) in PhCl, and then heating the mixture at 110 °C for 24 h gave 4ai in 55% yield. Other quindoline derivatives 4di, 4fi, and 4gi could also be synthesized by our method. Unlike the annulation of 2g–HCl–H2O with 3-methoxybenzothiophene (3a) (see 4ga in Table 3), the pre-removal of HCl and H2O from 2g–HCl–H2O as a commercial source is required here to obtain 4gi in reasonable yield. With 2g–HCl–H2O instead, the formation of 4gi resulted in only 1% NMR yield. These results inspired us to address cryptolepine derivatives, due to their potentialities as anti-malarial and/or anti-cancer drugs.

Table 5.
Indium-catalyzed annulation of o-acylanilines with 3-acetyloxyindole a.
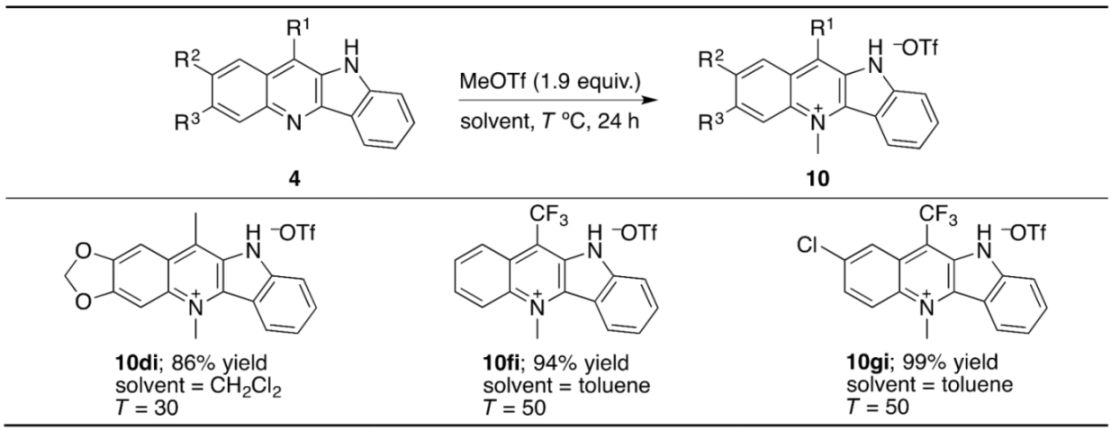
As previously demonstrated, the HOTf adduct of the 11-methylated cryptolepine (11-Me-10) shows higher anti-malarial and antitrypanosomal activities than that of the original cryptolepine (10) (Figure 3). Since the N-methylation of the pyridine ring of 4ai with methyl triflate (MeOTf) has been already reported [82], we targeted the synthesis of analogues thereof from other quindoline derivatives 4di, 4fi, and 4gi (Table 6). The N-methylation in accordance with the modified literature procedure successfully delivered 10di, 10fi, and 10gi, which are new compounds unreported in the literature [82]. Especially, 10fi, which has the 11-CF3 group instead of the 11-CH3 group in 11-Me-10, might be expected to be promising in view of anti-malarial and antitrypanosomal activities, due to the possible higher bioavailability. Moreover, since the acid-free cryptolepine derivatives have been the focus of examining anti-cancer activity (11 and 11-Me-11 in Figure 3), there should be a demand for the acid-free form. Accordingly, we confirmed that the neutralization of, for instance, 10fi with a Na2CO3 aqueous solution provides 11fi with no TfOH in quantitative yield (Scheme 2).
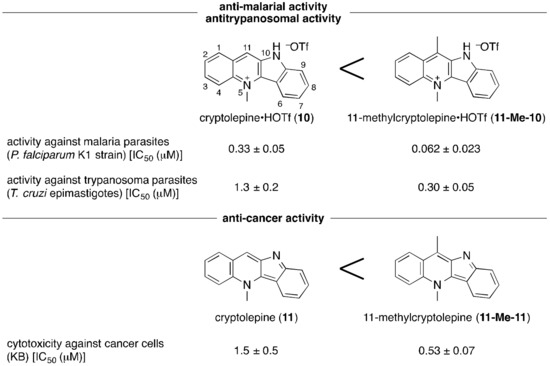
Figure 3.
Anti-malarial, antitrypanosomal and anti-cancer activities of cryptolepine, 11-methylcryptolepine, and their HOTf adducts [82].

Table 6.
N-Methylation of indolo[3,2-b]quinolines with MeOTf a.
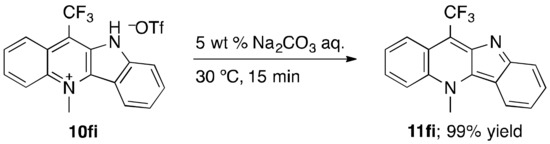
Scheme 2.
Neutralization of 10fi.
In order to get insight into the reaction pathway of the present annulation reaction, some experiments were performed (Scheme 3). At first, upon treating 2e with 3a at room temperature rather than the standard heating temperature, only the SNAr-based intermolecular N–C bond-forming reaction proceeded to furnish 6ea in 53% yield with 60% conversion of 2e, thus being not contaminated by 12ea derived from the C–C bond formation as a possible alternative first stage, and by final annulation product 4ea [Equation (1) in Scheme 3]. Subsequently, 6ea isolated from the reaction of Equation (1) was heated under the standard reaction conditions, and thereby 4ea was obtained highly efficiently via the intramolecular C–C bond-forming annulation [Equation (2) in Scheme 3]. On the other hand, Me2-2e, wherein the nitrogen atom is dimethylated and would thus no longer act as a nucleophilic site, did not participate in making a C–C bond with 3a, leading possibly to Me2-12ea. As a result, Me2-2e was recovered quantitatively, even under the standard heating reaction conditions [Equation (3) in Scheme 3]. Accordingly, these results strongly suggest that the annulation reaction proceeds in the order of the SNAr-based intermolecular N–C bond formation, followed by the SEAr-based intramolecular C–C bond formation. Experimental procedures for Equations (1) and (2) as well as spectral and analytical data (melting point, NMR, and HRMS), and NMR charts for products 6ea and 4ea are provided in Supplementary Materials.
