Effects of Vitamin D3 and Marine Omega-3 Fatty Acids Supplementation on Biomarkers of Systemic Inflammation: 4-Year Findings from the VITAL Randomized Trial
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Population
2.2. Blood Collection and Laboratory Analysis
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants
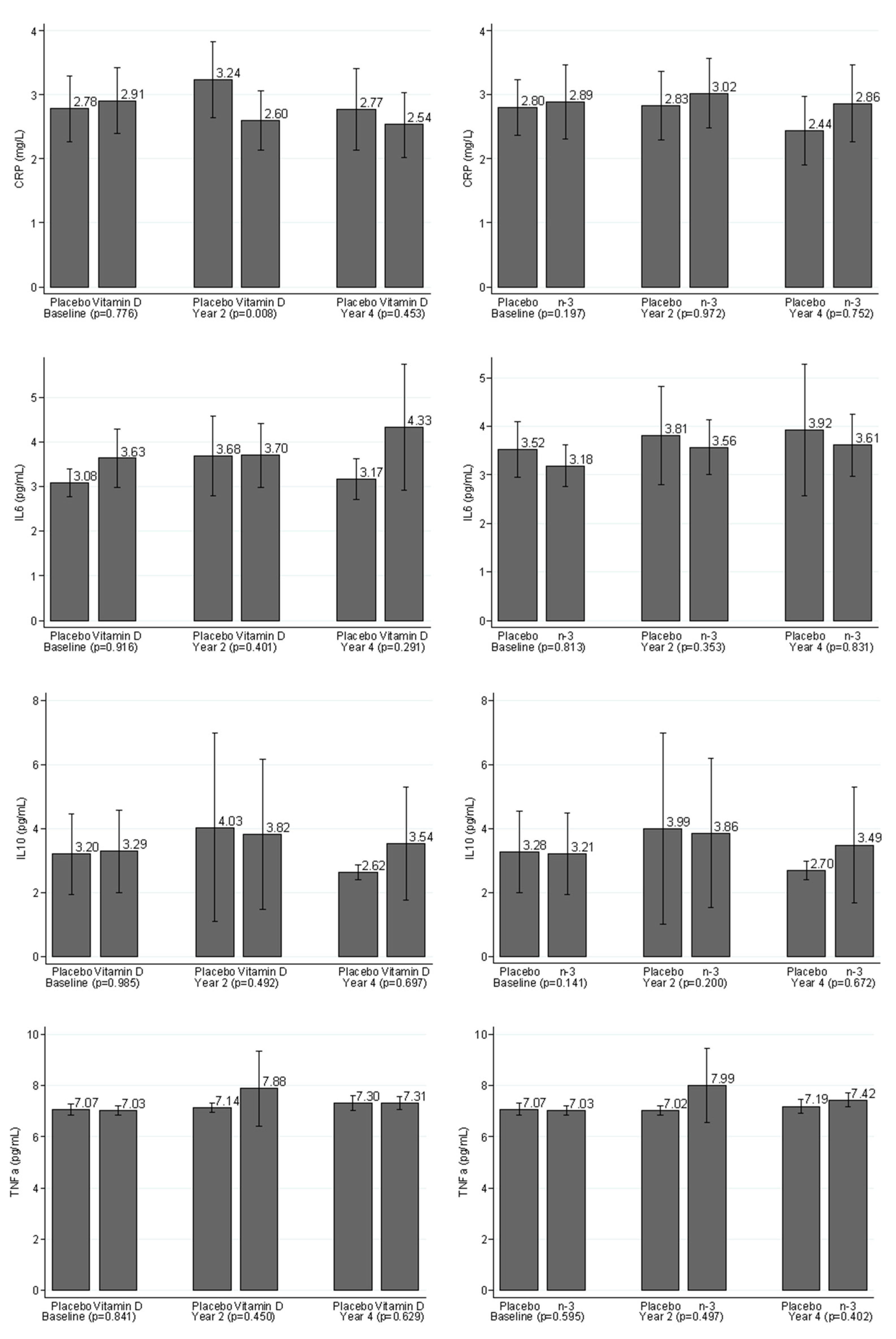
3.2. Comparisons of Levels of Inflammatory Markers between the Placebo Group and Treatment Group
3.3. Effects of Vitamin D3 Supplementation and Marine n-3 Fatty Acids on Changes in Markers of Inflammation in all Participants
3.4. Compliance-Adjusted Analysis
3.5. Exploratory Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Clinical Trial Registry
Abbreviations
References
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.-M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Batuca, J.; Alves, J.D. C-reactive protein in systemic lupus erythematosus. Autoimmunity 2009, 42, 282–285. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Katz, R.; Jenny, N.S.; Zakai, N.A.; LeWinter, M.M.; Barzilay, J.I.; Cushman, M. Metabolic Syndrome, Inflammation, and Incident Heart Failure in the Elderly. Circ. Heart Fail. 2008, 1, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Pepys, M.B.; Boeing, H.; Muche, R.; Brenner, H. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swardfager, W.; Lanctôt, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A Meta-Analysis of Cytokines in Alzheimer’s Disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Ostadmohammadi, V.; Doosti-Irani, A.; Ghayour-Mobarhan, M.; Ferns, G.; Akbari, H.; Ghaderi, A.; Talari, H.R.; Asemi, Z. The Effects of Vitamin D Supplementation on Biomarkers of Inflammation and Oxidative Stress in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2018, 50, 429–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamilian, H.; Amirani, E.; Milajerdi, A.; Kolahdooz, F.; Mirzaei, H.; Zaroudi, M.; Ghaderi, A.; Asemi, Z. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109651. [Google Scholar] [CrossRef]
- Akbari, M.; Ostadmohammadi, V.; Lankarani, K.B.; Tabrizi, R.; Kolahdooz, F.; Heydari, S.T.; Kavari, S.H.; Mirhosseini, N.; Mafi, A.; Dastorani, M.; et al. The Effects of Vitamin D Supplementation on Biomarkers of Inflammation and Oxidative Stress Among Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2018, 50, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Li, M.-S.; Lin, M.; Zhao, T.-Y.; Gao, P. Effect of fish oil supplement in maintenance hemodialysis patients: A systematic review and meta-analysis of published randomized controlled trials. Eur. J. Clin. Pharmacol. 2016, 72, 129–139. [Google Scholar] [CrossRef]
