PipeNig®-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-β-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Gas-Chromatographic Analyses of PipeNig®-FL
2.3. Cell Cultures
2.4. Cell Viability
2.5. Quantification of Adipocyte Lipid Accumulation and DNA Staining
2.6. Glucose Uptake Measurements
2.7. GLUT4 Translocation Analysis
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of PipeNig®-FL
3.2. Effects of PipeNig®-FL on 3T3-L1 Adipocyte Cell Viability
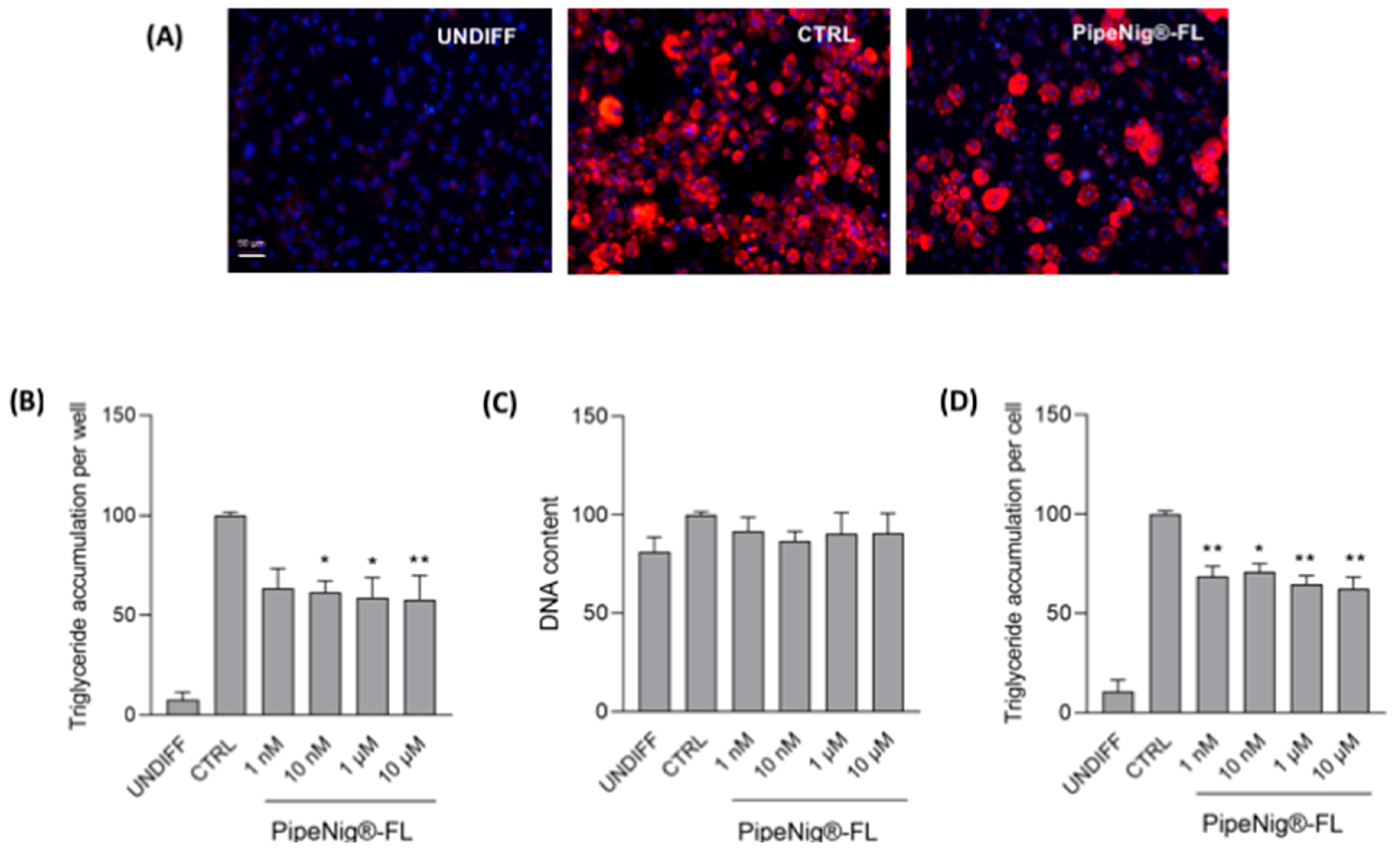
3.3. PipeNig®-FL Reduces Intracellular Lipid Accumulation in 3T3-L1 Cells without Altering the Cell Number
3.4. PipeNig®-FL Does Not Affect Cell Viability on C2C12 Muscle Cell
3.5. PipeNig®-FL Improves Glucose Uptake in C2C12 Myotubes
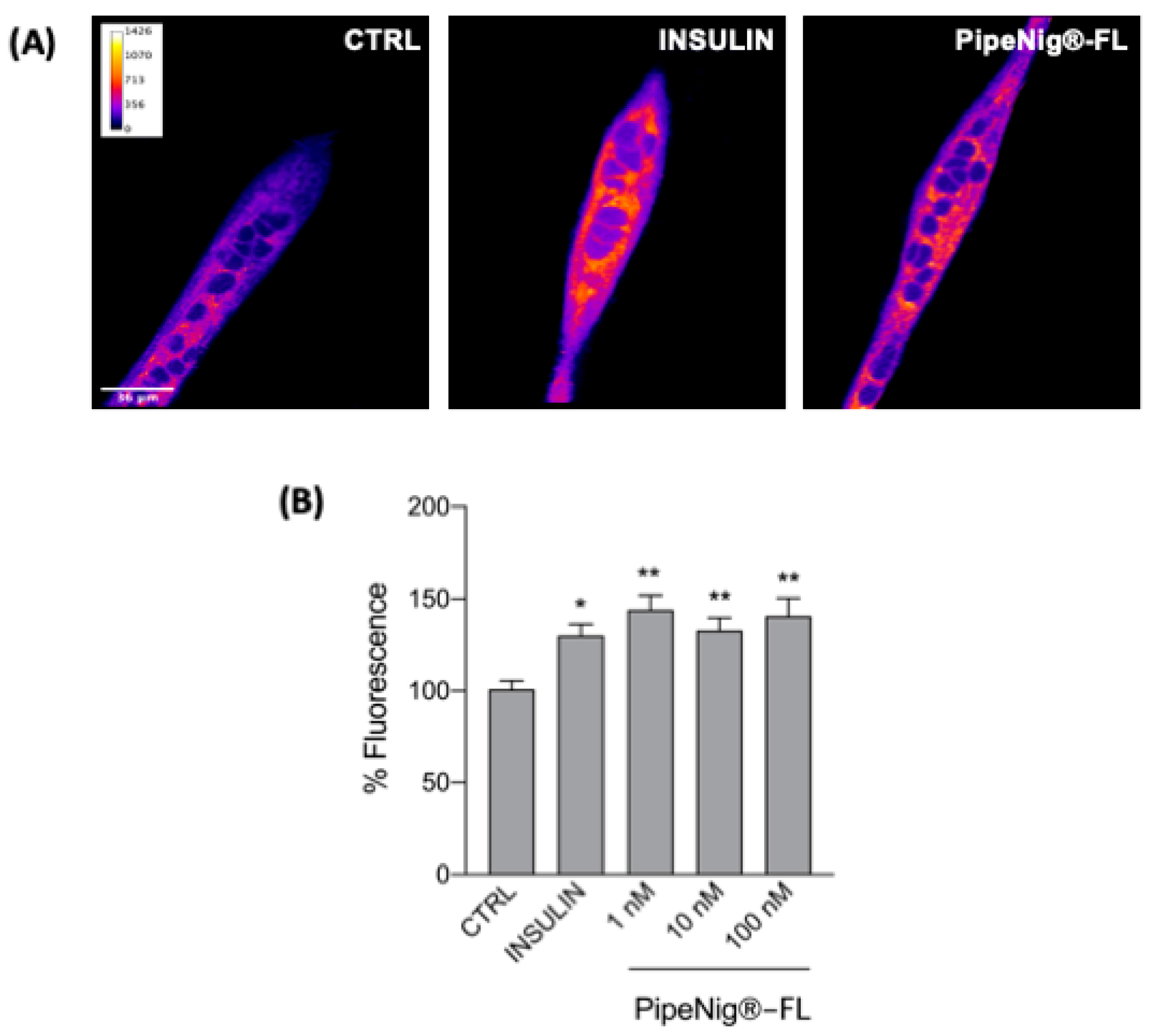
3.6. PipeNig®-FL Induces GLUT4 Translocation in C2C12 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reilly, M.P.; Rader, D.J. The Metabolic Syndrome. Circulation 2003, 108, 1546–1551. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Maccallini, C.; Amoia, P.; Amoroso, R. Multitarget PPARγ Agonists as Innovative Modulators of the Metabolic Syndrome. Eur. J. Med. Chem. 2019, 173, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Salinas, C.A.; Viveros-Ruiz, T. Recent Advances in Managing/Understanding the Metabolic Syndrome. F1000Res 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Silva Figueiredo, P.; Inada, A.C.; Ribeiro Fernandes, M.; Granja Arakaki, D.; Freitas, K.D.C.; Avellaneda Guimarães, R.D.C.; Aragão do Nascimento, V.; Aiko Hiane, P. An Overview of Novel Dietary Supplements and Food Ingredients in Patients with Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease. Molecules 2018, 23, 877. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gu, Z.; Wang, Y.; Wang, S.; Chen, H.; Zhang, H.; Chen, W.; Chen, Y.Q. Beyond Cannabis: Plants and the Endocannabinoid System. Trends Pharmacol. Sci. 2016, 37, 594–605. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F.A. Systematic Review on Black Pepper (Piper Nigrum L.): From Folk Uses to Pharmacological Applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Dagli, M.L.; Date, M.; Dekant, W.; Deodhar, C.; et al. RIFM Fragrance Ingredient Safety Assessment, β-Caryophyllene Alcohol, CAS Registry Number 472-97-9. Food Chem. Toxicol. 2018, 122, S566–S572. [Google Scholar] [CrossRef]
- Di Giacomo, S.; DI Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing Properties of β-Caryophyllene and β-Caryophyllene Oxide in Combination with Doxorubicin in Human Cancer Cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J.J.G. Chemosensitization of Hepatocellular Carcinoma Cells to Sorafenib by β-Caryophyllene Oxide-Induced Inhibition of ABC Export Pumps. Arch. Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef]
- Ambrož, M.; Šmatová, M.; Šadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Skarková, V.H.; Králová, V.; Skálová, L. Sesquiterpenes α-Humulene and β-Caryophyllene Oxide Enhance the Efficacy of 5-Fluorouracil and Oxaliplatin in Colon Cancer Cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lv, Y.; Tian, X.; Lou, J.; An, R.; Zhang, Q.; Li, M.; Xu, L.; Dong, Z. Neuroprotective Effect of β-Caryophyllene on Cerebral Ischemia-Reperfusion Injury via Regulation of Necroptotic Neuronal Death and Inflammation: In Vivo and in Vitro. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Viveros-Paredes, J.M.; González-Castañeda, R.E.; Gertsch, J.; Chaparro-Huerta, V.; López-Roa, R.I.; Vázquez-Valls, E.; Beas-Zarate, C.; Camins-Espuny, A.; Flores-Soto, M.E. Neuroprotective Effects of β-Caryophyllene against Dopaminergic Neuron Injury in a Murine Model of Parkinson’s Disease Induced by MPTP. Pharmaceuticals 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.S.; Mohamed, M.E. β-Caryophyllene as a Potential Protective Agent Against Myocardial Injury: The Role of Toll-Like Receptors. Molecules 2019, 24, 1929. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a Natural Bicyclic Sesquiterpene Attenuates Doxorubicin-Induced Chronic Cardiotoxicity via Activation of Myocardial Cannabinoid Type-2 (CB2) Receptors in Rats. Chem.