An Overview of the Versatility of the Parts of the Globe Artichoke (Cynara scolymus L.), Its By-Products and Dietary Supplements
Abstract
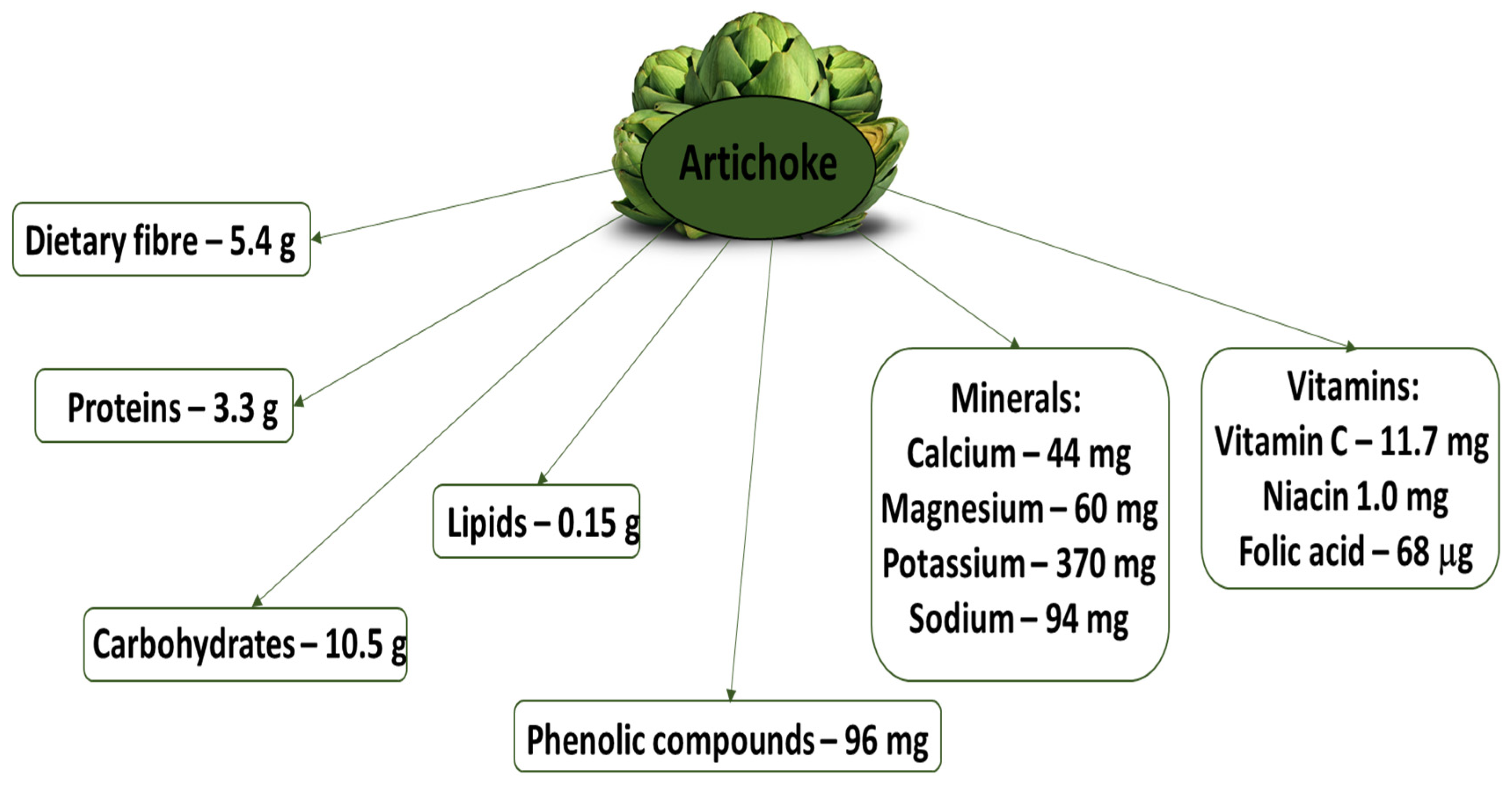
1. Introduction
2. Phytochemical Characteristics of C. scolymus
3. Biological Activity of Different Parts of C. scolymus
3.1. Antioxidant Activity
3.2. Effect on the Liver and Other Elements of Digestive System
3.3. Cardioprotective Action
3.4. Other Biological Properties
4. The Safety of Artichoke and the Metabolism of Its Phenolic Compounds
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Grabowska, A.; Caruso, G.; Mehrafarin, A.; Kalisz, A.; Gruszecki, R.; Kunicki, E.; Sekara, A. Application of modern agronomic and biotechnological strategies to valorize worldwide globe artichoke (Cynara cardunculus L.) potential-an analytical overview. Ital. J. Agron. 2018, 13, 2790289. [Google Scholar]
- Cavini, S.; Guzzetti, L.; Givoia, F.; Regonesi, M.E.; Di Gennaro, P.; Magoni, C.; Campne, L.; Labra, M.; Bruni, I. Artichoke (Cynara cadrunculus var. scolymus L.) by-products as a source of inulin: How to valorize an agricultural supply chain extracting an added-value compound. Nat. Prod. Res. 2022, 36, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAOSTAT). Crops Statistics. 2018. Available online: www.fao.org (accessed on 4 June 2018).