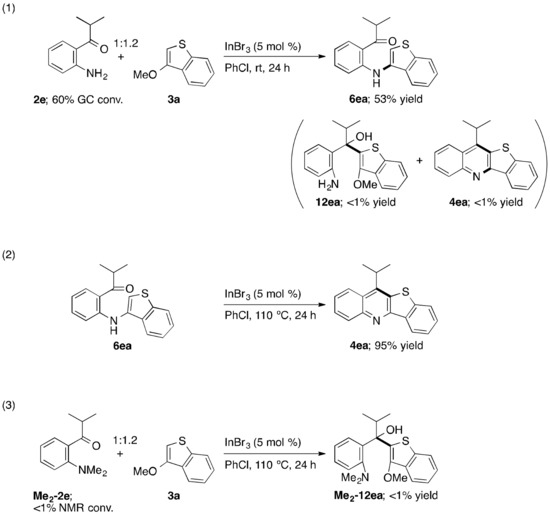
Scheme 3.
Control experiments for mechanistic studies.
On the basis of the above experimental results as well as the previous ones, a proposed reaction mechanism is illustrated in Scheme 4 that exemplifies the reaction of 2e with 3a. First up is the SNAr-based intermolecular amination of 3a by the nucleophilic attack of the nitrogen atom of 2e via previously proposed transition state A [54], followed by the release of the indium catalyst (In) and MeOH to give intermediate 6ea. Next is the nucleophilic attack of the thienyl ring to the carbonyl moiety activated by In as shown in transition state B, hereby providing 8ea, and then desired structure 4ea after aromatizing dehydration. The ring-closing C–C bond-forming process might be accelerated by the electron flow from the lone pair on the nitrogen atom. However, due to the fact that 6ea is the only intermediate confirmed during the annulation process [Equation (1) in Scheme 3], the rate-determining step is likely to be present at the intramolecular C–C bond-forming stage.
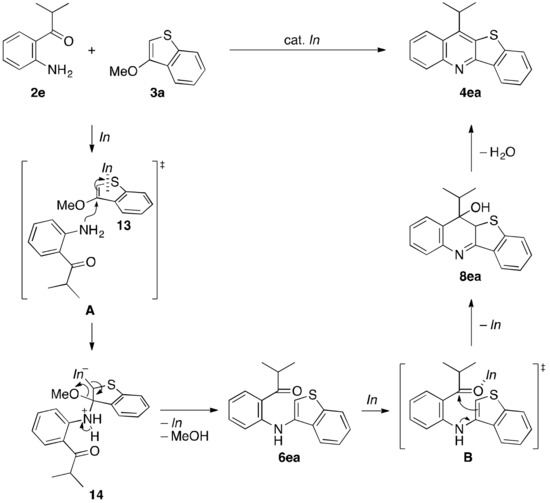
Scheme 4.
A proposed reaction mechanism.
3. Materials and Methods
3.1. General Remarks
All manipulations were conducted with a standard Schlenk technique under an argon atmosphere. Nuclear magnetic resonance (NMR) spectra were taken on a JEOL JMN-ECA 400 (1H, 400 MHz; 13C, 100 MHz; 19F, 376 MHz) or JEOL JMN-ECA 500 (1H, 500 MHz; 13C, 125 MHz; 19F, 471 MHz) spectrometer (JEOL, Tokyo, Japan) using tetramethylsilane (1H and 13C) or trichlorofluoromethane (19F) as an internal standard. Analytical gas chromatography (GC) was performed on a Shimadzu model GC-2014 instrument with a flame ionization detector (Shimadzu, Kyoto, Japan), equipped with a capillary column of InertCap 5 (5% diphenyl- and 95% dimethylpolysiloxane, 30 m × 0.25 mm × 0.25 µm) (GL Sciences, Tokyo, Japan), using nitrogen as carrier gas. Gas chromatography-mass spectrometry (GC-MS) analyses were performed with a Shimadzu model GCMS-QP2010 instrument (Shimadzu, Kyoto, Japan) equipped with a capillary column of InertCap 5 by electron ionization at 70 eV using helium as the carrier gas. High-resolution mass spectra (HRMS) were obtained with a JEOL JMS-T100GCV spectrometer (JEOL, Tokyo, Japan). All of the melting points were measured with a Yanaco Micro Melting Point MP-500P apparatus (Yanaco, Kyoto, Japan), and are uncorrected. Kugelrohr bulb-to-bulb distillation was carried out with a Sibata glass tube oven GTO-250RS apparatus (Sibata Scientific Technology, Soka, Japan). Chlorobenzene (PhCl), toluene (PhMe), and dichloromethane (CH2Cl2) were distilled under argon from CaCl2 just prior to use. Dibutyl ether (Bu2O) and 1,4-dioxane were distilled under argon from sodium just prior to use. 1,2-Diethoxyethane, nitromethane (MeNO2), butanol (BuOH) and o-dichlorobenzene (o-C6H4Cl2) were stored over molecular sieves 4Å (MS 4Å) under argon. Butyronitrile (PrCN) was distilled under argon from P2O5 just prior to use. MeOH was stored over molecular sieves 3Å (MS 3Å) under argon. The following indium salts and substrates were synthesized according to the respective literature methods: In(NTf2)3 [83,84], In(ONf)3 [56,85], 1-(2-aminophenyl)-2-methyl-1-propanone (2e) [86], 1-(2-aminophenyl)-2,2,2-trifluoroethanone (2f) [87], (2-aminophenyl)(4-methoxyphenyl)methanone (2j) [88], (2-aminophenyl)(2-methylphenyl)methanone (2l) [88], 3-methoxybenzo[b]thiophene (3a) [89], 2-methoxybenzo[b]thiophene (3b) [54], 2-methoxy-5-methylthiophene (3d) [54], 2-methoxybenzo[b]furan (3f) [54], 2-methoxy-5-phenylfuran (3g) [54], 2-methoxy-1-phenyl-1H-pyrrole (3h) [54]. Unless otherwise noted, other substrates and reagents were commercially available, and used as received without further purification.
