Hypovitaminosis D in Postherpetic Neuralgia—High Prevalence and Inverse Association with Pain: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
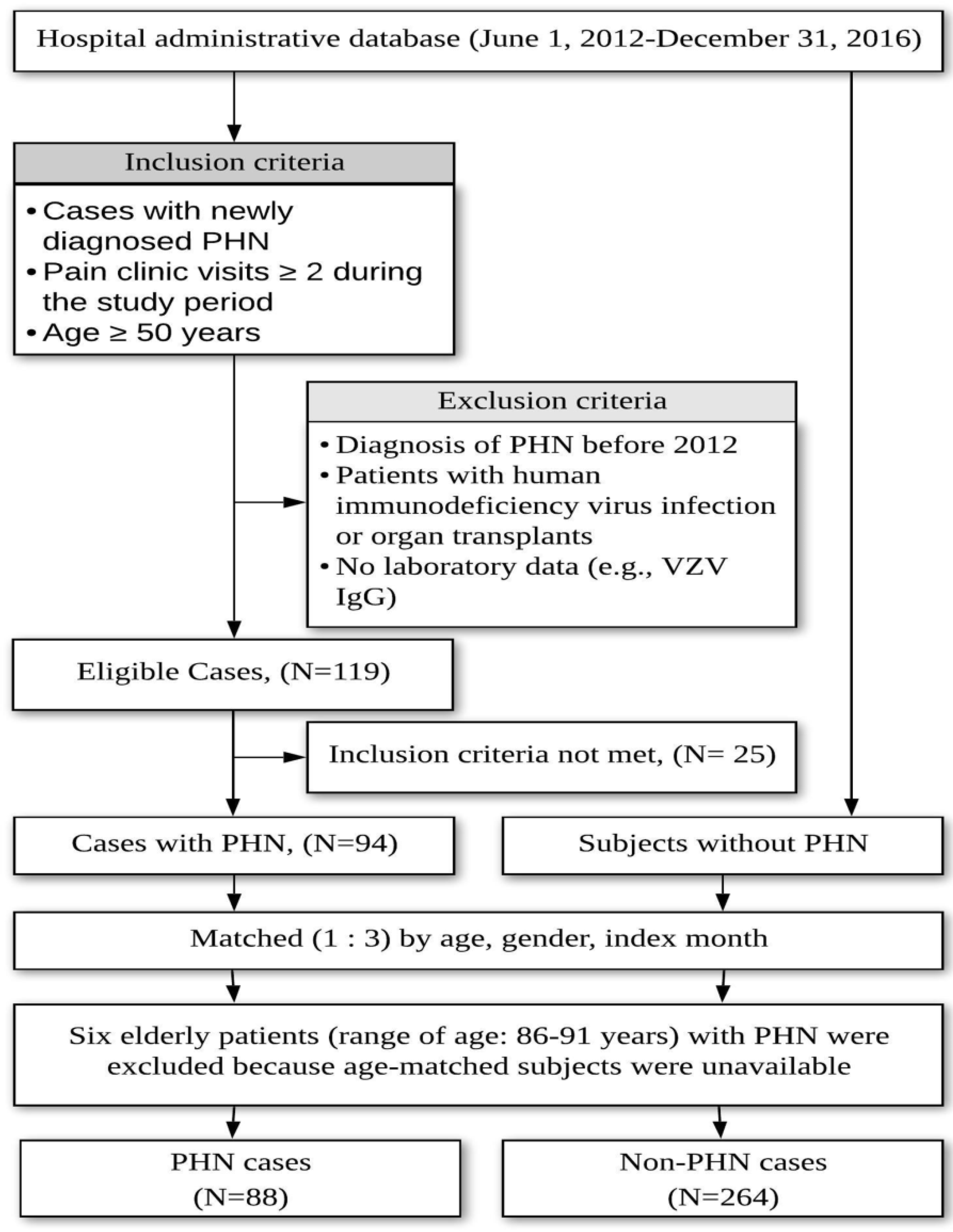
2.1. Autosearch and Chart Review Criteria for Postherpetic Neuralgia
2.2. Comorbidities
2.3. Specimen Collection, Handling, and Biochemical Determination
2.3.1. Determination of 25(OH)D
2.3.2. Zoster Immunity—VZV IgG/IgM Detected by ELISA
2.4. Sample Size
2.5. Statistical Analysis
3. Results
3.1. Part I Study
3.2. Part II Study
3.2.1. Comparison of Demographic and Clinical Characteristics Between Vitamin D-Deficient Patients and Vitamin D-Sufficient Patients
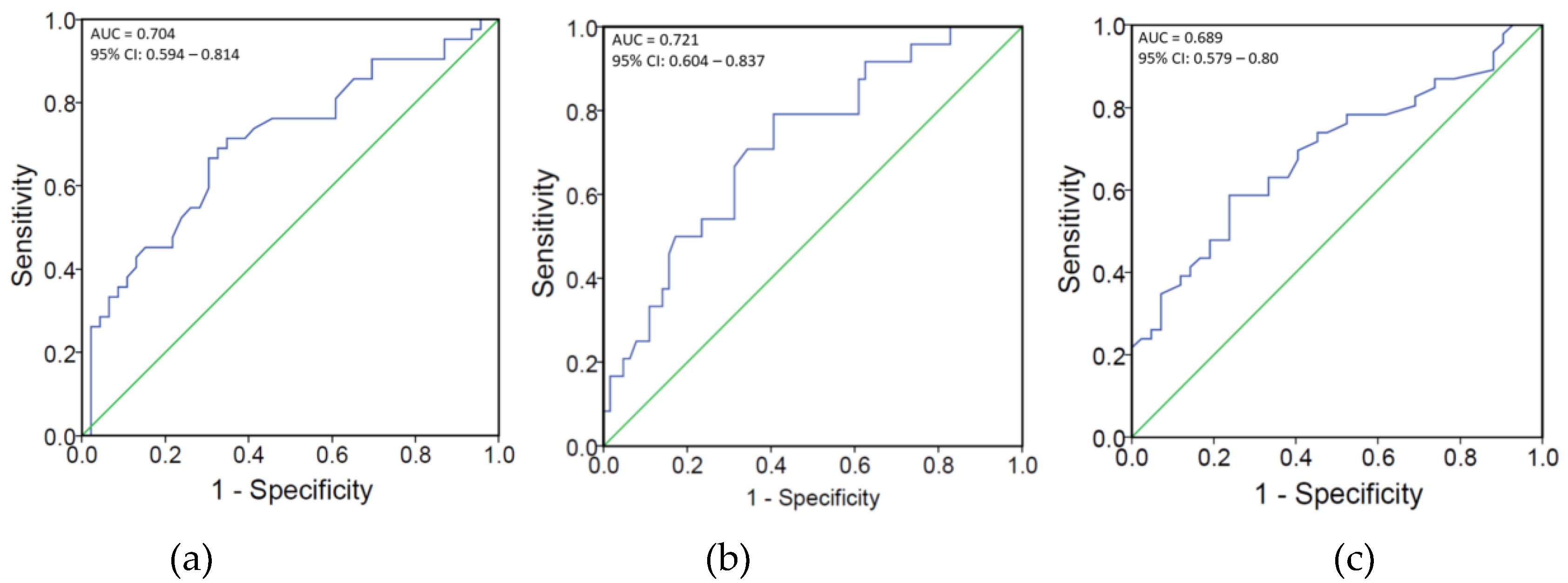
3.2.2. Correlations Between Pain and Serum 25(OH)D/VZV Igs in PHN
3.2.3. Proportions of 10 Items in the DN4 Questionnaire in Patients with Different Serum 25(OH)D/VZV Igs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Abbreviations
| 25(OH)D | 25-hydroxyvitamin D |
| AUC | area under the ROC curve |
| DN4 | Douleur Neuropathique 4 |
| ELISA | Enzyme Linked Immunosorbent Assay |
| ICD-9 | International Classification of Diseases, Ninth Revision, Clinical Modification |
| iNOS | inducible nitric oxide synthase |
| NMDA | N-methyl-D-aspartate |
| NO | nitric oxide |
| NRS | numeric rating pain scale |
| PHN | postherpetic neuralgia |
| ROC curve | receiver operating characteristic curve |
| ROS | reactive oxygen species |
| TLR | toll-like receptor |
| TRPA1 | transient receptor potential ankyrin 1 |
| VZV | varicella-zoster virus |
References
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Huang, Y.A.; Lai, Y.C.; Sun, C.K. Prevalence and predictors of hypovitaminosis D among the elderly in subtropical region. PLoS ONE 2017, 12, e0181063. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crack, L.R.; Jones, L.; Malavige, G.N.; Patel, V.; Ogg, G.S. Human antimicrobial peptides LL-37 and human beta-defensin-2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin. Exp. Dermatol. 2012, 37, 534–543. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Griffin, M.D.; Lutz, W.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 6800–6805. [Google Scholar] [CrossRef]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef]
- Lefebvre d’Hellencourt, C.; Montero-Menei, C.N.; Bernard, R.; Couez, D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J. Neurosci. Res. 2003, 71, 575–582. [Google Scholar] [CrossRef]
- Garcion, E.; Nataf, S.; Berod, A.; Darcy, F.; Brachet, P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res. Mol. Brain Res. 1997, 45, 255–267. [Google Scholar] [CrossRef]
