Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Handling, Analyses, and Extraction
3. Results
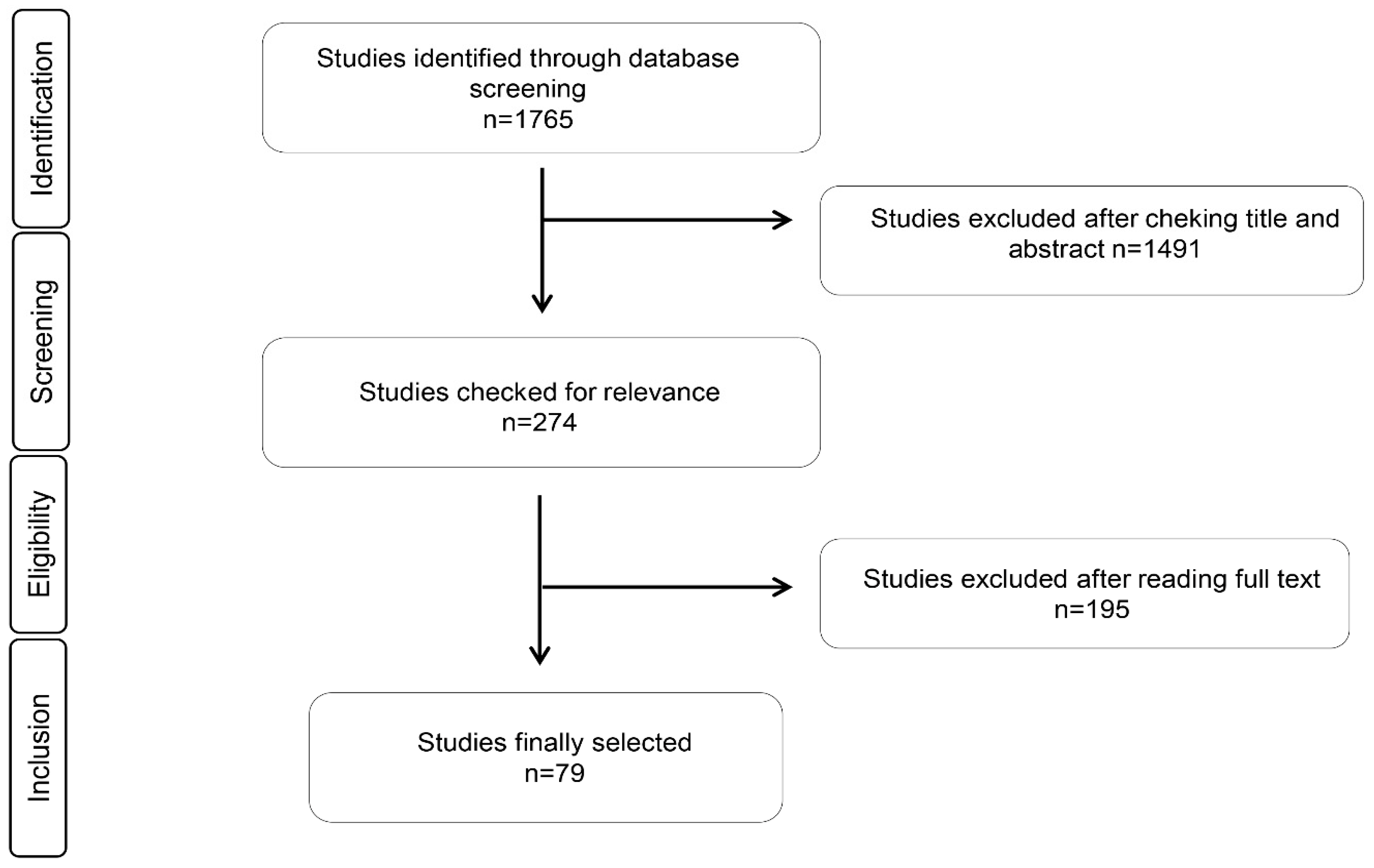
3.1. Study Identification and Selection
3.2. Antimicrobial Activity of Esential Oils
3.3. Antioxidant Activity of Essential Oils
3.4. Immunomodulatory Activity of Essential Oils in Cells and Animals
4. Discussion
4.1. Antimicrobial Activity of Esential Oils
4.2. Antioxidant Activity of Essential Oils
4.3. Immunomodulatory Activity Effects of Essential Oils in Cells and Animals
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, R.L.; Mondal, S. Current Issues in Food Safety With Reference to Human Health. In Food Safety and Human Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–14. [Google Scholar]
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, A.C.; Ayyıldız, S. Food additives: Colorants. In Science within Food: Up-to-Date Advances on Research and Educational Ideas; Formatex Research Center: Badajoz, Spain, 2017. [Google Scholar]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, A.E.; Stobberingh, E.E. Epidemiology of resistance to antibiotics: Links between animals and humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Rico-Campa, A.; Martinez-Gonzalez, M.A.; Alvarez-Alvarez, I.; Mendonca, R.D.; de la Fuente-Arrillaga, C.; Gomez-Donoso, C.; Bes-Rastrollo, M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019, 365, l1949. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Kon, K.V.; Rai, M.K. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev. Anti-Infect. Ther. 2012, 10, 775–790. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Koidis, A. Methods for extracting essential oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–38. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Sharma, M.; Koul, A.; Sharma, D.; Kaul, S.; Swamy, M.K.; Dhar, M.K. Metabolic Engineering Strategies for Enhancing the Production of Bio-active Compounds from Medicinal Plants. In Natural Bio-Active Compounds; Springer: Berlin, Germany, 2019; pp. 287–316. [Google Scholar]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2016, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Cava-Roda, R.M.; Taboada-Rodríguez, A.; Valverde-Franco, M.T.; Marín-Iniesta, F. Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157: H7 in milk. Food Bioprocess. Technol. 2012, 5, 2120–2131. [Google Scholar] [CrossRef]
- Alfonzo, A.; Martorana, A.; Guarrasi, V.; Barbera, M.; Gaglio, R.; Santulli, A.; Settanni, L.; Galati, A.; Moschetti, G.; Francesca, N. Effect of the lemon essential oils on the safety and sensory quality of salted sardines (Sardina pilchardus Walbaum 1792). Food Control 2017, 73, 1265–1274. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Jia, S.; Luo, Y. Antimicrobial effects of cinnamon bark oil on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Food Control 2017, 82, 316–324. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Anastasiou, C.; Buchbauer, G. Essential Oils as Immunomodulators: Some Examples. Open Chem. 2017, 15, 352–370. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, H.J.; Chao, J.; Liu, J.F. Essential oil of Artemisia argyi suppresses inflammatory responses by inhibiting JAK/STATs activation. J. Ethnopharmacol. 2017, 204, 107–117. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Cao, G.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2019, 97, 133–143. [Google Scholar] [CrossRef]
- Andrade, L.N.; De Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Aghraz, A.; Benameur, Q.; Gervasi, T.; Ait Dra, L.; Ben-Mahdi, M.; Larhsini, M.; Markouk, M.; Cicero, N. Antibacterial activity of Cladanthus arabicus and Bubonium imbricatum essential oils alone and in combination with conventional antibiotics against Enterobacteriaceae isolates. Lett. Appl. Microbiol. 2018, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, L.; Pena, A.; Velascd, J.; Baptista, J.G.; Rojas, L.; Aparicio, R.; Usubillaga, A. Chemical composition and antibacterial activity of the essential oil of Ruilopezia bracteosa. Nat. Prod. Commun. 2015, 10, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Anjum, A.A.; Ahmad, A.; Firyal, S.; Sana, S.; Latif, A.A. In vitro activity of Nigella sativa against antibiotic resistant Salmonella enterica. Environ. Toxicol. Pharmacol. 2018, 58, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Tabatabaei Yazdi, F.; Vasiee, A.; Mortazavi, S.A. Oliveria decumbens essential oil: Chemical compositions and antimicrobial activity against the growth of some clinical and standard strains causing infection. Microb. Pathog. 2018, 114, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Boonyanugomol, W.; Kraisriwattana, K.; Rukseree, K.; Boonsam, K.; Narachai, P. In vitro synergistic antibacterial activity of the essential oil from Zingiber cassumunar Roxb against extensively drug-resistant Acinetobacter baumannii strains. J. Infect. Public Health 2017, 10, 586–592. [Google Scholar] [CrossRef]