- Teterycz, D.; Michalak-Majewska, M. Artichoke (Cynara scolymus)—An innovative raw material with rich therapeutic properties. Zesz. Probl. Postep. Nauk. Rol. 2018, 593, 87–100. [Google Scholar]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Santo-Buelga, C.; Ferreira, I.C. Infusions of artichoke and milk thistle represent a good source of phenolic acids and flavonoids. Food Funct. 2015, 6, 56–62. [Google Scholar] [CrossRef]
- Sonnante, G.; De Paolis, A.; Lattanzio, V.; Paerrino, P. Genetic variation in wild and cultivated artichoke revealed by RAPD markers. Gen. Res. Crop. Evol. 2002, 49, 247–252. [Google Scholar] [CrossRef]
- Ceccarelli, J.E.; Curadi, M. Globe artichoke as a functional food. J. Nutr. Metab. 2010, 3, 197–201. [Google Scholar]
- Macchia, L.; Giliberti, L.A. Allergy to pomegranate and artichoke, novel food allergens of the Meditarranean diet. In Proceedings of the Food Allergy and Anaphylaxis Meeting (FAAM 2013), Nice, France, 7–9 February 2013. [Google Scholar]
- Salekzamani, S.; Ebrahimi-Mameghani, M.; Rezazadeh, K. The antioxidant activity of artichoke (Cynara scolymus): A sytemiatic review and meta-analysis of animal studies. Phytother. Res. 2019, 33, 55–71. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Corrado, G.; Colla, G.; Cardarelli, M.; Pascale, S.; Rouphael, Y. Phytochemical profile, mineral content, and bioactive compounds in leaves of seed-propagated artichoke hybrid cultivars. Molecules 2020, 25, 3795. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Villar, A.; Zangara, A.; Smith, C.R.; Risco, E. In vitro evaluation of prebiotic properties of a commercial artichoke inflorescence extract revealed bifidogenic effects. Nutrients 2020, 12, 1552. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Tucci, M.; De Palma, M. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunuculus L. var. scolumus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Lim, T.K. Cynara cardunculasi. In Edible Medicinal and Non-Medicinal Plants. Flowers; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2013; Volume 7, pp. 292–320. [Google Scholar]
- Lombardo, S.; Panino, G. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2010, 119, 1175–1181. [Google Scholar]
- Llorach, R.; Espin, J.C.; Tomas-Bargberan, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Rice-Evans, C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998, 29, 247–255. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.; Zapolski-Downar, A.; Naruszewicz, M.; Siennicka, A.; Krasnodebsk, B.; Kolodziej, B. Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci. 2002, 71, 2897–2908. [Google Scholar] [CrossRef]
- Vamanu, E.; Vamanu, A. Antioxidant and antimicrobial activities of ethanol extracts of Cynara scolumus (Cynara folium, Asteracea family). Trop. J. Harm. Res. 2011, 10, 777–783. [Google Scholar]
- Adzet, T.; Camarasa, J.; Carlos Laguna, J. Hepatoprotective activity of polyphenolic compounds from Cynara scolymus against CCl4 toxicity in isolated rat hepatocytes. J. Nat. Prod. 1987, 50, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R. Antioxidative and protective properties of extracts from leaves of the artichoke (Cynara scolymus L.) against hydroperoxide-induced oxidative stress in cultured rat hepatocytes. Toxicol. Appl. Pharmacol. 1997, 144, 279–286. [Google Scholar] [CrossRef]
- Perez-Garcia, F.; Adzet, T.; Canigueral, S. Activity of artichoke leaf extract on reactive oxygen species in human leukocytes. Free Rad. Res. 2000, 33, 661–665. [Google Scholar] [CrossRef]
- Rahimuddin, S.; Khoj, S.M.; Zuhair, M.M.; Howell, N.K.; Brown, J.E. Inhibition of lipid peroxidation in UVA-treated skin fibroblasts by luteolin and its glucosides. Eur. J. Lipid Sci. Technol. 2007, 109, 647–655. [Google Scholar] [CrossRef]
- Juzyszyn, Z.; Czerny, B.; Pawlik, A.; Drozdzik, M. The effect of artichoke (Cynara scolymus L.) extract on ROS generation in HUVEC cells. Phytother. Res. 2008, 22, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; D’antuono, I.; Linsalata, V.; Pizzi, F.; Minervini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: Influence of in vitro gastrointestinal digestion. J. Func. Foods 2014, 10, 456–464. [Google Scholar] [CrossRef]
- D’antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Func. 2015, 6, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Milackova, I.; Kapustova, K.; Mucaji, P.; Hosek, J. Artichoke leaf extract inhibits AKR1B1 and reduces NF-κB activity in human leukemic cells. Phytother. Res. 2017, 31, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Llorach, R.; Tomas-Barberan, F.A.; Ferreres, F. Functionalisation of commercial chicken soup with enriched polyphenol extract from vegetable by-products. Eur. Food Res. Technol. 2005, 220, 31–36. [Google Scholar] [CrossRef]
- Vacca, M.; Pinto, D.; Annunziato, A.; Ressa, A.; Calasso, M.; Pontonio, E.; Celano, G.; De Angelis, M. Gluten-free bread enriched with artichoke leaf extract in vitro exerted antioxidant and anti-inflammatory properties. Antioxidants 2023, 12, 845. [Google Scholar] [CrossRef]
- Shallan, M.A.; Ali, M.A.; Meshrf, W.A.; Marrez, D.A. In vitro antimicrobial, antioxidant and anticancer activities of globe artichoke (Cynara cardunculus var. scolymus L.) bracts and receptacles ethanolic extract. Biocat. Agricult. Biotechnol. 2020, 29, 101774. [Google Scholar] [CrossRef]
- Jimenez-Escrig, A.; Dragsted, L.O.; Danashvar, B.; Pulido, R.; Saura-Calixto, F. In vitro antioxidant activities of edible artichoke (Cynara scolymus L.) and effect on biomarker of antioxidants in rats. J. Agric. Food Chem. 2003, 51, 5540–5545. [Google Scholar] [CrossRef]
