Potential of Bioremediation and PGP Traits in Streptomyces as Strategies for Bio-Reclamation of Salt-Affected Soils for Agriculture
Abstract
1. Introduction
1.1. The Genus Streptomyces
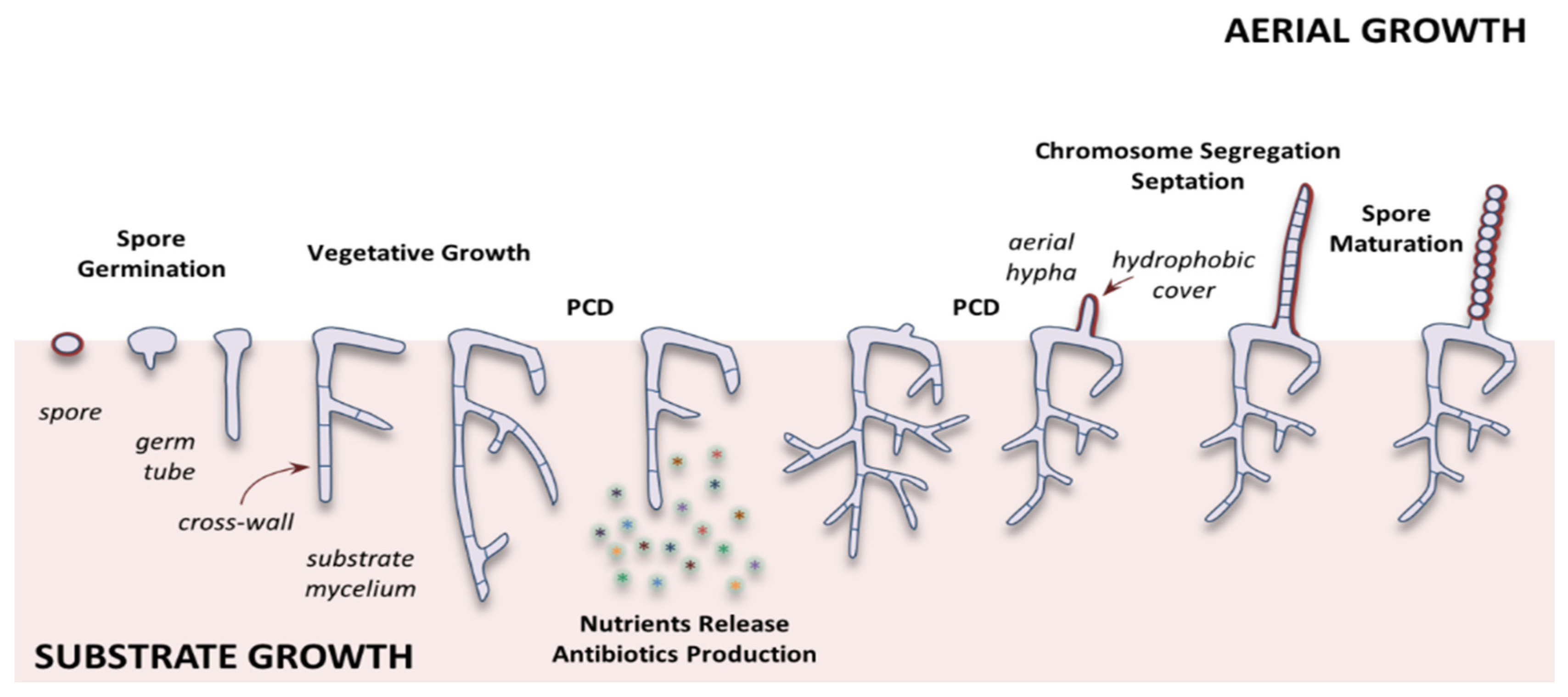
1.2. Streptomyces Life Cycle
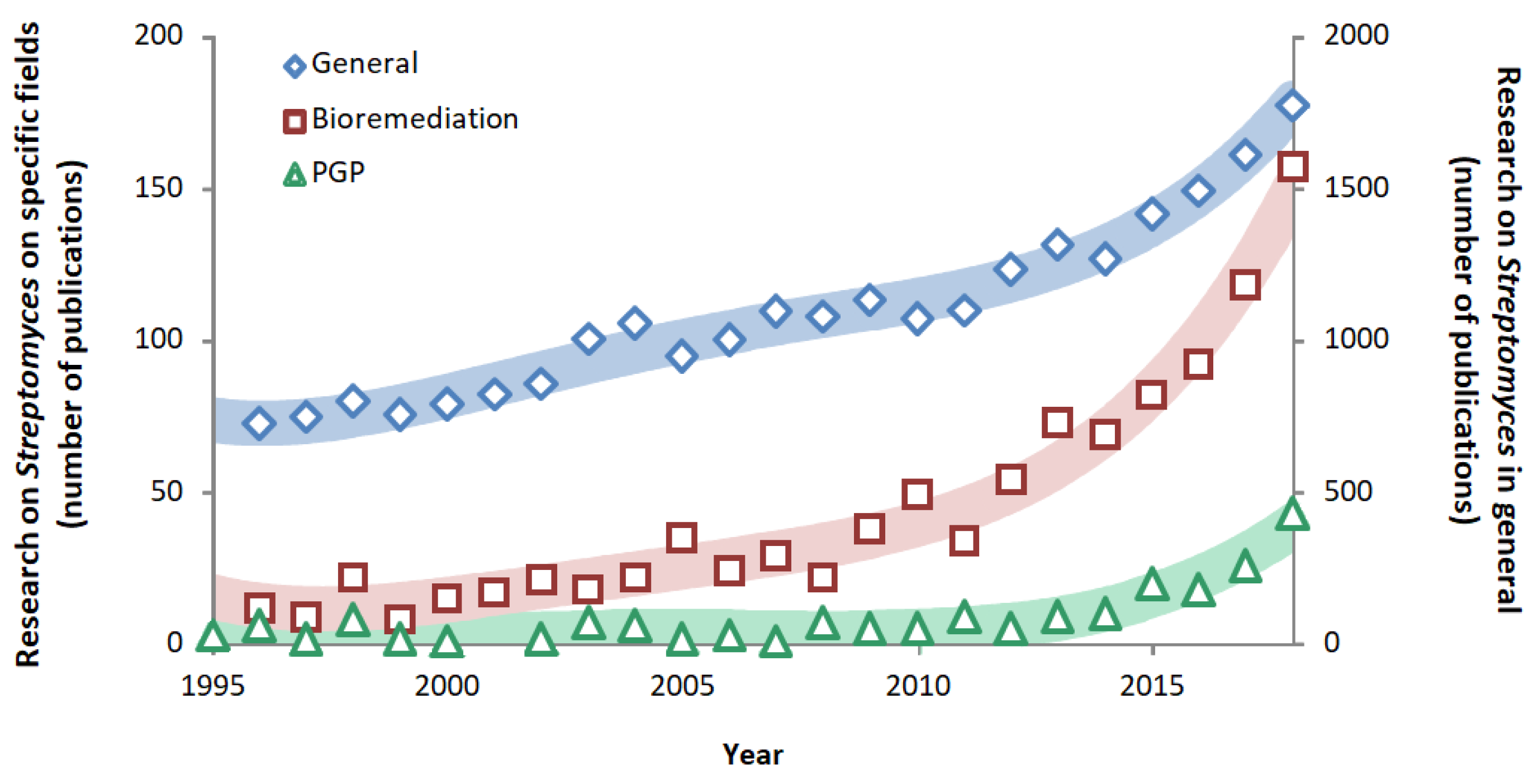
1.3. Streptomyces Applications
2. Streptomyces in Bioremediation
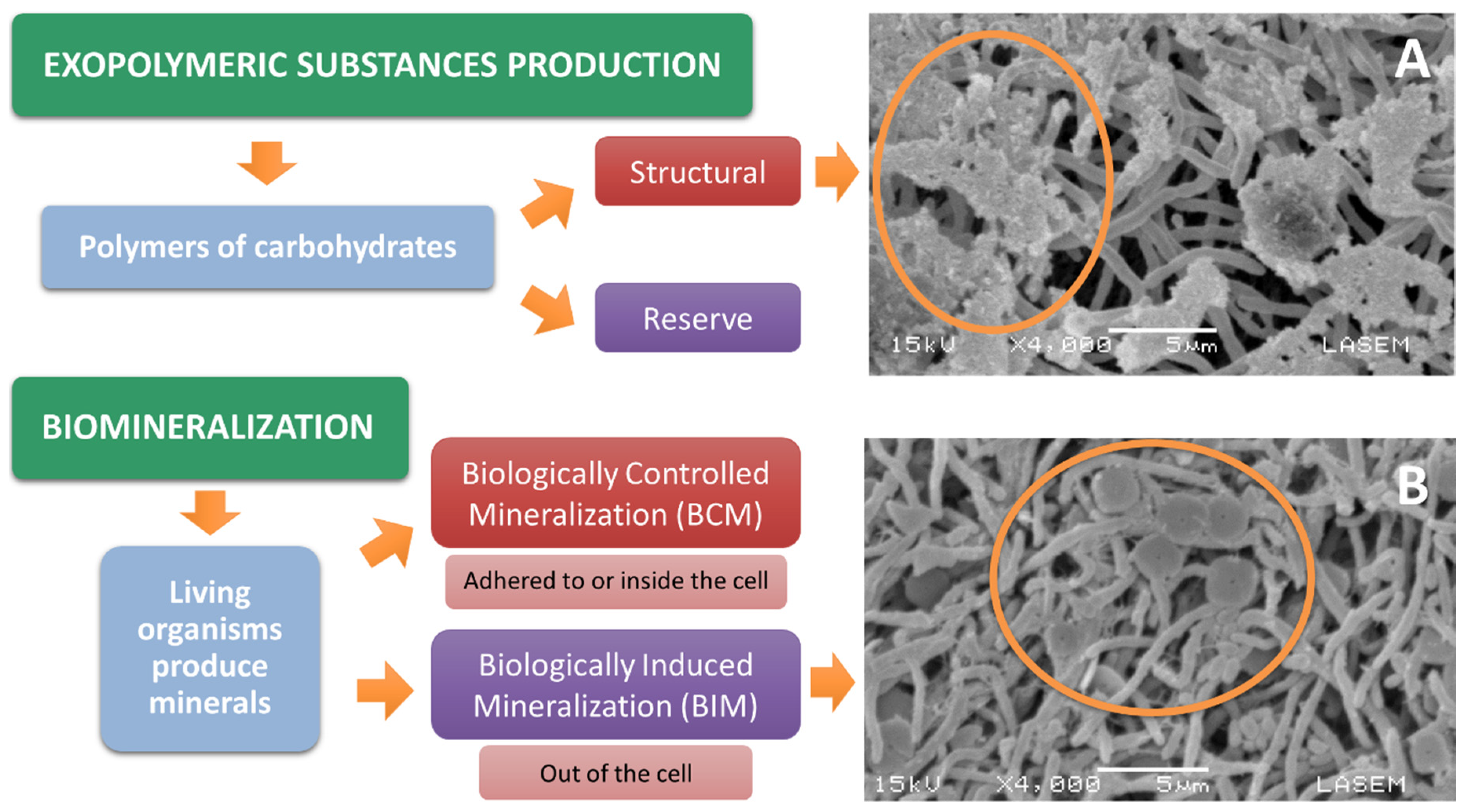
2.1. Microbial Mechanisms Used for Bioremediation
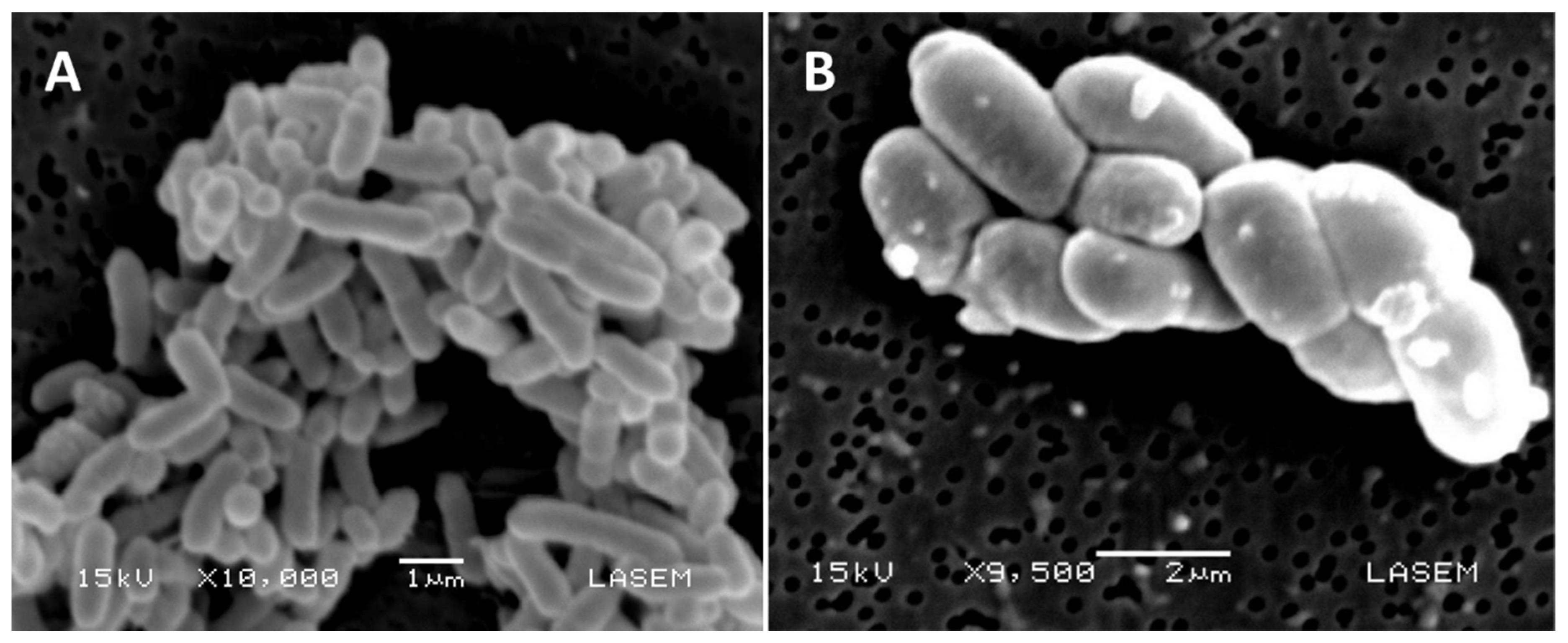
2.2. The Case of Boron-Mining Environmental Impact
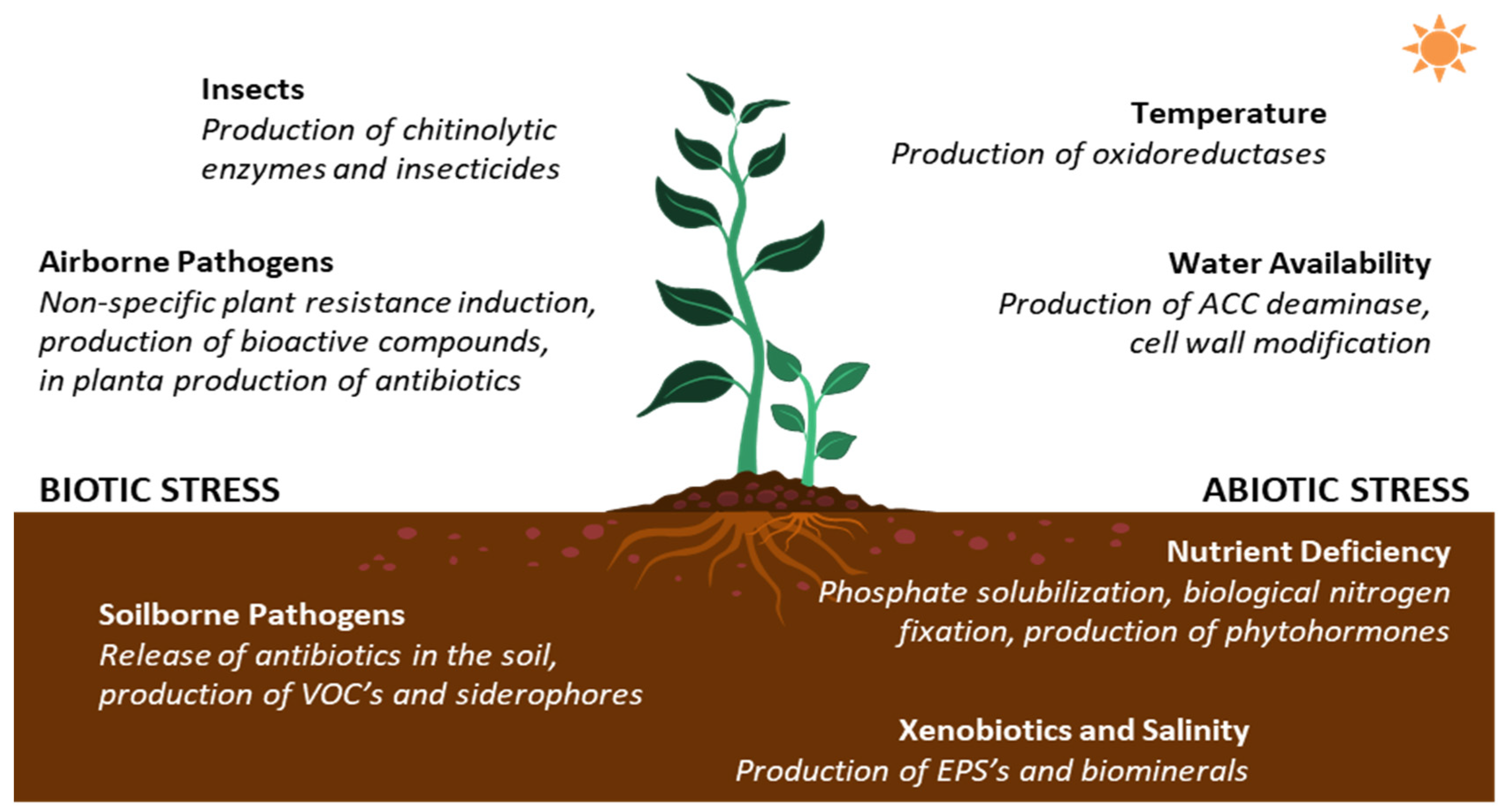
3. Streptomyces in Plant Growth Promotion
3.1. PGP Streptomyces against Biotic Stressors
3.2. PGP Streptomyces against Abiotic Stressors
4. Bio-Reclamation of Saline Soils
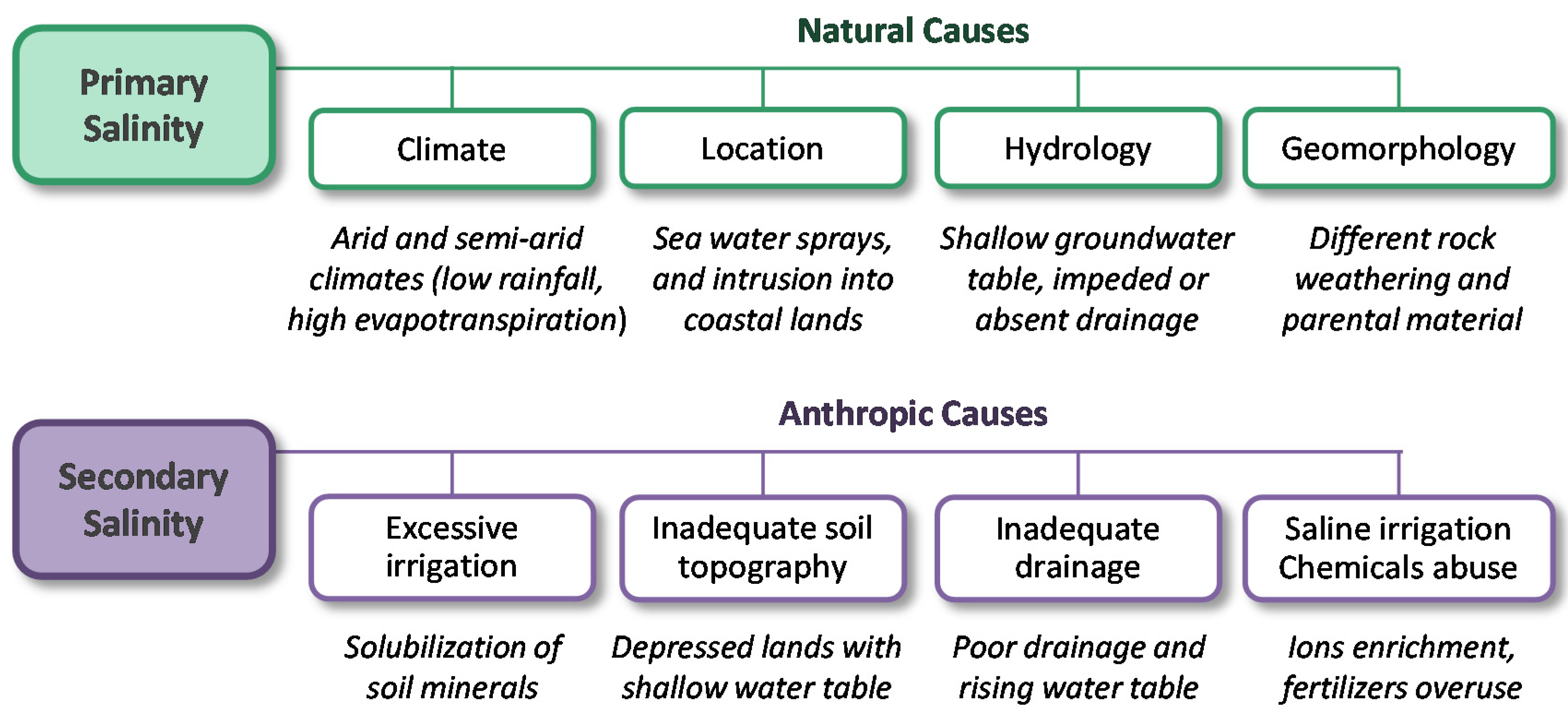
4.1. Soil Salinity, Causes and Effects
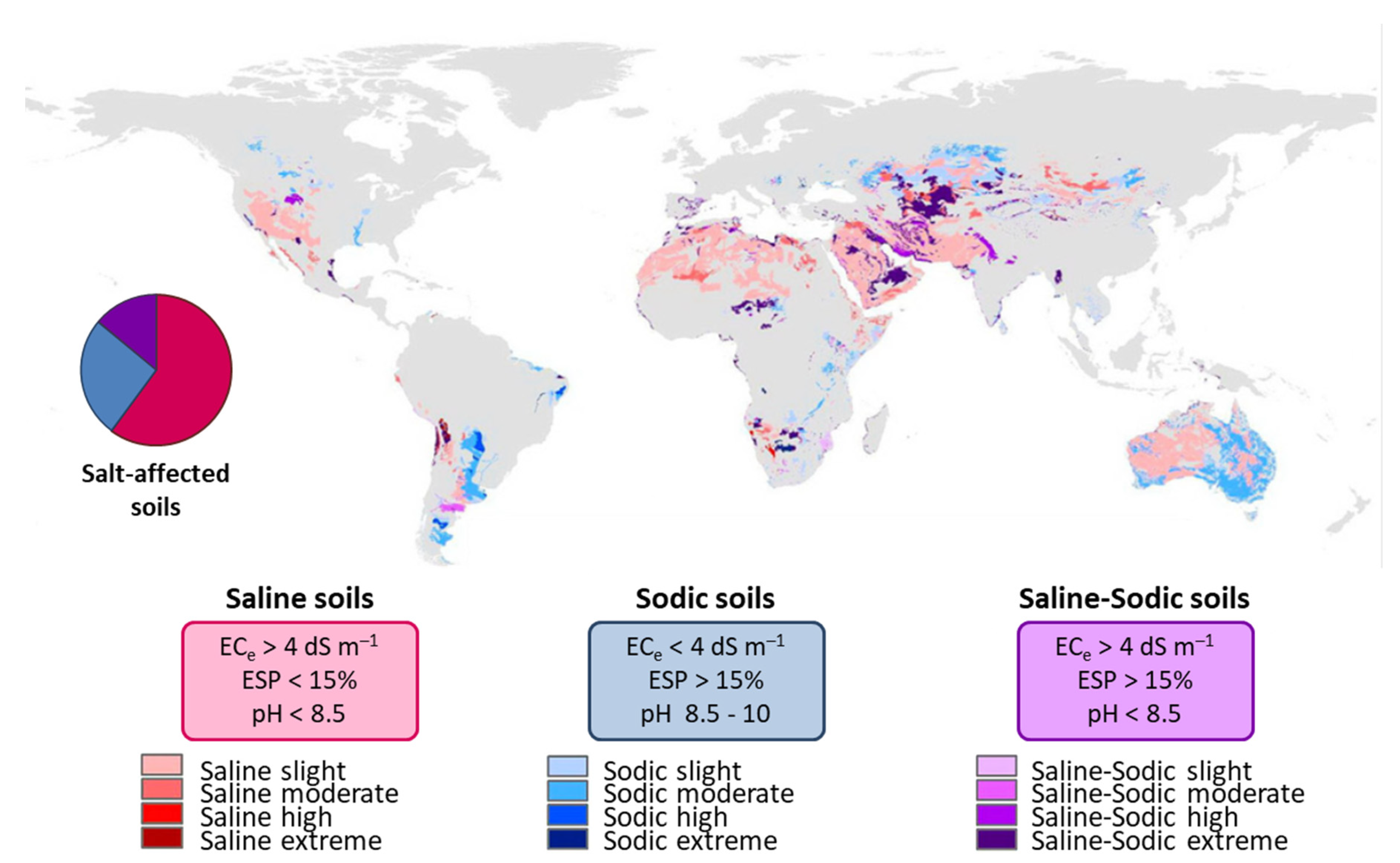
4.2. Salt-Affected Soils Classification and Distribution
4.3. Reclamation vs. Bio-Reclamation of Salt-Affected Soils
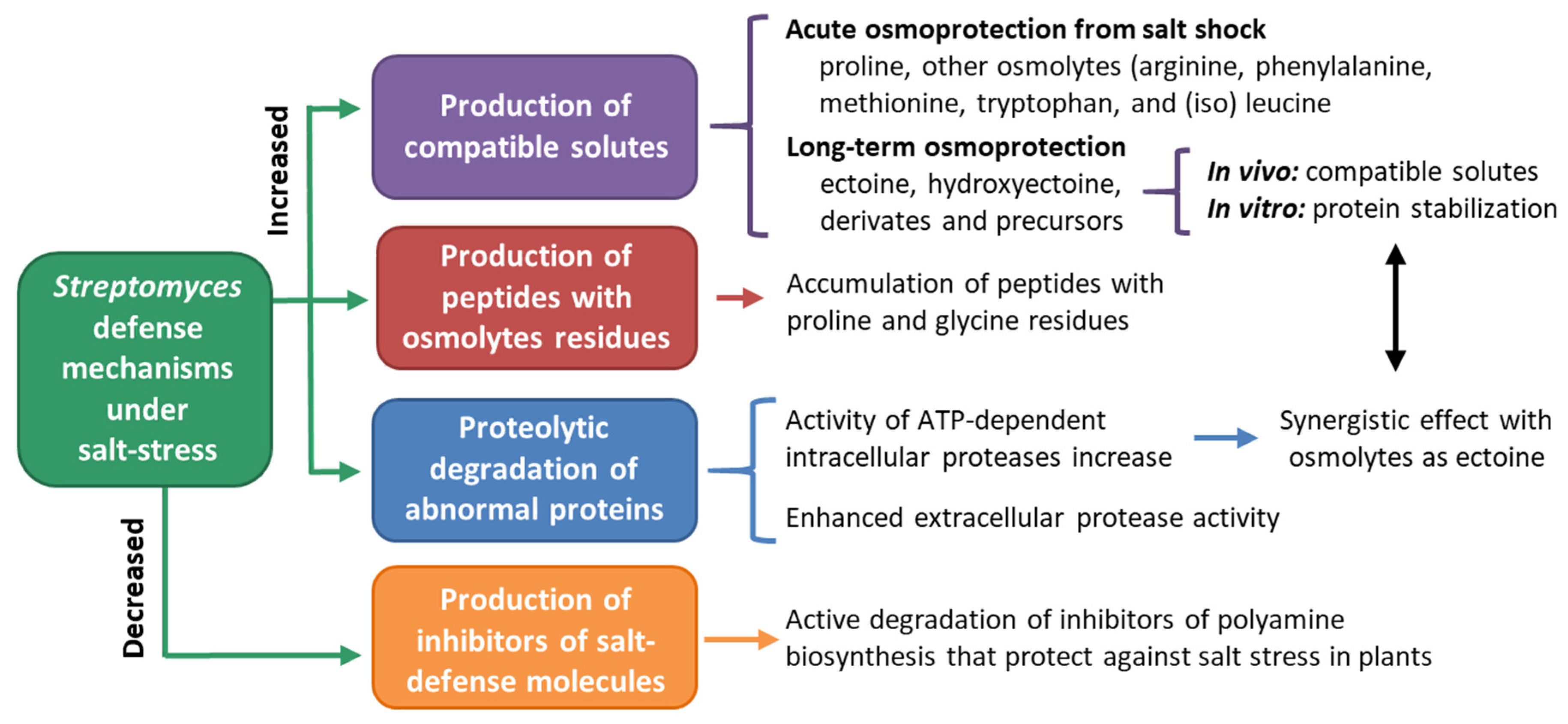
4.4. Streptomyces in Salt-Affected Soils
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Alexander, D. Bacteria and Archea. In Principles and Applications of Soil Microbiology; Sylvia, D., Fuhrmann, J., Hartel, P., Zuberer, D., Eds.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2005; pp. 101–139. [Google Scholar]
- Atlas, R.M.; Bartha, R. Ecología Microbiana y Microbiología Ambiental, 4th ed.; Addison Wesley: Madrid, Spain, 1998. [Google Scholar]
- Zuberer, D.A.; Wollum, A.G. Introduction and historical perspective. In Principles and Applications of Soil Microbiology; Sylvia, D., Fuhrmann, J., HArtel, P., Zuberer, D., Eds.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2005; pp. 3–25. [Google Scholar]
- Matsukawa, E.; Nakagawa, Y.; Iimura, Y.; Hayakawa, M. A new enrichment method for the selective isolation of streptomycetes from the root surfaces of herbaceous plants. Actinomycetologica 2007, 21, 66–69. [Google Scholar] [CrossRef]
- He, X.; Li, H.; Pan, Y.; Wang, L.; Tan, H.; Liu, G. SCO3129, a TetR family regulator, is responsible for osmotic stress in Streptomyces coelicolor. Synth. Syst. Biotechnol. 2018, 3, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Moraga, N.B.; Irazusta, V.; Amoroso, M.J.; Rajal, V.B. Bio-precipitates produced by two autochthonous boron tolerant Streptomyces strains. J. Environ. Chem. Eng. 2017, 5, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J. Actinomycetes in soils, composts and fodders. In Actinomycetales: Characteristics and Practical Importance; Sykes, G., Skinner, F.A., Eds.; Academic Press: London, UK, 1973; pp. 231–251. [Google Scholar]