3.2. Synthesis of Substrates
3.2.1. Synthesis of 1-(2-Amino-5-chlorophenyl)-2,2,2-trifluoroethanone (2g): Removal of HCl and H2O from 2g–HCl–H2O
A hydrochloride–hydrate adduct of 2g (407 mg, 1.46 mmol) was placed in a 15-mL screw-cap vial. To this, a saturated NaHCO3 aqueous solution (2.0 mL) was added, and the resulting mixture was stirred at room temperature for 3 min. The aqueous phase was extracted with EtOAc (5 mL × 3). The combined organic layer was washed with brine (2 mL) and then dried over anhydrous sodium sulfate (Na2SO4). Filtration and evaporation of the solvent left a residue, which was successively passed through a pad of silica gel using EtOAc to give analytically pure 2g in 99% yield (324 mg) as a yellow solid (m.p. 92–94 °C). Compound 2g has already appeared in the literature [87], and its spectral and analytical data are in good agreement with those reported. Accordingly, only the 1H-NMR data are provided here. 1H-NMR (400 MHz, CDCl3) δ 6.47 (bs, 2H), 6.70 (d, J = 8.9 Hz, 1H), 7.33 (dd, J = 9.0, 2.4 Hz, 1H), 7.66–7.75 (m, 1H).
3.2.2. Synthesis of 1-[2-(Dimethylamino)phenyl]-2-methyl-1-propanone (Me2-2e)
On the basis of the literature procedure that has been used when dimethylating closely related 1-(2-aminophenyl)ethanone derivatives [90], Me2-2e was prepared using the following reagents and conditions: 2e (163 mg, 1.00 mmol), MeI (426 mg, 3.00 mmol), K2CO3 (346 mg, 2.50 mmol), N,N-dimethylformamide (0.60 mL), 80 °C, 8 h, and was isolated by column chromatography on silica gel (n-hexane/EtOAc = 30/1) in 71% yield (136 mg) as a pale yellow oil. Compound Me2-2e has already appeared in the literature [91], and its spectral and analytical data are in good agreement with those reported. Accordingly, only the 1H-NMR data are provided here. 1H-NMR (500 MHz, CDCl3) δ 1.12 (d, J = 6.9 Hz, 6H), 2.76 (s, 6H), 3.66 (sept, J = 6.9 Hz, 1H), 6.95 (td, J = 7.4, 0.9 Hz, 1H), 7.00 (dd, J = 8.3, 0.6 Hz, 1H), 7.28 (dd, J = 7.7, 1.7 Hz, 1H), 7.34 (ddd, J = 8.6, 7.3, 1.7 Hz, 1H).
3.3. Indium-Catalyzed Annulation of o-Acylanilines with Alkoxyheteroarenes: An Experimental Procedure Exemplified by the Synthesis of 4aa
InBr3 (4.43 mg, 12.5 µmol) was placed in a 20-mL Schlenk tube, which was heated at 80 °C in vacuo for 15 min. The tube was cooled down to room temperature, and filled with argon. PhCl (0.20 mL) was added to the tube, and the mixture was then stirred at room temperature for 3 min. To this, 3-methoxybenzothiophene (3a) (49.3 mg, 0.300 mmol) and 1-(2-aminophenyl)ethanone (2a) (33.8 mg, 0.250 mmol) were added in that order, and the mixture was stirred at 110 °C for 24 h, followed by adding a saturated NaHCO3 aqueous solution (0.5 mL). The resulting mixture was stirred for 20 min, and the aqueous phase was then extracted with EtOAc (5 mL × 3). The combined organic layer was washed with brine (1 mL), and then dried over anhydrous sodium sulfate (Na2SO4). Filtration and evaporation of the solvent followed by column chromatography on silica gel (n-hexane/EtOAc = 10/1) gave 11-methyl[1]benzothieno[3,2-b]quinoline (4aa) in 90% yield (56.1 mg) as a pale yellow solid (m.p. 145–146 °C). Compound 4aa was characterized by 1H- and 13C-NMR spectroscopy and HRMS, as follows: 1H-NMR (400 MHz, CDCl3) δ 2.94 (s, 3H), 7.53–7.59 (m, 1H), 7.59–7.65 (m, 2H), 7.76 (ddd, J = 8.5, 6.9, 1.5 Hz, 1H), 7.87 (dd, J = 7.8, 0.7 Hz, 1H), 8.10–8.16 (m, 1H), 8.27–8.32 (m, 1H), 8.62–8.67 (m, 1H); 13C-NMR (100 MHz, CDCl3) δ 17.4, 122.8, 123.0, 124.0, 125.1, 125.9, 126.1, 128.5, 129.8, 130.1, 132.0, 135.1, 137.2, 140.7, 146.7, 153.3. HRMS (FD) Calcd for C16H11NS: M, 249.0612. Found: m/z 249.0619.
Besides a general experimental procedure for the synthesis of compounds 4, details of the reaction conditions, purification methods, spectral and analytical data (melting point, NMR, and HRMS), and NMR charts for all products 4 in Table 3, Table 4 and Table 5 are provided in Supplementary Materials.
3.4. N-Methylation of Indolo[3,2-b]quinolines with MeOTf: An Experimental Procedure Exemplified by the Synthesis of 10fi
Compound 10fi was synthesized based on the modified literature procedure [82], as follows: A flame-dried 20-mL Schlenk tube was charged with 4fi (28.6 mg, 0.100 mol) and toluene (0.60 mL). The resulting solution was degassed by three freeze-pump-thaw cycles, and the tube was then filled with argon. To this solution, MeOTf (31.2 mg, 0.190 mmol) that had been distilled by Kugelrohr at 90 °C/500 Pa prior to use was added, and the mixture was then stirred at 50 °C for 24 h. The resulting mixture, including a solid product, was filtered, and the solid was washed with Et2O (5 mL). The filtrate was concentrated, and the residue was filtered and then washed with Et2O (5 mL). This concentration–filtration–washing sequence was repeated once again, and the combined solid was dried in vacuo to give an analytically pure 5-methyl-11-trifluoromethyl-10H-quindolinium 1,1,1-trifluoromethanesulfonate (10fi) in 94% yield (42.4 mg) as a yellow solid (mp 281–282 °C). Compound 10fi was characterized by 1H-, 13C- and 19F-NMR spectroscopy and HRMS, as follows: 1H-NMR (500 MHz, dimethyl sulfoxide-d6) δ 5.13 (s, 3H), 7.62 (ddd, J = 8.3, 7.2, 1.1 Hz, 1H), 7.97 (dt, J = 8.3, 0.9 Hz, 1H), 8.06 (ddd, J = 8.3, 7.2, 1.1 Hz, 1H), 8.15 (ddd, J = 8.7, 6.8, 0.9 Hz, 1H), 8.28 (ddd, J = 9.0, 6.9, 1.3 Hz, 1H), 8.51–8.60 (m, 1H), 8.89 (d, J = 8.6 Hz, 1H), 8.97 (d, J = 9.2 Hz, 1H), 12.71 (s, 1H); 13C-NMR (125 MHz, dimethyl sulfoxide-d6) δ 41.7, 113.5, 113.7, 116.8 (q, J = 32.8 Hz), 119.2, 120.6 (q, J = 322.2 Hz), 121.5, 122.5, 123.2 (q, J = 275.9 Hz), 124.2 (q, J = 3.0 Hz), 127.1, 129.3, 130.8 (q, J = 1.2 Hz), 132.0, 135.1, 135.9, 142.7, 147.3; 19F-NMR (376 MHz, dimethyl sulfoxide-d6) δ –77.3, –53.4. HRMS (FD) Calcd for C17H12F3N2: M+, 301.0947. Found: m/z 301.0936.