- Goni, I.; Jimenez-Escrig, A.; Gudiel, M.; Saura-Calixto, F.D. Artichoke (Cynara scolymus L) modifies bacterial enzymatic activities and antioxidant status in rat cecum. Nutr. Res. 2005, 25, 607–615. [Google Scholar] [CrossRef][Green Version]
- Kaymaz, M.B.; Kandemir, F.M.; Pamukcu, E.; Eroksuz, Y.; Ozdemir, N. Effects of aqueous artichoke (Cynara scolymus) leaf extract on hepatic damage generated by alpha-amanitine. Kafkas Univ. Veter. Fakult. Der. 2017, 23, 155–160. [Google Scholar]
- Abdel-Kader, M.M.; El-Sayed, E.M.; Kassem, S.S.; El-Din Haggag, M.; El-Hawary, Z. Protective and antioxidant effects of Cynara scolumus leaves against carbon tetrachloride toxicity in rats. Res. J. Pharmaceutic. Biol. Chem. Sci. 2014, 5, 1373–1380. [Google Scholar]
- Al-Ahdab, M.A. Protective effect of artichoke (Cynara scolymus L.) leaves and pulp extracts against carbon tetracholoride-induced acute hepatoxicity in rats. World Appl. Sci. J. 2014, 32, 1004–1014. [Google Scholar]
- Najim, S.M.; Numan, I.T.; Hamad, M.N. The possible cardioprotective effects of different fractions of artichoke extracts against 5-Fu induced cardiotoxicity in albino rats. Int. J. Pharm. Pharmceut. Sci. 2015, 7, 165–169. [Google Scholar]
- Jaleel, G.A.A.; Saleh, D.O.; El-Awdan, S.A. Beneficial effect of artichoke leaf extract on ethylene glycol-induced urolithiasis in rats. Int. J. Pharm. Phytochem. Res. 2016, 8, 960–967. [Google Scholar]
- Khattab, H.A.; Wazzan, M.A.; Al-Ahdab, M.A. Nephroprotective potential of artichoke leaves extract against gentamicin in rats: Antioxidant mechanisms. Pak. J. Pharmaceut. Sci. 2016, 29, 1775–1782. [Google Scholar]
- Colak, E.; Ustuner, M.C.; Tekin, N.; Colak, E.; Burukoglu, D.; Degirmanci, I.; Gunes, H.V. The hepatocurative effects of Cynara scolymus L. leaf extract on carbon tetrachloride-induced oxidative stress and hepatic injury in rats. Sprigerplus 2016, 5, 216. [Google Scholar] [CrossRef] [PubMed]
- El-Boshy, M.; Ashshi, A.; Gaith, M.; Qusty, N.; Bokhary, T.; Altaweel, N.; Abdelhady, M. Studies on the protective effect of the artichoke (Cynara scolymus) leaf extract against cadmium toxicity-induced oxidative stress, hepatorenal damage, and immunosuppressive and hematological disorders in rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 12372–12383. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Soofiniya, Y. Hypolipidemic and hypoglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. J. Med. Plant Res. 2011, 5, 2717–2723. [Google Scholar]
- Magielse, J.; Verlaet, A.; Breynaert, A.; Keenoy, B.M.; Apers, S.; Pieters, L.; Hermans, N. Investigation of the in vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol. Nutr. Food Res. 2014, 58, 211–215. [Google Scholar] [CrossRef]
- Ben Salem, M.; Affes, H. Chemicals composition, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evid. Based Compl. Alternat. Med. 2017, 1, 4951937. [Google Scholar]
- Kucukgergin, C.; Aydin, A.F.; Ozdemirler-Erata, G.; Mehmetcik, G.; Kocak-Toker, N.; Uysal, M. Effect of artichoke leaf extract on hepatic and cardiac oxidative stress in rats fed on high cholesterol diet. Biol. Trace Elem. Res. 2010, 135, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Kusku-Kiraz, Z.; Mehmetcik, G.; Dogru-Abbasoglu, S.; Uysal, M. Artichoke leaf extract reduces oxidative stress and lipoprotein dyshomeostsis in rats fed on high cholesterol diet. Phytother. Res. 2010, 24, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Jafari-Dehkordi, E.; Seidkhani-Nahal, A. Lipid-lowering effect of artichoke on liver phosphatidate phosphohydralase and plasma lipids in hyperlipidemic rats. J. Med. Plant Res. 2011, 5, 4918–4924. [Google Scholar]
- Magied, M.M.A.; Hussien, S.; Zaki, S.M.; Said, R. Artichoke (Cynara scolymus L.) leaves and heads extract as hypoglycemic and hypocholesterolemic in rats. J. Food Nutr. Res. 2016, 4, 60–68. [Google Scholar]
- Mocelin, R.; Marcon, M.; Santo, G.D.; Zanatta, L.; Sachett, A.; Schönell, A.P.; Bevilaqua, F.; Giachini, M.; Chitolina, R.; Wildner, S.M.; et al. Hypolipidemic and antiathergenic effects of Cynara scolymus in cholesterol-fed rats. Braz. J. Pharm. 2016, 26, 233–239. [Google Scholar] [CrossRef]
- Ben Salem, M.; Affes, H.; Dhouibi, R.; Charfi, S.; Turki, M.; Hammami, S.; Ayedi, F.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Effect of artichoke (Cynara scolymus) on cardiac markers, lipid profile and antioxidants levels in tissue of HFD-induced obesity. Arch. Physiol. Biochem. 2022, 128, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, M.; Affes, H.; Dhouibi, R.; Charfi, S.; Turki, M.; Hammami, S.; Ayedi, F.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Preventive effect of artichoke (Cynara scolymus L.) in kidney dysfunction against high-diet induced obesity in rats. Arch. Physiol. Biochem. 2022, 128, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Florek, E.; Szukalska, M.; Markiewicz, K.; Miechowicz, I.; Gornowicz-Porowska, J.; Jelinska, A.; Kasprzyk-Pochopien, J.; Nawrot, J.; Sobczak, A.; Horoszkiewicz, M.; et al. Evaluation of the protective and regeneartive properties of commercially available artichoke leaf powder extract on plasma and liver oxidative stress parameters. Antioxidants 2023, 11, 1846. [Google Scholar] [CrossRef]
- Rezazadeh, K.; Rahmati-Yamchi, M.; Mohammadnejad, L.; Ebrahimi-Mameghani, M.; Delazar, A. Effects of artichoke leaf extract supplementation on metabolic parameters in women with metabolic syndrome: Influence of TCFL2-rs7903146 and FTO-rs9939609 polymorphisms. Phytother. Res. 2018, 32, 84–93. [Google Scholar] [CrossRef]
