Biocontrol of Cereal Crop Diseases Using Streptomycetes
Abstract
1. Introduction
2. Plant-Microbe Interactions and Their Effect on Plant Health
3. Streptomyces—Plant Interactions
3.1. Streptomyces in Disease Suppressive Soils
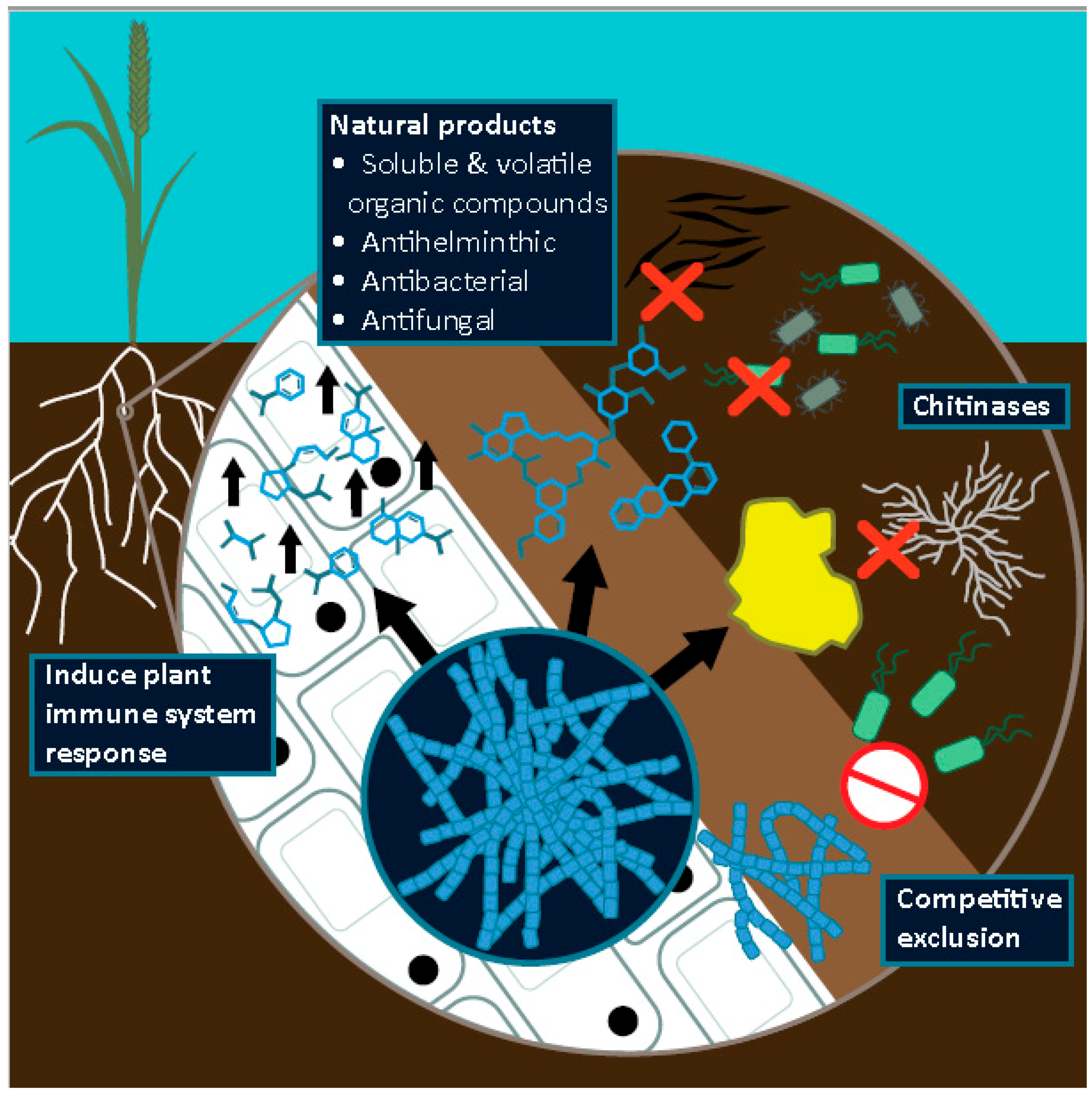
3.2. Antimicrobials against Phytopathogens of Cereal Crops
3.3. Enzymatic Control of Phytopathogens: Chitinases
3.4. Direct Inhibition by Volatile Organic Compounds
3.5. Antihelmintic Compounds
3.6. Indirect Inhibition of Phytopathogens of Cereal Crops
4. The Potential of Streptomyces Bacteria as Efficient Biocontrol Agents
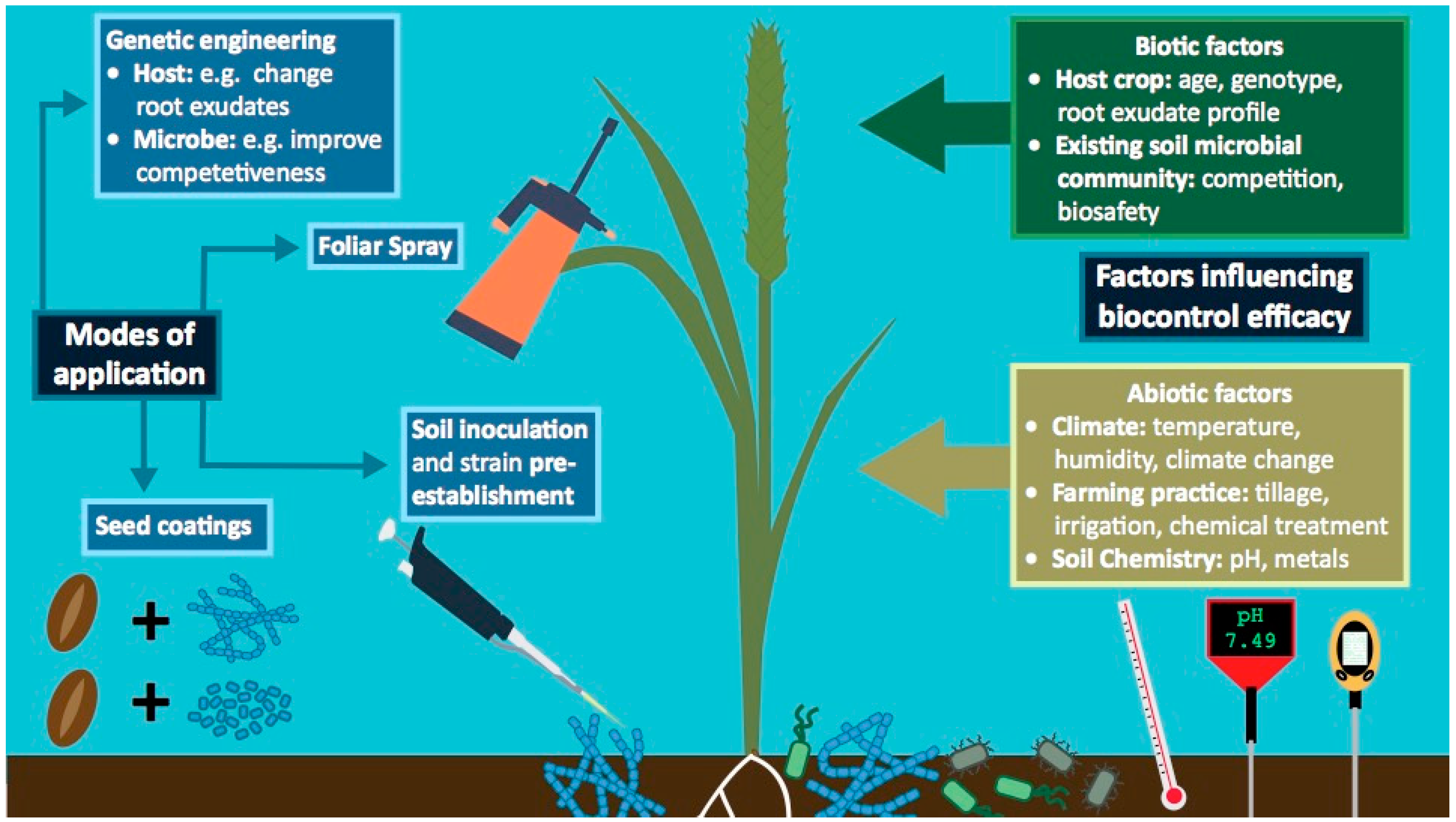
4.1. Abiotic Factors Influencing Biocontrol Efficacy
4.2. Optimising Biocontrol Delivery Systems Involving Streptomyces
4.3. Exploiting Plant Recruitment Mechanisms to Improve Biocontrol Agents
4.4. The Biosafety of Streptomycete-Based Biocontrol Agents
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- McKevith, B. Nutritional aspects of cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Crop Prospects and Food Situation, March 2019; FAO: Rome, Italy, 2019. [Google Scholar]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Beddington, J. Food security: Contributions from science to a new and greener revolution. Philos. Trans. R. Soc. Lond B Biol. Sci. 2010, 365, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.-N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- McDonald, B.A.; Stukenbrock, E.H. Rapid emergence of pathogens in agro-ecosystems: Global threats to agricultural sustainability and food security. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Doehlemann, G.; Okmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Leonard, K.J.; Szabo, L.J. Stem rust of small grains and grasses caused by Puccinia graminis. Mol. Plant Pathol. 2005, 6, 99–111. [Google Scholar] [CrossRef]
- Nalley, L.; Tsiboe, F.; Durand-Morat, A.; Shew, A.; Thoma, G. Economic and Environmental Impact of Rice Blast Pathogen (Magnaporthe oryzae); Alleviation in the United States. PLoS ONE 2016, 11, e0167295. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.J. Take-all of wheat. Physiol. Mol. Plant Pathol. 2003, 62, 73–86. [Google Scholar] [CrossRef]
- Kwak, Y.S.; Weller, D.M. Take-all of Wheat and Natural Disease Suppression: A Review. Plant Pathol. J. 2013, 29, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.T.; Michelsen, P.P.; Franco, C.M.M. Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol. Control 2004, 29, 359–366. [Google Scholar] [CrossRef]
- Hernandez-Restrepo, M.; Groenewald, J.Z.; Elliott, M.L.; Canning, G.; McMillan, V.E.; Crous, P.W. Take-all or nothing. Stud. Mycol. 2016, 83, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.; Ser, H.L.; Khan, T.M.; Chuah, L.H.; Pusparajah, P.; Chan, K.G.; Goh, B.H.; Lee, L.H. The potential of Streptomyces as biocontrol Agents against the Rice Blast Fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; McLaughlin, L.; Zepp, A.; Lakitan, B.; Kraus, T.; Kleinman, P.; Vancini, F.; Roach, W.J.; Graap, E.; Keeton, W.S.; et al. Environmental and economic effects of reducing pesticide use in agriculture. Agric. Ecosyst. Environ. 1993, 46, 273–288. [Google Scholar] [CrossRef]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a plant’s best friend? FEMS Microbiol. Ecol. 2016, 92, 119. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Hjelmso, M.H. Agricultural soils, pesticides and microbial diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. Camb. Philos. Soc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chellemi, D.O.; Gamliel, A.; Katan, J.; Subbarao, K.V. Development and Deployment of Systems-Based Approaches for the Management of Soilborne Plant Pathogens. Phytopathology 2016, 106, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.; Rutkoski, J. Advances and Challenges in Genomic Selection for Disease Resistance. Annu. Rev. Phytopathol. 2016, 54, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Goutam, U.; Kukreja, S.; Yadav, R.; Salaria, N.; Thakur, K.; Goyal, A.K. Recent trends and perspectives of molecular markers against fungal diseases in wheat. Front. Microbiol. 2015, 6, 861. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G.; Lagudah, E.S.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 2014, 5, 641. [Google Scholar] [CrossRef] [PubMed]
- Hiltner, L. Uber neure Erfahrungen und probleme auf dem gebeit der bodenback- teriologie und unter besonderer berucksichtigung der grundungung und brache. Deut. Landwirsch Ges. 1904, 98, 59–78. [Google Scholar]