- Chaib, F.; Allali, H.; Bennaceur, M.; Flamini, G. Chemical Composition and Antimicrobial Activity of Essential Oils from the Aerial Parts of Asteriscus graveolens (Forssk.) Less. and Pulicaria incisa (Lam.) DC.: Two Asteraceae Herbs Growing Wild in the Hoggar. Chem. Biodivers. 2017, 14, e1700092. [Google Scholar] [CrossRef]
- Chen, C.C.; Yan, S.H.; Yen, M.Y.; Wu, P.F.; Liao, W.T.; Huang, T.S.; Wen, Z.H.; Wang, H.M.D. Investigations of kanuka and manuka essential oils for in vitro treatment of disease and cellular inflammation caused by infectious microorganisms. J. Microbiol. Immunol. Infect. 2016, 49, 104–111. [Google Scholar] [CrossRef]
- Chiboub, W.; Sassi, A.B.; Amina, C.M.; Souilem, F.; El Ayeb, A.; Djlassi, B.; Ascrizzi, R.; Flamini, G.; Harzallah-Skhiri, F. Valorization of the Green Waste from Two Varieties of Fennel and Carrot Cultivated in Tunisia by Identification of the Phytochemical Profile and Evaluation of the Antimicrobial Activities of Their Essentials Oils. Chem. Biodivers. 2019, 16, e1800546. [Google Scholar] [CrossRef]
- Condò, C.; Anacarso, I.; Sabia, C.; Iseppi, R.; Anfelli, I.; Forti, L.; de Niederhäusern, S.; Bondi, M.; Messi, P. Antimicrobial activity of spices essential oils and its effectiveness on mature biofilms of human pathogens. Nat. Prod. Res. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- de Jesus, I.C.; Santos Frazao, G.G.; Blank, A.F.; de Aquino Santana, L.C. Myrcia ovata Cambessedes essential oils: A proposal for a novel natural antimicrobial against foodborne bacteria. Microb. Pathog. 2016, 99, 142–147. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Mang, S.M.; Belviso, S.; De Feo, V.; Camele, I. Antimicrobial activity and chemical composition of three essential oils extracted from Mediterranean aromatic plants. J. Med. Food 2016, 19, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Fadil, M.; Fikri-Benbrahim, K.; Rachiq, S.; Ihssane, B.; Lebrazi, S.; Chraibi, M.; Haloui, T.; Farah, A. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella typhimurium: Optimization of antibacterial activity by mixture design methodology. Eur. J. Pharm. Biopharm. 2018, 126, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, T.; Moradi, P.; Mahboubi, M.; Rahimi, E.; Momtaz, H.; Hamedi, B. Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine 2015, 22, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289. [Google Scholar] [CrossRef] [PubMed]
- Gadisa, E.; Weldearegay, G.; Desta, K.; Tsegaye, G.; Hailu, S.; Jote, K.; Takele, A. Combined antibacterial effect of essential oils from three most commonly used Ethiopian traditional medicinal plants on multidrug resistant bacteria. BMC Complement. Altern. Med. 2019, 19, 24. [Google Scholar] [CrossRef]
- Igwaran, A.; Iweriebor, B.C.; Ofuzim Okoh, S.; Nwodo, U.U.; Obi, L.C.; Okoh, A.I. Chemical constituents, antibacterial and antioxidant properties of the essential oil flower of Tagetes minuta grown in Cala community Eastern Cape, South Africa. Bmc Complement. Altern. Med. 2017, 17, 351. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Adwan, L.; K’Aibni, S.; Shraim, N.; Zaid, A.N. Chemical composition, anthelmintic, antibacterial and antioxidant effects of Thymus bovei essential oil. Bmc Complement. Altern. Med. 2016, 16, 418. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choi, H.; Lee, J.C.; Lee, Y.C.; Woo, E.R.; Lee, D.G. Antibacterial Activity of Hibicuslide C on Multidrug-Resistant Pseudomonas aeruginosa Isolates. Curr. Microbiol. 2016, 73, 519–526. [Google Scholar] [CrossRef]
- Linde, G.; Gazim, Z.; Cardoso, B.; Jorge, L.; Tešević, V.; Glamočlija, J.; Soković, M.; Colauto, N. Antifungal and antibacterial activities of Petroselinum crispum essential oil. Genet. Mol. Res. 2016. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Hosseini, H.; Nasrollahzadeh, J.; Khaneghah, A.M.; Rismanchi, M.; Chaves, R.D.; Shahraz, F.; Azizkhani, M.; Mahmoudzadeh, L.; Haslberger, A.G. Antibacterial activity of Carum copticum essential oil against Escherichia coli O157: H7 in meat: Stx genes expression. Curr. Microbiol. 2016, 73, 265–272. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Arnold, N.A.; Menichini, F.; Senatore, F. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three Origanum species growing wild in Lebanon and Greece. Nat. Prod. Res. 2016, 30, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fan, S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Montironi, I.D.; Cariddi, L.N.; Reinoso, E.B. Evaluation of the antimicrobial efficacy of Minthostachys verticillata essential oil and limonene against Streptococcus uberis strains isolated from bovine mastitis. Rev. Argent. Microbiol. 