- Elander, R.P. Microbial screening, selection and strain improvement. In Basic Biotechnology; Bu Lock, J., Kristiansen, B., Eds.; Academic Press: London, UK, 1987; pp. 217–251. [Google Scholar]
- Keiser, T.; Viv, M.J.; Buttner, M.J.; Chater, K.F. Preparation and analysis of genomic plasmid DNA. In Practical Streptomyces Genetics; Keiser, T., Viv, M.J., Buttner, M.J., Chater, K.F., Hopwood, D.A., Eds.; The John Innes Foundation: Norwich, UK, 2000; pp. 161–210. [Google Scholar]
- Goodfellow, M.; Mordaski, M.; Williams, S. The biology of actinomycetes. In Extremophiles: Microbial Life in Extreme Environments; Horikoshi, K., Grant, W., Eds.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Tokala, R.K.; Strap, J.L.; Jung, C.M.; Crawford, D.L.; Salove, M.H.; Deobald, L.A.; Bailey, J.F.; Morra, M.J. Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the Pea Plant (Pisum sativum). Appl. Environ. Microbiol. 2002, 68, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Aldesuquy, H.S.; Mansour, F.A.; Abo-Hamed, S.A. Effect of the culture filtrates of Streptomyces on growth and productivity of wheat plants. Folia Microbiol. (Praha) 1998, 43, 465–470. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Alekhya, G.; Prakash, B.; Kudapa, H.; Rathore, A.; Varshney, R.K. The extent of grain yield and plant growth enhancement by plant growth-promoting broad-spectrum Streptomyces sp. in chickpea. Springerplus 2015, 4, 31. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Vidya, M.S.; Rathore, A. Plant growth-promoting activities of Streptomyces spp. in sorghum and rice. Springer Plus 2013, 2, 574. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Vadlamudi, S.; Bandikinda, P.; Sathya, A.; Vijayabharathi, R.; Rupela, O.; Kudapa, H.; Katta, K.; Varshney, R.K. Evaluation of Streptomyces strains isolated from herbal vermicompost for their plant growth-promotion traits in rice. Microbiol. Res. 2014, 169, 40–48. [Google Scholar] [CrossRef]
- Hernández, A.; Rives, N.; Caballero, A.; Hernández, A.; Heydrich, M. Caracterización de rizobacterias asociadas al cultivo del maíz en la producción de metabolitos del tipo AIA, sideróforos y ácido salicílico. Rev. Colomb. Biotecnol. 2004, VI, 6–13. [Google Scholar]
- Ravel, J.; Amoroso, M.J.; Colwell, R.R.; Hill, R.T. Mercury resistant actinomycetes form Chesapeake Bay. FEMS Microbiol. Lett. 1998, 162, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Watve, M.G.; Tickoo, R.; Jog, M.M.; Bhole, B.D. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 2001, 176, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Omura, S. Isolation of new actinomycete strains for the screening of new bioactive compounds. J. Gen. Appl. Microbiol. 2003, 49, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Cañaveras, J.C.; Hoyos, M.; Sanchez-Moral, S.; Sanz-Rubio, E.; Bedoya, J.; Soler, V.; Groth, I.; Schumann, P.; Laiz, L.; Gonzalez, I.; et al. Microbial communities associated with hydromagnesite and needle-fiber aragonite deposits in a karstic cave (Altamira, Northern Spain). Geomicrobiol. J. 1999, 16, 9–25. [Google Scholar]