- Gaiero, J.R.; McCall, C.A.; Thompson, K.A.; Day, N.J.; Best, A.S.; Dunfield, K.E. Inside the root microbiome: Bacterial root endophytes and plant growth promotion. Am. J. Bot. 2013, 100, 1738–1750. [Google Scholar] [CrossRef]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the plant microbiome: Looking back and future perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Garrido-Oter, R.; Munch, P.C.; Weiman, A.; Droge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Del Rio, T.G. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.E.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner Plant Values: Diversity, Colonization and Benefits from Endophytic Bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease Suppressive Soils: New Insights from the Soil Microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.A. Streptomyces in Nature and Medicine: The Antibiotic Makers; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Chater, K.F. Streptomyces inside-out: A new perspective on the bacteria that provide us with antibiotics. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Chater, K.F. Recent advances in understanding Streptomyces. F1000 Res. 2016, 5, 2795. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, A.; Worsley, S.F.; Hutchings, M.I.; van Wezel, G.P. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol. Rev. 2017, 41, 392–416. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Schrey, S.D.; Tarkka, M.T. Friends and foes: Streptomycetes as modulators of plant disease and symbiosis. Antonie Van Leeuwenhoek 2008, 94, 11–19. [Google Scholar] [CrossRef]
- Rey, T.; Dumas, B. Plenty Is No Plague: Streptomyces Symbiosis with Crops. Trends Plant Sci. 2017, 22, 30–37. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS ONE 2013, 8, e56329. [Google Scholar] [CrossRef] [PubMed]
- Weinert, N.; Piceno, Y.; Ding, G.-C.; Meincke, R.; Heuer, H.; Berg, G.; Schloter, M.; Andersen, G.; Smalla, K. PhyloChip hybridization uncovered an enormous bacterial diversity in the rhizosphere of different potato cultivars: Many common and few cultivar-dependent taxa. FEMS Microbiol. Ecol. 2011, 75, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Johnson, C.; Santos-Medellin, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Effects of jasmonic acid signalling on the wheat microbiome differ between body sites. Sci. Rep. 2017, 7, 41766. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.T.; Franco, C.M. Visualization of an endophytic Streptomyces species in wheat seed. Appl. Environ. Microbiol. 2003, 69, 4260–4262. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Bonaldi, M.; Chen, X.; Kunova, A.; Pizzatti, C.; Saracchi, M.; Cortesi, P. Colonization of lettuce rhizosphere and roots by tagged Streptomyces. Front. Microbiol. 2015, 6, 25. [Google Scholar] [CrossRef]
- Chen, X.; Pizzatti, C.; Bonaldi, M.; Saracchi, M.; Erlacher, A.; Kunova, A.; Berg, G.; Cortesi, P. Biological Control of Lettuce Drop and Host Plant Colonization by Rhizospheric and Endophytic Streptomycetes. Front. Microbiol. 2016, 7, 714. [Google Scholar] [CrossRef]
- Tokala, R.K.; Strap, J.L.; Jung, C.M.; Crawford, D.L.; Salove, M.H.; Deobald, L.A.; Bailey, J.F.; Morra, M. Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl. Environ. Microbiol. 2002, 68, 2161–2171. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Damodharan, K.; Yang, S.H.; Suh, J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef]
- Chaurasia, A.; Meena, B.R.; Tripathi, A.N.; Pandey, K.K.; Rai, A.B.; Singh, B. Actinomycetes: An unexplored microorganisms for plant growth promotion and biocontrol in vegetable crops. World J. Microbiol. Biotechnol. 2018, 34, 132. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Madaan, S.; Archana, G. Antibiotic producing endophytic Streptomyces spp. colonize above-ground plant parts and promote shoot growth in multiple healthy and pathogen-challenged cereal crops. Microbiol. Res. 2018, 215, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, Y.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Jiang, D.; Hsiang, T. Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol. Control 2011, 58, 139–148. [Google Scholar] [CrossRef]
- Tian, X.L.; Cao, L.X.; Tan, H.M.; Zeng, Q.G.; Jia, Y.Y.; Han, W.Q.; Zhou, S.N. Study on the communities of endophytic fungi and endophytic actinomycetes from rice and their antipathogenic activities in vitro. World J. Microbiol. Biotechnol. 2004, 20, 303–309. [Google Scholar] [CrossRef]
- Jung, B.; Park, S.; Lee, Y.; Lee, J. Biological Efficacy of Streptomyces sp. Strain BN1 Against the Cereal Head Blight Pathogen Fusarium graminearum. Plant Pathol. J. 2013, 29, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Han, S.; Hong, H.J.; Cho, H.; Kim, D.; Kwon, Y.; Kwon, S.K.; Crusemann, M.; Bok Lee, Y.; Kim, J.F.; et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Yekkour, A.; Sabaou, N.; Zitouni, A.; Errakhi, R.; Mathieu, F.; Lebrihi, A. Characterization and antagonistic properties of Streptomyces strains isolated from Saharan soils, and evaluation of their ability to control seedling blight of barley caused by Fusarium culmorum. Lett. Appl. Microbiol. 2012, 55, 427–435. [Google Scholar] [CrossRef]
- LahdenperÄ, M.L.; Simon, E.; Uoti, J. Mycostop-A Novel Biofungicide Based on Streptomyces Bacteria. In Developments in Agricultural and Managed Forest Ecology; Beemster, A.B.R., Bollen, G.J., Gerlagh, M., Ruissen, M.A., Schippers, B., Tempel, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 23, pp. 258–263. [Google Scholar]