2016, 48, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutlu-Ingok, A.; Karbancioglu-Guler, F. Cardamom, Cumin, and Dill Weed Essential Oils: Chemical Compositions, Antimicrobial Activities, and Mechanisms of Action against Campylobacter spp. Molecules 2017, 22, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoh, S.O.; Iweriebor, B.C.; Okoh, O.O.; Okoh, A.I. Bioactive constituents, radical scavenging, and antibacterial properties of the leaves and stem essential oils from Peperomia pellucida (L.) Kunth. Pharmacogn. Mag. 2017, 13, S392. [Google Scholar] [CrossRef] [PubMed]
- Okoh, S.O.; Iweriebor, B.C.; Okoh, O.O.; Nwodo, U.U.; Okoh, A.I. Antibacterial and Antioxidant Properties of the Leaves and Stem Essential Oils of Jatropha gossypifolia L. Biomed Res. Int. 2016, 2016, 9392716. [Google Scholar] [CrossRef] [Green Version]
- Oukerrou, M.A.; Tilaoui, M.; Mouse, H.A.; Leouifoudi, I.; Jaafari, A.; Zyad, A. Chemical composition and cytotoxic and antibacterial activities of the essential oil of Aloysia citriodora palau grown in Morocco. Adv. Pharmacol. Sci. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Paredes, A.; Leyton, Y.; Riquelme, C.; Morales, G. A plant from the altiplano of Northern Chile Senecio nutans, inhibits the Vibrio cholerae pathogen. SpringerPlus 2016, 5, 1788. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.; Baek, K.-H. Antibacterial activity and action mechanism of the essential oil from Enteromorpha linza L. against foodborne pathogenic bacteria. Molecules 2016, 21, 388. [Google Scholar] [CrossRef]
- Pereira, C.A.; Costa, A.C.; Liporoni, P.C.; Rego, M.A.; Jorge, A.O. Antibacterial activity of Baccharis dracunculifolia in planktonic cultures and biofilms of Streptococcus mutans. J. Infect. Public Health 2016, 9, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porfírio, E.M.; Melo, H.M.; Pereira, A.M.G.; Cavalcante, T.T.A.; Gomes, G.A.; Carvalho, M.G.D.; Costa, R.A.; Júnior, F.E.A.C. In vitro antibacterial and antibiofilm activity of Lippia alba essential oil, citral, and carvone against Staphylococcus aureus. Sci. World J. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakkas, H.; Gousia, P.; Economou, V.; Sakkas, V.; Petsios, S.; Papadopoulou, C. In vitro antimicrobial activity of five essential oils on multidrug resistant Gram-negative clinical isolates. J. Intercult. Ethnopharmacol. 2016, 5, 212. [Google Scholar] [CrossRef]
- Salem, M.Z.; Elansary, H.O.; Ali, H.M.; El-Settawy, A.A.; Elshikh, M.S.; Abdel-Salam, E.M.; Skalicka-Woźniak, K. Bioactivity of essential oils extracted from Cupressus macrocarpa branchlets and Corymbia citriodora leaves grown in Egypt. Bmc Complement. Altern. Med. 2018, 18, 23. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Sharafati Chaleshtori, F.; Saholi, M.; Sharafati Chaleshtori, R. Chemical Composition, Antioxidant and Antibacterial Activity of Bunium persicum, Eucalyptus globulus, and Rose Water on Multidrug-Resistant Listeria Species. J. Evid.-Based Integr. Med. 2018, 23, 2515690X17751314. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, A.; Mohammadzadeh, A.; Salehi, T.Z.; Mahmoodi, P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 2018, 124, 379–388. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Hoseini-Alfatemi, S.; Sharifi-Rad, M.; Sharifi-Rad, M.; Iriti, M.; Sharifi-Rad, M.; Sharifi-Rad, R.; Raeisi, S. Phytochemical compositions and biological activities of essential oil from Xanthium strumarium L. Molecules 2015, 20, 7034–7047. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. In Vitro Evaluation of the Antioxidant, Cytoprotective, and Antimicrobial Properties of Essential Oil from Pistacia vera L. Variety Bronte Hull. Int. J. Mol. Sci. 2017, 18, 1212. [Google Scholar] [CrossRef] [Green Version]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.M.; Alsaadi, A.I.; Youssef, E.G.; Khitrov, G.; Noreddin, A.M.; Husseiny, M.I.; Ibrahim, A.S. Calli Essential Oils Synergize with Lawsone against Multidrug Resistant Pathogens. Molecules 2017, 22, 2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibyangye, J.; Okech, M.A.; Nyabayo, J.M.; Nakavuma, J.L. In vitro antibacterial activity of Ocimum suave essential oils against uropathogens isolated from patients in selected hospitals in Bushenyi district, Uganda. Br. Microbiol. Res. J. 2015, 8, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touihri, I.; Boukhris, M.; Marrakchi, N.; Luis, J.; Hanchi, B.; Kallech-Ziri, O. Chemical Composition and Biological Activities of Allium roseum L. var. grandiflorum Briq. Essential Oil. J. Oleo Sci. 2015, 2015, ess15055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ušjak, L.; Petrović, S.; Drobac, M.; Soković, M.; Stanojković, T.; Ćirić, A.; Niketić, M. Edible wild plant Heracleum pyrenaicum subsp. orsinii as a potential new source of bioactive essential oils. J. Food Sci. Technol. 