- Xu, T.; Sun, Y.; Sun, W.; Yao, L.; Sun, L.; Liu, L.; Ma, J.; Wang, L. Effect of omega-3 fatty acid supplementation on serum lipids and vascular inflammation in patients with end-stage renal disease: A meta-analysis. Sci. Rep. 2016, 6, 39346. [Google Scholar] [CrossRef]
- O’Mahoney, L.; Matu, J.; Price, O.J.; Birch, K.M.; Ajjan, R.A.; Farrar, D.; Tapp, R.; West, D.J.; Deighton, K.; Campbell, M.D. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Xin, W.; Wei, W.; Li, X. Effects of fish oil supplementation on inflammatory markers in chronic heart failure: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2012, 12, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, M.; Zhu, X.; Gao, P.; Yang, S.; Han, Y.; Chen, X.; Xiao, L.; Yuan, S.; Liu, F.; et al. Effects of Omega-3 Fatty Acids on Markers of Inflammation in Patients With Chronic Kidney Disease: A Controversial Issue. Ther. Apher. Dial. 2018, 22, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Senftleber, N.K.; Nielsen, S.M.; Andersen, J.R.; Bliddal, H.; Tarp, S.; Lauritzen, L.; Furst, D.E.; Suarez-Almazor, M.E.; Lyddiatt, A.; Christensen, R. Marine Oil Supplements for Arthritis Pain: A Systematic Review and Meta-Analysis of Randomized Trials. Nutrients 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124.e4. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Costenbader, K.H.; MacFarlane, L.A.; Lee, I.-M.; Buring, J.E.; Mora, S.; Bubes, V.; Kotler, G.; Camargo, C.A.; Manson, J.E.; Cook, N.R. Effects of One Year of Vitamin D and Marine Omega-3 Fatty Acid Supplementation on Biomarkers of Systemic Inflammation in Older US Adults. Clin. Chem. 2019, 65, 1508–1521. [Google Scholar] [CrossRef]
- Myburgh, P.H.; Towers, G.W.; Kruger, I.M.; Nienaber-Rousseau, C. CRP Genotypes Predict Increased Risk to Co-Present with Low Vitamin D and Elevated CRP in a Group of Healthy Black South African Women. Int. J. Environ. Res. Public Health 2018, 15, 111. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef]
- Heikkila, K.; Harris, R.; Lowe, G.; Rumley, A.; Yarnell, J.; Gallacher, J.; Ben-Shlomo, Y.; Ebrahim, S.; Lawlor, D.A. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: Findings from two prospective cohorts and a meta-analysis. Cancer Causes Control 2009, 20, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Amani, R.; Fazelian, S.; Paknahad, Z.; Kheiri, S.; Khajehali, L. Effect of Vitamin D supplement on mood status and inflammation in Vitamin D deficient Type 2 diabetic women with anxiety: A randomized clinical trial. Int. J. Prev. Med. 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Ebrahimi, F.A.; Aghadavod, E.; Talaee, R.; Jafarnejad, S.; Dizaji, S.H.; Asemi, Z. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J. Affect. Disord. 