The Distinct Regulation of the Vitamin D and Aryl Hydrocarbon Receptors in COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethics Statement
2.3. Virology
2.4. Isolation of RNA and Expression Screens
2.5. Statistical Analysis
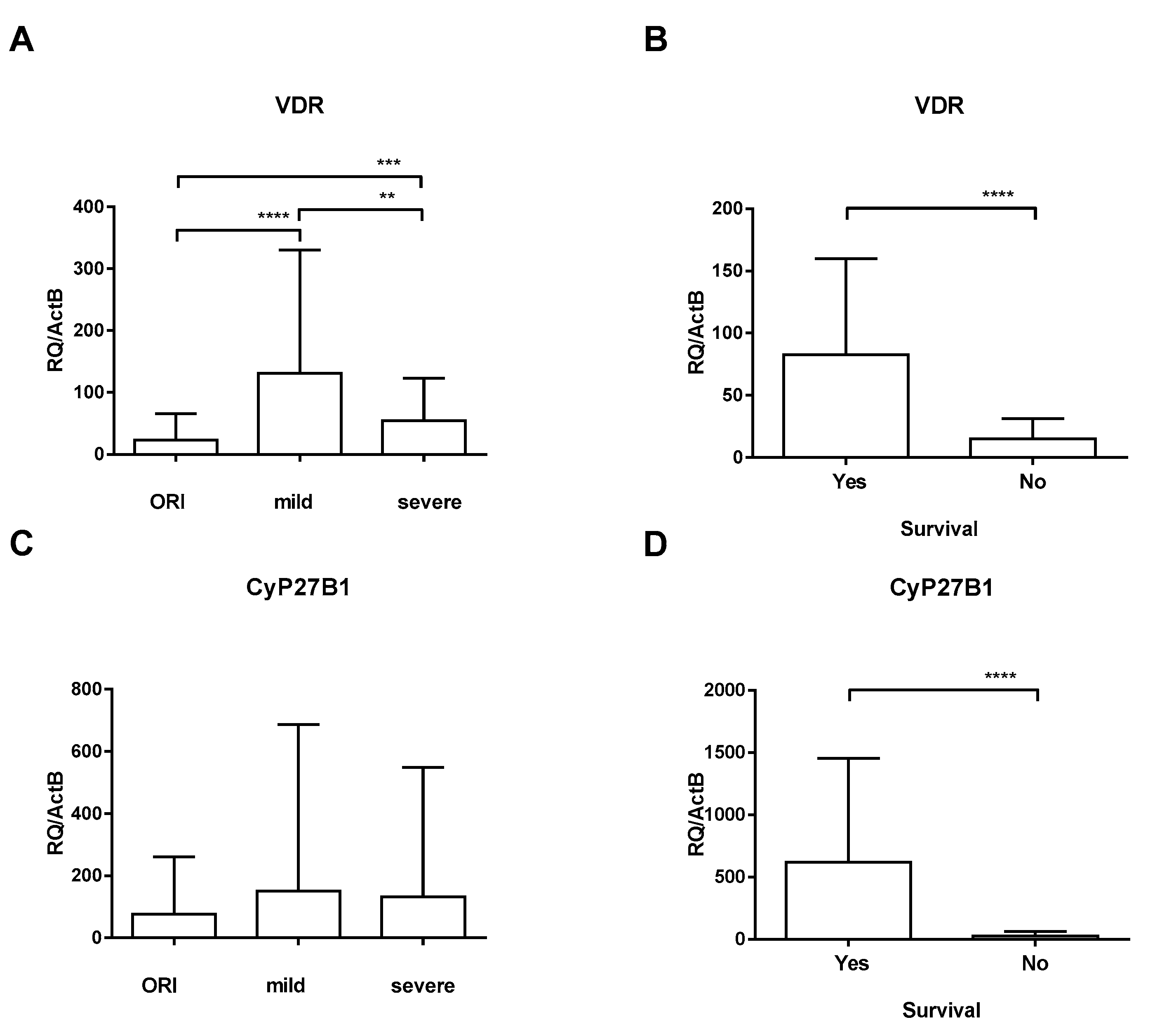
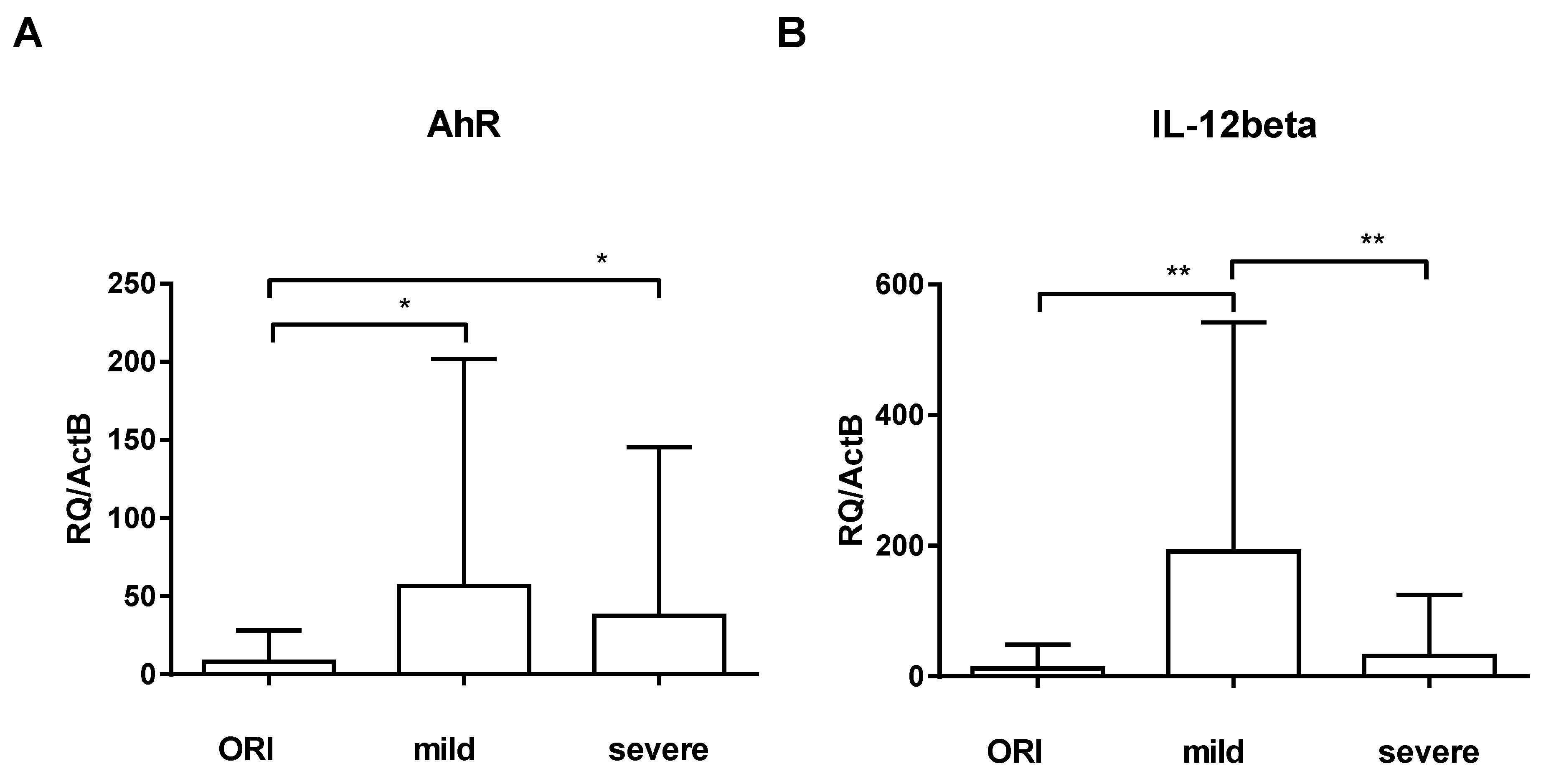
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Lu, X.; Xu, C.; Sun, W.; Pan, B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020, 92, 548–551. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Deng, Y.; Li, W. Coronavirus Disease 2019 (COVID-19): What we know? J. Med. Virol. 2020, 20, 697–706. [Google Scholar] [CrossRef]
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Frerichs, I. Mortality in COVID-19 is not merely a question of resource availability. Lancet Respir. Med. 2020, 8, 832–833. [Google Scholar] [CrossRef] [PubMed]
- de la Rica, R.; Borges, M.; Gonzalez-Freire, M. COVID-19: In the Eye of the Cytokine Storm. Front. Immunol. 2020, 11, 558898. [Google Scholar] [CrossRef]
- Reddy, K.; Rogers, A.J.; McAuley, D.F. Delving beneath the surface of hyperinflammation in COVID-19. Lancet Rheumatol. 2020, 2, e578–e579. [Google Scholar] [CrossRef] [PubMed]
- Cavender, D.E. Interactions between endothelial cells and the cells of the immune system. Int. Rev. Exp. Pathol. 1991, 32, 57–94. [Google Scholar] [CrossRef]
- Glaros, T.; Larsen, M.; Li, L. Macrophages and fibroblasts during inflammation, tissue damage and organ injury. Front. Biosci. 2009, 14, 3988–3993. [Google Scholar] [CrossRef]
- Kmiec, Z.; Cyman, M.; Slebioda, T.J. Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Adv. Med. Sci. 2017, 62, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Dejana, E.; Fiocchi, C. Immune regulation by microvascular endothelial cells: Directing innate and adaptive immunity, coagulation, and inflammation. J. Immunol. 2007, 178, 6017–6022. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.; Rismani, M.; Pourmontaseri, H.; Mirzaii, E.; Niknia, S.; Miladpour, B. The role of vitamin D receptor and IL-6 in COVID-19. Mol. Genet. Genomic Med. 2023, 11, e2172. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Morello, G.; Malaguarnera, R.; Piro, S.; Furno, D.L.; Malaguarnera, L. Candidate genes of SARS-CoV-2 gender susceptibility. Sci. Rep. 2021, 11, 21968. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Gilani, S.J.; Bin-Jumah, M.N.; Nadeem, M.S.; Kazmi, I. Vitamin D attenuates COVID-19 complications via modulation of proinflammatory cytokines, antiviral proteins, and autophagy. Expert Rev. Anti-Infect. Ther. 2022, 20, 231–241. [Google Scholar] [CrossRef]
- Russo, C.; Valle, M.S.; Malaguarnera, L.; Romano, I.R.; Malaguarnera, L. Comparison of Vitamin D and Resveratrol Performances in COVID-19. Nutrients 2023, 15, 2639. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Paolino, S.; Smith, V. Evidences for a protective role of vitamin D in COVID-19. RMD Open 2020, 6, e001454. [Google Scholar] [CrossRef]
- Gunville, C.F.; Mourani, P.M.; Ginde, A.A. The role of vitamin D in prevention and treatment of infection. Inflamm. Allergy Drug Targets 2013, 12, 239–245. [Google Scholar] [CrossRef]
- Siddiqui, M.; Manansala, J.S.; Abdulrahman, H.A.; Nasrallah, G.K.; Smatti, M.K.; Younes, N.; Althani, A.A.; Yassine, H.M. Immune Modulatory Effects of Vitamin D on Viral Infections. Nutrients 2020, 12, 2879. [Google Scholar] [CrossRef]
- Ciarambino, T.; Para, O.; Giordano, M. Immune system and COVID-19 by sex differences and age. Womens Health 2021, 17, 17455065211022262. [Google Scholar] [CrossRef]
- Vadakedath, S.; Kandi, V.; Mohapatra, R.K.; Pinnelli, V.B.K.; Yegurla, R.R.; Shahapur, P.R.; Godishala, V.; Natesan, S.; Vora, K.S.; Sharun, K.; et al. Immunological aspects and gender bias during respiratory viral infections including novel Coronavirus disease-19 (COVID-19): A scoping review. J. Med. Virol. 2021, 93, 5295–5309. [Google Scholar] [CrossRef]
- Dunn, S.E.; Perry, W.A.; Klein, S.L. Mechanisms and consequences of sex differences in immune responses. Nat. Rev. Nephrol. 2024, 20, 37–55. [Google Scholar] [CrossRef]
- Cutolo, M.; Smith, V.; Paolino, S. Understanding immune effects of oestrogens to explain the reduced morbidity and mortality in female versus male COVID-19 patients. Comparisons with autoimmunity and vaccination. Clin. Exp. Rheumatol. 2020, 38, 383–386. [Google Scholar] [CrossRef]