2017, 54, 2193–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utegenova, G.A.; Pallister, K.B.; Kushnarenko, S.V.; Ozek, G.; Ozek, T.; Abidkulova, K.T.; Kirpotina, L.N.; Schepetkin, I.A.; Quinn, M.T.; Voyich, J.M. Chemical Composition and Antibacterial Activity of Essential Oils from Ferula L. Species against Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 1679. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.; Mendes, A.; Martins da Costa, P.; Belo, A.D. Chemical composition, antibacterial, antibiofilm and synergistic properties of essential oils from Eucalyptus globulus Labill. and seven Mediterranean aromatic plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, L.; Tang, X.; Peng, L.; Li, X.; Zhao, G.; Zhong, L. Chemical Composition, Antimicrobial and Antioxidant Activities of the Flower Volatile Oils of Fagopyrum esculentum, Fagopyrum tataricum and Fagopyrum Cymosum. Molecules 2018, 23, 182. [Google Scholar] [CrossRef] [Green Version]
- Bag, A.; Chattopadhyay, R.R. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef] [Green Version]
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehsani, A.; Alizadeh, O.; Hashemi, M.; Afshari, A.; Aminzare, M. Phytochemical, antioxidant and antibacterial properties of Melissa officinalis and Dracocephalum moldavica essential oils. Vet. Res. Forum 2017, 8, 223–229. [Google Scholar] [PubMed]
- Hu, Q.P.; Cao, X.M.; Hao, D.L.; Zhang, L.L. Chemical Composition, Antioxidant, DNA Damage Protective, Cytotoxic and Antibacterial Activities of Cyperus rotundus Rhizomes Essential Oil against Foodborne Pathogens. Sci. Rep. 2017, 7, 45231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaradat, N.; Adwan, L.; K’Aibni, S.; Zaid, A.N.; Shtaya, M.J.Y.; Shraim, N.; Assali, M. Variability of Chemical Compositions and Antimicrobial and Antioxidant Activities of Ruta chalepensis Leaf Essential Oils from Three Palestinian Regions. Biomed Res. Int. 2017, 2017, 2672689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, M. Chemical composition and antimicrobial, antioxidant activities and anti-inflammatory potential of Achillea millefolium L., Anethum graveolens L., and Carum copticum L. essential oils. J. Herb. Med. 2015, 5, 217–222. [Google Scholar] [CrossRef]
- Marin, I.; Sayas-Barbera, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Foods 2016, 5, 18. [Google Scholar] [CrossRef]
- Marrelli, M.; Araniti, F.; Abenavoli, M.R.; Statti, G.; Conforti, F. Potential Health Benefits of Origanum heracleoticum Essential Oil: Phytochemical and Biological Variability among Different Calabrian Populations. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Okoh, S.O.; Iweriegbor, B.C.; Okoh, O.O.; Nwodo, U.U.; Okoh, A.I. Bactericidal and antioxidant properties of essential oils from the fruits Dennettia tripetala G. Baker. BMC Complement. Altern. Med. 2016, 16, 486. [Google Scholar] [CrossRef] [Green Version]
- Ouedrhiri, W.; Balouiri, M.; Bouhdid, S.; Harki, E.H.; Moja, S.; Greche, H. Antioxidant and antibacterial activities of Pelargonium asperum and Ormenis mixta essential oils and their synergistic antibacterial effect. Environ. Sci. Pollut. Res. Int. 2018, 25, 29860–29867. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Izadi, A.; Malek Poor, F.; Hamedi, B. Chemical composition, antioxidant and antibacterial activities of essential oils from Ferulago angulata. Pharm. Biol. 2016, 54, 2515–2520. [Google Scholar] [CrossRef] [Green Version]
- Poaty, B.; Lahlah, J.; Porqueres, F.; Bouafif, H. Composition, antimicrobial and antioxidant activities of seven essential oils from the North American boreal forest. World J. Microbiol. Biotechnol. 2015, 31, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Semeniuc, C.A.; Socaciu, M.I.; Socaci, S.A.; Muresan, V.; Fogarasi, M.; Rotar, A.M. Chemometric Comparison and Classification of Some Essential Oils Extracted from Plants Belonging to Apiaceae and Lamiaceae Families Based on Their Chemical Composition and Biological Activities. Molecules 2018, 23, 2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeri, A.; Akhtari, J.; Soheili, V.; Taghizadeh, S.F.; Sahebkar, A.; Shaddel, R.; Asili, J. Identification and biological activity of the volatile compounds of Glycyrrhiza triphylla Fisch. & C.A.Mey. Microb. Pathog. 2017, 109, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules 2015, 20, 14402–14424. [Google Scholar] [CrossRef]