2018, 238, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Papageorgiou, M.; Deshmukh, H.; Kilpatrick, E.S.; Mann, V.; Corless, L.; Abouda, G.; Rigby, A.S.; Atkin, S.L.; Sathyapalan, T. A Randomized, Controlled Trial of Vitamin D Supplementation on Cardiovascular Risk Factors, Hormones, and Liver Markers in Women with Polycystic Ovary Syndrome. Nutrients 2019, 11, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Wang, B.; Han, W.; Zhu, Z.; Wang, X.; Jin, X.; Antony, B.; Cicuttini, F.; Wluka, A.; Winzenberg, T.; et al. Vitamin D supplementation and inflammatory and metabolic biomarkers in patients with knee osteoarthritis: Post hoc analysis of a randomised controlled trial. Br. J. Nutr. 2018, 120, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirhosseini, N.; Rainsbury, J.; Kimball, S.M. Vitamin D Supplementation, Serum 25(OH)D Concentrations and Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.-Q.; Li, X.-X.; Qiu, S.-Q.; Yu, Y.; Li, M.-G.; Yang, L.-T.; Li, L.-J.; Wang, S.; Zheng, P.-Y.; Yang, P.-C. Vitamin D contributes to mast cell stabilization. Allergy 2017, 72, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, J.; Pang, X.; Wang, S.; Wu, D.; Zhang, X.; Feng, L. Angiotensin II induces C-reactive protein expression via AT1-ROS-MAPK-NF-κB signal pathway in hepatocytes. Cell. Physiol. Biochem. 2013, 32, 569–580. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.; Yan, W.; Lu, L.; Tao, Y.; Li, F.; Wang, Y.; Cai, W. Effect of a fish oil-based lipid emulsion on intestinal failure-associated liver disease in children. Eur. J. Clin. Nutr. 2018, 72, 1364–1372. [Google Scholar] [CrossRef]
- Peres, A.; Dorneles, G.P.; Boeira, M.C.R.; Schipper, L.L.; Beretta, C.D.L.; Vilela, T.; Andrade, V.M.; Romão, P.R.T. Acute fish oil supplementation modulates the inflammatory response after strenuous exercise in obese men: A cross-over study. Prostaglandins Leukot. Essent. Fat. Acids 2018, 137, 5–11. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Effect of ω-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy: A meta-analysis of randomized control trials in China. Medicine 2018, 97, e0472. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, E.; Jamilian, M.; Dadpour, B.; Nezami, Z.; Vahedpoor, Z.; Mahmoodi, S.; Aghadavod, E.; Taghizadeh, M.; Hassan, A.B.; Asemi, Z. The effects of fish oil on gene expression in patients with polycystic ovary syndrome. Eur. J. Clin. Investig. 2018, 48, e12893. [Google Scholar] [CrossRef] [PubMed]
- Mateș, L.; Popa, D.-S.; Rusu, M.E.; Fizeșan, I.; Leucuța, D. Walnut Intake Interventions Targeting Biomarkers of Metabolic Syndrome and Inflammation in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2022, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Zhao, F.; Song, S.; Li, Y.; Xu, X.; Zhou, G.; Li, C. Fish oil, lard and soybean oil differentially shape gut microbiota of middle-aged rats. Sci. Rep. 2017, 7, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrington, J.L.; Chapkin, R.S.; Switzer, K.C.; Morris, J.S.; Mcmurray, D.N. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin. Exp. Immunol. 2001, 125, 499–507. [Google Scholar] [CrossRef]