- Adesina, M.F.; Lembke, A.; Costa, R.; Speksnijder, A.; Smalla, K. Screening of bacterial isolates from various European soils for in vitro antagonistic activity towards Rhizoctonia solani and Fusarium oxysporum: Site-dependent composition and diversity revealed. Soil Biol. Biochem. 2007, 39, 2818–2828. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef]
- Bressan, W. Biological control of maize seed pathogenic fungi by use of actinomycetes. Biol. Control 2003, 48, 233–240. [Google Scholar] [CrossRef]
- Sahli, A.A.A. Biocontrol of Fusarium udum diseases for some wheat cultivars by Streptomyces spororaveus. Afr. J. Microbiol. Res. 2012, 6. [Google Scholar] [CrossRef]
- Wu, Z.M.; Yang, Y.; Li, K.T. Antagonistic activity of a novel antifungalmycin N2 from Streptomyces sp. N2 and its biocontrol efficacy against Rhizoctonia solani. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.M.; Crawford, D.L. Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl. Environ. Microbiol. 1995, 61, 3119–3128. [Google Scholar] [PubMed]
- Wan, M.; Li, G.; Zhang, J.; Jiang, D.; Huang, H.-C. Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biol. Control 2008, 46, 552–559. [Google Scholar] [CrossRef]
- Xu, B.; Chen, W.; Wu, Z.-m.; Long, Y.; Li, K.-t. A Novel and Effective Streptomyces sp. N2 Against Various Phytopathogenic Fungi. Appl. Biochem. Biotechnol. 2015, 177, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, B.; Crawford, D.L. Properties of the chitinase of the antifungal biocontrol agent Streptomyces lydicus WYEC108. Enzym. Microb. Technol. 1997, 20, 489–493. [Google Scholar] [CrossRef]
- Bignell, D.R.; Seipke, R.F.; Huguet-Tapia, J.C.; Chambers, A.H.; Parry, R.J.; Loria, R. Streptomyces scabies 87–22 contains a coronafacic acid-like biosynthetic cluster that contributes to plant-microbe interactions. Mol. Plant Microbe Interact. 2010, 23, 161–175. [Google Scholar] [CrossRef]
- Fyans, J.K.; Altowairish, M.S.; Li, Y.; Bignell, D.R. Characterization of the Coronatine-Like Phytotoxins Produced by the Common Scab Pathogen Streptomyces scabies. Mol. Plant Microbe Interact. 2015, 28, 443–454. [Google Scholar] [CrossRef]
- Fyans, J.K.; Bown, L.; Bignell, D.R. Isolation and characterization of plant-pathogenic Streptomyces species associated with common scab-infected potato tubers in Newfoundland. Phytopathology 2016, 106, 123–131. [Google Scholar] [CrossRef]
- Kers, J.A.; Cameron, K.D.; Joshi, M.V.; Bukhalid, R.A.; Morello, J.E.; Wach, M.J.; Gibson, D.M.; Loria, R. A large, mobile pathogenicity island confers plant pathogenicity on Streptomyces species. Mol. Microbiol. 2005, 55, 1025–1033. [Google Scholar] [CrossRef]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as symbionts: An emerging and widespread theme? Fems Microbiol. Rev. 2012, 36, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, E.; Mendes, R.; Bakker, P.A.; Raaijmakers, J.M. Fungal invasion of the rhizosphere microbiome. ISME J. 2016, 10, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, P.; Ward, J.; Barbella, A.; Solares, N.; Izyumin, D.; Burman, P.; Chellemi, D.O.; Subbarao, K.V. Soil Microbiomes Associated with Verticillium Wilt-Suppressive Broccoli and Chitin Amendments are Enriched with Potential Biocontrol Agents. Phytopathology 2018, 108, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Kinkel, L.L.; Schlatter, D.C.; Bakker, M.G.; Arenz, B.E. Streptomyces competition and co-evolution in relation to plant disease suppression. Res. Microbiol. 2012, 163, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Tahvonen, R.k. The suppressiveness of Finnish light coloured Sphagnum peat. J. Sci. Agric. Soc. Finl. 1982, 54, 345–356. [Google Scholar] [CrossRef][Green Version]
- Chater, K.F.; Biro, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Schrempf, H. Recognition and degradation of chitin by Streptomycetes. Antonie Van Leeuwenhoek 2001, 79, 285–289. [Google Scholar] [CrossRef]
- Hoster, F.; Schmitz, J.E.; Daniel, R. Enrichment of chitinolytic microorganisms: Isolation and characterization of a chitinase exhibiting antifungal activity against phytopathogenic fungi from a novel Streptomyces strain. Appl. Microbiol. Biotechnol. 2005, 66, 434–442. [Google Scholar] [CrossRef]
- Quecine, M.C.; Araujo, W.L.; Marcon, J.; Gai, C.S.; Azevedo, J.L.; Pizzirani-Kleiner, A.A. Chitinolytic activity of endophytic Streptomyces and potential for biocontrol. Lett. Appl. Microbiol. 2008, 47, 486–491. [Google Scholar] [CrossRef]
- Gomes, R.C.; Semêdo, L.T.A.S.; Soares, R.M.A.; Alviano, C.S.; And, L.F.L.; Coelho, R.R.R. Chitinolytic activity of actinomycetes from a cerrado soil and their potential in biocontrol. Lett. Appl. Microbiol. 2000, 30, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Joo, G.J. Purification and characterization of an extracellular chitinase from the antifungal biocontrol agent Streptomyces halstedii. Biotechnol. Lett. 2005, 27, 1483–1486. [Google Scholar] [CrossRef]