- Chen, H.C.; Chang, W.T.; Hseu, Y.C.; Chen, H.Y.; Chuang, C.H.; Lin, C.C.; Lee, M.S.; Lin, M.K. Immunosuppressive Effect of Litsea cubeba L. Essential Oil on Dendritic Cell and Contact Hypersensitivity Responses. Int. J. Mol. Sci. 2016, 17, 1319. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef] [Green Version]
- Krifa, M.; El Mekdad, H.; Bentouati, N.; Pizzi, A.; Ghedira, K.; Hammami, M.; El Meshri, S.E.; Chekir-Ghedira, L. Immunomodulatory and anticancer effects of Pituranthos tortuosus essential oil. Tumour Biol. 2015, 36, 5165–5170. [Google Scholar] [CrossRef]
- Ma, Q.; Jiang, J.G.; Yuan, X.; Qiu, K.; Zhu, W. Comparative antitumor and anti-inflammatory effects of flavonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from Cirsium japonicum DC. Food Chem. Toxicol. 2019, 125, 422–429. [Google Scholar] [CrossRef]
- Oüzek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Oüzek, T.; Baser, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Yoo, S.-A.; Kim, W.-U.; Cho, C.-S.; Woo, J.-M.; Yoon, C.-H.; Yoo, S.; Kim, W.; Cho, C.; Woo, J.; et al. Anti-inflammatory effects of essential oils extracted from Chamaecyparis obtusa on murine models of inflammation and RAW 264.7 cells. Mol. Med. Rep. 2016, 13, 3335–3341. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Zhu, L.; Zeng, D.; Long, W.; Zhu, S.-M. Chemical composition and anti-inflammatory activities of essential oil from Trachydium roylei. J. Food Drug Anal. 2016, 24, 602–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. 2018, 98, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Altop, A.; Erener, G.; Duru, M.E.; Isik, K. Effects of essential oils from Liquidambar orientalis Mill. leaves on growth performance, carcass and some organ traits, some blood metabolites and intestinal microbiota in broilers. Br. Poult. Sci. 2018, 59, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cetin, E.; Yibar, A.; Yesilbag, D.; Cetin, I.; Cengiz, S.S. The effect of volatile oil mixtures on the performance and ilio-caecal microflora of broiler chickens. Br. Poult. Sci. 2016, 57, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Cairo, P.L.G.; Gois, F.D.; Sbardella, M.; Silveira, H.; de Oliveira, R.M.; Allaman, I.B.; Cantarelli, V.S.; Costa, L.B. Effects of dietary supplementation of red pepper (Schinus terebinthifolius Raddi) essential oil on performance, small intestinal morphology and microbial counts of weanling pigs. J. Sci. Food Agric. 2018, 98, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Ma, X.; Geng, S.; Jiang, X.; Huang, Q.; Hu, C.; Han, X. Intestinal Microbiome-Metabolome Responses to Essential Oils in Piglets. Front. Microbiol. 2018, 9, 1988. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.D.L.; Baldisserotto, B.; Sutili, F.; Gressler, L.; Battisti, E.; Heinzmann, B.; De Vargas, A.C. Plant essential oils against Aeromonas hydrophila: In vitro activity and their use in experimentally infected fish. J. Appl. Microbiol. 2015, 119, 47–54. [Google Scholar] [CrossRef]
- Sell, C.S. The Chemistry of Fragrances: From Perfumer to Consumer; Royal Society of Chemistry: London, UK, 2006; Volume 38. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)control of Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef] [Green Version]
- Winkelstroter, L.K.; Teixeira, F.B.; Silva, E.P.; Alves, V.F.; De Martinis, E.C. Unraveling microbial biofilms of importance for food microbiology. Microb. Ecol. 2014, 68, 35–46. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tian, X. Quorum Sensing and Bacterial Social Interactions in Biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Hadi, S.; Bhat, S.; Azmi, A.; Hanif, S.; Shamim, U.; Ullah, M. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.; Gardini, F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Tenore, G.C.; Ciampaglia, R.; Arnold, N.A.; Piozzi, F.; Napolitano, F.; Rigano, D.; Senatore, F. Antimicrobial and antioxidant properties of the essential oil of Salvia lanigera from Cyprus. Food Chem. Toxicol. 2011, 49, 238–243. [Google Scholar] [CrossRef]
- de Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira Júnior, R.G.; Gama e Silva, M.; de Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araújo, M.T.; Coutinho, H.D.M.; et al. Essential Oils and Their Major Compounds in the Treatment of Chronic Inflammation: A Review of Antioxidant Potential in Preclinical Studies and Molecular Mechanisms. Oxidative Med. Cell. Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef] [Green Version]
- Thapa, D.; Louis, P.; Losa, R.; Zweifel, B.; Wallace, R.J. Essential oils have different effects on human pathogenic and commensal bacteria in mixed faecal fermentations compared with pure cultures. Microbiology 2015, 161, 441–449. [Google Scholar] [CrossRef]