Besides a general experimental procedure for the synthesis of compounds 10, details of the reaction conditions, spectral and analytical data (melting point, NMR, and HRMS), and NMR charts for all products 10 in Table 6 are provided in Supplementary Materials.
3.5. An Experimental Procedure for the Synthesis of 11fi by Neutralizing 10fi
Compound 11fi was synthesized based on the literature procedure [82], as follows: 10fi (22.5 mg, 0.0500 mmol) was placed in a 15-mL screw-cap vial. To this, a 5 wt % Na2CO3 aqueous solution (2.0 mL) was added, and the resulting mixture was stirred at 30 °C for 15 min. The aqueous phase was extracted with CHCl3 (4 mL × 4), and the combined organic layer was dried over anhydrous sodium sulfate (Na2SO4). Filtration and evaporation of the solvent followed by column chromatography on silica gel (CHCl3/Et3N = 50/1) gave 5-methyl-11-trifluoromethyl-5H-quindoline (11fi) in 99% yield (14.9 mg) as a dark navy solid [m.p. 251–253 °C (decomp.)]. Compound 11fi was characterized by 1H-, 13C- and 19F-NMR spectroscopy and HRMS, as follows: 1H-NMR (400 MHz, dimethyl sulfoxide-d6) δ 5.02 (s, 3H), 7.12 (ddd, J = 8.4, 6.6, 1.1 Hz, 1H), 7.65 (ddd, J = 8.4, 6.8, 1.1 Hz, 1H), 7.72 (dd, J = 8.6, 0.8 Hz, 1H), 8.86 (ddd, J = 8.6, 6.8, 1.0 Hz, 1H), 7.96 (ddd, J = 8.9, 6.9, 1.1 Hz, 1H), 8.47–8.52 (m, 1H), 8.55 (dd, J = 8.5, 0.7 Hz, 1H), 8.70 (d, J = 8.9 Hz, 1H); 13C-NMR (100 MHz, dimethyl sulfoxide-d6) δ 40.3, 113.6, 116.1 (q, J = 29.2 Hz), 117.4, 117.9, 119.5, 120.1 (q, J = 1.4 Hz), 124.0 (q, J = 3.8 Hz), 125.0 (q, J = 277.3 Hz), 125.7, 125.9, 127.8, 131.6, 132.7, 142.3, 143.8, 162.9; 19F-NMR (471 MHz, dimethyl sulfoxide-d6) δ –50.8. HRMS (FD) Calcd for C17H11F3N2: M, 300.0874. Found: m/z 300.0870.
4. Conclusions
We disclosed here that the indium-catalyzed tandem N–C and C–C bond-forming reaction of o-acylanilines with MeO–heteroarenes is a practical methodology to synthesize a range of HA[b]Qs with tricyclic and tetracyclic [2,3-b] and [3,2-b] skeletons fused with sulfur-, oxygen-, and nitrogen-based five-membered heteroaryl rings. Indolo[3,2-b]quinolines, which are also the frameworks constructed by our method, were readily converted to cryptolepine derivatives that have not yet been prepared. Mechanistic investigations revealed that the central pyridyl ring is constructed by the sequence of the intermolecular N–C bond-formation, followed by the C–C bond-forming ring closure.
Supplementary Materials
Supplementary materials are available online: experimental details for the synthesis of each product as well as 1H-, 13C- and 19F-NMR spectra and HRMS data. References [53,82] are cited in the supplementary materials.
Acknowledgments
We thank Meiji University for partially covering the costs to publish in open access.