- Choi, S.R.; Roh, D.H.; Yoon, S.Y.; Choi, H.S.; Kang, S.Y.; Han, H.J.; Beitz, A.J.; Lee, J.H. Astrocyte D-serine modulates the activation of neuronal NOS leading to the development of mechanical allodynia in peripheral neuropathy. Mol. Pain 2019, 15, 1744806919843046. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Zhi, C.; Shen, M.; Sun, W.; Miao, D.; Yuan, X. 1,25(OH)2D3 Deficiency Induces Colon Inflammation via Secretion of Senescence-Associated Inflammatory Cytokines. PLoS ONE 2016, 11, e0146426. [Google Scholar] [CrossRef] [PubMed]
- Arenas, O.M.; Zaharieva, E.E.; Para, A.; Vasquez-Doorman, C.; Petersen, C.P.; Gallio, M. Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat. Neurosci. 2017, 20, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Nakamura, S.; Zhao, M.; So, K.; Inoue, K.; Numata, T.; Takahashi, N.; Shirakawa, H.; Mori, Y.; Nakagawa, T.; et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat. Commun. 2016, 7, 12840. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, H.K.; Kim, J.H.; Chung, K.; Chung, J.M. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain 2007, 133, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kim, H.K.; Chung, J.M.; Chung, K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 2007, 131, 262–271. [Google Scholar] [CrossRef]
- Guida, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Piscitelli, F.; De Filippis, F.; Paino, S.; Ricciardi, F.; Siniscalco, D.; Marabese, I.; et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav. Immun. 2019, in press. [Google Scholar] [CrossRef]
- Poisbeau, P.; Aouad, M.; Gazzo, G.; Lacaud, A.; Kemmel, V.; Landel, V.; Lelievre, V.; Feron, F. Cholecalciferol (Vitamin D3) Reduces Rat Neuropathic Pain by Modulating Opioid Signaling. Mol. Neurobiol. 2019, 56, 7208–7221. [Google Scholar] [CrossRef]
- Soderstrom, L.H.; Johnson, S.P.; Diaz, V.A.; Mainous, A.G. Association between vitamin D and diabetic neuropathy in a nationally representative sample: Results from 2001–2004 NHANES. Diabet. Med. 2012, 29, 50–55. [Google Scholar] [CrossRef]
- Shillo, P.; Selvarajah, D.; Greig, M.; Gandhi, R.; Rao, G.; Wilkinson, I.D.; Anand, P.; Tesfaye, S. Reduced vitamin D levels in painful diabetic peripheral neuropathy. Diabet. Med. 2019, 36, 44–51. [Google Scholar] [CrossRef]
- Yesil, H.; Sungur, U.; Akdeniz, S.; Gurer, G.; Yalcin, B.; Dundar, U. Association between serum vitamin D levels and neuropathic pain in rheumatoid arthritis patients: A cross-sectional study. Int. J.Rheum. Dis. 2018, 21, 431–439. [Google Scholar] [CrossRef]
- Yawn, B.P.; Itzler, R.F.; Wollan, P.C.; Pellissier, J.M.; Sy, L.S.; Saddier, P. Health care utilization and cost burden of herpes zoster in a community population. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2009; Volume 84, pp. 787–794. [Google Scholar]