- Itoh, Y.; Takahashi, K.; Takizawa, H.; Nikaidou, N.; Tanaka, H.; Nishihashi, H.; Watanabe, T.; Nishizawa, Y. Family 19 chitinase of Streptomyces griseus HUT6037 increases plant resistance to the fungal disease. Biosci. Biotechnol. Biochem. 2003, 67, 847–855. [Google Scholar] [CrossRef]
- Cordovez, V.; Carrion, V.J.; Etalo, D.W.; Mumm, R.; Zhu, H.; van Wezel, G.P.; Raaijmakers, J.M. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front. Microbiol. 2015, 6, 1081. [Google Scholar] [CrossRef]
- Wheatley, R.E. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 2002, 81, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef]
- Burg, R.W.; Miller, B.M.; Baker, E.E.; Birnbaum, J.; Currie, S.A.; Hartman, R.; Kong, Y.L.; Monaghan, R.L.; Olson, G.; Putter, I.; et al. Avermectins, new family of potent anthelmintic agents: Producing organism and fermentation. Antimicrob. Agents Chemother. 1979, 15, 361–367. [Google Scholar] [CrossRef]
- Huang, W.K.; Sun, J.H.; Cui, J.K.; Wang, G.F.; Kong, L.A.; Peng, H.; Chen, S.L.; Peng, D.L. Efficacy evaluation of fungus Syncephalastrum racemosum and nematicide avermectin against the root-knot nematode Meloidogyne incognita on cucumber. PLoS ONE 2014, 9, e89717. [Google Scholar] [CrossRef]
- Kumar, M.; Gantasala, N.P.; Roychowdhury, T.; Thakur, P.K.; Banakar, P.; Shukla, R.N.; Jones, M.G.; Rao, U. De novo transcriptome sequencing and analysis of the cereal cyst nematode, Heterodera avenae. PLoS ONE 2014, 9, e96311. [Google Scholar] [CrossRef]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Nijs, L.d.; Hockland, S.; Maafi, Z.T. Current Nematode Threats to World Agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar]
- Nour, S.M.; Lawrence, J.R.; Zhu, H.; Swerhone, G.D.; Welsh, M.; Welacky, T.W.; Topp, E. Bacteria associated with cysts of the soybean cyst nematode (Heterodera glycines). Appl. Environ. Microbiol. 2003, 69, 607–615. [Google Scholar] [CrossRef]
- Samac, D.A.; Kinkel, L.L. Suppression of the root-lesion nematode (Pratylenchus penetrans) in alfalfa (Medicago sativa) by Streptomyces spp. Plant Soil 2001, 235, 35–44. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.M.; Li, Y.H.; Ding, S.L.; Yuan, H.X.; Riley, I.T.; Li, H.L. Biocontrol of cereal cyst nematode by Streptomyces anulatus isolate S07. Australas. Plant Pathol. 2016, 45, 57–64. [Google Scholar] [CrossRef]
- Archetti, M.; Ubeda, F.; Fudenberg, D.; Green, J.; Pierce, N.E.; Yu, D.W. Let the right one in: A microeconomic approach to partner choice in mutualisms. Am. Nat. 2011, 177, 75–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Van Wees, S.C.; Van der Ent, S.; Pieterse, C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef]
- Kurth, F.; Mailander, S.; Bonn, M.; Feldhahn, L.; Herrmann, S.; Grosse, I.; Buscot, F.; Schrey, S.D.; Tarkka, M.T. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol. Plant Microbe Interact. 2014, 27, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Lehr, N.A.; Schrey, S.D.; Hampp, R.; Tarkka, M.T. Root inoculation with a forest soil streptomycete leads to locally and systemically increased resistance against phytopathogens in Norway spruce. New Phytol. 2008, 177, 965–976. [Google Scholar] [CrossRef]
- Conn, V.M.; Walker, A.R.; Franco, C.M. Endophytic Actinobacteria induce defense pathways in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008, 21, 208–218. [Google Scholar] [CrossRef]
- Tarkka, M.T.; Lehr, N.A.; Hampp, R.; Schrey, S.D. Plant behavior upon contact with Streptomycetes. Plant Signal. Behav. 2008, 3, 917–919. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Verhagen, B.W.; Glazebrook, J.; Zhu, T.; Chang, H.S.; van Loon, L.C.; Pieterse, C.M. The transcriptome of rhizobacteria-induced systemic resistance in arabidopsis. Mol. Plant Microbe Interact. 2004, 17, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Kinkel, L.L.; Samac, D.A. Biological Control of Phytophthora Root Rots on Alfalfa and Soybean with Streptomyces. Biol. Control 2002, 23, 285–295. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Himmelstein, J.C.; Maul, J.E.; Everts, K.L. Impact of Five Cover Crop Green Manures and Actinovate on Fusarium Wilt of Watermelon. Plant Dis. 2014, 98, 965–972. [Google Scholar] [CrossRef]
- Sabaratnam, S.; Traquair, J.A. Mechanism of antagonism by Streptomyces griseocarneus (strain Di944) against fungal pathogens of greenhouse-grown tomato transplants. Can. J. Plant Pathol. 2015, 37, 197–211. [Google Scholar] [CrossRef]
- Zhang, S.; Vallad, G.E.; White, T.L.; Huang, C.-H. Evaluation of Microbial Products for Management of Powdery Mildew on Summer Squash and Cantaloupe in Florida. Plant Dis. 2011, 95, 461–468. [Google Scholar] [CrossRef]