- Haferburg, G.; Kloess, G.; Schmitz, W.; Kothe, E. “Ni-struvite”—A new biomineral formed by a nickel resistant Streptomyces acidiscabies. Chemosphere 2008, 72, 517–523. [Google Scholar] [CrossRef]
- Flärdh, K. Growth polarity and cell division in Streptomyces. Curr. Opin. Microbiol. 2003, 6, 564–571. [Google Scholar] [CrossRef]
- Chater, K.F. A morphological and genetic mapping study of white colony mutants of Streptomyces coelicolor. J. Gen. Microbiol. 1972, 72, 9–28. [Google Scholar] [CrossRef]
- Claessen, D.; Rozen, D.E.; Kuipers, O.P.; Søgaard-Andersen, L.; van Wezel, G.P. Bacterial solutions to multicellularity: A tale of biofilms, filaments and fruiting bodies. Nat. Rev. Microbiol. 2014, 12, 115. [Google Scholar] [CrossRef]
- Elliot, M.A.; Buttner, M.J.; Nodwell, J.R. Multicellular development in Streptomyces. In Myxobacteria; Whitworth, D.E., Ed.; American Society of Microbiology: Washington, DC, USA, 2008; pp. 419–438. ISBN 9781555814205. [Google Scholar]
- Wildermuth, H.; Hopwood, D.A. Septation during sporulation in Streptomyces coelicolor. J. Gen. Microbiol. 1970, 60, 51–59. [Google Scholar] [CrossRef][Green Version]
- Jakimowicz, D.; van Wezel, G.P. Cell division and DNA segregation in Streptomyces: How to build a septum in the middle of nowhere? Mol. Microbiol. 2012, 85, 393–404. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.; Chen, M.; Crawford, R.; Ivanova, E. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Yu, H.; Yue, Z. Production of extracellular polymeric substances from Rhodopseudomonas acidophila in presence of toxic substances. Appl. Microbiol. Biotechnol. 2005, 69, 216–222. [Google Scholar] [CrossRef]
- Mendez, C.; Braña, A.F.; Manzanal, M.B.; Hardisson, C. Role of substrate mycelium in colony development in Streptomyces. Can. J. Microbiol. 1985, 31, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, H. Development and organization of the aerial mycelium in Streptomyces coelicolor. J. Gen. Microbiol. 1970, 60, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bibb, M.J. Regulation of secondary metabolism in streptomycetes. Curr. Opin. Microbiol. 2005, 8, 208–215. [Google Scholar] [CrossRef]
- van Wezel, G.P.; McDowall, K.J. The regulation of the secondary metabolism of Streptomyces: New links and experimental advances. Nat. Prod. Rep. 2011, 28, 1311–1333. [Google Scholar] [CrossRef]