- Rieder, F.J.J.; Groschel, C.; Kastner, M.T.; Kosulin, K.; Laengle, J.; Zadnikar, R.; Marculescu, R.; Schneider, M.; Lion, T.; Bergmann, M.; et al. Human cytomegalovirus infection downregulates vitamin-D receptor in mammalian cells. J. Steroid Biochem. Mol. Biol. 2017, 165, 356–362. [Google Scholar] [CrossRef]
- Robak, O.; Kastner, M.T.; Stecher, C.; Schneider, M.; Andreas, M.; Greinix, H.; Kallay, E.; Honsig, C.; Steininger, C. Cytomegalovirus Infection Downregulates Vitamin D Receptor in Patients Undergoing Hematopoietic Stem Cell Transplantation. Transplantation 2021, 105, 1595–1602. [Google Scholar] [CrossRef]
- Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 6 February 2024).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Rheum. Dis. Clin. N. Am. 2012, 38, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, S.S.; Nouri, M.; Sakhinia, E.; Babaloo, Z.; Jadideslam, G.; Shahriar, A.; Farhadi, J.; Khabbazi, A. The expression and methylation status of vitamin D receptor gene in Behcet’s disease. Immun. Inflamm. Dis. 2019, 7, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Davola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef]
- Turski, W.A.; Wnorowski, A.; Turski, G.N.; Turski, C.A.; Turski, L. AhR and IDO1 in pathogenesis of COVID-19 and the "Systemic AhR Activation Syndrome:" a translational review and therapeutic perspectives. Restor. Neurol. Neurosci. 2020, 38, 343–354. [Google Scholar] [CrossRef]
- Giovannoni, F.; Bosch, I.; Polonio, C.M.; Torti, M.F.; Wheeler, M.A.; Li, Z.; Romorini, L.; Rodriguez Varela, M.S.; Rothhammer, V.; Barroso, A.; et al. AHR is a Zika virus host factor and a candidate target for antiviral therapy. Nat. Neurosci. 2020, 23, 939–951. [Google Scholar] [CrossRef]
- Hariharan, A.; Hakeem, A.R.; Radhakrishnan, S.; Reddy, M.S.; Rela, M. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology 2021, 29, 91–100. [Google Scholar] [CrossRef]
- Giovannoni, F.; Li, Z.; Garcia, C.C.; Quintana, F.J. A potential role for AHR in SARS-CoV-2 pathology. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, L.; Tian, L.; Yin, S.; Ma, X.; Cheng, S.; Tang, W.; Yu, J.; Ma, W.; Zhou, X.; et al. Aryl Hydrocarbon Receptor Promotes IL-10 Expression in Inflammatory Macrophages Through Src-STAT3 Signaling Pathway. Front. Immunol. 2018, 9, 2033. [Google Scholar] [CrossRef]