| Parameter | Inclusion Criteria |
|---|---|
| Population | Studies performed in cells and animals, including humans |
| Intervention | Treatment with essential oil |
| Comparison | Essential oil vs. control |
| Outcome | Antimicrobial, antioxidant, and immunomodulatory effects |
| Article | Plant Derived EOs | Main Components of EOs | Bacteria | MIC/MBC/IC50 |
|---|---|---|---|---|
| Aghraz et al. [25] | Cladanthus arabicus and Bubonium imbricatum | Cladanthus arabicus: oxygenated monoterpenes (61.4%): cis-chrysanthenyl acetate (31.4%) and thymolisobutyrate (3.4%); Bubonium imbricatum: monoterpenes hydrocarbons (75.8%): sabinene (31.1%), β-pinene (16.7%), myrcene (12.3%), and α-pinene (5.3%) | E. coli, K. pneumoniae, E. cloacae, P. mirabilis, Salmonella spp. | MIC 200–800 μg/mL for C. arabicus, MIC 400–1600 μg/mL for B. imbricatum |
| Alarcon et al. [26] | Ruilopezia bracteosa | β-myrcene (34.2%), α-pinene (24.3%), 7-epi-α-selinene (9.1%), and β-pinene (8.5%) | S. aureus, E. faecalis, K. pneumoniae, E. coli, S. Typhi, P. aeruginosa | MIC 10 μg/mL |
| Ashraf et al. [27] | Nigella sativa | Hymoquinone, dithymoquinone, thymohydroquinone, and thymol | S. Enterica | MIC ≥1000.0 ± 322.7 μg/mL |
| Behbahani et al. [28] | Oliveria decumbens | Thymol (28.45%) γ-terpinene (22.2%), ρ-cymene (17.90%), myristicin (13.55%), carvacrol (8.50%), and limonene (2.60%) | P. aerogenes, E. coli, S. pyogenes, S. epidermidis | MIC, 1–8 mg/mL; MBC 1–16 mg/mL |
| Boonyanugomol et al. [29] | Zingiber cassumunar | sabinene, γ-terpinene, α-terpinene, terpinene-4-ol, and (E)-1–(3,4-dimethoxyphenyl)butadiene | Acinetobacter baumannii | MIC/MBC: 7.00–9.24 mg/mL |
| Chaib et al. [30] | Asteriscus graveolens and Pulicaria incisa | Asteriscus graveolens: cis-chrysanthenyl acetate (31.1%), myrtenyl acetate (15.1%), and kessane (11.5%); Pulicaria incisa: chrysanthenone (45.3%) and 2,6-dimethylphenol (12.6%) | K. pneumoniae, E. coli, A. baumannii, P. aeruginosa, L. monocytogenes, S. aureus, P. mirabilis | MIC: 19–1250 μg/mL |
| Chen et al. [31] | Kunzea ericoides and Leptospermum scoparium | - | T. mucoides, C. tropicalis, S. aureus, S. mutans, S. sobrinus, E. coli | MIC 0.78%–3.13% |
| Chiboub et al. [32] | Foeniculum vulgare MILL, Daucus carota L. subsp. sativus | Daucus carota: isospathulenol, caryophyllene oxide, and δ-elemene Foeniculum vulgare MILL: (E)-anethole p-anisaldehyde, p-acetonylanisole, limonen, exo-fenchol acetate, and methyl chavicol | S. aureus, B. subtilis, B. amyloliquefaciens, S. Enterica, E. coli, C. albicans | MIC: 6.25–50 mg/mL |
| Condo et al. [33] | Pimpinella anisum L., Cinnamomum zeylanicum, Syzygium aromaticum, and Cuminum cyminum L. | Pimpinella anisum L: trans-anethole ((E)-1-methoxy-4-(1-propenyl) benzene); Cinnamomum zeylanicum: cinnamaldehyde; Syzygium aromaticum: eugenol; Cuminum cyminum: cuminaldehyde (4-isopropylbenzaldehyde), and cuminyl alcohol (4-isopropyl-benzyl-alcohol) | S. aureus, S. epidermidis, E. faecalis, S. pyogenes, E. coli, P. aeruginosa, A. hydrophila, P. mirabilis, K. pneumoniae, C. albicans | |
| De Jesus et al. [34] | Myrcia ovata Cambessedes | Geranial (40%), neral (28%), citronella (9%) | P. aeruginosa, S. aureus, B. cereus, B. subtilis, E. faecalis, S. marcescens, E. coli, S. enteritidis | MIC: 0.78–25 μL/mL |
| Elshafie et al. [35] | Verbena officinalis, Majorana hortensis, and Salvia officinalis | Verbena officinalis: Isobornyl formate (45.4%), (E)-citral (47.5%); Majorana hortensis: 1,8-cineole (33.5%), β-phellandrene (9.1%), α-pinene (9%), limonene (6.4%); Salvia officinalis: Trans-thurjone (37.9%), canfor (13.9%), and borneol (7.6%) | B. megaterium, B. mojavensis, C. michiganensis, E. coli, X. campestris, P. savastanoi, P. syringae pv. phaseolicola | MIC: 1000–10,000 mg/L |
| Fadil et al. [36] | Mixture of Thymus vulgaris, Rosmarinus officinalis L., and Myrtus communis L. | T. vulgaris: Thymol (37.54%), p-cymene (14.49%), c-terpinene (11.15%), linalool (4.71%), and carvacrol (4.62%); R. officinalis: α-pinene (48.58%), 1,8-cineole (33.4%) and camphene (8.69%); M. communis: borneol (27.15%), 1,8-cineole (21.33%), α-pinene (11.09%), myrtenyl acetate (6.45%), trans-pinocarveol (4.82%), and α -terpineol (4.83%) | S. Typhimurium | Thyme MIC: 0.25% (V/V); myrtle MIC: 0.5% (V/V); Rosemary MIC: 2% (V/V) |
| Falsafi et al. [37] | Satureja bachtiarica Bunge | Carvacrol (45.5%), thymol (27.9%), p-cymene (4.4%), γ-terpinene (4.0%), α-pinene (1.5%), 1,8-cineole (1.3%), α-terpinene (1.2%), and E-caryophyllene (1.1%) | H. pylori | MIC: 0.035 μL/mL |