- Rezazadeh, K.; Rezazadeh, F.; Ebrahimi-Mamaghani, M. The effect of artichoke leaf extract supplementation on lipid and CETP response in metabolic syndrome with respect to Taq 1B CETP polymorphism: A randomized controlled clinical trial. Eur. J. Integr. Med. 2018, 17, 112–118. [Google Scholar] [CrossRef]
- Rezazadeh, K.; Aliashrafi, S.; Asghari-Jafarabadi, M.; Ebrahimi-Mameghni, M. Antioxidant response to artichoke leaf extract supplementation in metabolic syndrome: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2018, 37, 790–796. [Google Scholar] [CrossRef]
- Skarpanska-Stejnborn, A.; Pilaczynska-Szczesniak, L.; Basta, P.; Deskur-Smielcka, E.; Horoszkiewicz-Hassan, M. The influence of supplementation with artichoke (Cynara scolymus L.) extract on selected redox parameters in rowers. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Lohr, G.; Deters, A.; Hensel, A. In vitro investigations of Cynara scolymus L. extract on cell physiology of HepG2 liver cells. Braz. J. Pharmaceut. Sci. 2009, 45, 201–208. [Google Scholar] [CrossRef]
- Gebhardt, R.; Fausel, M. Antioxidant and hepatoprotective effects of artichoke extracts and constituents in cultured rat hepatocytes. Toxic Vitro 1997, 11, 669–672. [Google Scholar] [CrossRef]
- Frigerio, J.; Tedesco, E.; Benetti, F.; Insolia, V.; Nicotra, G.; Mezzasalma, V.; Pagliari, S.; Labra, M.; Campone, L. Anticholesterolemic activity of three vegetal extracts (artichoke, caigua, and fenugreek) and their unique blend. Front. Pharmacol. 2021, 12, 726199. [Google Scholar] [CrossRef]
- Mathew, A.; Deng, Z.; Nelson, C.J.; Mayberry, T.G.; Bai, Q.; Lequio, M.; Fajardo, E.; Xiao, H.; Wakefield, M.R.; Fang, Y. Artichoke as a melanoma growth inhibitor. Med. Oncol. 2023, 40, 262. [Google Scholar] [CrossRef]
- Villarini, M.; Acito, M.; di Vito, R.; Vannini, S.; Dominici, L.; Fatigoni, C.; Pagiotti, R.; Moretti, M. Pro-apoptotic activity of artichoke leaf extracts in human HT-29 and RKO colon cancer cells. Int. J. Environ. Res. Public Health 2021, 18, 4166. [Google Scholar] [CrossRef]
- Miccadei, S.; Di Venere, D.; Cardinali, A.; Romano, F.; Durazzo, A.; Foddai, M.; Fraioli, R.; Mbarhan, S.; Maiani, G. Antioxidative and apoptotic properties of polyphenolic extracts from edible part of artichoke (Cynara scolymus L.) on cultured rat hepatocytes and on human hepatoma cells. Nutr. Cancer 2008, 60, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Di Venere, D.; Mardente, S.; Miccadei, S. Artichoke polyphenols sensitize human breast cancer cells to chemotherapeutic drugs via a ROS-mediated downregulation of flap endonuclease 1. Oxid. Med. Cell. Longev. 2020, 1, 7965435. [Google Scholar] [CrossRef]
- El Morsy, E.M.; Kamel, R. Protective effect of artichoke leaf extract against paracetamol-induced hepatotoxicity in rats. Pharm. Biol. 2015, 53, 167–173. [Google Scholar] [CrossRef]
- Song, S.; Zhao, L.; Feng, L.; Wang, W.; Cong, T.; He, H. Using aging rats model to investigate anti-oxidative ability of artichoke (Cynara scolymus L.) leaf extract. Acta Hort. 2012, 944, 1–7. [Google Scholar]
- Deng, A.; Liu, F.; Tang, X.; Wang, Y.; Xie, P.; Yang, Q.; Xiao, B. Water extract from artichoke ameliorates high-fat diet-induced non-alcoholic fatty liver disease in rats. BMC Complement Med. Ther. 2022, 22, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.Y.; Kim, S.Y.; Choi, M.S. Kuteolin-enriched artichoke leaf extract alleviates the metabolic syndrome in mice with high-fat diet-induced obesity. Nutrients 2018, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Mehmetcik, G.; Ozdemirler, G.; Kocak-Toker, N.; Cevikbas, U.; Uysal, M. Effect of pretreatment with artichoke extract on carbon tetrachloride-induced liver injury and oxidative stress. Exper. Toxicol. Path. 2008, 60, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Doostkam, A.; Fathalipour, M.; Anbardar, M.H.; Purkhosrow, A.; Mirkhani, H. Therapeutic effects of milk thistle (Silybum marianum L.) and artichoke (Cynara scolymus L.) on nonalcoholic fatty liver disease in type 2 diabetic rats. Can. J. Gastroenteral. Heapatol. 2022, 11, 2868904. [Google Scholar] [CrossRef] [PubMed]
- Nasef, M.A.; Yousef, M.I.; Ghareeb, D.A.; Augustyniak, M.; Aboul-Souds, M.A.M.; Wakil, A.E. Hepatoprotective effects of a chemically-characterized extract from artichoke (Cynara scolymus L.) against AFB1-induced toxicity in rats. Drug Chem. Toxicol. 2023, 46, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Heidarian, E.; Ghatreh-Samani, K. Modulatory effects of artichoke (Cynara scolymus L.) leaf extract against oxidative stress and hepatic TNF-α gene expression in acute diazinon-induced liver injury in rats. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 20180180. [Google Scholar] [CrossRef]
- Celepi, S.; Colak, B.; Celepi, P.; Bigat, I.; Batur, H.G.; Soysal, F.; Karakurt, S.; Hucumenoglu, S.; Kismet, K.; Sahin, M. Effects of artichoke leaf extract on hepatic ischemia-reperfusion injury. Rev. Assoc. Med. Bras. 2022, 68, 87–93. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Yousef, M.; Ghareeb, D.A.; Augustyniak, M.; Giesy, J.P.; Aboul-Soud, M.A.M.; Wakil, A.E. Artichoke leaf extract-mediated neuroprotection against effects of aflatoxin in male rats. BioMed Res. Int. 2022, 19, 4421828. [Google Scholar] [CrossRef]
- Piccinini, A.; Oliviera, M.P.; Silva, M.R.; Bett, G.S.; Becker, I.B.; Mendes, T.F.; Salla, D.H.; Silva, L.E.; Vilela, T.C.; Moraes, F.M.; et al. Effects of ethanolic extract of Cynara cardunculus (artichoke) leaves on neuroinflammatory and neurochemical parameters in a diet-induced mice obesity model. Neurochem. Res. 2022, 47, 1888–1903. [Google Scholar] [CrossRef]