- Nehmar, R.; Fauconnier, L.; Alves-Filho, J.; Togbe, D.; DeCauwer, A.; Bahram, S.; Le Bert, M.; Ryffel, B.; Georgel, P. Aryl hydrocarbon receptor (Ahr)-dependent Il-22 expression by type 3 innate lymphoid cells control of acute joint inflammation. J. Cell Mol. Med. 2021, 25, 4721–4731. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, Y.X.; Jiang, L.J.; Chen, Q.; Wang, T.; Ye, D.W. Targeting JAK-STAT Signaling to Control Cytokine Release Syndrome in COVID-19. Trends Pharmacol. Sci. 2020, 41, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Penco, F.; Mollica, H.; Bocca, P.; Prigione, I.; Corcione, A.; Cangelosi, D.; Schena, F.; Del Zotto, G.; Amaro, A.; et al. Spontaneous NLRP3 inflammasome-driven IL-1-beta secretion is induced in severe COVID-19 patients and responds to anakinra treatment. J. Allergy Clin. Immunol. 2022, 150, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ni Choileain, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Kolls, J.K.; Garry, R.F. Role of the T cell vitamin D receptor in severe COVID-19. Nat. Immunol. 2022, 23, 5–6. [Google Scholar] [CrossRef]
- Notz, Q.; Herrmann, J.; Schlesinger, T.; Kranke, P.; Sitter, M.; Helmer, P.; Stumpner, J.; Roeder, D.; Amrein, K.; Stoppe, C.; et al. Vitamin D deficiency in critically ill COVID-19 ARDS patients. Clin. Nutr. 2022, 41, 3089–3095. [Google Scholar] [CrossRef]
- Apaydin, T.; Polat, H.; Dincer Yazan, C.; Ilgin, C.; Elbasan, O.; Dashdamirova, S.; Bayram, F.; Tukenmez Tigen, E.; Unlu, O.; Tekin, A.F.; et al. Effects of vitamin D receptor gene polymorphisms on the prognosis of COVID-19. Clin. Endocrinol. 2022, 96, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, R.; Shushizadeh, M.H.; Barazandehrokh, M.; Choopani, S.; Azarnezhad, A.; Paknahad, S.; Pirhoushiaran, M.; Makani, S.Z.; Yeganeh, R.Z.; Al-Kateb, A.; et al. Association of Vitamin D receptor gene polymorphisms and clinical/severe outcomes of COVID-19 patients. Infect. Genet. Evol. 2021, 96, 105098. [Google Scholar] [CrossRef] [PubMed]

| SARS-CoV-2-Infected | ||||||
|---|---|---|---|---|---|---|
| ORIs | Mild | Severe | ||||
| N | % | N | % | N | % | |
| Number of patients | 71 | 29.6 | 64 | 26.7 | 105 | 43.8 |
| Gender | ||||||
| Male | 31 | 43.7 | 30 | 46.9 | 47 | 40.0 |
| Female | 40 | 56.3 | 34 | 53.1 | 58 | 60.0 |