- Massengill, J.S.; Kittredge, J.L. Practical considerations in the pharmacological treatment of postherpetic neuralgia for the primary care provider. J. Pain Res. 2014, 7, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Drolet, M.; Brisson, M.; Schmader, K.E.; Levin, M.J.; Johnson, R.; Oxman, M.N.; Patrick, D.; Blanchette, C.; Mansi, J.A. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: A prospective study. CMAJ 2010, 182, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Yawn, B.P.; Hales, C.M.; Wollan, P.C.; Bialek, S.R.; Zhang, J.; Kurland, M.J.; Harpaz, R. Herpes zoster vaccine effectiveness and manifestations of herpes zoster and associated pain by vaccination status. Hum. Vaccines Immunother. 2015, 11, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- van Seventer, R.; Bach, F.W.; Toth, C.C.; Serpell, M.; Temple, J.; Murphy, T.K.; Nimour, M. Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: A randomized double-blind trial. Eur. J. Neurol. 2010, 17, 1082–1089. [Google Scholar] [CrossRef]
- Stamm, T.A.; Pieber, K.; Crevenna, R.; Dorner, T.E. Impairment in the activities of daily living in older adults with and without osteoporosis, osteoarthritis and chronic back pain: A secondary analysis of population-based health survey data. BMC Musculoskelet. Disord. 2016, 17, 139. [Google Scholar] [CrossRef]
- Chao, C.T.; Lee, S.Y.; Yang, W.S.; Yen, C.J.; Chiang, C.K.; Huang, J.W.; Hung, K.Y. Serum vitamin D levels are positively associated with varicella zoster immunity in chronic dialysis patients. Sci. Rep. 2014, 4, 7371. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chang, C.Y.; Feng, P.H.; Chu, C.C.; So, E.C.; Hu, M.L. Plasma vitamin C is lower in postherpetic neuralgia patients and administration of vitamin C reduces spontaneous pain but not brush-evoked pain. Clin. J. Pain 2009, 25, 562–569. [Google Scholar] [CrossRef]
- Timmerman, H.; Steegers, M.A.H.; Huygen, F.; Goeman, J.J.; van Dasselaar, N.T.; Schenkels, M.J.; Wilder-Smith, O.H.G.; Wolff, A.P.; Vissers, K.C.P. Investigating the validity of the DN4 in a consecutive population of patients with chronic pain. PLoS ONE 2017, 12, e0187961. [Google Scholar] [CrossRef]
- Bartley, J. Post herpetic neuralgia, schwann cell activation and vitamin D. Med. Hypotheses 2009, 73, 927–929. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chu, C.C.; Lin, Y.S.; So, E.C.; Shieh, J.P.; Hu, M.L. Nutrient deficiencies as a risk factor in Taiwanese patients with postherpetic neuralgia. Br. J. Nutr. 2011, 106, 700–707. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chu, C.C.; So, E.C.; Hsing, C.H.; Hu, M.L. Treatment of postherpetic neuralgia with intravenous administration of vitamin C. Anesth. Analg. 2006, 103, 1616–1617. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Lan, K.M.; Wang, L.K.; Chen, J.Y. Treatment of Postherpetic Neuralgia with Intravenous Administration of Zinc Sulfate: A Case Report. A A Pract. 2018, 11, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, T.; Linde, A.; Olding-Stenkvist, E.; Wahren, B. Antiviral IgM and IgG subclasses in varicella zoster associated neurological syndromes. J. Neurol. Neurosurg. Psychiatry 1989, 52, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Chiang, C.K.; Huang, J.W.; Hung, K.Y. Vitamin D is closely linked to the clinical courses of herpes zoster: From pathogenesis to complications. Med. Hypotheses 2015, 85, 452–457. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chang, C.Y.; Lin, Y.S.; Hu, M.L. Nutritional factors in herpes zoster, postherpetic neuralgia, and zoster vaccination. Popul. Health Manag. 2012, 15, 391–397. [Google Scholar] [CrossRef]
- Yawn, B.P.; Saddier, P.; Wollan, P.C.; St Sauver, J.L.; Kurland, M.J.; Sy, L.S. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2007; Volume 82, pp. 1341–1349. [Google Scholar]
- Chen, J.Y.; Lan, K.M.; Sheu, M.J.; Tseng, S.F.; Weng, S.F.; Hu, M.L. Peptic ulcer as a risk factor for postherpetic neuralgia in adult patients with herpes zoster. J. Med. Virol. 2015, 87, 222–229. [Google Scholar] [CrossRef]
- Mansueto, P.; Seidita, A.; Vitale, G.; Gangemi, S.; Iaria, C.; Cascio, A. Vitamin D Deficiency in HIV Infection: Not Only a Bone Disorder. BioMed Res. Int. 2015, 2015, 735615. [Google Scholar] [CrossRef]
- Stein, E.M.; Shane, E. Vitamin D in organ transplantation. Osteoporos. Int. 2011, 22, 2107–2118. [Google Scholar] [CrossRef]
- Levis, S.; Gomez, A.; Jimenez, C.; Veras, L.; Ma, F.; Lai, S.; Hollis, B.; Roos, B.A. Vitamin d deficiency and seasonal variation in an adult South Florida population. J. Clin. Endocrinol. Metab. 2005, 90, 1557–1562. [Google Scholar] [CrossRef]
- Mut Surmeli, D.; Surmeli, Z.G.; Bahsi, R.; Turgut, T.; Selvi Oztorun, H.; Atmis, V.; Varli, M.; Aras, S. Vitamin D deficiency and risk of Helicobacter pylori infection in older adults: A cross-sectional study. Aging Clin. Exp. Res. 2018, 31, 985–991. [Google Scholar] [CrossRef]
- Koivula, M.K.; Matinlassi, N.; Laitinen, P.; Risteli, J. Four automated 25-OH total vitamin D immunoassays and commercial liquid chromatography tandem-mass spectrometry in Finnish population. Clin. Lab. 2013, 59, 397–405. [Google Scholar] [CrossRef] [PubMed]