| Fournomiti et al. [38] | Origanum vulgare, Salvia officinalis, Thymus vulgaris | Origanum vulgare: Carvacrol and thymol; Salvia officinalis: 1,8-cineole, α-thujone and camphor; Thymus vulgaris: thymol and carvacrol | E. coli, K. oxytoca, K. pneumoniae | MIC oregano: 0.9 mg/mL; 73.5 µg/mL; MIC thyme: 8.1 µg/mL; 9.5 µg/mL; 28.6 µg/mL against K. oxytoca, K. pneumoniae and E. coli, respectively |
| Gadisa et al. [39] | Blepharis cuspidata, Boswellia ogadensis, and Thymus schimper | E. coli, K. pneumoniae and MRSA | MIC: 0.39–6.25 μL/mL/MBC (0.78–12.5 μL/mL) against MDR E. coli and K. pneumoniae | |
| Igwaran et al. [40] | Tagetes minuta | β-Ocimene (14.40%), m-tert-butyl-Phenol (9.41%), 2,6-dimethyl-, (E)-5,7-Octadien-4-one (7.14%), 1,2, 3,4,4a,5,6,7-octa hydro-4a-methyl-naphthalene (5.58%), and spathulenol (4.56%) | S. uberis, E. cloacae, S. aureus, M. smegmatis, L. ivanovii, Vibrio spp., E. coli | MIC (S. aureus, M. smegatis, and S. uberis): 0.125 mg/mL; L. ivanovii, Vibrio spp., E. cloacae and E. coli: 0.06 mg/mL. MBC (E. cloacae and E. coli): 0.06 mg/mL; MBC S. uberis: 0.5 mg/mL; Vibrio spp.: 0.125 mg/mL |
| Jaradat et al. [41] | Thymus bovei | trans-geraniol (35.38%), α-citral (20.37%), β-citral (14.76%), cis-geraniol (7.38%), and 3-octanol (4.38%) | S. aureus, E. coli, P. aeruginosa, C. albicans | MIC: 0.25–0.5 mg/mL |
| Lee et al. [42] | Hibicuslide C | - | P. aeruginosa strains | MIC range: 5.0–10.0 µg/mL |
| Linde et al. [43] | Petroselinum crispum | Apiol (50.3%), myristicin (14.0%), and β-phellandrene (14.6%) | B. cereus, E. cloacae, L. monocytogenes, E. coli, P. aeruginosa, S. Typhimurium, S. aureus | MICs 0.04–1.0 mg/mL. MBCs 0.15–10.0 mg/mL |
| Mahmoudzadeh et al. [44] | Carum copticum | thymol (36.4%), p-Cymene (31.4%), and γ-Terpinene (21.73%) | E. coli | MIC 0.05%–1.75%; MBC 0.052%–3.25% |
| Man et al. [45] | Boswellia sacra, Myrtus communis, Thymus vulgaris, Citrus limon, Origanum vulgare, and Lavandula angustifolia | Boswellia sacra: β-pinene (25.6%), α-terpinene (18.6%); Myrtus communis: β-pinene (25%), eucalyptol (28.7%); Thymus vulgaris: linalool (56.5%), geranyl propionate (16.3%); Citrus limon: limonene (36.9%), β-pinene (15%), α-pinene (19.2%); Origanum vulgare: carvacrol (80.5%); Lavandula angustifolia: linalyl-butyrate (26.5%), and linalool (25%) | S. aureus, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa | MICs/MBCs 0.1% to >50% |
| Marrelli et al. [46] | Origanum dictamnus, Origanum libanoticum and Origanum microphyllum | O. dictamnus: p-cymene (32.7%), γ-terpinene (12.4%), carvacrol (14.7%), and linalool (7.8%); O. microphyllum: Terpinen-4-ol (16.2%), carvacrol (13.3%), sabinene (7.5%), and trans-sabinene hydrate (7.1%); Origanum libanoticum: linalool (6.5%), thymol methyl ether (9.8%), (E)-b-caryophyllene (7.7%), and hexadecanoic acid (11.3%) | B. cereus, B. subtilis, S. epidermidis, S. aureus, S. faecalis, E. coli | O. dictamnus MIC: 25–50 mg/mL |
| Meng et al. [47] | Juniperus rigida | Caryophyllene (13.11%) and α-caryophyllene (11.72%) | K. pneumoniae | MIC/MBC: 3.125 mg/mL |
| Montironi et al. [48] | Minthostachys verticillata | Pulegone (51.7%) and menthone (37.8%) | S. uberis | MIC: 14.3–114.5 mg/mL/MBC: 114.5–229 mg/mL |
| Mutlu-Ingok et al. [49] | Cardamom, cumin, and dill weed | Cumin: p-mentha-1,3-dien-7-al (26.7%), cumin aldehyde (24.1%), γ-terpinene (16.9%), and β-pinene (14.4%); Cardamom: α-terpinly acetate (43.4%) and 1,8-cineole (29.2%); Dill weed: carvone (41.6%), and limonene (27.4%) | C. jejuni, C. coli | MIC/MBC: 0.05 L/mL, cumin, Cardamon/cumin MIC/MBC: 0.025 L/mL |
| Okoh et al. [50] | Peperomia pellucida | Linalool, d-limonene, β-caryophyllene, and linalyl acetate were the major compounds | S. aureus, L. ivanovii, M. smegmatis, S. uberis, E. cloacae, E. coli, V. paraheamolyticus | MIC: 0.15–0.20 mg/mL |
| Okoh et al. [51] | Jatropha gossypifolia | Phytol, germacrene D, 𝛼-copaene, 𝛼-terpinene, and limonene were the major compounds | E. coli, E. faecium, and S. aureus | MIC/MBC: 0.025–0.10 mg/; MBC: 0.05–0.10 mg/mL |
| Oukerrou et al. [52] | Aloysia citriodora | 𝛽-spathulenol (15.61%), ar-curcumene (14.15%), trans-caryophyllene oxide (14.14%), and neral (10.02%) | E. coli, S. aureus, P. aeruginosa | MIC: 2.84–8.37 mg/mL |
| Paredes et al. [53] | Senecio nutans | Methyl cinnamate (44.9%), p-cymenol (27.2%), | Vibrio cholerae | MIC: 0.4 mg/mL |
| Patra et al. [54] | Enteromorpha linza | Hexadecanoic acid, nonadecadiene, tetradecanoic acid, tridecanol, and azetidine | B. cereus, S. aureus | MIC/MBC: 12.5–25 mg/mL |
| Pereira et al. [55] | Baccharis dracunculifolia | - | Streptococcus mutans | MIC: 6% |
| Porfirio et al. [56] | Lippia alba | Geranial, neral, p-cymene, geranic acid, carvone, and limonene were the major compounds | S. aureus | MIC 0.5–1 mg/mL; MBC: 0.5–2 mg/mL |