- Tahvonen, R.; Hannukkala, A.; Avikainen, H. Effect of seed dressing treatment of Streptomyces griseoviridis on barley and spring wheat in field experiments. Agric. Food Sci. 1995, 4, 419–427. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Pershina, E.V.; Ivanova, E.A.; Korvigo, I.O.; Chirak, E.L.; Sergaliev, N.H.; Abakumov, E.V.; Provorov, N.A.; Andronov, E.E. Investigation of the core microbiome in main soil types from the East European plain. Sci. Total Environ. 2018, 631, 1421–1430. [Google Scholar] [CrossRef]
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: What lies ahead? Ecosphere 2015, 6, art130. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Mavrodi, O.V.; Elbourne, L.D.H.; Tetu, S.; Bonsall, R.F.; Parejko, J.; Yang, M.; Paulsen, I.T.; Weller, D.M.; Thomashow, L.S. Long-Term Irrigation Affects the Dynamics and Activity of the Wheat Rhizosphere Microbiome. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Babin, D.; Deubel, A.; Jacquiod, S.; Sørensen, S.J.; Geistlinger, J.; Grosch, R.; Smalla, K. Impact of long-term agricultural management practices on soil prokaryotic communities. Soil Biol. Biochem. 2019, 129, 17–28. [Google Scholar] [CrossRef]
- Turner, T.R.; Ramakrishnan, K.; Walshaw, J.; Heavens, D.; Alston, M.; Swarbreck, D.; Osbourn, A.; Grant, A.; Poole, P.S. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. ISME J. 2013, 7, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Bressan, M.; Roncato, M.-A.; Bellvert, F.; Comte, G.; el Zahar Haichar, F.; Achouak, W.; Berge, O. Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. ISME J. 2009, 3, 1243–1257. [Google Scholar] [CrossRef]
- Micallef, S.A.; Shiaris, M.P.; Colón-Carmona, A. Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. J. Exp. Bot. 2009, 60, 1729–1742. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Edwards, J.A.; Santos-Medellín, C.M.; Liechty, Z.S.; Nguyen, B.; Lurie, E.; Eason, S.; Phillips, G.; Sundaresan, V. Compositional shifts in root-associated bacterial and archaeal microbiota track the plant life cycle in field-grown rice. PLoS Biol. 2018, 16, e2003862. [Google Scholar] [CrossRef]
- Bakker, M.G.; Chaparro, J.M.; Manter, D.K.; Vivanco, J.M. Impacts of bulk soil microbial community structure on rhizosphere microbiomes of Zea mays. Plant Soil 2015, 392, 115–126. [Google Scholar] [CrossRef]
- Rocca, J.D.; Simonin, M.; Blaszczak, J.R.; Ernakovich, J.G.; Gibbons, S.M.; Midani, F.S.; Washburne, A.D. The Microbiome Stress Project: Toward a Global Meta-Analysis of Environmental Stressors and Their Effects on Microbial Communities. Front. Microbiol. 2019, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruyter-Spira, C.; Bouwmeester, H.J. Engineering the plant rhizosphere. Curr. Opin. Biotechnol. 2015, 32, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Entry, J.A.; Strasbaugh, C.A.; Sojka, R.E. Wood Chip-Polyacrylamide Medium for Biocontrol Bacteria Decreases Verticillium dahliae Infection on Potato. Biocontrol Sci. Technol. 2000, 10, 677–686. [Google Scholar] [CrossRef]
- Lumaret, J.-P.; Errouissi, F.; Floate, K.; Rombke, J.; Wardhaugh, K. A Review on the Toxicity and Non-Target Effects of Macrocyclic Lactones in Terrestrial and Aquatic Environments. Curr. Pharm. Biotechnol. 2012, 13, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Dennert, F.; Imperiali, N.; Staub, C.; Schneider, J.; Laessle, T.; Zhang, T.; Wittwer, R.; van der Heijden, M.G.A.; Smits, T.H.M.; Schlaeppi, K.; et al. Conservation tillage and organic farming induce minor variations in Pseudomonas abundance, their antimicrobial function and soil disease resistance. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Shen, Q.; Zhang, R.; Chen, W. Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil 2013, 366, 453–466. [Google Scholar] [CrossRef]
- Shen, Z.; Xue, C.; Penton, C.R.; Thomashow, L.S.; Zhang, N.; Wang, B.; Ruan, Y.; Li, R.; Shen, Q. Suppression of banana Panama disease induced by soil microbiome reconstruction through an integrated agricultural strategy. Soil Biol. Biochem. 2019, 128, 164–174. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Feng, Y.; Yang, X.; Li, X.; Shen, Q. Tobacco bacterial wilt can be biologically controlled by the application of antagonistic strains in combination with organic fertilizer. Biol. Fertil. Soils 2013, 49, 447–464. [Google Scholar] [CrossRef]
- Pane, C.; Spaccini, R.; Piccolo, A.; Scala, F.; Bonanomi, G. Compost amendments enhance peat suppressiveness to Pythium ultimum, Rhizoctonia solani and Sclerotinia minor. Biol. Control 2011, 56, 115–124. [Google Scholar] [CrossRef]
- Watanabe, N.; Lewis, J.A.; Papavizas, G.C. Influence of Nitrogen Fertilizers on Growth, Spore Production and Germination, and Biocontrol Potential of Trichoderma and Gliocladium. J. Phytopathol. 2008, 120, 337–346. [Google Scholar] [CrossRef]
- Ketabchi, S.; Charehgani, H.; Majzoob, S. Impact of Rhizosphere Antagonistic Bacteria & Urea Fertilizer on Root Knot Nematode (Meloidogyne incognita) Under Green House Conditions. J. Anim. Plant Sci. 2016, 26, 1780–1786. [Google Scholar]