- van Rijckevorsel, G.G.; Bovee, L.P.; Damen, M.; Sonder, G.J.; Schim van der Loeff, M.F.; van den Hoek, A. Increased seroprevalence of IgG-class antibodies against cytomegalovirus, parvovirus B19, and varicella-zoster virus in women working in child day care. BMC Public Health 2012, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Kim, Y.S.; Nahm, F.S.; Yoo da, H.; Choi, E.; Lee, P.B.; Choo, H.; Park, Z.Y.; Yang, C.S. The positive duration of varicella zoster immunoglobulin M antibody test in herpes zoster. Medicine 2016, 95, e4616. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. A Review of the Evidence Supporting the Vitamin D-Cancer Prevention Hypothesis in 2017. Anticancer Res. 2018, 38, 1121–1136. [Google Scholar] [PubMed]
- Han, G.Y.; Choi, Y.A.; Lee, K.Y.; Park, Y.O.; Cho, S.U.; Shim, M.; Kim, B.; Kim, S.O. The Comparison of the Blood Level of 25-Hydroxyvitamin D3 in Healthy Adult and Patients with Herpes Zoster. Korean J. Fam. Pract. 2016, 6, 288–292. [Google Scholar] [CrossRef]
- Gilden, D.H.; Cohrs, R.J.; Mahalingam, R. VZV vasculopathy and postherpetic neuralgia: Progress and perspective on antiviral therapy. Neurology 2005, 64, 21–25. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Boontanrart, M.; Hall, S.D.; Spanier, J.A.; Hayes, C.E.; Olson, J.K. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J. Neuroimmunol. 2016, 292, 126–136. [Google Scholar] [CrossRef]
- Jiao, K.P.; Li, S.M.; Lv, W.Y.; Jv, M.L.; He, H.Y. Vitamin D3 repressed astrocyte activation following lipopolysaccharide stimulation in vitro and in neonatal rats. Neuroreport 2017, 28, 492–497. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef]
- Djouhri, L.; Koutsikou, S.; Fang, X.; McMullan, S.; Lawson, S.N. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J. Neurosci. 2006, 26, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Lv, M.M.; Wang, S.; Chen, L.; Qian, N.S.; Tang, Y.; Zhang, X.D.; Ren, P.C.; Gao, C.J.; Sun, X.D.; et al. Spinal astrocytic activation is involved in a virally-induced rat model of neuropathic pain. PLoS ONE 2011, 6, e23059. [Google Scholar] [CrossRef] [PubMed]
- Forbes, H.J.; Bhaskaran, K.; Thomas, S.L.; Smeeth, L.; Clayton, T.; Mansfield, K.; Minassian, C.; Langan, S.M. Quantification of risk factors for postherpetic neuralgia in herpes zoster patients: A cohort study. Neurology 2016, 87, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Di Bartolo, G.; Malaguarnera, G.; Bella, R.; Lanza, G.; Malaguarnera, M. Vitamin D Serum Levels in Patients with Statin-Induced Musculoskeletal Pain. Dis. Mark. 2019, 2019, 3549402. [Google Scholar] [CrossRef]
- Gokcek, E.; Kaydu, A. Assessment of Relationship between Vitamin D Deficiency and Pain Severity in Patients with Low Back Pain: A Retrospective, Observational Study. Anesth. Essays Res. 2018, 12, 680–684. [Google Scholar] [CrossRef]