-Biol. Interact. 2019, 304, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.A.; Bustanji, Y.K.; Abdalla, S.S. Hypocholesterolemic Effect of β-Caryophyllene in Rats Fed Cholesterol and Fat Enriched Diet. J. Clin. Biochem. Nutr. 2018, 62, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Protects against Diet-Induced Dyslipidemia and Vascular Inflammation in Rats: Involvement of CB2 and PPAR-γ Receptors. Chem.-Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a Natural Sesquiterpene, Modulates Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Acta Histochem. 2014, 116, 1469–1479. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a Natural Sesquiterpene Lactone Attenuates Hyperglycemia Mediated Oxidative and Inflammatory Stress in Experimental Diabetic Rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef]
- Da Silva Oliveira, G.L.; Machado, K.C.; Machado, K.C.; da Silva, A.P.D.S.C.L.; Feitosa, C.M.; de Castro Almeida, F.R. Non-Clinical Toxicity of β-Caryophyllene, a Dietary Cannabinoid: Absence of Adverse Effects in Female Swiss Mice. Regul. Toxicol. Pharmacol. 2018, 92, 338–346. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jia, Y.; Lee, J.H.; Jun, H.-J.; Lee, H.-S.; Hwang, K.-Y.; Lee, S.-J. Trans-Caryophyllene Is a Natural Agonistic Ligand for Peroxisome Proliferator-Activated Receptor-α. Bioorganic Med. Chem. Lett. 2014, 24, 3168–3174. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Alleviates Diet-Induced Neurobehavioral Changes in Rats: The Role of CB2 and PPAR-γ Receptors. Biomed. Pharmacother. 2019, 110, 145–154. [Google Scholar] [CrossRef]
- Pomatto, V.; Cottone, E.; Cocci, P.; Mozzicafreddo, M.; Mosconi, G.; Nelson, E.R.; Palermo, F.A.; Bovolin, P. Plasticizers Used in Food-Contact Materials Affect Adipogenesis in 3T3-L1 Cells. J. Steroid Biochem. Mol. Biol. 2018, 178, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.P.; Femminò, S.; Antoniotti, S.; Querio, G.; Alloatti, G.; Levi, R. Catestatin Induces Glucose Uptake and GLUT4 Trafficking in Adult Rat Cardiomyocytes. Available online: https://www.hindawi.com/journals/bmri/2018/2086109/cta/ (accessed on 9 October 2019). [CrossRef]