- Cicek, B.; Genc, S.; Yeni, Y.; Kuzucu, M.; Cetin, A.; Yildirim, S.; Bolat, I.; Kantarci, M.; Hacimuftuoglu, A.; Lazopoulos, G.; et al. Artichoke (Cynara scolymus) methanolic leaf extract alleviates diethylnitrosamine-induced toxicity in BALB-c mouse brain: Involvement of oxidative stress and apoptotically related Klotho/PPARɤ signaling. J. Pers. Med. 2022, 4, 2012. [Google Scholar] [CrossRef]
- Ardalani, H.; Jandaghi, P.; Meraji, A.; Hassanpour Moghdam, M. The effect of Cynara scolymus on blood pressure and BMI in hypertensive patients: A randomized, double-blind, placebo-controlled, clinical trial. Complement Med. Res. 2020, 27, 40–46. [Google Scholar] [CrossRef]
- Holtmann, G.; Adam, B.; Haag, S.; Collet, W.; Grunewald, E.; Windeck, T. Efficacy of artichoke leaf extract in the treatment of patients with functional dyspepsia: A six-week placebo-controlled, double-blind, multicenter trial. Aliment. Pharmacol. Ther. 2003, 18, 1099–1105. [Google Scholar] [CrossRef]
- Ebrahimi-Mamaeghani, M.; Asghari-Jafarabadi, M.; Rezazadeh, K. TCFL2-rs7903146 polymorphism modulates the effect of artichoke leaf extract supplementation on insulinresistance in metabolic syndrome: A randomized, double-blind, placebo-controlled trial. J. Integr. Med. 2018, 16, 329–334. [Google Scholar] [CrossRef]
- Rondanelli, M.; Giacosa, A.; Opizzi, A.; Faliva, M.A.; Sala, P.; Perna, S.; Riva, A.; Morazzoni, P.; Bombardelli, E. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolemia: A double-blind, randomized, placebo-controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 7–15. [Google Scholar] [CrossRef]
- Rondanelli, M.; Castellazzi, A.M.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Naso, M.; Nichetti, M.; Tagliacarne, C.; Valsecchi, C.; et al. Natural killer response and lipo-metabolic profile in adults with low HDL-cholesterol and mild hypercholesterolemia: Beneficial effects of artichoke leaf extract supplementation. Evid. Based Complement. Alternat. Med. 2019, 1, 2069701. [Google Scholar] [CrossRef] [PubMed]
- Marakis, G.; Walker, A.F.; Middleton, R.W.; Booth, J.C.; Wright, J.; Pike, D.J. Artichoke leaf extract reduces mild dyspepsia in an open study. Phytomedicine 2002, 9, 694–699. [Google Scholar] [CrossRef]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Wallis, C.; Simpson, H.C. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trail. Phytomedicine 2008, 15, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.I.; Mohamed, T.K.; Ekshamy, A.I.; El-Toumy, S.A.; Abdel Lateef, A.M.; Farrag, A.R. Chemical constituents and anti-ulcerogenic potential of the scales of Cynara scolymus (artichoke) heads. J. Sci. Food Agric. 2013, 93, 2494–2501. [Google Scholar] [CrossRef]
- Fantini, N.; Colomobo, G.; Giori, A.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Carai, M.A. Evidence of glycemia-lowering effect by a Cynara scolymus L. extract in normal and obese rats. Phytother. Res. 2011, 25, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Jiang, S.L.; Liu, S.B.; Peng, J.B.; Hu, S.; Wang, X.; Zhou, W.; Liu, T.; Guo, J.W.; Zhou, H.H.; et al. Metabolomics of artichoke bud extract in spontaneously hypertensive rats. ACS Omega 2021, 6, 18610–18622. [Google Scholar] [CrossRef]
- Roghani-Dehkordi, F.; Kamkhah, A.F. Artichoke leaf juice contains antihypertensive effect in patients with mild hypertension. J. Diet. Suppl. 2009, 6, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Elasayed, E.G.; Abdou, A.G.; Maher, T.D.; Motawea, S.M. Hepatoprotective effect of artichoke leaf extracts in comparison with silymarin on acetaminophen-induced hepatotoxicity in mice. J. Imunoassay Immunochem. 2020, 41, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.C.; Jhuang, J.H.; Yao, H.T. Artichoke leaf extract supplementation lowers hepatic oxidative stress and inflammation and increases multidrug resistance-associated protein 2 in mice fed a high-fat and high-cholesterol diet. Food Funct. 2021, 12, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Arfuso, F.; Giudice, E.; Rizzo, M.; Piccione, G.; Mhalhel, K.; Levandi, M. Antioxidant and hepatoprotective effect of a nutritional supplement with silymarin phytosome, choline chloride, L-cystine, artichoke, and vitamin E in dogs. Antioxidants 2022, 11, 2339. [Google Scholar] [CrossRef] [PubMed]
- Sumer, E.; Sentruk, G.E.; DEmirel, O.U.; Yesilada, E. Comparative biochemical and histopathological evaluations proved that receptacle is the most effective part of Cynara scolymus against liver and kidney damages. J. Ethnopharmacol. 2020, 249, 112458. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Atkin, S.L.; Butler, A.E.; Jafari, R.; Badeli, R.; Sahebkar, A. Efficacy of artichoke leaf extract in non-alcoholic fatty liver disease: A pilot double-blind randomized controlled trial. Phytother. Res. 2018, 32, 1382–1387. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Ataee, M.; Bahrami, G.; Heydarpour, F.; Aneva, I.Y.; Farzaaei, M.H.; Ahmadi-Juoibari, T. The effects of co-administration of artichoke leaf extract supplementation with metformin and vitamin E in patients with monalcoholic fatty liver disease: A randomized clinical trial. Phytother. Res. 2021, 35, 6324–6334. [Google Scholar] [CrossRef]
- Moradi, S.; Shokri-Mashhadi, N.; Saraf-Bank, S.; Mohammadi, H.; Zobeiri, M.; Clark, C.C.T.; Rouhani, M.H. The effects of Cynara scolymus L. supplementation on liver enzymes: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14726. [Google Scholar] [CrossRef]
- Moradi, M.; Sohrabi, G.; Golbidi, M.; Yarmohammadi, S.; Hemati, N.; Campbell, M.S.; Moradi, S.; Kermani, M.A.H.; Farzaei, M.H. Effects of artichoke on blood pressure: A systematic review and meta-analysis. Comp. Ther. Med. 2021, 57, 102668. [Google Scholar] [CrossRef]