- Siddiqui, I.A.; Shaukat, S.S. Zinc and glycerol enhance the production of nematicidal compounds in vitro and improve the biocontrol of Meloidogyne javanica in tomato by fluorescent pseudomonads. Lett. Appl. Microbiol. 2002, 35, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Antoraz, S.; SantamarÃa, R.n.I.; DÃaz, M.; Sanz, D.; RodrÃguez, H.c. Toward a new focus in antibiotic and drug discovery from the Streptomyces arsenal. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Vörös, M.; Manczinger, L.; Kredics, L.; Szekeres, A.; Shine, K.; Alharbi, N.S.; Khaled, J.M.; Vágvölgyi, C. Influence of agro-environmental pollutants on a biocontrol strain of Bacillus velezensis. Microbiol. Open 2019, 8, e00660. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chhatpar, H.S. Combined use of Streptomyces sp. A6 and chemical fungicides against fusarium wilt of Cajanus cajan may reduce the dosage of fungicides required in the field. Crop. Prot. 2011, 30, 770–775. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Preininger, C.; Sauer, U.; Bejarano, A.; Berninger, T. Concepts and applications of foliar spray for microbial inoculants. Appl. Microbiol. Biotechnol. 2018, 102, 7265–7282. [Google Scholar] [CrossRef] [PubMed]
- Jambhulkar, P.P.; Sharma, P.; Yadav, R. Delivery Systems for Introduction of Microbial Inoculants in the Field. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 199–218. [Google Scholar]
- Pill, W.G. Advances in Fluid Drilling. HortTechnology 1991, 1, 59–65. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, S.M. Effects of root-dip treatment with certain phosphate solubilizing microorganisms on the fusarial wilt of tomato. Bioresour. Technol. 2002, 85, 213–215. [Google Scholar] [CrossRef]
- Hardaker, J.M.; Hardwick, R.C. A Note on Rhizobium Inoculation of Beans (Phaseolus vulgaris) using the Fluid Drill Technique. Exp. Agric. 1978, 14, 17. [Google Scholar] [CrossRef]
- Clarkson, J.P.; Payne, T.; Mead, A.; Whipps, J.M. Selection of fungal biological control agents of Sclerotium cepivorum for control of white rot by sclerotial degradation in a UK soil. Plant Pathol. 2002, 51, 735–745. [Google Scholar] [CrossRef]
- Callan, N.W.; Mathre, D.; Miller, J.B. Bio-priming Seed Treatment for Biological Control of Pythium ultimum Pre-emergence Damping-off in sh2 Sweet Corn. Plant Dis. 1990, 74, 368–372. [Google Scholar] [CrossRef]
- Ardakani, S.S.; Heydari, A.; Khorasani, N.; Arjmandi, R. Development of New Bioformulations of Pseudomonas fluorescens and Evaluation of These Products Against Damping-off of Cotton Seedlings. J. Plant Pathol. 2010, 92, 83–88. [Google Scholar]
- El-Mougy, N.S.; Abdel-Kader, M.M. Long-term activity of bio-priming seed treatment for biological control of faba bean root rot pathogens. Australas. Plant Pathol. 2008, 37, 464. [Google Scholar] [CrossRef]
- Sutruedee, P.; Dusit, A.; Wilawan, C.; Tiyakhon, C.; Natthiya, B. Bioformulation Pseudomonas fluorescens SP007s against dirty panicle disease of rice. Afr. J. Microbiol. Res. 2013, 7, 5274–5283. [Google Scholar] [CrossRef]
- Yadav, R.S.; Singh, V.; Pal, S.; Meena, S.K.; Meena, V.S.; Sarma, B.K.; Singh, H.B.; Rakshit, A. Seed bio-priming of baby corn emerged as a viable strategy for reducing mineral fertilizer use and increasing productivity. Sci. Hortic. 2018, 241, 93–99. [Google Scholar] [CrossRef]
- Singh, P.J.; Mehrotra, R.S. Biological control of Rhizoctonia bataticola on gram by coating seed with Bacillus and Streptomyces spp. and their influence on plant growth. Plant Soil 1980, 56, 475–483. [Google Scholar] [CrossRef]
- Misk, A.; Franco, C. Biocontrol of chickpea root rot using endophytic Actinobacteria. BioControl 2011, 56, 811–822. [Google Scholar] [CrossRef]
- El-Abyad, M.S.; El-Sayed, M.A.; El-Shanshoury, A.R.; El-Sabbagh, S.M. Towards the biological control of fungal and bacterial diseases of tomato using antagonistic Streptomyces spp. Plant Soil 1993, 149, 185–195. [Google Scholar] [CrossRef]
- Müller, H.; Berg, G. Impact of formulation procedures on the effect of the biocontrol agent Serratia plymuthica HRO-C48 on Verticillium wilt in oilseed rape. BioControl 2008, 53, 905–916. [Google Scholar] [CrossRef]
- Sabaratnam, S.; Traquair, J.A. Formulation of a Streptomyces Biocontrol Agent for the Suppression of Rhizoctonia Damping-off in Tomato Transplants. Biol. Control 2002, 23, 245–253. [Google Scholar] [CrossRef]
- Ryan, P.R.; Dessaux, Y.; Thomashow, L.S.; Weller, D.M. Rhizosphere engineering and management for sustainable agriculture. Plant Soil 2009, 321, 363–383. [Google Scholar] [CrossRef]
- Quiza, L.; St-Arnaud, M.; Yergeau, E. Harnessing phytomicrobiome signaling for rhizosphere microbiome engineering. Front. Plant Sci. 2015, 6, 507. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.E.Z.; Heulin, T.; Guyonnet, J.P.; Achouak, W. Stable isotope probing of carbon flow in the plant holobiont. Curr. Opin. Biotechnol. 2016, 41, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Del Rio, T.G.; Jones, C.D.; Tringe, S.G. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef]