Author Contributions
T.T. conceived the idea of this study and designed the experiments; K.Y., M.S. and Y.Y. performed the experiments; K.Y., M.S. and Y.Y. analyzed the data; T.T. contributed reagents/materials/analysis tools; K.Y. with the assistance of T.T. wrote the paper and prepared the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Lal, B.; Bhise, N.B.; Gidwani, R.M.; Lakdawala, A.D.; Joshi, K.; Patvardhan, S. Isolation, synthesis and biological activity of Evolitrine and analogs. ARKIVOC 2005, 2005, 77–97. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Loke, P.L.; Malone, J.F.; McRoberts, W.C.; Hamilton, J.T.G. Synthesis, structure and stereochemistry of quinoline alkaloids from Choisya ternata. Org. Biomol. Chem. 2007, 5, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, S.; Wan, S.; Ren, S.; Li, W.; Jiang, T. Efficient synthesis of jusbetonin, an indolo[3,2-b]quinoline glycoside, and its derivatives. Carbohydr. Res. 2009, 344, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Takeuchi, Y.; Hashigaki, K.; Ikeda, Y.; Chang, M.R.; Takeuchi, K.; Matsushima, M.; Tsuruo, T.; Tashiro, T.; Tsukagoshi, S.; et al. Synthesis and Antitumor Activity of Fused Tetracyclic Quinoline Derivatives. 1. J. Med. Chem. 1989, 32, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Tseng, C.-H.; Chen, Y.-L.; Lu, C.-M.; Kao, C.-L.; Wu, M.-H.; Tzeng, C.-C. Identification of benzofuro[2,3-b]quinoline derivatives as a new class of antituberculosis agents. Eur. J. Med. Chem. 2010, 45, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, P.T.; Ajay, A.K.; Bhat, M.K.; Parameswaran, P.S.; Tilve, S.G. Iodine catalyzed one-pot synthesis of chloro-substituted linear and angular indoloquinolines and in vitro antiproliferative activity study of different indoloquinolines. Med. Chem. Res. 2013, 22, 88–93. [Google Scholar] [CrossRef]
- Du, G.; Huang, S.-M.; Zhai, P.; Chen, S.-B.; Hua, W.-Z.; Tan, J.-H.; Ou, T.-M.; Huang, S.-L.; Li, D.; Gu, L.-Q.; et al. Synthesis and evaluation of new BODIPY-benzofuroquinoline conjugates for sensitive and selective DNA detection. Dyes Pigment. 2014, 107, 97–105. [Google Scholar] [CrossRef]
- Sokhanvar, M.; Pordel, M. Synthesis, spectroscopic characterization and DFT calculations of a new highly fluorescent heterocyclic system: Imidazo[4,5-a]quinindoline. ARKIVOC 2014, 2014, 328–341. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, X.; Zhao, N.; Xu, S.; An, Z.; Zhuang, X.; Lan, Z.; Wen, L.; Wan, X. Reductive Ring Closure Methodology toward Heteroacenes Bearing a Dihydropyrrolo[3,2-b]pyrrole Core: Scope and Limitation. J. Org. Chem. 2014, 79, 11339–11348. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, P.; Thiruvengadam, T.K.; Ramasamy, K. Synthese von Furo[2,3-b]chinolinen. Monatshefte Chem. 1977, 108, 725–733. [Google Scholar] [CrossRef]
- Gee, M.B.; Lee, W.J.; Yum, E.K. Synthesis of Pyrrolo[2,3-b]quinolines by Palladium-catalyzed Heteroannulation. Bull. Korean Chem. Soc. 2003, 24, 1193–1196. [Google Scholar] [CrossRef]
- Dutta, B.; Some, S.; Ray, J.K. Thermal cyclization of 3-arylamino-3-(2-nitrophenyl)-propenal Schiff base hydrochlorides followed by triethyl phosphite mediated deoxygenation: A facile synthesis of quindolines. Tetrahedron Lett. 2006, 47, 377–379. [Google Scholar] [CrossRef]
- Vera-Luque, P.; Alajarín, R.; Alvarez-Builla, J.; Vaquero, J.J. An Improved Synthesis of α-Carbolines under Microwave Irradiation. Org. Lett. 2006, 8, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Aillaud, I.; Bossharth, E.; Conreaux, D.; Desbordes, P.; Monteiro, N.; Balme, G. A Synthetic Entry to Furo[2,3-b]pyridin-4(1H)-ones and Related Furoquinolinones via Iodocyclization. Org. Lett. 2006, 8, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.N.S.S.P.; Srihari, E.; Ravinder, M.; Kumar, K.P.; Murthy, U.S.N.; Rao, V.J. DBU Promoted Facile Synthesis of New Thieno[2,3-b]pyridine/quinoline Derivatives and Their Antimicrobial Evaluation. J. Heterocycl. Chem. 2013, 50, E131–E135. [Google Scholar] [CrossRef]
- Bogányi, B.; Kámán, J. A concise synthesis of indoloquinoline skeletons applying two consecutive Pd-catalyzed reactions. Tetrahedron 2013, 69, 9512–9519. [Google Scholar] [CrossRef]
- Wu, B.; Yoshikai, N. Versatile Synthesis of Benzothiophenes and Benzoselenophenes by Rapid Assembly of Arylzinc Reagents, Alkynes, and Elemental Chalcogens. Angew. Chem. Int. Ed. 2013, 52, 10496–10499. [Google Scholar] [CrossRef] [PubMed]
- Barl, N.M.; Sansiaume-Dagousset, E.; Monzón, G.; Wagner, A.J.; Knochel, P. Preparation and Reactions of Heteroarylmethylzinc Reagents. Org. Lett. 2014, 16, 2422–2425. [Google Scholar] [CrossRef] [PubMed]
- Iaroshenko, V.O.; Gevorgyan, A.; Mkrtchyan, S.; Grigoryan, T.; Movsisyan, E.; Villinger, A.; Langer, P. Regioselective Direct Arylation of Fused 3-Nitropyridines and Other Nitro-Substituted Heteroarenes: The Multipurpose Nature of the Nitro Group as a Directing Group. ChemCatChem 2015, 7, 316–324. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, S. Rh(III)-catalyzed 7-azaindole synthesis via C–H activation/annulative coupling of aminopyridines with alkynes. Chem. Commun. 2015, 51, 11202–11205. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, B.; Song, X.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed Synthesis of Quinolines from Propargyl Silyl Ethers and Anthranils through the Umpolung of a Gold Carbene Carbon. Angew. Chem. Int. Ed. 2016, 55, 12688–12692. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.D.; Garud, D.R.; Udagawa, T.; Koketsu, M. Synthesis of thieno[2,3-b]quinoline and selenopheno[2,3-b]quinoline derivatives via iodocyclization reaction and a DFT mechanistic study. Org. Biomol. Chem. 2018, 16, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bierer, D.E.; Dubenko, L.G.; Zhang, P.; Lu, Q.; Imbach, P.A.; Garofalo, A.W.; Phuan, P.-W.; Fort, D.M.; Litvak, J.; Gerber, R.E.; et al. Antihyperglycemic Activities of Cryptolepine Analogues: An Ethnobotanical Lead Structure Isolated from Cryptolepis sanguinolenta. J. Med. Chem. 1998, 41, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Rádl, S.; Konvička, P.; Váchal, P. A New Approach to the Synthesis of Benzofuro[3,2-b]quinolines, Benzothieno[3,2-b]quinolines and Indolo[3,2-b]quinolines. J. Heterocycl. Chem. 2000, 37, 855–862. [Google Scholar] [CrossRef]