- Amini, M.R.; Sheikhhossein, F.; Talebyan, A.; Bazshahi, E.; Djafari, F.; Hekmatdoost, A. Effects of artichoke supplementation on liver enzymes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. Res. 2022, 11, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.R.; Sheikhhossein, F.; Alvani, M.; Shoura, S.M.S.; Sohrabnavi, A.; Heidarian, E.; Hekmatdoost, A. Anti-hypertensive effects of rtaichoke supplementation in adults: A systematic review and dose-response meta-anlysis of randomized controlled trials. Clin. Nutr. Res. 2022, 26, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.M.; Farag, M.A. Therapeutic potential of artichoke in the treatment of fatty liver: A systematic review and meta-analysis. J. Med. Food 2022, 10, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wei, R.; Deng, A.; Lei, T. Protective effects of ethanolic extracts from artichoke, an edible herbal medicine, against acute alcohol-induced liver injury in mice. Nutrients 2017, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, D.; Park, S.J.; Kim, K.S.; Park, G.D.; Kim, O.K.; Lee, J. Artichoke extract directly suppresses inflammation and apoptosis in hepatocytes during the development of non-alcoholic fatty liver disease. J. Med. Food 2021, 24, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Fonts, S.; Garcia-Alday, I.; Petrangolini, G.; Riva, A.; Frattini, E.; Allegrini, P.; Togni, S.; Vitale, J. Efficacy of artichoke and ginger extracts with simethicone to treat gastrointestinal symptoms in endurance athletes: A pilot study. Minerva Gastroenterol. 2022, 68, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.F.; Middleton, R.W.; Petrowicz, O. Artichoke leaf extract reduces symptoms of irritable bowel syndrome in a post-marketing surveillance study. Phytother. Res. 2001, 15, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate model of mice colitis. Artificial neural network modelling of inflammatory markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef]
- Sahebkar, A.; Pirro, M.; Banach, M.; Mikhailidis, D.; Atkin, S.L.; Cicero, A.F.G. Lipid-lowering activity of artichoke extracts: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2549–2556. [Google Scholar] [CrossRef]
- Arnaboldi, L.; Corsini, A.; Bellosta, S. Artichoke and bergamot extracts: A new opportunity for the management of dyslipidemia and related risk factors. Minerva Med. 2022, 113, 141–157. [Google Scholar] [CrossRef]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef]
- Shimoda, H.; Ninomiya, K.; Nishida, N.; Yoshino, T.; Morikawa, T.; Matsuda, H.; Yoshikawa, M. Anti-hyperlipidemic sesquiterpenes and new sesquiterpene glycosides from the leaves of artichoke (Cynara scolymus L.): Structure requirement and mode of action. Bioorg. Med. Chem. Lett. 2003, 13, 223–228. [Google Scholar] [CrossRef]
- Cicero, A.R.G.; Fogacci, F.; Tocci, G.; D’Addato, S.; Grandi, E.; Banach, M.; Borghi, C. Three arms, double-blind, non-inferiority, randomized clinical study testing the lipid-lowering effect of a novel dietary supplement containing red yeast rice and artichoke extracts compared to Armolipid Plus® and placebo. Arch. Med. Sci. 2023, 19, 1169–1179. [Google Scholar] [CrossRef]
- Mahboubi, M. Cynara scolymus (artichoke) and its efficacy in management of obesity. Bull. Fac. Pharm. 2018, 56, 115–120. [Google Scholar] [CrossRef]
- Feiden, T.; Valduga, E.; Zeni, J.; Steffens, J. Bioactive compounds from artichoke and application potential. FTB 2023, 3, 312–327. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Abbas, H.; Zewail, M.; Noureldin, M.H.; Ali, M.M.; Shamaa, M.M.; Khattab, M.A.; Inbrahim, N. Neuroprotective effect of artichoke-based nanoformulation in sporadic Alzheimer’s disease mouse model: Focus on antioxidant, anti-inflammatory, and amyloidogenic pathways. Pharmaceuticals 2022, 15, 1202. [Google Scholar] [CrossRef]
- Jalili, C.; Moradi, S.; Babaei, A.; Boozari, B.; Asbaghi, O.; Lazaridi, A.V.; Kermani, M.A.H.; Miraghajani, M. Effects of Cynara scolymus L. on glycemic indices: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2020, 52, 102496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zhang, H.X.; Lo, R. Antifungal activity of Cynara scilymus L. extract. Fitoterapia 2005, 76, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A.I.M.; Goda, M.S.; Farrag, A.F.S.; Nasr, A.M.; Swidan, S.A.; Nafie, M.S.; Abdel-Kader, M.S.; Badr, J.M.; Abdelhameed, R.F.A. Silver nanoparticles formulation of flower head’s polyphenols of Cynara scolymus L.: A promising candidate against prostate (PC-3) cancer cell line through apoptosis activation. Molecules 2022, 27, 6304. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A.I.M.; Farrag, A.F.S.; Nasr, A.M.; Swidan, S.A.; Nafie, M.S.; Abdel-Kader, M.S.; Goda, M.S.; Badr, J.M.; Abdelhameed, R.F.A. Comparative estimation of the cytotoxic activity of different parts of Cynara scolymus L.: Crude extracts versus green synthesized silver nanoparticles with apoptotic investigation. Pharmaceutics 2022, 14, 2185. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Gulihaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and anti-inflammatory protective properties of human enriched serum following artichoke leaf extract absorption: Results from an innovative ex vivo clinical trial. Nutrients 2021, 13, 2653. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Science Medicines Health, EMA/HMPC/150209/2009; 13 September 2011. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-cynara-scolymus-l-folium_en.pdf (accessed on 13 February 2009).