- Allchorne, A.J.; Broom, D.C.; Woolf, C.J. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol. Pain 2005, 1, 36. [Google Scholar] [CrossRef]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef]
- Lee, P.; Chen, R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch. Intern. Med. 2008, 168, 771–772. [Google Scholar] [CrossRef]
- Kim, Y.G.; Paek, J.O.; Kim, J.S.; Yu, H.J. Clinical significance of serum varicella zoster virus immunoglobulin M and G in varicella and herpes zoster. Korean J. Dermatol. 2015, 53, 441–448. [Google Scholar]
- Higa, K.; Dan, K.; Manabe, H.; Noda, B. Factors influencing the duration of treatment of acute herpetic pain with sympathetic nerve block: Importance of severity of herpes zoster assessed by the maximum antibody titers to varicella-zoster virus in otherwise healthy patients. Pain 1988, 32, 147–157. [Google Scholar] [CrossRef]
- Acosta, E.P.; Balfour, H.H., Jr. Acyclovir for treatment of postherpetic neuralgia: Efficacy and pharmacokinetics. Antimicrob. Agents Chemother. 2001, 45, 2771–2774. [Google Scholar] [CrossRef] [PubMed]
- Quan, D.; Hammack, B.N.; Kittelson, J.; Gilden, D.H. Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch. Neurol. 2006, 63, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Wang, L.K.; Hung, K.C.; Wu, Z.F.; Chang, C.Y.; Chen, J.Y. Patient characteristics and analgesic efficacy of antiviral therapy in postherpetic neuralgia. Med. Hypotheses 2019, 131, 109323. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
| Predictors | PHN (n = 88) | Controls (n = 264) | Univariate OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 65.3 (9.4) | 65.3 (9.0) | 0.997 | |||
| Age groups | ||||||
| ≥60 years | 64 (72.7%) | 192 (72.7%) | ||||
| 50–59 years | 24 (27.3%) | 72 (27.3%) | ||||
| Gender | ||||||
| Male, n (%) | 47 (53.4%) | 141 (53.4%) | ||||
| Body mass index, mean (SD) | 23.68 (3.26) | 23.99 (3.07) | 0.426 | |||
| Body mass index (kg/m2) | 1.29 (0.54-3.06) | 0.563 | 1.01 (0.36-2.79) | 0.990 | ||
| <18.5 or ≥30 | 8 (9.1%) | 19 (7.2%) | ||||
| 18.5~30 | 80 (90.0%) | 245 (92.8%) | ||||
| 25(OH)D (nmol/L), mean (SD) | 68.96(18.72) | 75.13 (17.47) | 0.005 | |||
| Vitamin D status | 3.31 (1.92-5.72) | <0.001 | 3.12 (1.73-5.61) | <0.001 * | ||
| Sufficiency, n (%) | 23 (26.1%) | 140 (51.9%) | ||||
| Hypovitaminosis D, n (%) | 65 (73.9%) | 124 (47.0%) | ||||
| Comorbidities | ||||||
| Hypertension | 33 (37.5%) | 84 (31.8%) | 1.35 (0.78-2.37) | 0.279 | 1.14 (0.59-2.17) | 0.702 |
| Diabetes mellitus | 26 (29.5%) | 42 (15.9%) | 2.22 (1.26-3.90) | 0.005 | 1.97 (0.96-4.06) | 0.065 |