- Strekowski, L.; Lee, H.; Lin, S.-Y.; Czarny, A.; Van Derveer, D. Synthesis and Conformation of 2-Aminophenyldiarylperfluoroalkylmethanes (Molecular Propellers). J. Org. Chem. 2000, 65, 7703–7706. [Google Scholar] [CrossRef] [PubMed]
- Tugusheva, N.Z.; Ryabova, S.Y.; Solov’eva, N.P.; Anisimova, O.S.; Granik, V.G. Investigation of the Reaction of N-Acetylindoxyl with substituted anilines. Synthesis of derivatives of indolo[3,2-b]quinolines. Chem. Heterocycl. Compd. 2001, 37, 885–893. [Google Scholar] [CrossRef]
- Patteux, C.; Levacher, V.; Dupas, G. A Novel Traceless Solid-Phase Friedländer Synthesis. Org. Lett. 2003, 5, 3061–3063. [Google Scholar] [CrossRef] [PubMed]
- Engqvist, R.; Bergman, J. An improved synthesis of neocryptolepine. Org. Prep. Proced. Int. 2004, 36, 386–390. [Google Scholar] [CrossRef]
- Iaroshenko, V.O.; Wang, Y.; Zhang, B.; Volochnyuk, D.; Sosnovskikh, V.Y. Facile Synthesis of Fluorinated Benzofuro- and Benzothieno[2,3-b]pyridines, α-Carbolines and Nucleosides Containing the α-Carboline Framework. Synthesis 2009, 2393–2402. [Google Scholar] [CrossRef]
- Ali, S.; Li, Y.-X.; Anwar, S.; Yang, F.; Chen, Z.-S.; Liang, Y.-M. One-Pot Access to Indolo[2,3-b]quinolines by Electrophile-Triggered Cross-Amination/Friedel–Crafts Alkylation of Indoles with 1-(2-Tosylaminophenyl)ketones. J. Org. Chem. 2012, 77, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Vaghei, R.; Malaekehpoor, S.M. N-Bromosuccinimide as an efficient catalyst for the synthesis of indolo[2,3-b]quinolines. Tetrahedron Lett. 2012, 53, 4751–4753. [Google Scholar] [CrossRef]
- Lian, Y.; Hummel, J.R.; Bergman, R.G.; Ellman, J.A. Facile Synthesis of Unsymmetrical Acridines and Phenazines by a Rh(III)-Catalyzed Amination/Cyclization/Aromatization Cascade. J. Am. Chem. Soc. 2013, 135, 12548–12551. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, H.; Yu, Y.; Wu, W.; Jiang, H. Palladium-Catalyzed Intermolecular Aerobic Oxidative Cyclization of 2-Ethynylanilines with Isocyanides: Regioselective Synthesis of 4-Halo-2-aminoquinolines. J. Org. Chem. 2013, 78, 10319–10328. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhou, Y.; Huang, H.; Yang, Y.; Liu, Y.; Li, Y. Copper-Catalyzed Synthesis of Substituted Quinolines via C–N Coupling/Condensation from ortho-Acylanilines and Alkenyl Iodides. J. Org. Chem. 2015, 80, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, M.; Wojciechowski, K. Simple synthesis 11-substituted norcryptotackieine derivatives. RSC Adv. 2015, 5, 94296–94303. [Google Scholar] [CrossRef]
- Yan, Z.; Wan, C.; Wan, J.; Wang, Z. An efficient iron-promoted synthesis of 6H-indolo[2,3-b]quinolines and neocryptolepine derivatives. Org. Biomol. Chem. 2016, 14, 4405–4408. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, B. Tandem Rh(III)-Catalyzed C–H Amination/Annulation Reactions: Synthesis of Indoloquinoline Derivatives in Water. Org. Lett. 2016, 18, 2820–2823. [Google Scholar] [CrossRef] [PubMed]
- Senadi, G.C.; Dhandabani, G.K.; Hu, W.-P.; Wang, J.-J. Metal-free annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines: Efficient syntheses of acridines. Green Chem. 2016, 18, 6241–6245. [Google Scholar] [CrossRef]
- Janni, M.; Thirupathi, A.; Arora, S.; Peruncheralathan, S. Chemoselective Ullmann coupling at room temperature: A facile access to 2-aminobenzo[b]thiophenes. Chem. Commun. 2017, 53, 8439–8442. [Google Scholar] [CrossRef] [PubMed]
- Obata, N.; Mizuno, H.; Koitabashi, T.; Takizawa, T. The Cycloaddition Reaction of Isonitrile to Ketenimine Formed by the Reaction of Isonitrile with Carbene. Bull. Chem. Soc. Jpn. 1975, 48, 2287–2293. [Google Scholar] [CrossRef]
- Szmuszkovicz, J.; Baczynskyj, L.; Chidester, C.C.; Duchamp, D.J. Reaction of 2-Amino-7-chloro-5-phenyl-3H-[1,4]benzodiazepine with 1,3-Dicarbonyl Compounds. J. Org. Chem. 1976, 41, 1743–1747. [Google Scholar] [CrossRef]
- Sunder, S.; Peet, N.P. Synthesis of Benzofuro[3,2-b]quinoline-6(11H)one and Derivatives. J. Heterocycl. Chem. 1978, 15, 1379–1382. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Chang, M.-R.; Hashigaki, K.; Tashiro, T.; Tsuruo, T.; Tsukagoshi, S.; Yamato, M. Synthesis and Antitumor Activity of Fused Quinoline Derivatives. III. Novel N-Glycosylaminoindolo[3,2-b]quinolines. Chem. Pharm. Bull. 1992, 40, 1481–1485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, Y.; Goon, S.; Meth-Cohn, O. Carbenes from Vilsmeier reagents by the action of bases in POCl3; the umpolung of Vilsmeier reagents. Chem. Commun. 1996, 1395–1396. [Google Scholar] [CrossRef]
- Du, W.; Curran, D.P. Synthesis of Carbocyclic and Heterocyclic Fused Quinolines by Cascade Radical Annulations of Unsaturated N-Aryl Thiocarbamates, Thioamides, and Thioureas. Org. Lett. 2003, 5, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, P.T.; Parameswaran, P.S.; Tilve, S.G. Double reductive cyclization: A facile synthesis of the indoloquinoline alkaloid cryptotackieine. Tetrahedron Lett. 2007, 48, 7870–7872. [Google Scholar] [CrossRef]
- Schmittel, M.; Steffen, J.-P.; Rodríguez, D.; Engelen, B.; Neumann, E.; Cinar, M.E. Thermal C2–C6 Cyclization of Enyne–Carbodiimides: Experimental Evidence Contradicts a Diradical and Suggests a Carbene Intermediate. J. Org. Chem. 2008, 73, 3005–3016. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Ishida, T.; Takemoto, Y. Synthesis of Indolo[2,3-b]quinolines by Palladium-catalyzed Annulation of Unsaturated Isothioureas. Chem. Lett. 2009, 38, 772–773. [Google Scholar] [CrossRef]