- Kim, N.-H.; Jegal, J.; Kim, Y.N.; Heo, J.-D.; Rho, J.-R.; Yang, M.H.; Jeong, E.J. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, T. Medicinal Properties of Terpenes Found in Cannabis Sativa and Humulus Lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Arizuka, N.; Murakami, T.; Suzuki, K. The Effect of β-Caryophyllene on Nonalcoholic Steatohepatitis. J. Toxicol. Pathol. 2017, 30, 263–273. [Google Scholar] [CrossRef]
- Butt, M.S.; Pasha, I.; Sultan, M.T.; Randhawa, M.A.; Saeed, F.; Ahmed, W. Black Pepper and Health Claims: A Comprehensive Treatise. Crit. Rev. Food Sci. Nutr. 2013, 53, 875–886. [Google Scholar] [CrossRef]
- Bagheri, H.; Abdul Manap, M.Y.B.; Solati, Z. Response Surface Methodology Applied to Supercritical Carbon Dioxide Extraction of Piper Nigrum L. Essential Oil. LWT-Food Sci. Technol. 2014, 57, 149–155. [Google Scholar] [CrossRef]
- Hafidi, M.E.; Buelna-Chontal, M.; Sánchez-Muñoz, F.; Carbó, R. Adipogenesis: A Necessary but Harmful Strategy. Int. J. Mol. Sci. 2019, 20, 3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Levy, R.M. β-Caryophyllene Promotes Osteoblastic Mineralization, and Suppresses Osteoclastogenesis and Adipogenesis in Mouse Bone Marrow Cultures in Vitro. Exp. Ther. Med. 2016, 12, 3602–3606. [Google Scholar] [CrossRef] [PubMed]
- Suijun, W.; Zhen, Y.; Ying, G.; Yanfang, W. A Role for Trans-Caryophyllene in the Moderation of Insulin Secretion. Biochem. Biophys. Res. Commun. 2014, 444, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.J.; Chai, S.Y.; Albiston, A.L. GLUT4 Associated Proteins as Therapeutic Targets for Diabetes. Recent. Pat. Endocr. Metab. Immune. Drug Discov. 2011, 5, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gannon, N.P.; Conn, C.A.; Vaughan, R.A. Dietary Stimulators of GLUT4 Expression and Translocation in Skeletal Muscle: A Mini-Review. Mol. Nutr. Food Res. 2015, 59, 48–64. [Google Scholar] [CrossRef] [PubMed]