| Malignancy | 15 (17.0%) | 18 (6.8%) | 2.71 (1.31-5.59) | 0.007 | 3.21 (1.38-7.48) | 0.007 * |
| Chronic liver disease | 10 (11.4%) | 28 (10.6%) | 1.08 (0.51-2.28) | 0.846 | 1.24 (0.52-2.93) | 0.630 |
| Chronic kidney disease | 2 (2.3%) | 6 (2.3%) | 1.00 (0.20-4.95) | 1.000 | 0.75 (0.13-4.48) | 0.757 |
| Autoimmune diseases | 8 (9.1%) | 10 (3.8%) | 2.40 (0.95-6.08) | 0.065 | 2.85 (0.98-8.27) | 0.055 |
| H. pylori-related PUD | 23 (26.1%) | 25 (9.5%) | 3.15 (1.70-5.84) | <0.001 | 3.47 (1.71-7.03) | 0.001 * |
| Antiviral therapy | 38 (43.2%) | - | ||||
| Average spontaneous pain, mean (SD) (NRS 0–10) | 5.84 (1.46) | - | ||||
| Brush-evoked pain, mean (SD) (NRS 0–10) | 3.14 (3.10) | - |
| Hypovitaminosis D (n = 65) | Sufficiency of vitamin D (n = 23) | p | |
|---|---|---|---|
| Age group | 0.075 | ||
| ≥60 years, n (%) | 44 (67.7) | 20 (87.0) | |
| Gender | 0.071 | ||
| Male, n (%) | 31 (47.7) | 16 (69.6) | |
| Body mass index (kg/m2) | 0.357 | ||
| <18.5 or ≥30, n (%) | 7 (10.8) | 1 (4.3) | |
| VZV-IgG (mIU/mL), mean (SD) | 4239 (1382) | 4281 (1066) | 0.955 |
| VZV-IgG, positive, n (%) | 65 (100) | 23 (100) | 1.0 |
| VZV-IgM, mean (SD) | 0.63 (0.45) | 0.40 (0.25) | 0.016 * |
| VZV-IgM, positive, n (%) | 8 (12.3) | 1 (4.3) | 0.279 |
| Comorbidities, n (%) | |||
| Hypertension | 25 (38.5) | 8 (34.8) | 0.754 |
| Diabetes mellitus | 21 (32.3) | 5 (21.7) | 0.340 |
| Malignancy | 12 (18.5) | 3 (13.0) | 0.553 |
| Chronic liver disease | 8 (12.3) | 2 (8.7) | 0.639 |
| Chronic kidney disease | 2 (3.1) | 0 (0.0) | 0.416 |
| Autoimmune diseases | 6 (9.2) | 2 (8.7) | 0.939 |
| Helicobacter pylori-related PUD | 19 (29.2) | 4 (17.4) | 0.267 |
| Vitamin D supplements★, n (%) | 1 (1.5) | 4 (17.4) | 0.005 * |
| Average spontaneous pain, mean (SD) (NRS 0–10) | 6.1 (2.1) | 5.3 (1.8) | 0.021 * |
| Brush-evoked pain, mean (SD) (NRS 0–10) | 4.3 (6.8) | 2.5 (8.3) | 0.007 * |
| Correlation | Spearman’s Correlation Coefficient | p |
|---|---|---|
| Spontaneous pain (NRS 0-10) vs. | ||
| brush-evoked pain (NRS 0–10) | 0.196 | 0.067 |
| 25(OH)D (nmol/L) | −0.329 * | 0.002 |
| VZV IgG(mIU/ml) | 0.249 | 0.019 |
| VZV IgM | 0.363 * | 0.001 |
| Brush-evoked pain (NRS 0-10) vs. | ||
| 25(OH)D (nmol/L) | −0.311 * | 0.003 |
| VZV IgG(mIU/ml) | −0.181 | 0.092 |
| VZV IgM | −0.183 | 0.088 |
| Cutoff | 25(OH)D | p | Insufficiency Deficiency | p | ||
|---|---|---|---|---|---|---|
| >67.0 nmol/L (n = 46) | ≤ 67.0nmol/L (n = 42) | 50–75nmol/L (n = 55) | <50.0 nmol/L (n = 10) | |||
| Burning pain, n (%) | 28 (56.0) | 22 (44.0) | 0.422 | 14 (25.5) | 2 (20.0) | 0.713 |
| Painful cold, n (%) | 2 (11.8) | 15 (88.2) | <0.001 * | 10 (18.2) | 6 (60.0) | 0.005 * |