- Manteca, A.; Ye, J.; Sánchez, J.; Jensen, O.N. Phosphoproteome analysis of Streptomyces development reveals extensive protein phosphorylation accompanying bacterial differentiation. J. Proteome Res. 2011, 10, 5481–5492. [Google Scholar] [CrossRef]
- Bibb, M.J.; Domonkos, A.; Chandra, G.; Buttner, M.J. Expression of the chaplin and rodlin hydrophobic sheath proteins in Streptomyces venezuelae is controlled by sigma(BldN) and a cognate anti-sigma factor, RsbN. Mol. Microbiol. 2012, 84, 1033–1049. [Google Scholar] [CrossRef]
- Keijser, B.J.F.; Noens, E.E.E.; Kraal, B.; Koerten, H.K.; van Wezel, G.P. The Streptomyces coelicolor ssgB gene is required for early stages of sporulation. FEMS Microbiol. Lett. 2003, 225, 59–67. [Google Scholar] [CrossRef]
- Claessen, D.; Rink, R.; de Jong, W.; Siebring, J.; de Vreugd, P.; Boersma, F.G.H.; Dijkhuizen, L.; Wosten, H.A.B. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 2003, 17, 1714–1726. [Google Scholar] [CrossRef]
- Wildermuth, H.; Wehrli, E.; Horne, R.W. The surface structure of spores and aerial mycelium in Streptomyces coelicolor. J. Ultrastruct. Res. 1971, 35, 168–180. [Google Scholar] [CrossRef]
- Claessen, D.; de Jong, W.; Dijkhuizen, L.; Wösten, H.A.B. Regulation of Streptomyces development: Reach for the sky! Trends Microbiol. 2006, 14, 313–319. [Google Scholar] [CrossRef]
- Wösten, H.A.; van Wetter, M.A.; Lugones, L.G.; van der Mei, H.C.; Busscher, H.J.; Wessels, J.G. How a fungus escapes the water to grow into the air. Curr. Biol. 1999, 9, 85–88. [Google Scholar] [CrossRef]
- Dragos, A.; Kovacs, A.T.; Claessen, D. The role of functional amyloids in multicellular growth and development of Gram-positive bacteria. Biomolecules 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Flärdh, K.; Leibovitz, E.; Buttner, M.J.; Chater, K.F. Generation of a non-sporulating strain of Streptomyces coelicolor A3(2) by the manipulation of a developmentally controlled ftsZ promoter. Mol. Microbiol. 2000, 38, 737–749. [Google Scholar] [CrossRef]
- Celler, K.; Koning, R.I.; Koster, A.J.; van Wezel, G.P. Multidimensional view of the bacterial cytoskeleton. J. Bacteriol. 2013, 195, 1627–1636. [Google Scholar] [CrossRef]
- Jones, S.E.; Ho, L.; Rees, C.A.; Hill, J.E.; Nodwell, J.R.; Elliot, M.A. Streptomyces exploration is triggered by fungal interactions and volatile signals. Elife 2017, 6, e21738. [Google Scholar] [CrossRef]
- Schatz, A.; Bugie, E.; Waksman, S.A. Streptomycin, a substance exhibiting antibiotic activity against Gram-Positive and Gram-Negative bacteria. Proc. Soc. Exp. Biol. Med. 1944, 55, 66–69. [Google Scholar] [CrossRef]
- Elsevier, B.V. ScienceDirect. Available online: https://www.sciencedirect.com/ (accessed on 10 October 2019).