- Haddadin, M.J.; El-Nachef, C.; Kisserwani, H.; Chaaban, Y.; Kurth, M.J.; Fettinger, J.C. 2-Benzyliden-2H-thieto[3,2-b]quinoline: A new heterocycle and its rearrangement to 2-phenylthieno[3,2-b]quinoline. Tetrahedron Lett. 2010, 51, 6687–6689. [Google Scholar] [CrossRef]
- Chen, K.; Tang, X.-Y.; Shi, M. Rh(II)-Catalyzed formation of pyrrolo[2,3-b]quinolines from azide-methylenecyclopropanes and isonitriles. Chem. Commun. 2016, 52, 1967–1970. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-Z.; Hu, X.-B.; Xu, Q.; Shi, M. Thermally induced formal [3+2] cyclization of ortho-aminoaryl-tethered alkylidenecyclopropanes: Facile synthesis of furoquinoline and thienoquinoline derivatives. Chem. Commun. 2016, 52, 2701–2704. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.-S.; Wang, M.-X.; Xu, D.-C.; Xie, J.-W. Synthesis of 2,3-Dihydrothieno(2,3-b)quinolines and Thieno(2,3-b)quinolines via an Unexpected Domino Aza-MBH/Alkylation/Aldol Reaction. J. Org. Chem. 2016, 81, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-C.; Liu, T.; Cheng, Y.-F.; Chang, H.-H.; Li, X.; Zhou, R.; Wei, W.-L.; Qiao, Y. AlCl3-Catalyzed Intramolecular Cyclization of N-Arylpropynamides with N-Sulfanylsuccinimides: Divergent Synthesis of 3-Sulfenyl Quinolin-2-ones and Azaspiro[4,5]trienones. J. Org. Chem. 2017, 82, 13459–13467. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, K.; Yoshimura, Y.; Akehi, M.; Tsuchimoto, T. A Heteroarylamine Library: Indium-Catalyzed Nucleophilic Aromatic Substitution of Alkoxyheteroarenes with Amines. Adv. Synth. Catal. 2018. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Hatanaka, K.; Shirakawa, E.; Kawakami, Y. Indium triflate-catalysed double addition of heterocyclic arenes to alkynes. Chem. Commun. 2003, 2454–2455. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Matsubayashi, H.; Kaneko, M.; Nagase, Y.; Miyamura, T.; Shirakawa, E. Indium-Catalyzed Annulation of 2-Aryl- and 2-Heteroarylindoles with Propargyl Ethers: Concise Synthesis and Photophysical Properties of Diverse Aryl- and Heteroaryl-Annulated[a]carbazoles. J. Am. Chem. Soc. 2008, 130, 15823–15835. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimoto, T.; Kanbara, M. Reductive Alkylation of Indoles with Alkynes and Hydrosilanes under Indium Catalysis. Org. Lett. 2011, 13, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimoto, T.; Igarashi, M.; Aoki, K. Exclusive Synthesis of β-Alkylpyrroles under Indium Catalysis: Carbonyl Compounds as Sources of Alkyl Groups. Chem. Eur. J. 2010, 16, 8975–8979. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, S.; Hondo, T.; Tsuchimoto, T. Easy Access to a Library of Alkylindoles: Reductive Alkylation of Indoles with Carbonyl Compounds and Hydrosilanes under Indium Catalysis. Adv. Synth. Catal. 2016, 358, 1136–1149. [Google Scholar] [CrossRef]
- Nomiyama, S.; Ogura, T.; Ishida, H.; Aoki, K.; Tsuchimoto, T. Indium-Catalyzed Regioselective β-Alkylation of Pyrroles with Carbonyl Compounds and Hydrosilanes and Its Application to Construction of a Quaternary Carbon Center with a β-Pyrrolyl Group. J. Org. Chem. 2017, 82, 5178–5197. [Google Scholar] [CrossRef] [PubMed]
- Yanada, R.; Hashimoto, K.; Tokizane, R.; Miwa, Y.; Minami, H.; Yanada, K.; Ishikura, M.; Takemoto, Y. Indium(III)-Catalyzed Tandem Reaction with Alkynylbenzaldehydes and Alkynylanilines to Heteroaromatic Compounds. J. Org. Chem. 2008, 73, 5135–5138. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Tsuji, H.; Yamagata, K.-I.; Endo, K.; Tanaka, I.; Nakamura, M.; Nakamura, E. Efficient Formation of Ring Structures Utilizing Multisite Activation by Indium Catalysis. J. Am. Chem. Soc. 2008, 130, 17161–17167. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yamamoto, K.; Yamanobe, A.; Ito, K.; Kinoshita, H.; Ichikawa, J.; Hosomi, A. Indium(III)-catalyzed Coupling between Alkynes and Aldehydes to α,β-Unsaturated Ketones. Chem. Lett. 2010, 39, 766–767. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Okita, A.; Yasuda, M.; Baba, A. Indium Tribromide Catalyzed Cross-Claisen Condensation between Carboxylic Acids and Ketene Silyl Acetals Using Alkoxyhydrosilanes. Angew. Chem. Int. Ed. 2011, 50, 8623–8625. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Okita, A.; Yasuda, M.; Baba, A. Synthesis of a Wide Range of Thioethers by Indium Triiodide Catalyzed Direct Coupling between Alkyl Acetates and Thiosilanes. Org. Lett. 2012, 14, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Loh, T.-P. Asymmetric Hetero-Diels–Alder Reaction of Danishefsky’s Dienes with α-Carbonyl Esters Catalyzed by an Indium(III)–PyBox Complex. Org. Lett. 2013, 15, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Nishimoto, Y.; Yasuda, M.; Baba, A. Indium Chloride Catalyzed Alkylative Rearrangement of Propargylic Acetates Using Alkyl Chlorides, Alcohols, and Acetates: Facile Synthesis of α-Alkyl-α,β-Unsaturated Carbonyl Compounds. Org. Lett. 2014, 16, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, N.; Prajapati, D. Indium-catalyzed, novel route to β,β-disubstituted indanones via tandem Nakamura addition–hydroarylation–decarboxylation sequence. Chem. Commun. 2015, 51, 3347–3350. [Google Scholar] [CrossRef] [PubMed]
- Hamachi, Y.; Katano, M.; Ogiwara, Y.; Sakai, N. Production of Quaternary α-Aminonitriles by Means of Indium-Catalyzed Three-Component Reaction of Alkynes, Amines, and Trimethylsilyl Cyanide. Org. Lett. 2016, 18, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Pathipati, S.R.; van der Werf, A.; Eriksson, L.; Selander, N. Diastereoselective Synthesis of Cyclopenta[c]furans by a Catalytic Multicomponent Reaction. Angew. Chem. Int. Ed. 2016, 55, 11863–11866. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Wang, S.-W.; Long, Y. Indium-mediated Organic Reactions and their Application in Organic Synthesis. Curr. Org. Chem. 2016, 20, 2865–2880. [Google Scholar] [CrossRef]
- For the synthesis of HA[b]Qs with one type of a heteroaryl ring, see: References 10–16, 18–21, 25–28, 30–36, 38, 40–50, 52 and 53.