- Fritsche, K.; Byrge, M.; Feng, C. Dietary omega-3 polyunsaturated fatty acids from fish oil reduce interleukin-12 and interferon-gamma production in mice. Immunol. Lett. 1999, 65, 167–173. [Google Scholar] [CrossRef]
- Koppelmann, T.; Pollak, Y.; Ben-Shahar, Y.; Gorelik, G.; Sukhotnik, I. The Mechanisms of the Anti-Inflammatory and Anti-Apoptotic Effects of Omega-3 Polyunsaturated Fatty Acids during Methotrexate-Induced Intestinal Damage in Cell Line and in a Rat Model. Nutrients 2021, 13, 888. [Google Scholar] [CrossRef]
- Bonatto, S.J.R.; Folador, A.; Aikawa, J.; Yamazaki, R.K.; Pizato, N.; Oliveira, H.H.; Vecchi, R.; Curi, R.; Calder, P.; Fernandes, L.C. Lifelong exposure to dietary fish oil alters macrophage responses in Walker 256 tumor-bearing rats. Cell. Immunol. 2004, 231, 56–62. [Google Scholar] [CrossRef]
- Petursdottir, D.H.; Olafsdottir, I.; Hardardottir, I. Dietary Fish Oil Increases Tumor Necrosis Factor Secretion but Decreases Interleukin-10 Secretion by Murine Peritoneal Macrophages. J. Nutr. 2002, 132, 3740–3743. [Google Scholar] [CrossRef] [Green Version]
- Karonova, T.; Stepanova, A.; Bystrova, A.; Jude, E.B. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients 2020, 12, 2518. [Google Scholar] [CrossRef]
- Ghadiri-Anari, A.; Mozafari, Z.; Gholami, S.; Khodaei, S.-A.; Aboutorabi-Zarchi, M.; Sepehri, F.; Nadjarzade, A.; Rahmanian, M.; Namiranian, N. Dose vitamin D supplementations improve peripheral diabetic neuropathy? A before-after clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Baxter, C.; Romero, B.D.; McLeod, D.S.A.; English, D.R.; Armstrong, B.K.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; O’Connell, R.; et al. The D-Health Trial: A randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. 2022, 10, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.; Simpson, J.A.; Kotowicz, M.; Young, D.; Nicholson, G. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total (N = 1054) | Vitamin D3 | n-3 FAs | ||||
|---|---|---|---|---|---|---|---|
| Vitamin D3 (N = 520) | Placebo (N = 534) | p | n-3 FAs (N = 527) | Placebo (N = 527) | p | ||
| Age—yr | 64.9 ± 6.5 | 64.7 ± 6.3 | 65.1 ± 6.6 | 0.600 | 64.8 ± 6.5 | 64.9 ± 6.4 | 0.777 |
| Female sex—N (%) | 515 (49) | 256 (49) | 259 (49) | 0.813 | 260 (49) | 255 (48) | 0.758 |
| Race—N (%) | 0.622 | 0.390 | |||||
| White | 871 (84) | 429 (84) | 442 (85) | 429 (83) | 442 (85) | ||
| Black | 88 (9) | 42 (8) | 46 (9) | 43 (8) | 45 (9) | ||
| Others | 73 (7) | 40 (8) | 33 (6) | 42 (8) | 31 (6) | ||