- Jones, S.E.; Elliot, M.A. Streptomyces exploration: Competition, volatile communication and new bacterial behaviours. Trends Microbiol. 2017, 25, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D.; et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.A. Highlights of Streptomyces genetics. Heredity (Edinb.) 2019, 123, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Teixeira, M.F.S.; Converti, A.; Porto, A.L.F.; Sarubbo, L.A. Production of a new lipoprotein biosurfactant by Streptomyces sp. DPUA1566 isolated from lichens collected in the Brazilian Amazon using agroindustry wastes. Biocatal. Agric. Biotechnol. 2019, 17, 142–150. [Google Scholar] [CrossRef]
- Baoune, H.; Aparicio, J.D.; Pucci, G.; Ould El Hadj-Khelil, A.; Polti, M.A. Bioremediation of petroleum-contaminated soils using Streptomyces sp. Hlh1. J. Soils Sediments 2019, 19, 2222–2230. [Google Scholar] [CrossRef]
- Boudh, S.; Tiwari, S.; Singh, J.S. Microbial-Mediated Lindane Bioremediation. In Agro-Environmental Sustainability: Volume 2: Managing Environmental Pollution; Singh, J.S., Seneviratne, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 213–233. ISBN 978-3-319-49727-3. [Google Scholar]
- Medina, R.; Gara, P.M.D.; Fernández-González, A.J.; Rosso, J.A.; Del Panno, M.T. Remediation of a soil chronically contaminated with hydrocarbons through persulfate oxidation and bioremediation. Sci. Total Environ. 2018, 618, 518–530. [Google Scholar] [CrossRef]
- de Santos, A.A.; da Silveira, J.A.G.; Bonifacio, A.; Rodrigues, A.C.; do Figueiredo, M.V.B. Antioxidant response of cowpea co-inoculated with plant growth-promoting bacteria under salt stress. Brazilian J. Microbiol. Public. Brazilian Soc. Microbiol. 2018, 49, 513–521. [Google Scholar] [CrossRef]
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci. Rep. 2018, 8, 1950. [Google Scholar] [CrossRef]
- Tolba, S.T.M.; Ibrahim, M.; Amer, E.A.M.; Ahmed, D.A.M. First insights into salt tolerance improvement of Stevia by plant growth-promoting Streptomyces species. Arch. Microbiol. 2019, 201, 1295–1306. [Google Scholar] [CrossRef]
- Xu, L.; Naylor, D.; Dong, Z.; Simmons, T.; Pierroz, G.; Hixson, K.K.; Kim, Y.-M.; Zink, E.M.; Engbrecht, K.M.; Wang, Y.; et al. Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E4284–E4293. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Bioremediation. Contam. Site Clean-up Inf. 2020. Available online: https://clu-in.org/greenremediat (accessed on 9 February 2020).
- Vidali, M. Bioremediation. An overview*. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. The Role of Microorganisms in Bioremediation—A Review. Open J. Environ. Biol. 2017, 2, 38–46. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Benimeli, C.S.; Cuozzo, S.A.; Amoroso, M.J. Isolation of pesticide-degrading actinomycetes from a contaminated site: Bacterial growth, removal and dechlorination of organochlorine pesticides. Int. Biodeterior. Biodegrad. 2010, 64, 434–441. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Sáez, J.M.; Benimeli, C.S.; Amoroso, M.J. Lindane biodegradation by defined consortia of indigenous Streptomyces strains. Water Air Soil Pollut. 2011, 222, 217–231. [Google Scholar] [CrossRef]
- Alvarez, A.; Saez, J.M.; Davila Costa, J.S.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Briceño, G.E.; Saez, J.M.; Benimeli, C.S.; Diez, M.C.; Amoroso, M.J. Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. Biomed Res. Int. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Alvarez, A.; Saez, J.M.; Benimeli, C.S.; Amoroso, M.J. Methoxychlor bioremediation by defined consortium of environmental Streptomyces strains. Int. J. Environ. Sci. Technol. 2014, 11, 1147–1156. [Google Scholar] [CrossRef]
- Benimeli, C.S.; Fuentes, M.S.; Abate, C.M.; Amoroso, M.J. Bioremediation of lindane-contaminated soil by Streptomyces sp. M7 and its effects on Zea mays growth. Int. Biodeterior. Biodegrad. 2007, 61, 233–239. [Google Scholar] [CrossRef]
- Cuozzo, S.A.; Fuentes, M.S.; Bourguignon, N.; Benimeli, C.S.; Amoroso, M.J. Chlordane biodegradation under aerobic conditions by indigenous Streptomyces strains. Int. Biodeterior. Biodegrad. 2012, 66, 19–24. [Google Scholar] [CrossRef]
- Schippers, A. Microorganisms involved in bioleaching and nucleic acid-based molecular methods for their identification and quantification. In Microbial Processing of Metal Sulfides; Donati, E.R., Sand, W., Eds.; Springer Netherlands: Dordrecht, The Netherland, 2007; pp. 3–33. ISBN 978-1-4020-5589-8. [Google Scholar]
- Polti, M.A.; Atjián, M.C.; Amoroso, M.J.; Abate, C.M. Soil chromium bioremediation: Synergic activity of actinobacteria and plants. Int. Biodeterior. Biodegrad. 2011, 65, 1175–1181. [Google Scholar] [CrossRef]
- Polti, M.A.; Amoroso, M.J.; Abate, C.M. Intracellular chromium accumulation by Streptomyces sp. MC1. Water Air Soil Pollut. 2011, 214, 49–57. [Google Scholar] [CrossRef]
- Albarracín, V.; Winik, B.; Kothe, E.; Amoroso, M.J.; Abate, J.C. Copper bioaccumulation by actinobacteria Amycolatopsis sp. AB0. J. Basic Microbiol. 2008, 48, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Polti, M.; Amoroso, M.J.; Abate, C.M. Chromium (VI) resistance and removal by actinomycete strains isolated from sediments. Chemosphere 2008, 67, 660–667. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Gupta, A.; Joia, J.; Sood, A.; Sood, R.; Sidhu, C.; Kaur, G. Microbes as potential tool for remediation of heavy metals: A review. J. Microb. Biochem. Technol. 2016, 8, 364–372. [Google Scholar] [CrossRef]
- Moraga, N.B.; Poma, H.R.; Amoroso, M.J.; Rajal, V.B. Isolation and characterization of indigenous Streptomyces and Lentzea strains from soils containing boron compounds in Argentina. J. Basic Microbiol. 2014, 54, 568–577. [Google Scholar] [CrossRef]
- Moraga, N.B.; Amoroso, M.J.; Rajal, V.B. Streptomyces from soils contaminated with boron compounds. In Actinobacteria. Application in Bioremediation and Production of Industrial Enzymes; Amoroso, M.J., Benimeli, C.S., Cuozzo, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 136–164. [Google Scholar]
- Majzlik, P.; Strasky, A.; Adam, V.; Nemec, M.; Trnkova, L.; Zehnalek, J.; Hubalek, J.; Provaznik, I.; Kizek, R. Influence of zinc(II) and copper(II) ions on Streptomyces bacteria revealed by electrochemistry. Int. J. Electrochem. Sci. 2011, 6, 2171–2191. [Google Scholar]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Thavamani, P.; Samkumar, R.A.; Satheesh, V.; Subashchandrabose, S.R.; Ramadass, K.; Naidu, R.; Venkateswarlu, K.; Megharaj, M. Microbes from mined sites: Harnessing their potential for reclamation of derelict mine sites. Environ. Pollut. 2017, 230, 495–505. [Google Scholar] [CrossRef]
- Schütze, E.; Weist, A.; Klose, M.; Wach, T.; Schumann, M.; Nietzsche, S.; Merten, D.; Baumert, J.; Majzlan, J.; Kothe, E. Taking nature into lab: Biomineralization by heavy metal-resistant streptomycetes in soil. Biogeosciences 2013, 10, 3605–3614. [Google Scholar] [CrossRef]
- Pérez-González, T.; Valverde-Tercedor, C.; Jiménez-López, C. Biomineralización bacteriana de magnetita y aplicaciones. Semin. SEM 2012, 7, 58–74. [Google Scholar]
- Handley-Sidhu, S.; Renshaw, J.C.; Moriyama, S.; Stolpe, B.; Mennan, C.; Bagheriasl, S.; Yong, P.; Stamboulis, A.; Paterson-Beedle, M.; Sasaki, K.; et al. Uptake of Sr2+ and Co2+ into biogenic hydroxyapatite: Implications for biomineral ion exchange synthesis. Environ. Sci. Technol. 2011, 45, 6985–6990. [Google Scholar] [CrossRef] [PubMed]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef]
- Pal, A.; Paul, A. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49–64. [Google Scholar] [CrossRef]
- Martínez, F.L.; Orce, I.G.; Rajal, V.B.; Irazusta, V.P. Salar del Hombre Muerto, source of lithium-tolerant bacteria. Environ. Geochem. Health 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Moritz, R.; Kirschner, C.; Borchard, W.; Maibaum, R.; Wingender, J.; Flemming, H. The role of intermolecular interactions: Studies on model systems for bacterial biofilms. Int. Biodeterior. Biodegrad. 1999, 26, 3–16. [Google Scholar] [CrossRef]
- Centro de Información Minera de Argentina. Boratos. Panor. Merc. Rocas Miner. Ind. 2018. Available online: http://bit.ly/2SDslO9 (accessed on 9 February 2020).