| Electric sharp pain, n (%) | 35 (53.8) | 30 (46.2) | 0.619 | 9 (16.4) | 2 (20.0) | 0.778 |
| Tingling, n (%) | 35 (47.9) | 38 (52.1) | 0.073 | 33 (60.0) | 7 (70.0) | 0.550 |
| Pins and needles, n (%) | 36 (49.3) | 37 (50.7) | 0.220 | 31 (56.4) | 6 (60.0) | 0.831 |
| Numbness, n (%) | 19 (54.3) | 16 (45.7) | 0.759 | 17 (30.9) | 4 (40.0) | 0.572 |
| Itching, n (%) | 15 (32.6) | 13 (31.0) | 0.868 | 17 (30.9) | 2 (20.0) | 0.485 |
| Hypoesthesia to touch, n (%) | 17 (50.0) | 17 (50.0) | 0.735 | 21 (38.2) | 4 (40.0) | 0.913 |
| Hypoesthesia to pinprick, n (%) | 13 (46.4) | 15 (53.6) | 0.453 | 19 (34.5) | 3 (30.0) | 0.780 |
| Brush-evoked pain, n (%) | 27 (42.2) | 37 (57.8) | 0.002 * | 43 (78.2) | 10 (100.0) | 0.225 |
| DN4 ≥4, n (%) | 39 (84.8) | 35 (83.3) | 0.853 | 45(81.8) | 9 (90.0) | 0.526 |
| Cutoff | VZV IgM | p | |
|---|---|---|---|
| ≥0.6 (n = 37) | <0.6 (n = 51) | ||
| Burning pain, n (%) | 19 (38.0) | 31 (62.0) | 0.378 |
| Painful cold, n (%) | 7 (41.2) | 10 (58.8) | 0.936 |
| Electric sharp pain, n (%) | 27 (41.5) | 38 (58.5) | 0.871 |
| Tingling, n (%) | 30 (41.1) | 43 (58.9) | 0.691 |
| Pins and needles, n (%) | 32 (43.8) | 41 (56.2) | 0.453 |
| Numbness, n (%) | 14 (40.0) | 21 (60.0) | 0.752 |
| Itching, n (%) | 12 (32.4) | 16 (31.4) | 0.916 |
| Hypoesthesia to touch, n (%) | 13 (38.2) | 21 (61.8) | 0.566 |
| Hypoesthesia to pinprick, n (%) | 12 (42.9) | 16 (57.1) | 0.916 |
| Brush-evoked pain, n (%) | 28 (43.8) | 36 (56.3) | 0.597 |
| DN4 ≥4, n (%) | 30 (81.1) | 44 (86.3) | 0.511 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Y.; Lin, Y.-T.; Wang, L.-K.; Hung, K.-C.; Lan, K.-M.; Ho, C.-H.; Chang, C.-Y. Hypovitaminosis D in Postherpetic Neuralgia—High Prevalence and Inverse Association with Pain: A Retrospective Study. Nutrients 2019, 11, 2787. https://doi.org/10.3390/nu11112787
Chen J-Y, Lin Y-T, Wang L-K, Hung K-C, Lan K-M, Ho C-H, Chang C-Y. Hypovitaminosis D in Postherpetic Neuralgia—High Prevalence and Inverse Association with Pain: A Retrospective Study. Nutrients. 2019; 11(11):2787. https://doi.org/10.3390/nu11112787
Chicago/Turabian StyleChen, Jen-Yin, Yao-Tsung Lin, Li-Kai Wang, Kuo-Chuan Hung, Kuo-Mao Lan, Chung-Han Ho, and Chia-Yu Chang. 2019. "Hypovitaminosis D in Postherpetic Neuralgia—High Prevalence and Inverse Association with Pain: A Retrospective Study" Nutrients 11, no. 11: 2787. https://doi.org/10.3390/nu11112787
APA StyleChen, J.-Y., Lin, Y.-T., Wang, L.-K., Hung, K.-C., Lan, K.-M., Ho, C.-H., & Chang, C.-Y. (2019). Hypovitaminosis D in Postherpetic Neuralgia—High Prevalence and Inverse Association with Pain: A Retrospective Study. Nutrients, 11(11), 2787. https://doi.org/10.3390/nu11112787