| BMI—kg/m2 | 28.2 ± 5.3 | 28.1 ± 5.3 | 28.3 ± 5.4 | 0.296 | 28.7 ± 5.4 | 27.8 ± 5.3 | 0.007 |
| Current smoking—N (%) | 57 (5) | 30 (6) | 27 (5) | 0.593 | 26 (5) | 31 (6) | 0.473 |
| Medication use—N (%) | |||||||
| Hypertension | 432 (41) | 204 (39) | 228 (43) | 0.253 | 220 (42) | 212 (40) | 0.616 |
| Diabetes | 89 (8) | 49 (9) | 40 (7) | 0.259 | 45 (9) | 44 (8) | 0.912 |
| Cholesterol | 364 (35) | 185 (36) | 179 (34) | 0.483 | 193 (37) | 171 (32) | 0.154 |
| 25(OH)D—ng/mL | 28.1 ± 9.1 | 27.6 ± 8.8 | 28.7 ± 9.3 | 0.072 | 28.3 ± 9.5 | 28.0 ± 8.6 | 0.766 |
| 25(OH)D < 20 ng/mL—% | 175 (17) | 84 (16) | 91 (17) | 0.709 | 90 (17) | 85 (16) | 0.669 |
| n−3 index—% | 2.9 ± 1.0 | 2.9 ± 1.0 | 3.0 ± 1.0 | 0.472 | 3.0 ± 1.0 | 2.9 ± 1.0 | 0.441 |
| SBP—mmHg | 124.2 ± 14.3 | 123.7 ± 14.3 | 124.6 ± 14.6 | 0.325 | 124.2 ± 14.3 | 124.2 ± 14.6 | 0.837 |
| DBP—mmHg | 76.2 ± 9.2 | 75.8 ± 9.1 | 76.6 ± 9.3 | 0.092 | 76.6 ± 9.0 | 75.8 ± 9.4 | 0.163 |
| Ln hs-CRP | Ln IL-6 | Ln IL-10 | Ln TNF-α | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |
| Model 1 | N = 2700 | N = 1927 | N = 2660 | N = 2710 | ||||
| Year 2 | −0.16 (0.06) | 0.010 | −0.04 (0.05) | 0.340 | −0.00 (0.02) | 0.887 | 0.00 (0.02) | 0.878 |
| Year 4 | −0.02 (0.07) | 0.762 | 0.06 (0.05) | 0.272 | −0.00 (0.03) | 0.954 | 0.01 (0.02) | 0.539 |
| Model 2 | N = 2647 | N = 1882 | N = 2607 | N = 2657 | ||||
| Year 2 | −0.16 (0.06) | 0.010 | −0.05 (0.05) | 0.269 | −0.00 (0.02) | 0.926 | 0.00 (0.02) | 0.775 |
| Year 4 | −0.02 (0.07) | 0.739 | 0.06 (0.05) | 0.281 | −0.00 (0.03) | 0.999 | 0.01 (0.02) | 0.497 |
| Model 3 | N = 2626 | N = 1866 | N = 2587 | N = 2636 | ||||
| Year 2 | −0.17 (0.06) | 0.007 | −0.05 (0.05) | 0.267 | −0.00 (0.02) | 0.962 | 0.00 (0.02) | 0.837 |
| Year 4 | −0.02 (0.07) | 0.753 | 0.06 (0.05) | 0.292 | 0.00 (0.03) | 0.907 | 0.01 (0.02) | 0.495 |
| hs-CRP | IL-6 | IL-10 | TNF-α | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |
| Model 1 | N = 2700 | N = 1927 | N = 2660 | N = 2710 | ||||
| Year 2 | −0.06 (0.06) | 0.330 | 0.04 (0.05) | 0.357 | 0.03 (0.02) | 0.160 | 0.03 (0.02) | 0.076 |
| Year 4 | −0.04 (0.07) | 0.631 | 0.04 (0.05) | 0.459 | 0.01 (0.03) | 0.661 | 0.04 (0.02) | 0.035 |
| Model 2 | N = 2647 | N = 1882 | N = 2607 | N = 2657 | ||||
| Year 2 | −0.06 (0.06) | 0.336 | 0.06 (0.05) | 0.245 | 0.03 (0.02) | 0.145 | 0.03 (0.02) | 0.054 |
| Year 4 | −0.03 (0.07) | 0.637 | 0.05 (0.05) | 0.367 | 0.01 (0.03) | 0.653 | 0.04 (0.02) | 0.037 |
| Model 3 | N = 2626 | N = 1866 | N = 2587 | N = 2636 | ||||
| Year 2 | −0.05 (0.06) | 0.445 | 0.05 (0.05) | 0.258 | 0.03 (0.02) | 0.140 | 0.03 (0.02) | 0.049 |
| Year 4 | −0.04 (0.07) | 0.632 | 0.05 (0.05) | 0.352 | 0.01 (0.03) | 0.648 | 0.04 (0.02) | 0.043 |
| hs-CRP | IL-6 | IL-10 | TNF-α | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |
| Model 1 | N = 2530 | N = 1795 | N = 2492 | N = 2540 | ||||
| Year 2 | −0.13 (0.06) | 0.033 | −0.07 (0.05) | 0.184 | 0.00 (0.02) | 0.835 | 0.00 (0.02) | 0.829 |
| Year 4 | 0.01 (0.08) | 0.945 | 0.06 (0.06) | 0.268 | −0.01 (0.03) | 0.718 | 0.01 (0.02) | 0.813 |
| Model 2 | N = 2482 | N = 1755 | N = 2444 | N = 2492 | ||||
| Year 2 | −0.13 (0.06) | 0.043 | −0.07 (0.05) | 0.160 | 0.00 (0.02) | 0.843 | 0.01 (0.02) | 0.718 |
| Year 4 | 0.01 (0.08) | 0.912 | 0.07 (0.06) | 0.253 | −0.01 (0.03) | 0.773 | 0.01 (0.02) | 0.725 |
| Model 3 | N = 2461 | N = 1739 | N = 2424 | N = 2471 | ||||
| Year 2 | −0.14 (0.06) | 0.027 | −0.07 (0.05) | 0.149 | 0.01 (0.02) | 0.824 | 0.00 (0.02) | 0.809 |
| Year 4 | 0.01 (0.08) | 0.898 | 0.07 (0.06) | 0.258 | −0.00 (0.03) | 0.878 | 0.01 (0.02) | 0.713 |
| hs-CRP | IL-6 | IL-10 | TNF-α | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |
| Model 1 | N = 2533 | N = 1873 | N = 2593 | N = 2643 | ||||
| Year 2 | −0.06 (0.06) | 0.342 | 0.05 (0.05) | 0.345 | 0.03 (0.02) | 0.126 | 0.03 (0.02) | 0.054 |
| Year 4 | −0.04 (0.07) | 0.577 | 0.06 (0.06) | 0.307 | 0.01 (0.03) | 0.628 | 0.05 (0.02) | 0.027 |
| Model 2 | N = 2585 | N = 1833 | N = 2545 | N = 2595 | ||||
| Year 2 | −0.06 (0.06) | 0.360 | 0.06 (0.05) | 0.228 | 0.04 (0.02) | 0.116 | 0.04 (0.02) | 0.041 |
| Year 4 | −0.04 (0.07) | 0.604 | 0.06 (0.06) | 0.245 | 0.01 (0.03) | 0.628 | 0.05 (0.02) | 0.029 |
| Model 3 | N = 2564 | N = 1817 | N = 2525 | N = 2574 | ||||
| Year 2 | −0.04 (0.06) | 0.475 | 0.06 (0.05) | 0.239 | 0.04 (0.02) | 0.110 | 0.04 (0.02) | 0.037 |
| Year 4 | −0.04 (0.08) | 0.595 | 0.07 (0.06) | 0.231 | 0.01 (0.03) | 0.621 | 0.04 (0.02) | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Zhu, H.; Chen, L.; Huang, Y.; Christen, W.; Cook, N.R.; Copeland, T.; Mora, S.; Buring, J.E.; Lee, I.-M.; et al. Effects of Vitamin D3 and Marine Omega-3 Fatty Acids Supplementation on Biomarkers of Systemic Inflammation: 4-Year Findings from the VITAL Randomized Trial. Nutrients 2022, 14, 5307. https://doi.org/10.3390/nu14245307
Dong Y, Zhu H, Chen L, Huang Y, Christen W, Cook NR, Copeland T, Mora S, Buring JE, Lee I-M, et al. Effects of Vitamin D3 and Marine Omega-3 Fatty Acids Supplementation on Biomarkers of Systemic Inflammation: 4-Year Findings from the VITAL Randomized Trial. Nutrients. 2022; 14(24):5307. https://doi.org/10.3390/nu14245307
Chicago/Turabian StyleDong, Yanbin, Haidong Zhu, Li Chen, Ying Huang, William Christen, Nancy R. Cook, Trisha Copeland, Samia Mora, Julie E. Buring, I-Min Lee, and et al. 2022. "Effects of Vitamin D3 and Marine Omega-3 Fatty Acids Supplementation on Biomarkers of Systemic Inflammation: 4-Year Findings from the VITAL Randomized Trial" Nutrients 14, no. 24: 5307. https://doi.org/10.3390/nu14245307