| Compound | R.T. | Content (mg g−1) | Relative % |
|---|---|---|---|
| α-Phellandrene | 5.35 | 0.47 | 0.11 |
| α-Pinene | 5.55 | 4.06 | 0.95 |
| 4-ethyl-octane | 6.02 | 0.64 | 0.15 |
| Sabinene | 6.62 | 2.93 | 0.69 |
| β-Pinene | 6.79 | 2.76 | 0.65 |
| β-Myrcene | 7.11 | 1.10 | 0.12 |
| δ-3-Carene | 7.79 | 3.85 | 0.90 |
| Limonene | 8.55 | 7.84 | 1.10 |
| δ-Elemene | 24.79 | 2.21 | 0.52 |
| α-Copaene | 27.15 | 3.37 | 0.75 |
| Isocaryophyllene | 28.86 | 1.18 | 0.28 |
| β-Caryophyllene | 29.94 | 814.44 | 87.61 |
| δ-Cadinol | 31.49 | 0.70 | 0.16 |
| α-Caryophyllene | 31.75 | 6.13 | 1.43 |
| δ-Cadinene | 35.61 | 0.74 | 0.17 |
| Caryophyllene oxide | 38.94 | 0.79 | 0.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geddo, F.; Scandiffio, R.; Antoniotti, S.; Cottone, E.; Querio, G.; Maffei, M.E.; Bovolin, P.; Gallo, M.P. PipeNig®-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-β-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes. Nutrients 2019, 11, 2788. https://doi.org/10.3390/nu11112788
Geddo F, Scandiffio R, Antoniotti S, Cottone E, Querio G, Maffei ME, Bovolin P, Gallo MP. PipeNig®-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-β-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes. Nutrients. 2019; 11(11):2788. https://doi.org/10.3390/nu11112788
Chicago/Turabian StyleGeddo, Federica, Rosaria Scandiffio, Susanna Antoniotti, Erika Cottone, Giulia Querio, Massimo E. Maffei, Patrizia Bovolin, and Maria Pia Gallo. 2019. "PipeNig®-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-β-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes" Nutrients 11, no. 11: 2788. https://doi.org/10.3390/nu11112788
APA StyleGeddo, F., Scandiffio, R., Antoniotti, S., Cottone, E., Querio, G., Maffei, M. E., Bovolin, P., & Gallo, M. P. (2019). PipeNig®-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-β-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes. Nutrients, 11(11), 2788. https://doi.org/10.3390/nu11112788