| Median age in years | 47 | 42 | 56 | |||
| (range) | (22–76) | (18–79) | (29–74) | |||
| BMI on admission | 23.1 | |||||
| (range) | (20.0–33.1) | |||||
| Co-morbidities | N | % | ||||
| Obesity (BMI ≥ 30 kg/m2) | 53 | 50.5 | ||||
| Arterial hypertension | 103 | 98.1 | ||||
| Diabetes mellitus | 38 | 36.2 | ||||
| Ischemic cardiopathy | 14 | 13.3 | ||||
| Cerebro-vascular disease | 17 | 16.2 | ||||
| Chronic kidney failure | 10 | 9.5 | ||||
| Chronic respiratory disease | 45 | 42.9 | ||||
| Immunocompromised status | 18 | 17.1 | ||||
| Main delays | Median | Range | ||||
| Days between disease onset and hospital admission | 2 | (0–12) | ||||
| Days between disease onset and ICU admission | 8 | (6–11) | ||||
| Days between disease onset and intubation | 10 | (3–22) | ||||
| Days between disease onset and ECMO (n = 77) | 18 | (4–36) | ||||
| Median | Range | |||||
| Duration of IMV | 15 | (12–44) | ||||
| Days between disease onset and IMV | 6 | (2–16) | ||||
| Length of ICU stay, days | 19 | (12–45) | ||||
| N | % | |||||
| On ECMO | 77 | 37.4 | ||||
| Gender | ||||||
| Female | 27 | 21.3 | ||||
| Male | 100 | 78.7 | ||||
| ICU Mortality | 36 | 30.0 | ||||
| Mean | SD | |||||
| SAPS II | 27.8 | ±3.4 | ||||
| SOFA | 4.6 | ±1.9 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robak, O.; Kastner, M.-T.; Voill-Glaninger, A.; Viveiros, A.; Steininger, C. The Distinct Regulation of the Vitamin D and Aryl Hydrocarbon Receptors in COVID-19. Nutrients 2024, 16, 598. https://doi.org/10.3390/nu16050598
Robak O, Kastner M-T, Voill-Glaninger A, Viveiros A, Steininger C. The Distinct Regulation of the Vitamin D and Aryl Hydrocarbon Receptors in COVID-19. Nutrients. 2024; 16(5):598. https://doi.org/10.3390/nu16050598
Chicago/Turabian StyleRobak, Oliver, Marie-Theres Kastner, Astrid Voill-Glaninger, André Viveiros, and Christoph Steininger. 2024. "The Distinct Regulation of the Vitamin D and Aryl Hydrocarbon Receptors in COVID-19" Nutrients 16, no. 5: 598. https://doi.org/10.3390/nu16050598
APA StyleRobak, O., Kastner, M.-T., Voill-Glaninger, A., Viveiros, A., & Steininger, C. (2024). The Distinct Regulation of the Vitamin D and Aryl Hydrocarbon Receptors in COVID-19. Nutrients, 16(5), 598. https://doi.org/10.3390/nu16050598