- Haichar, F.Z.; Roncato, M.A.; Achouak, W. Stable isotope probing of bacterial community structure and gene expression in the rhizosphere of Arabidopsis thaliana. FEMS Microbiol. Ecol. 2012, 81, 291–302. [Google Scholar] [CrossRef]
- Jousset, A.; Rochat, L.; Lanoue, A.; Bonkowski, M.; Keel, C.; Scheu, S. Plants respond to pathogen infection by enhancing the antifungal gene expression of root-associated bacteria. Mol. Plant Microbe Interact. 2011, 24, 352–358. [Google Scholar] [CrossRef]
- Lanoue, A.; Burlat, V.; Henkes, G.J.; Koch, I.; Schurr, U.; Rose, U.S. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol. 2010, 185, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Daddaoua, A.; Matilla, M.A.; Krell, T.; Chini, A.; Morel, B. An auxin controls bacterial antibiotics production. Nucleic Acids Res. 2018, 46, 11229–11238. [Google Scholar] [CrossRef]
- Van der Meij, A.; Willemse, J.; Schneijderberg, M.A.; Geurts, R.; Raaijmakers, J.M.; van Wezel, G.P. Inter- and intracellular colonization of Arabidopsis roots by endophytic actinobacteria and the impact of plant hormones on their antimicrobial activity. Antonie Van Leeuwenhoek 2018, 111, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Quintana, N.; El Kassis, E.G.; Kim, H.K.; Choi, Y.H.; Sugiyama, A.; Verpoorte, R.; Martinoia, E.; Manter, D.K.; Vivanco, J.M. An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol. 2009, 151, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Jiang, T.; Liu, Y.-X.; Bai, Y.-C.; Reed, J.; Qu, B.; Goossens, A.; Nützmann, H.-W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 364, eaau6389. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.G.; Murrell, J.C. Stable isotope probing-linking microbial identity to function. Nat. Rev. Microbiol. 2005, 3, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Camilios-Neto, D.; Bonato, P.; Wassem, R.; Tadra-Sfeir, M.Z.; Brusamarello-Santos, L.C.; Valdameri, G.; Donatti, L.; Faoro, H.; Weiss, V.A.; Chubatsu, L.S.; et al. Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. BMC Genom. 2014, 15, 378. [Google Scholar] [CrossRef] [PubMed]
- Mateus, I.D.; Masclaux, F.G.; Aletti, C.; Rojas, E.C.; Savary, R.; Dupuis, C.; Sanders, I.R. Dual RNA-seq reveals large-scale non-conserved genotype x genotype-specific genetic reprograming and molecular crosstalk in the mycorrhizal symbiosis. ISME J. 2019. [Google Scholar] [CrossRef]
- Berry, D.; Stecher, B.; Schintlmeister, A.; Reichert, J.; Brugiroux, S.; Wild, B.; Wanek, W.; Richter, A.; Rauch, I.; Decker, T.; et al. Host-compound foraging by intestinal microbiota revealed by single-cell stable isotope probing. Proc. Natl. Acad. Sci. USA 2013, 110, 4720–4725. [Google Scholar] [CrossRef]
- Musat, N.; Musat, F.; Weber, P.K.; Pett-Ridge, J. Tracking microbial interactions with NanoSIMS. Curr. Opin. Biotechnol. 2016, 41, 114–121. [Google Scholar] [CrossRef]
- Watrous, J.D.; Alexandrov, T.; Dorrestein, P.C. The evolving field of imaging mass spectrometry and its impact on future biological research. J. Mass Spectrom. 2011, 46, 209–222. [Google Scholar] [CrossRef]
- Vurukonda, S.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Winding, A.; Binnerup, S.J.; Pritchard, H. Non-target effects of bacterial biological control agents suppressing root pathogenic fungi. FEMS Microbiol. Ecol. 2004, 47, 129–141. [Google Scholar] [CrossRef]
- Deising, H.B.; Gase, I.; Kubo, Y. The unpredictable risk imposed by microbial secondary metabolites: How safe is biological control of plant diseases? J. Plant Dis. Prot. 2017, 124, 413–419. [Google Scholar] [CrossRef]
- Zotchev, S.B. Polyene Macrolide Antibiotics and their Applications in Human Therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Ole Becker, J.; Berg, G.; Hauschild, R.; Jehle, J.; Köhl, J.; Smalla, K. Biocontrol of plant diseases is not an unsafe technology! J. Plant Dis. Prot. 2018, 125, 121–125. [Google Scholar] [CrossRef]
- Hennessy, R.C.; Glaring, M.A.; Olsson, S.; Stougaard, P. Transcriptomic profiling of microbe-microbe interactions reveals the specific response of the biocontrol strain P. fluorescens In5 to the phytopathogen Rhizoctonia solani. BMC Res. Notes 2017, 10, 376. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Tyc, O.; van den Berg, M.; Gerards, S.; van Veen, J.A.; Raaijmakers, J.M.; de Boer, W.; Garbeva, P. Impact of interspecific interactions on antimicrobial activity among soil bacteria. Front. Microbiol. 2014, 5, 567. [Google Scholar] [CrossRef]
- Wyss, P.; Boller, T.; Wiemken, A. Testing the effect of biological control agents on the formation of vesicular arbuscular mycorrhiza. Plant Soil 1992, 147, 159–162. [Google Scholar] [CrossRef]
- Richter, D.L.; Zuellig, T.R.; Bagley, S.T.; Bruhn, J.N. Effects of red pine (Pinus resinosa Ait.) mycorrhizoplane-associated actinomycetes on in vitro growth of ectomycorrhizal fungi. Plant Soil 1989, 115, 109–116. [Google Scholar] [CrossRef]