- Zahran, H.H. Diversity, adaptation and activity of the bacterial flora in saline environments. Biol. Fertil. Soils 1997, 25, 211–223. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica (Cairo) 2012, 2012, 15. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Damodharan, K.; Yang, S.H.; Suh, J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of “Micro Tom” tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef]
- Srivastava, S.; Patel, J.S.; Singh, H.B.; Sinha, A.; Sarma, B.K. Streptomyces rochei SM3 induces stress tolerance in chickpea against Sclerotinia sclerotiorum and NaCl. J. Phytopathol. 2015, 163, 583–592. [Google Scholar] [CrossRef]
- Hasegawa, S.; Meguro, A.; Nishimura, T.; Kunoh, H. Drought tolerance of tissue-cultured seedlings of mountain laurel (Kalmia latifolia L.) induced by an endophytic actinomycete I. Enhancement of osmotic pressure in leaf cells. Actinomycetologica 2004, 18, 43–47. [Google Scholar] [CrossRef][Green Version]
- Hasegawa, S.; Meguro, A.; Toyoda, K.; Nishimura, T.; Kunoh, H. Drought tolerance of tissue-cultured seedlings of mountain laurel (Kalmia latifolia L.) induced by an endophytic actinomycete II. Acceleration of callose accumulation and lignification. Actinomycetologica 2005, 19, 13–17. [Google Scholar] [CrossRef][Green Version]
- Yandigeri, M.S.; Meena, K.K.; Singh, D.; Malviya, N.; Singh, D.P.; Solanki, M.K.; Yadav, A.K.; Arora, D.K. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012, 68, 411–420. [Google Scholar] [CrossRef]
- Selvakumar, G.; Bhatt, R.M.; Upreti, K.K.; Bindu, G.H.; Shweta, K. Citricoccus zhacaiensis B-4 (MTCC 12119) a novel osmotolerant plant growth promoting actinobacterium enhances onion (Allium cepa L.) seed germination under osmotic stress conditions. World J. Microbiol. Biotechnol. 2015, 31, 833–839. [Google Scholar] [CrossRef]
- Kaur, T.; Vasudev, A.; Sohal, S.K.; Manhas, R.K. Insecticidal and growth inhibitory potential of Streptomyces hydrogenans DH16 on major pest of India, Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). BMC Microbiol. 2014, 14, 227. [Google Scholar] [CrossRef]
- Gurney, K.A.; Mantle, P.G. Biosynthesis of 1-N-methylalbonoursin by an endophytic Streptomyces sp. isolated from perennial ryegrass. J. Nat. Prod. 1993, 56, 1194–1198. [Google Scholar] [CrossRef]
- Castillo, U.F.; Strobel, G.A.; Ford, E.J.; Hess, W.M.; Porter, H.; Jensen, J.B.; Albert, H.; Robison, R.; Condron, M.A.M.; Teplow, D.B.; et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscansa. Microbiology 2002, 148, 2675–2685. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Kumari, B.R.; Sathya, A.; Srinivas, V.; Abhishek, R.; Sharma, H.C.; Gopalakrishnan, S. Biological activity of entomopathogenic actinomycetes against lepidopteran insects (Noctuidae: Lepidoptera). Can. J. Plant Sci. 2014, 94, 759–769. [Google Scholar] [CrossRef]
- Saeed, E.E.; Sham, A.; Salmin, Z.; Abdelmowla, Y.; Iratni, R.; El-Tarabily, K.; AbuQamar, S. Streptomyces globosus UAE1, a potential effective biocontrol agent for black scorch disease in date palm plantations. Front. Microbiol. 2017, 8, 1455. [Google Scholar] [CrossRef]
- Loria, R.; Kers, J.; Joshi, M. Evolution of plant pathogenicity in Streptomyces. Annu. Rev. Phytopathol. 2006, 44, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Loria, R.; Bignell, D.R.D.; Moll, S.; Huguet-Tapia, J.C.; Joshi, M.V.; Johnson, E.G.; Seipke, R.F.; Gibson, D.M. Thaxtomin biosynthesis: The path to plant pathogenicity in the genus Streptomyces. Antonie Van Leeuwenhoek 2008, 94, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Díaz-Cruz, G.; Cheng, Z.; Bignell, D.R.D. Virulence mechanisms of plant-pathogenic Streptomyces species: An updated review. Microbiology 2019, 165, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Bignell, D.R.D.; Fyans, J.K.; Cheng, Z. Phytotoxins produced by plant pathogenic Streptomyces species. J. Appl. Microbiol. 2014, 116, 223–235. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; Sivasithamparam, K. Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol. Biochem. 2006, 38, 1505–1520. [Google Scholar] [CrossRef]
- Cohen, M.F.; Mazzola, M. Resident bacteria, nitric oxide emission and particle size modulate the effect of Brassica napus seed meal on disease incited by Rhizoctonia solani and Pythium spp. Plant Soil 2006, 286, 75–86. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Tabatabaei, B.E.S.; Venturi, V. Virulence attenuation of Pectobacterium carotovorum using N-Acyl-homoserine lactone degrading bacteria isolated from potato rhizosphere. Plant Pathol. J. 2011, 27, 242–248. [Google Scholar] [CrossRef]
- Cao, L.; Qiu, Z.; You, J.; Tan, H.; Zhou, S. Isolation and characterization of endophytic streptomycete antagonists of fusarium wilt pathogen from surface-sterilized banana roots. FEMS Microbiol. Lett. 2005, 247, 147–152. [Google Scholar] [CrossRef]
- Getha, K.; Vikineswary, S.; Wong, W.H.; Seki, T.; Ward, A.; Goodfellow, M. Evaluation of Streptomyces sp. strain g10 for suppression of Fusarium wilt and rhizosphere colonization in pot-grown banana plantlets. J. Ind. Microbiol. Biotechnol. 2005, 32, 24–32. [Google Scholar] [CrossRef]
- Taechowisan, T.; Peberdy, J.F.; Lumyong, S. Isolation of endophytic actinomycetes from selected plants and their antifungal activity. World J. Microbiol. Biotechnol. 2003, 19, 381–385. [Google Scholar] [CrossRef]
- Miyashita, K.; Fujii, T.; Sawada, Y. Molecular cloning and characterization of chitinase genes from Streptomyces lividans 66. Microbiology 1991, 137, 2065–2072. [Google Scholar] [CrossRef]
- Abd-Allah, E.F. Streptomyces plicatus as a model biocontrol agent. Folia Microbiol. (Praha) 2001, 46, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Joo, G.-J. Production of an anti-fungal substance for biological control of Phytophthora capsici causing phytophthora blight in red-peppers by Streptomyces halstedii. Biotechnol. Lett. 2005, 27, 201–205. [Google Scholar] [CrossRef]
- Mahadevan, B.; Crawford, D.L. Properties of the chitinase of the antifungal biocontrol agent Streptomyces lydicus WYEC108. Enzyme Microb. Technol. 1997, 20, 489–493. [Google Scholar] [CrossRef]
- Errakhi, R.; Bouteau, F.; Lebrihi, A.; Barakate, M. Evidences of biological control capacities of Streptomyces spp. against Sclerotium rolfsii responsible for damping-off disease in sugar beet (Beta vulgaris L.). World J. Microbiol. Biotechnol. 2007, 23, 1503–1509. [Google Scholar] [CrossRef]
- Trejo-Estrada, S.R.; Paszczynski, A.; Crawford, D.L. Antibiotics and enzymes produced by the biocontrol agent Streptomyces violaceusniger YCED-9. J. Ind. Microbiol. Biotechnol. 1998, 21, 81–90. [Google Scholar] [CrossRef]
- Barakate, M.; Ouhdouch, Y.; Oufdou, K.; Beaulieu, C. Characterization of rhizospheric soil streptomycetes from Moroccan habitats and their antimicrobial activities. World J. Microbiol. Biotechnol. 2002, 18, 49–54. [Google Scholar] [CrossRef]
- Emmert, E.A.; Handelsman, J. Biocontrol of plant disease: A (Gram-) positive perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Gopalakrishnan, S.; Sathya, A.; Kumar, M.V.; Srinivas, V.; Mamta, S. Streptomyces sp. as plant growth-promoters and host-plant resistance inducers against Botrytis cinerea in chickpea. Biocontrol Sci. Technol. 2018, 28, 1140–1163. [Google Scholar] [CrossRef]