- Englisch, W.; Beckers, M.; Unkauf, M.; Ruepp, M.; Zinserling, V. Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung 2000, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Bugianesi, R.; Romano, F.; Di Venere, D.; Miccadei, S.; Durazzo, A.; Foddai, M.S.; Ctasta, G.; Linsalata, V.; Maiani, G. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: A pilot study. Br. J. Nutr. 2007, 97, 963–969. [Google Scholar] [CrossRef] [PubMed]

| Artichoke Preparations | Dosage and Duration | Models | Biological Activity | References |
|---|---|---|---|---|
| In vitro model | ||||
| Leaf extract | 50 µg/mL; 2 h | Human monocyte leukemia cell line exposed to LPS | Antioxidant activity | [27] |
| Leaf extract | 1–100 µg/mL; 2 h | Human liver cells HepG2-ethanol-induced cell toxicity | Antioxidant activity; hepatoprotective activity | [56] |
| Leaf extract | 25–100 µg/mL; 24 h | Monocytes and endothelial cells exposed to ox-LDL, LPS and TNFα | Antioxidant activity | [18] |
| Leaf extract | 100 µg/mL and 1 ng/mL | Human leukocytes exposed to N-formyl-methionyl-leucyl-phenylalanine, phorbol myristate acetate, and H2O2 | Antioxidant activity | [22] |
| Leaf extract | 1–20 µg/mL | LDL | Antioxidant activity | [17] |
| Leaf extract | 0.001–1 mg/mL; 40 min | Culture rat hepatocytes exposed to t-BHP | Antioxidant activity; hepatoprotective activity | [21] |
| Leaf extract | 0.005 and 0.5 mg/mL; 40 min | Culture rat hepatocytes exposed to H2O2 | Antioxidant activity; hepatoprotective activity | [57] |
| Leaf extract | 50–250 µg/mL; 48 h | Human hepatic cell line (HepG2) | Anticholesterolemic activity | [58] |
| Leaf extract | 50 µg/mL; 72 h | Melanoma cell line HTB-72 | Anti-cancer activity | [59] |
| Leaf extract | 1–4 µg/mL; 4 h | Human HT-29 and RKO colon cancer cells | Anti-cancer activity | [60] |
| Head extract | 0.18–1.44 µg/mL; 1–4 h | Human LDL | Antioxidant activity | [26] |
| Head extract | 0.75–24 µg/mL; 30 min | Human intestinal cell line (HT-29) exposed to H2O2 | Antioxidant activity | [25] |
| Head extract | 400–1200 µM; 24 h | Human hepatoma HepG2 cells and cultured rat hepatocytes exposed to glucose oxidase | Antioxidant activity; hepatoprotective activity | [61] |
| Head extract | 12.5–50 µM; 24 h | Human breast cell lines (MCF7 and MDA-MB231) | Anti-cancer activity | [62] |
| Head extract | 5 µg/L; 24 or 48 h | Colonic microbiota | Prebiotic effect | [12] |
| In vivo model | ||||
| Leaf extract | 200 and 400 mg/kg; 4 weeks | Diabetic rats | Antioxidant activity; hypoglycaemic action | [43] |
| Leaf extract | 200 and 400 mg/kg; 60 days | Rats with obesity | Antioxidant activity; renoprotective activity | [50] |
| Leaf extract | 300 mg/kg; 4 weeks | Rats treated with Cd | Antioxidant activity; renoprotective; hepatoprotective activity; reducing hematological disturbances | [40] |
| Leaf extract | 1500 mg/kg; 2 weeks | Rats with α-amanitine induced hepatotoxicity | Antioxidant activity; hepatoprotective activity | [33] |
| Leaf extract | 1500 mg/kg; 2 weeks | Rats with CCl4 induced hepatotoxicity | Antioxidant activity; hepatoprotective activity | [39] |
| Leaf extract | 125–500 mg/kg; 4 weeks | Rats with ethylene glycol-induced urolithiasis | Antioxidant activity | [37] |
| Leaf extract | 200–600 mg/kg; 10 days | Rats with gentamicin-induced nephrotoxicity | Antioxidant activity; renoprotective activity | [38] |
| Leaf extract | 150–600 mg/kg; 30 days | Hypercholesterolemic rats | Antioxidant activity | [48] |
| Leaf extract | 1500 and 3000 mg/kg; 42 days | Hypercholesterolemic rats | Antioxidant activity | [47] |
| Leaf extract | 1500 mg/kg; 2 weeks | Rats with paracetamol-induced liver injury | Antioxidant activity; hepatoprotective activity | [63] |
| Leaf extract | 200 mg/kg; 30 days | Rats with 5-flurouracil induced cardiotoxicity | Antioxidant activity | [36] |
| Leaf extract | 20% and 40% of diets; 4 weeks | Rats treated with CCl4 | Antioxidant activity | [34] |
| Leaf extract | 200 and 1000 mg/kg; 3 weeks | Diabetic rats | Antioxidant activity | [42] |
| Leaf extract | 20–80 mg/kg; 36 days | Aging rats | Antioxidant activity | [64] |
| Leaf extract | 200 and 400 mg/kg; 21 days | Diabetes rats | Antioxidant activity | [41] |
| Leaf extract | 0.4, 0.8, and 1.6 g/kg bw; 8 weeks | Rats treated with a high-fat diet | Antioxidant activity; hepatoprotective activity; anti-inflammatory effect; anti-insulin resistance effect | [65] |
| Leaf extract-rich in luteolin | 0.005%; 16 weeks | Mice with high-fat diet-induced obesity | Anti-obesity effect | [66] |
| Leaf extract | 1500 mg/kg; 2 weeks | Hypercholesterolemic rats | Antioxidant activity; hepatoprotective activity | [44] |
| Leaf extract | 1500 mg/kg; 2 weeks | Hypercholesterolemic rats | Antioxidant activity | [45] |
| Leaf extract | 1500 mg/kg; 2 weeks | Rats treated with CCl4 | Antioxidant activity | [67] |
| Leaf extract | 200 and 400 mg/kg; 6 weeks | Rats treated with CCl4 | Antioxidant activity; hepatoprotective activity | [35] |
| Leaf extract | 200 and 400 mg/kg; 60 days | Wistar rats with a high-fat diet | Antioxidant activity; cardioprotective action | [49] |
| Leaf extract | 0.5, 1, and 1.5 g/kg bw/day; 2 weeks | Rats treated with CCl4 | Antioxidant activity | [51] |