- For the synthesis of HA[b]Qs with two types of heteroaryl rings, see: References 17, 22, 37, 39 and 51.
- For the synthesis of HA[b]Qs with three types of heteroaryl rings, see: References 24 and 29. The synthesis of HA[b]Qs with three types of heteroaryl rings is also achieved in references 4 and 23 but uses the same synthetic method as in reference 42 cited within reference 72.
- Ansah, C.; Mensah, K.B. A review of the anticancer potential of the antimalarial herbal Cryptolepis sanguinolenta and its major alkaloid cryptolepine. Ghana Med. J. 2013, 47, 137–147. [Google Scholar] [PubMed]
- Gibeau, A.L.; Snyder, J.K. Indium(III)-Catalyzed Hydrative Cyclization of 1,7-Diynyl Ethers. Org. Lett. 2011, 13, 4280–4283. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, S.; Pan, Y.; Xu, Y.; Wang, H. The indium-catalysed hydration of alkynes using substoichiometric amounts of PTSA as additive. Tetrahedron 2013, 69, 3775–3781. [Google Scholar] [CrossRef]
- Avery, M.A.; Verlander, M.S.; Goodman, M. Synthesis of 6-Aminoisoproterenol. J. Org. Chem. 1980, 45, 2750–2753. [Google Scholar] [CrossRef]
- Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Terrier, F. Modern Nucleophilic Aromatic Substitution; Wiley-VCH: Weinheim, Germany, 2013; pp. 205–278. ISBN 978-3-527-31861-2. [Google Scholar]
- Boahen, Y.O.; Mann, J. Synthesis and evaluation of quindoline and its analogue as potential anticancer agents. Chem. Nat. Compd. 2014, 50, 494–497. [Google Scholar] [CrossRef]
- Arzel, E.; Rocca, P.; Grellier, P.; Labaeïd, M.; Frappier, F.; Guéritte, F.; Gaspard, C.; Marsais, F.; Godard, A.; Quéguiner, G. New Synthesis of Benzo-δ-carbolines, Cryptolepines, and Their Salts: In Vitro Cytotoxic, Antiplasmodial, and Antitrypanosomal Activities of δ-Carbolines, Benzo-δ-carbolines, and Cryptolepines. J. Med. Chem. 2001, 44, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.G.; Hartley, J.P.; Griffin, D. Counterion effects in indium-catalysed aromatic electrophilic substitution reactions. Tetrahedron Lett. 2002, 43, 4789–4791. [Google Scholar] [CrossRef]
- Nakamura, M.; Endo, K.; Nakamura, E. A Modular Approach to α-Arylated Carbonyl Compounds via Indium Tris(bistriflylamide)-Catalyzed Regioselective Addition of β-Ketoesters to 1,3-Diynes. Adv. Synth. Catal. 2005, 347, 1681–1686. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Matsubayashi, H.; Kaneko, M.; Shirakawa, E.; Kawakami, Y. Easy Access to Aryl- and Heteroaryl-Annulated[a]carbazoles by the Indium-Catalyzed Reaction of 2-Arylindoles with Propargyl Ethers. Angew. Chem. Int. Ed. 2005, 44, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Wang, H.-Y.; Winston-McPherson, G.N.; Geng, H.-M.J.; Guzei, I.A.; Tang, W. Copper-catalyzed tandem annulation/arylation for the synthesis of diindolylmethanes from propargylic alcohols. Chem. Commun. 2014, 50, 12293–12296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Miao, Z.; Sheng, C.; Yao, J.; Zhuang, C.; Zhang, W. An alternative route for synthesis of o-trifluoroacetylanilines as useful fluorine-containing intermediates. J. Fluorine Chem. 2010, 131, 800–804. [Google Scholar] [CrossRef]
- Gavai, A.V.; Delucca, G.V.; O’Malley, D.; Gill, P.; Quesnelle, C.A.; Fink, B.E.; Zhao, Y.; Lee, F.Y. Bis(fluoroalkyl)alkanoylamino-1,4-benzodiazepinone Compounds as Notch Inhibitors and Their Preparation. WO Patent 2014047372, 27 March 2014. [Google Scholar]
- Chabert, J.F.D.; Joucla, L.; David, E.; Lemaire, M. An efficient phosphine-free palladium coupling for the synthesis of new 2-arylbenzo[b]thiophenes. Tetrahedron 2004, 60, 3221–3230. [Google Scholar] [CrossRef]
- Ilangovan, A.; Satish, G. Direct Amidation of 2′-Aminoacetophenones Using I2-TBHP: A Unimolecular Domino Approach toward Isatin and Iodoisatin. J. Org. Chem. 2014, 79, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Jean, M.; Renault, J.; van de Weghe, P. Palladium-catalyzed arylation of vinylic acetates. Phosphine ligand influenced regioselectivity. Tetrahedron Lett. 2009, 50, 6546–6548. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).