- Vatsa-Portugal, P.; Aziz, A.; Rondeau, M.; Villaume, S.; Morjani, H.; Clément, C.; Ait Barka, E. How Streptomyces anulatus primes grapevine defenses to cope with gray mold: A study of the early responses of cell suspensions. Front. Plant Sci. 2017, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Baz, M.; Tran, D.; Kettani-Halabi, M.; Samri, S.E.; Jamjari, A.; Biligui, B.; Meimoun, P.; El-Maarouf-Bouteau, H.; Garmier, M.; Saindrenan, P.; et al. Calcium- and ROS-mediated defence responses in BY2 tobacco cells by nonpathogenic Streptomyces sp. J. Appl. Microbiol. 2012, 112, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, C.; Amenós, M.; Corrales, I.; Doncheva, S.; Barceló, J. Root behavior in response to aluminum toxicity. In Plant-Environment Interactions. Signaling and Communication in Plants; Baluška, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 21–43. ISBN 978-3-540-89230-4. [Google Scholar]
- Dahal, B.; NandaKafle, G.; Perkins, L.; Brözel, V.S. Diversity of free-Living nitrogen fixing Streptomyces in soils of the badlands of South Dakota. Microbiol. Res. 2017, 195, 31–39. [Google Scholar] [CrossRef] [PubMed]
- MacKellar, D.; Lieber, L.; Norman, J.S.; Bolger, A.; Tobin, C.; Murray, J.W.; Oksaksin, M.; Chang, R.L.; Ford, T.J.; Nguyen, P.Q.; et al. Streptomyces thermoautotrophicus does not fix nitrogen. Sci. Rep. 2016, 6, 20086. [Google Scholar] [CrossRef]
- Aly, M.M.; Tork, S.; Al-Garni, S.M.; Kabli, S.A. Production and characterization of phytase from Streptomyces luteogriseus R10 isolated from decaying wood samples. Int. J. Agric. Biol. 2015, 17, 515–522. [Google Scholar]
- Ghorbani-Nasrabadi, R.; Greiner, R.; Alikhani, H.A.; Hamedi, J. Identification and determination of extracellular phytate-degrading activity in actinomycetes. World J. Microbiol. Biotechnol. 2012, 28, 2601–2608. [Google Scholar] [CrossRef]
- Farhat, M.B.; Boukhris, I.; Chouayekh, H. Mineral phosphate solubilization by Streptomyces sp. CTM396 involves the excretion of gluconic acid and is stimulated by humic acids. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef]
- Jog, R.; Pandya, M.; Nareshkumar, G.; Rajkumar, S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology 2014, 160, 778–788. [Google Scholar] [CrossRef]
- Rashad, F.M.; Fathy, H.M.; El-Zayat, A.S.; Elghonaimy, A.M. Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments in Egypt. Microbiol. Res. 2015, 175, 34–47. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Positive role of 1-aminocyclopropane-1-carboxylate deaminase-producing endophytic Streptomyces sp. GMKU 336 on flooding resistance of mung bean. Agric. Nat. Resour. 2018, 52, 330–334. [Google Scholar] [CrossRef]
- Doaa, D.B.; El-Saeed, S.A. Assessment of the optimum conditions for production and purification of superoxide dismutase from Actinomycetes. Egypt. J. Exp. Biol. 2016, 12, 163–173. [Google Scholar]
- Tan, L.T.-H.; Chan, K.-G.; Khan, T.M.; Bukhari, S.I.; Saokaew, S.; Duangjai, A.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM212 as a source of antioxidants with radical scavenging and metal chelating properties. Front. Pharmacol. 2017, 8, 276. [Google Scholar] [CrossRef]
- Wang, Z.; Solanki, M.K.; Yu, Z.-X.; Yang, L.-T.; An, Q.-L.; Dong, D.-F.; Li, Y.-R. Draft genome analysis offers insights into the mechanism by which Streptomyces chartreusis WZS021 increases drought tolerance in sugarcane. Front. Microbiol. 2019, 9, 3262. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Copeland, J.; Wang, P.W.; Guttman, D.S.; Kotanen, P.M.; Johnson, M.T.J. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl. Acad. Sci. USA 2018, 115, E1157–E1165. [Google Scholar] [CrossRef]
- Chhabra, R. Soil Salinity and Water Quality; A A Balkema Publishers: Rotterdam, The Netherlands, 1996; ISBN 9789054107279. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Handbook for Saline Soil Management; Vargas, R., Pankova, E.I., Balyuk, S.A., Krasilnikov, P.V., Khasankhanova, G., Eds.; Food and Agriculture Organization of the United Nations and Lomonosov Moscow State University: Rome, Italy, 2018; ISBN 9789251301418. [Google Scholar]
- Taleisnik, E.; Lavado, R. Ambientes Salinos y Alcalinos de la Argentina: Recursos y Aprovechamiento Productivo; EUDEBA: Buenos Aires, Argentina, 2016. [Google Scholar]
- Sparks, D. The Chemistry of Saline and Sodic soils. In Environmental Soil Chemistry; Academic Press Inc.: San Diego, CA, USA, 2003; pp. 285–300. ISBN 9780126564464. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Plant Responses to Saline and Sodic Conditions. In Agricultural Salinity Assessment and Management; Wallender, W.W., Tanji, K.K., Eds.; ASCE: Reston, VA, USA, 2011; pp. 169–205. ISBN 9780784411698. [Google Scholar]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- López-Berenguer, C.; García-Viguera, C.; Carvajal, M. Are root hydraulic conductivity responses to salinity controlled by aquaporins in broccoli plants? Plant Soil 2006, 279, 13–23. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Ors, S.; Suarez, D.L. Spinach biomass yield and physiological response to interactive salinity and water stress. Agric. Water Manag. 2017, 190, 31–41. [Google Scholar] [CrossRef]
- Giuffrida, F.; Scuderi, D.; Giurato, R.; Leonardi, C. Physiological response of broccoli and cauliflower as affected by NaCl salinity. Acta Hortic. 2013, 1005, 435–442. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; Desutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Khan, M.S.A. Effects of salt and water stress on leaf production, sodium and potassium ion accumulation in soybean. J. Plant Sci. (Science Publ. Group) 2014, 2, 209. [Google Scholar] [CrossRef][Green Version]
- Queiroz, H.M.; Sodek, L.; Haddad, C.R.B. Effect of salt on the growth and metabolism of Glycine max. Brazilian Arch. Biol. Technol. 2012, 55, 809–817. [Google Scholar] [CrossRef]
- Ullah, A.; Li, M.; Noor, J.; Tariq, A.; Liu, Y.; Shi, L. Effects of salinity on photosynthetic traits, ion homeostasis and nitrogen metabolism in wild and cultivated soybean. PeerJ 2019, 7, e8191. [Google Scholar] [CrossRef]
- Sumner, M.E.; Naidu, R. Sodic Soils: Distribution, Properties, Management and Environmental Consequences; Oxford University Press Inc.: New York, NY, USA, 1998; ISBN 9780195096552. [Google Scholar]
- Gambrell, R.; Wiesepape, J.B.; Patrick, W.H., Jr.; Duff, M.C. The effects of pH, redox, and salinity on metal release from a contaminated sediment. Water Air Soil Pollut. 1991, 57–58, 359–367. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Tiller, K.G. Chloro-complexation of cadmium in soil solutions of saline-sodic soils increases phyto-availability of cadmium. Trans. 15th World Congr. Soil Sci. 1994, 3b, 195–196. [Google Scholar]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 2011, 4, 2669–2681. [Google Scholar] [CrossRef]
- Richards, L. Diagnosis and Improvement of Saline Alkali Soils; Laboratory, U.S., Ed.; Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Keren, R.; Myamoto, S. Reclamation of saline, sodic and boron-affected soils. Agric. Salin. Assess. Manag. 1990, 71, 410–431. [Google Scholar]
- Zaman, M.; Shahid, S.A.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing AG: Cham, Switzerland, 2018; p. 17. ISBN 9783319961903. [Google Scholar]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- FAO/IIASA/ISRIC/ISS-CAS/JRC. Harmonized World Soil Database (version 1.1); Food and Agriculture Organization of the United Nations, FAO: Rome, Italy; IIASA: Laxenburg, Austria, 2009. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Advances in the Assessment and Mmonitoring of Salinization and Status of Biosaline Agriculture; FAO: Rome, Italy, 2010; ISBN 9789251064399. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT. Stat. Div. UN Food Agric. Organ. 2016. Available online: http://www.fao.org/faostat/en/ (accessed on 14 December 2019).