| Leaf extract (Atheromod-B®, Barij Essence Pharmaceutical Co., Tehran, Iran) | 60 mg/kg/day; 8 weeks | Type 2 diabetic rats | Antioxidant activity; cardioprotective action; hepatoprotective effect (no date) | [68] |
| Leaf extract | 100 mg/kg/day; 42 days | Male albino rats treated with aflatoxin B1 | Antioxidant activity; hepatoprotective effect | [69] |
| Leaf extract | 1500 mg/kg/day; 15 days | Rats | Antioxidant activity; hepatoprotective effect | [70] |
| Leaf extract | 30 mg/kg | Rats | Antioxidant activity; hepatoprotective effect | [71] |
| Leaf extract | 100 mg/kg; 42 days | Rats treated with aflatoxin B1 | Neuroprotectory effect | [72] |
| Leaf extract | 1600 mg/kg/day; 4 weeks | Mice with obesity | Antioxidant activity; hepatoprotective effect; anti-obesity potential | [73] |
| Leaf extract | 0.8 and 1.6 g/kg/day; 2 weeks | Mice treated with diethylnitrosamine | Neuroprotectory effect | [74] |
| Leaf extract | 500 mg twice daily, 8 weeks | Hypertensive patients | Improving BMI and systolic blood pressure | [75] |
| Leaf extract | 1800 mg/day; 12 weeks | Patients with metabolic syndrome | Antioxidant activity | [52] |
| Leaf extract | 1200 mg/day; 5 weeks | Members of a rowing team | Antioxidant activity | [55] |
| Leaf extract (HeparSL® forte, MCM Klosterfrau Vert. Gmbh, Berlin, Germany) | 320 mg/day; 6 weeks | Patients with functional dyspepsia | Alleviating symptoms and improving the disease-specific quality of life | [76] |
| Leaf extract | 1800 mg/day; 12 weeks | Patients with metabolic syndrome | Hypoglycemic action | [77] |
| Leaf extract | 250 mg/day; 8 weeks | Overweight subjects with mild hypercholesterolemia | Cardioprotective action | [78] |
| Leaf extract | 250 mg, twice daily; 60 days | Adults with low HDL-cholesterol and mild hypercholesterolemia | Cardioprotective action | [79] |
| Leaf extract | 320 or 640 mg/day; 2 months | Otherwise healthy subjects suffering from dyspepsia | Alleviating symptoms and improving the disease-specific quality of life | [80] |
| Leaf extract | 1280 mg/day; 3 months | Healthy adults with mild to moderate hypercholesterolemia | Cardioprotective action | [81] |
| Head extract | 1500 and 3000 mg/kg; 42 days | Hypercholesterolemic rats | Antioxidant activity | [47] |
| Head extract | 14% of diets; 3 weeks | Normal rats | Antioxidant activity | [32] |
| Head extract | 14% of diets; 3 weeks | Normal rats | Antioxidant activity | [31] |
| Head extract | 200 and 400 mg/kg; 2 weeks | Rats | Antiulcerogenic activity | [82] |
| Head extract | 500–1500 mg/kg | Normal and obese rats | Hypoglycemic action | [83] |
| Pulp (hearts and heads) extract | 200 and 400 mg/kg; 6 weeks | Rats treated with CCl4 | Antioxidant activity | [35] |
| Artichoke pellets (made by mixing dried and crushed artichoke) | 10% of diets; 45 days | Hyperlipidemic rats | Antioxidant activity | [46] |
| Bud extract | 50 and 100 mg/kg/day; 4 weeks | Hypertensive rats | Anti-hypertension effect | [84] |
| Leaf juice | 50 and 100 mg juice concentrate; 12 weeks | Patients with mild hypertension | Anti-hypertension effect | [85] |
| Country | Dietary Supplement | Diseases | Advance Effect |
|---|---|---|---|
| Austria | 1–2 coated tables or capsules (350 mg), three times daily | Digestive complaints, dyspepsia | No advance effect known |
| Belgium | Three coated tables or capsules (200 mg), two times daily | Digestive complaints, dyspepsia | No advance effect known |
| Bulgaria | 1–2 coated tablets, three times daily, or 1–2 teaspoon of solution, three times daily | Dyspepsia, and enhancing the fatty acid metabolism | Hypersensibility, diarrhea, nausea, flatulence |
| France | 1–2 coated tablets (200 mg), three times daily, or 1 ampule (10 mL), three times daily | Digestive and urinary complaints, dyspepsia | No advance effect known |
| Germany | One coated tablet (300 mg), 1–2 times daily | Digestive complaints, dyspepsia | Slight diarrhea, epigastric complaints |
| Poland | Two tablets, one a day (digestive disorders) or 2 tablets, three times daily (hyperlipidaemia) | Digestive complaints and hepatobiliary disturbances, mild to moderate hyperlipidemia | Mild gastro-intestinal disturbances |
| Spain | 600–1500 mg a day–capsules | Dyspepsia | No advance effect known |
| United Kingdom | one capsule, twice daily | Digestive complaints | No advance effect known |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olas, B. An Overview of the Versatility of the Parts of the Globe Artichoke (Cynara scolymus L.), Its By-Products and Dietary Supplements. Nutrients 2024, 16, 599. https://doi.org/10.3390/nu16050599
Olas B. An Overview of the Versatility of the Parts of the Globe Artichoke (Cynara scolymus L.), Its By-Products and Dietary Supplements. Nutrients. 2024; 16(5):599. https://doi.org/10.3390/nu16050599
Chicago/Turabian StyleOlas, Beata. 2024. "An Overview of the Versatility of the Parts of the Globe Artichoke (Cynara scolymus L.), Its By-Products and Dietary Supplements" Nutrients 16, no. 5: 599. https://doi.org/10.3390/nu16050599
APA StyleOlas, B. (2024). An Overview of the Versatility of the Parts of the Globe Artichoke (Cynara scolymus L.), Its By-Products and Dietary Supplements. Nutrients, 16(5), 599. https://doi.org/10.3390/nu16050599