- Schrey, S.D.; Erkenbrack, E.; Fruh, E.; Fengler, S.; Hommel, K.; Horlacher, N.; Schulz, D.; Ecke, M.; Kulik, A.; Fiedler, H.P.; et al. Production of fungal and bacterial growth modulating secondary metabolites is widespread among mycorrhiza-associated streptomycetes. BMC Microbiol. 2012, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.K.; Klubek, B.; Varsa, E.C. Identification and use of actinomycetes for enhanced nodulation of soybean co-inoculated with Bradyrhizobium japonicum. Can. J. Microbiol. 2003, 49, 483–491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samac, D.A.; Willert, A.M.; McBride, M.J.; Kinkel, L.L. Effects of antibiotic-producing Streptomyces on nodulation and leaf spot in alfalfa. Appl. Soil Ecol. 2003, 22, 55–66. [Google Scholar] [CrossRef]
- Maier, A.; Riedlinger, J.; Fiedler, H.-P.; Hampp, R. Actinomycetales bacteria from a spruce stand: Characterization and effects on growth of root symbiotic and plant parasitic soil fungi in dual culture. Mycol. Prog. 2004, 3, 129–136. [Google Scholar] [CrossRef]
- Schrey, S.D.; Schellhammer, M.; Ecke, M.; Hampp, R.; Tarkka, M.T. Mycorrhiza helper bacterium Streptomyces AcH 505 induces differential gene expression in the ectomycorrhizal fungus Amanita muscaria. New Phytol. 2005, 168, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottova, D.; Kristufek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef]
- Schmidt, R.; Koberl, M.; Mostafa, A.; Ramadan, E.M.; Monschein, M.; Jensen, K.B.; Bauer, R.; Berg, G. Effects of bacterial inoculants on the indigenous microbiome and secondary metabolites of chamomile plants. Front. Microbiol. 2014, 5, 64. [Google Scholar] [CrossRef]
- Prévost, K.; Couture, G.; Shipley, B.; Brzezinski, R.; Beaulieu, C. Effect of chitosan and a biocontrol streptomycete on field and potato tuber bacterial communities. BioControl 2006, 51, 533–546. [Google Scholar] [CrossRef]
- Conn, V.M.; Franco, C.M. Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl. Environ. Microbiol. 2004, 70, 6407–6413. [Google Scholar] [CrossRef] [PubMed]
- Scherwinski, K.; Wolf, A.; Berg, G. Assessing the Risk of Biological Control Agents on the Indigenous Microbial Communities: Serratia plymuthica HRO-C48 and Streptomyces sp. HRO-71 as Model Bacteria. BioControl 2007, 52, 87–112. [Google Scholar] [CrossRef]
- Ford, S.A.; King, K.C. Harnessing the Power of Defensive Microbes: Evolutionary Implications in Nature and Disease Control. PLoS Pathog. 2016, 12, e1005465. [Google Scholar] [CrossRef] [PubMed]
- Bouffaud, M.L.; Renoud, S.; Dubost, A.; Moenne-Loccoz, Y.; Muller, D. 1-Aminocyclopropane-1-carboxylate deaminase producers associated to maize and other Poaceae species. Microbiome 2018, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Sisaphaithong, T.; Kondo, D.; Matsunaga, H.; Kobae, Y.; Hata, S. Expression of plant genes for arbuscular mycorrhiza-inducible phosphate transporters and fungal vesicle formation in sorghum, barley, and wheat roots. Biosci. Biotechnol. Biochem. 2012, 76, 2364–2367. [Google Scholar] [CrossRef]
- Glassop, D.; Smith, S.E.; Smith, F.W. Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots. Planta 2005, 222, 688–698. [Google Scholar] [CrossRef]
| Pathogen | Cereal Crop Host | Symptoms | Impact | Streptomyces as Biocontrol |
|---|---|---|---|---|
| Magnaporthe oryzae (Rice blast) | Rice, Wheat | Panicle, leaf and head blast | Yield losses and mycotoxin contamination | Greenhouse and in vitro studies [18,65,66] |
| Fusarium spp. | All cereals | Head, root, crown and stem blight in addition to wilt and grain contamination | Yield losses and mycotoxin contamination | Greenhouse, in vitro and field studies [67,68,69,70,71,72,73,74] |
| Rhizoctonia solani | All cereals | Seedling damping off, and infection of stems, roots and foliage | Yield losses and reduction in grain quality | In vitro and growth chamber studies [64,66,71,75,76,77,78] |
| Gaumannomyces graminis (Wheat Take-all) | Wheat, Barley, Rye, Rice, Oat, Maize | Root lesions and rot that spreads upwards to aerial parts of the plant | Yield losses | In vitro and greenhouse studies [15,57] |
| Pythium spp. | Wheat, Barley, Rice, Maize | Seed damping off, as well as root and stem rot | Yield losses | In vitro and growth chamber studies [76,79] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newitt, J.T.; Prudence, S.M.M.; Hutchings, M.I.; Worsley, S.F. Biocontrol of Cereal Crop Diseases Using Streptomycetes. Pathogens 2019, 8, 78. https://doi.org/10.3390/pathogens8020078
Newitt JT, Prudence SMM, Hutchings MI, Worsley SF. Biocontrol of Cereal Crop Diseases Using Streptomycetes. Pathogens. 2019; 8(2):78. https://doi.org/10.3390/pathogens8020078
Chicago/Turabian StyleNewitt, Jake T., Samuel M. M. Prudence, Matthew I. Hutchings, and Sarah F. Worsley. 2019. "Biocontrol of Cereal Crop Diseases Using Streptomycetes" Pathogens 8, no. 2: 78. https://doi.org/10.3390/pathogens8020078
APA StyleNewitt, J. T., Prudence, S. M. M., Hutchings, M. I., & Worsley, S. F. (2019). Biocontrol of Cereal Crop Diseases Using Streptomycetes. Pathogens, 8(2), 78. https://doi.org/10.3390/pathogens8020078