- Ladeiro, B. Saline agriculture in the 21st century: Using salt contaminated resources to cope food requirements. J. Bot. 2012, 2012, 310705. [Google Scholar] [CrossRef]
- Keren, R. Salt-affected Soils, Reclamation. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Academic Press Inc.: San Diego, CA, USA, 2005; pp. 454–461. ISBN 9780123485304. [Google Scholar]
- Bradshaw, A.D. Ecological principles and land reclamation practice. Landsc. Plan. 1984, 11, 35–48. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abrol, I.P. Salt-Affected Soils: Their Reclamation and Management for Crop Production. In Advances in Soil Science; Lal, R., Ed.; Springer: New York, NY, USA, 1990; pp. 224–261. [Google Scholar]
- Pollmann, K.; Kutschke, S.; Matys, S.; Raff, J.; Hlawacek, G.; Lederer, F.L. Bio-recycling of metals: Recycling of technical products using biological applications. Biotechnol. Adv. 2018, 36, 1048–1062. [Google Scholar] [CrossRef]
- Arora, S.; Vanza, M.J.; Mehta, R.; Bhuva, C.; Patel, P.N. Halophilic microbes for bio-remediation of salt affected soils. African J. Microbiol. Res. 2014, 8, 3070–3078. [Google Scholar]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Roberson, E.B.; Firestone, M.K. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Jeschke, W.D.; Wolf, O. Effect of NaCI salinity on growth, development, ion distribution, and ion translocation in castor bean (Ricinus communis L.). J. Plant Physiol. 1988, 132, 45–53. [Google Scholar] [CrossRef]
- Oren, A. Industrial and environmental applications of halophilic microorganisms. Environ. Technol. 2010, 31, 825–834. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-p45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Arora, S.; Vanza, M. Microbial Approach for Bioremediation of Saline and Sodic Soils. In Bioremediation of Salt Affected Soils: An Indian Perspective; Arora, S., Singh, A.K., Singh, Y.P., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 87–100. ISBN 9783319482569. [Google Scholar]
- Ventosa, A.; Márquez, M.C.; Garabito, M.J.; Arahal, D.R. Moderately halophilic gram-positive bacterial diversity in hypersaline environments. Extremophiles 1998, 2, 297–304. [Google Scholar] [CrossRef]
- Oren, A. Diversity of halophilic microorganisms : Environments, phylogeny, physiology, and applications. J. Ind. Microbiol. Biotechnol. 2002, 28, 56–63. [Google Scholar] [CrossRef]
- Vasavada, S.H.; Thumar, J.T.; Singh, S.P. Secretion of a potent antibiotic by salt-tolerant and alkaliphilic actinomycete Streptomyces sannanensis strain RJT-1. Curr. Sci. 2006, 91, 1393–1397. [Google Scholar]
- Kannabiran, K.; Deepika Lakshmipathy, T.; Arun Prasad, A.; Kannabiran, K. Production of biosurfactant and heavy metal resistance activity of Streptomyces Sp. VITDDK3-a novel halo tolerant actinomycetes isolated from Saltpan soil. Adv. Biol. Res. (Rennes) 2010, 4, 108–115. [Google Scholar]
- Margesin, M.R. Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl. Microbiol. Biotechnol. 2001, 56, 650–663. [Google Scholar] [CrossRef]
- Bursy, J.; Kuhlmann, A.U.; Pittelkow, M.; Hartmann, H.; Jebbar, M.; Pierik, A.J.; Bremer, E. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl. Environ. Microbiol. 2008, 74, 7286–7296. [Google Scholar] [CrossRef]
- Empadinhas, N.; Da Costa, M.S. Osmoadaptation mechanisms in prokaryotes: Distribution of compatible solutes. Int. Microbiol. 2008, 11, 151–161. [Google Scholar]
- Killham, K.; Firestone, M.K. Salt stress control of intracellular solutes in streptomycetes indigenous to saline soils. Appl. Environ. Microbiol. 1984, 47, 301–306. [Google Scholar] [CrossRef]
- Kol, S.; Elena Merlo, M.; Scheltema, R.A.; De Vries, M.; Vonk, R.J.; Kikkert, N.A.; Dijkhuizen, L.; Breitling, R.; Takano, E. Metabolomic characterization of the salt stress response in Streptomyces coelicolor. Appl. Environ. Microbiol. 2010, 76, 2574–2581. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Miyazaki, A.; Takahashi, T.; Michael, A.; Kusano, T. The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett. 2006, 580, 6783–6788. [Google Scholar] [CrossRef]
- Sadeghi, A.; Soltani, B.M.; Jouzani, G.S.; Karimi, E.; Nekouei, M.K.; Sadeghizadeh, M. Taxonomic study of a salt tolerant Streptomyces sp. strain C-2012 and the effect of salt and ectoine on lon expression level. Microbiol. Res. 2014, 169, 232–238. [Google Scholar] [CrossRef]
- Sobczyk, A.; Bellier, A.; Viala, J.; Mazodier, P. The Ion gene, encoding an ATP-dependent protease, is a novel member of the HAIR/HspR stress-response regulon in actinomycetes. Microbiology 2002, 148, 1931–1937. [Google Scholar] [CrossRef]
- Thumar, J.T.; Singh, S.P. Organic solvent tolerance of an alkaline protease from salt-tolerant alkaliphilic Streptomyces clavuligerus strain Mit-1. J. Ind. Microbiol. Biotechnol. 2009, 36, 211–218. [Google Scholar] [CrossRef]
- Vicente, M.F.; Gorrochategui, J.; Cabello, A.; Gonza, A.; Genilloud, O.; Basilio, A.; Gonza, I. Patterns of antimicrobial activities from soil actinomycetes isolated under different conditions of pH and salinity. J. Appl. Microbiol. 2003, 95, 814–823. [Google Scholar]
- Sajid, I.; Blandine, Æ.C.; Fondja, F. Antifungal and antibacterial activities of indigenous Streptomyces isolates from saline farmlands : Prescreening, ribotyping and metabolic diversity. World J. Microbiol. Biotechnol. 2009, 25, 601–610. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Pande, S.; Sharma, M.; Humayun, P.; Kiran, B.K.; Sandeep, D.; Vidya, M.S.; Deepthi, K.; Rupela, O. Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 2011, 30, 1070–1078. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2008, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, Y. Characterization of nitrogen-fixing moderate halophilic cyanobacteria isolated from saline soils of Songnen Plain in China. Prog. Natural Sci. 2008, 18, 769–773. [Google Scholar] [CrossRef]
- Colwell, R.R. Microbial diversity: The importance of exploration and conservation. J. Ind. Microbiol. Biotechnol. 1997, 18, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W. Host specificity in microbe-microbe interactions: Biological control agents vary in specificity for hosts, pathogen control, ecological habitat, and environmental conditions. Bioscience 1996, 46, 406–409. [Google Scholar] [CrossRef]
- Gardner, J.M.; Chandler, J.L.; Feldman, A.W. Growth promotion and inhibition by antibiotic-producing fluorescent pseudomonads on citrus roots. Plant Soil 1984, 77, 103–113. [Google Scholar] [CrossRef]
- O’Neill, G.A.; Radley, R.A.; Chanway, C.P. Variable effects of emergence-promoting rhizobacteria on conifer seedling growth under nursery conditions. Biol. Fertil. Soils 1992, 13, 45–49. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T. Influence of salinity on the biological and biochemical activity of a calciorthird soil. Plant Soil 1996, 178, 255–263. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Mao, X.; Li, R. Biodegradation of phenanthrene by a halophilic bacterial consortium under aerobic conditions. Curr. Microbiol. 2009, 58, 205–210. [Google Scholar] [CrossRef]
- Amoozegar, M.A.; Hamedi, J.; Dadashipour, M.; Shariatpanahi, S. Effect of salinity on the tolerance to toxic metals and oxyanions in native moderately halophilic spore-forming bacilli. World J. Microbiol. Biotechnol. 2005, 21, 1237–1243. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.A.; Alvarez-Bernal, D.; Ramos-Valdivia, A.C.; Dendooven, L. Bioremediation of polycyclic aromatic hydrocarbon-contaminated saline-alkaline soils of the former Lake Texcoco. Chemosphere 2006, 62, 1749–1760. [Google Scholar] [CrossRef]
- Ngwenya, B.T. Bacterial Mineralization. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128035818. Available online: http://bit.ly/2OGxVxJ (accessed on 14 December 2019).
- Theng, B.K.; Yuan, G. Nanoparticles in the soil environment. Elements 2008, 4, 395–399. [Google Scholar] [CrossRef]
- Prozorov, T.; Bazylinski, D.A.; Mallapragada, S.K.; Prozorov, R. Novel magnetic nanomaterials inspired by magnetotactic bacteria: Topical review. Mater. Sci. Eng. R Reports 2013, 74, 133–172. [Google Scholar] [CrossRef]
- Hennebel, T.; De Gusseme, B.; Boon, N.; Verstraete, W. Biogenic metals in advanced water treatment. Trends Biotechnol. 2009, 27, 90–98. [Google Scholar] [CrossRef]
| Potential | Species | Characteristics/Purposes |
|---|---|---|
| Bioremediation | ||
| Streptomyces albogriseolus 053 HQ538724.1 and S. lincolnensis 128 HQ538726.1 | Formation of boron minerals by the cells [6] | |
| Streptomyces sp. DPUA1566 | Production of a new biosurfactant lipoprotein for use in agro-industrial waste [53] | |
| Streptomyces sp. Hlh1 | Degradation of petroleum compounds in contaminated soils [54] | |
| Streptomyces sp. strain M7 | Possible lindane degradation [55] | |
| Streptomyces antioxidans MUSC164T | Remediation of soils chronically contaminated with hydrocarbons [56] | |
| Plant Growth Promotion | ||
| Streptomyces T5 | Increase of superoxide dismutase, catalase and phenol peroxidase activities in nodules of cowpea plants exposed to salt stress [57] | |
| Streptomyces sp. GMKU 336 | Increase of salt-stress resistance of Oryza sativa L. cv. KDML105 [58] | |
| Streptomyces spp. | Increase of salt tolerance of Stevia [59] | |
| Streptomyces coelicolor (Sc1) and Streptomyces ambofaciens (Sc2) | Colonization of roots during drought to improve plant growth [60] | |
| Region | Population (Millions) | Land Area with Irrigation a (thousand ha) | Arable Land b (thousand ha) | Permanent Crops c (thousand ha) | Salt-Affected Land d (thousand ha) |
|---|---|---|---|---|---|
| World | 7043 | 324,548 | 1,395,490 | 162,100 | 971 |
| Africa | 1077 | 15,265 | 230,862 | 33,571 | 295 |
| North America | 346 | 27,730 | 194,640 | 7526 | 63 |
| Central America | 163 | 7306 | 28,195 | 5178 | 4 |
| South America | 400 | 15,880 | 133,326 | 14,199 | 57 |
| Asia | 4240 | 228,667 | 480,140 | 83,495 | 291 |
| Europe | 738 | 25,414 | 274,151 | 15,211 | 2 |
| Oceania | 37 | 3261 | 48,702 | 1603 | 144 |
| Former USSR | 117 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano-Armada, N.; Yañez-Yazlle, M.F.; Irazusta, V.P.; Rajal, V.B.; Moraga, N.B. Potential of Bioremediation and PGP Traits in Streptomyces as Strategies for Bio-Reclamation of Salt-Affected Soils for Agriculture. Pathogens 2020, 9, 117. https://doi.org/10.3390/pathogens9020117
Romano-Armada N, Yañez-Yazlle MF, Irazusta VP, Rajal VB, Moraga NB. Potential of Bioremediation and PGP Traits in Streptomyces as Strategies for Bio-Reclamation of Salt-Affected Soils for Agriculture. Pathogens. 2020; 9(2):117. https://doi.org/10.3390/pathogens9020117
Chicago/Turabian StyleRomano-Armada, Neli, María Florencia Yañez-Yazlle, Verónica P. Irazusta, Verónica B. Rajal, and Norma B. Moraga. 2020. "Potential of Bioremediation and PGP Traits in Streptomyces as Strategies for Bio-Reclamation of Salt-Affected Soils for Agriculture" Pathogens 9, no. 2: 117. https://doi.org/10.3390/pathogens9020117
APA StyleRomano-Armada, N., Yañez-Yazlle, M. F., Irazusta, V. P., Rajal, V. B., & Moraga, N. B. (2020). Potential of Bioremediation and PGP Traits in Streptomyces as Strategies for Bio-Reclamation of Salt-Affected Soils for Agriculture. Pathogens, 9(2), 117. https://doi.org/10.3390/pathogens9020117