| Puškárová et al. [57] | O. vulgare, T. vulgaris, S. sclarea, L. angustifolia, E. Caryophyllata, and T. plicata | - | E. coli, S. Typhimurium, Y. enterocolitica, S. aureus, L. monocytogenes, E. faecalis, B. cereus, A. protophormiae, P. fragi | MIC/MBC: 0.025%–0.5% |
| Sakkas et al. [58] | Ocimum basilicum L., Matricaria chamomilla, L.Thymus capitatus, L., Melaleuca alternifolia, Thymus vulgaris, L. | Ocimum basilicum L.: estragole; Matricaria chamomilla, L.: bisabolol and trans-b-farnesene; Thymus capitatus, L.: carvacrol and thymol; Melaleuca alternifolia: terpinen-4-ol and p-cymene; Thymus vulgaris, L: thymol, p-cymene, and linalool | A. baumannii, E. coli, K. pneumoniae and P. aeruginosa | MIC/MBC values ranged from 0.12% to 1.50% (v/v) for tea tree oil, 0.25%–4% (v/v) for origanum and thyme oil, 0.50% to >4% for basil oil |
| Salem et al. [59] | Cupressus macrocarpa Hartw and Corymbia citriodora (Hook.) | Terpinen-4-ol (23.7%), α-phellandrene (19.2%), α-citronellol (17.3%), and citronellal were the major constituents of C. macrocarpa, and α-citronellal (56%), α-citronellol (14.7%), citronellol acetate (12.3%), isopulegol, and eucalyptol were the primary constituents of C. citriodora | B. cereus, L. monocytogenes, M. flavus, S. aureus, D. solani, E. coli, P. atrosepticum, P. carotovorum subsp. Carotovorum, P. aeruginosa | MIC C. citriodora leaves 0.06–0.20 mg/mL, MBC: 0.12–0.41 mg/mL; MIC C. macrocarpa: 0.07–0.31 mg/mL, MBC: 0.15–0.63 mg/mL |
| Semeniuc et al. [60] | Parsley, lovage, basil, and thyme | β-myrcene, β-phellandrene, γ-terpinene, and α-pinene were the major compounds | B. cereus, S. aureus, P. aeruginosa, E. coli, S. Typhimurium | B. cereus MIC Basil: 10.8 μL/mL; thyme: 0.56 μL/mL; S. aureus MIC Basil: 2.45 μL/mL and thyme 0.06 μL/mL. P. aeruginosa MIC Basil 10.80 μL/mL and thyme 0.27 μL/mL. S. Typhimurium MIC Basil: 22.68 μL/mL and thyme: 0.56 μL/mL |
| Sharafiti Chaleshtori et al. [61] | Bunium persicum, Eucalyptus globulus, and rose water | B. persicum, β-pinene (11.72%), p-cymene (15.47%), g-terpinene (18.32%), cumin aldehyde (38.4%), p-mentha-1,3-dien-7-al (5.37%), and p-mentha1,4-dien-7-al (2.86%); E. globulus, limonene (9.4%) and 1,8-cineole (70.3%); rose water, linalool (6.6%), terpineol (3.3%), carvone (0.31%), citronellol (6.85%), trans-geraniol (7.11%), phenylethanol (66.84%), eugenol (4.53%), cytronellol, hydroxyl (1.15%), and geranic acid (1.2%) | Listeria spp. | MIC: 0.351–2.812 mg/mL MBC: 0.703–5.625 mg/mL |
| Sharifi et al. [62] | Thymus daenensis; Satureja hortensis | T. daenensis: carvacol (40%–69%), followed by γ-terpinene (30%–28%) and α-terpinene (5%–52%); S. hortensis: thymol (41%–28%), γ-terpinene (37%–63%), p-cymene (2%–12%) and α-terpinene (3%–52%) | S. aureus | MICs of T. daenensis: 0.0625 μL/mL; S. hortensis 0.125 μL/mL; MBC 0.125 μL/mL |
| Sharifi-Rad et al. [63] | Xanthium strumarium L. | cis-β-guaiene (34.2%), limonene (20.3%), borneol (11.6%), and bornyl acetate (4.5%) | K. pneumoniae, E. coli, P. aeruginosa, S. aureus, S. epidermis, B. subtilis | MIC S. aureus: 0.5 μg/mL; MIC B. subtilis 1.3 μg/mL; MIC K. pneumoniae 4.8 μg/mL |
| Smeriglio et al. [64] | Pistacia vera L. | 4-Carene, α-pinene, and δ-3-Carene were the major compounds | S. aureus, S. aureus MRSA, three clinical isolates of S. aureus, E. coli and P. aeruginosa | MIC/MBC: 7.11 mg/mL inhibited the growth of all the tested strains, with the exception of Pseudomonas |
| Snoussi et al. [65] | Petroselinum crispum, Ocimum basilicum | P. crispum: 1,3,8-p-menthatriene (24.2%), β-phellandrene (22.8%), apiol (13.2%), myristicin (12.6%), and terpinolene (10.3%); O. basilicum: linalool (42.1%), (E)-methylcinnamate (16.9%), and 1,8-cineole (7.6%) | V. alginolyticus, V. alginolyticus, V. parahaemolyticus, V. parahaemolyticus, Vibrio vulnificus, V. vulnificus, V. cholerae, A. hydrophila | P. crispum: MIC: 0.011–0.044 mg/mL MBC:2.81–11.25 mg/mL; O. basilicum MIC 0.019–0.039 mg/mL; MBC 2.5–10 mg/mL |
| Soliman et al. [66] | Calligonum comosum | Benzaldehyde derivative was the major compound | P. aeruginosa, K. pneumoniae, A. baumannii, and E. coli | MIC: 180.0–200.0 µg/mL |
| Tibyangye et al. [67] | Ocimum suave | Linalool and geraniol were the major compounds | E. coli, K. pneumoniae, S. aureus, E. faecalis, M. morganii, Citrobacter spp., Enterobacter spp. and P. aeruginosa | MIC: 0.78–22 μg/mL |
| Touihri et al. [68] | Allium roseum | Methyl methanethiosulfinate, 3-vinyl-1,2-dithiacyclohex-5-ene, and diallyl trisulfide were the major compounds | S. aureus, K. pneumoniae, E. coli, E. faecalis, S. Typhimurium | MIC: 0.078–2.5 mg/mL |
| Ušjak et al. [69] | Heracleum pyrenaicum subsp. orsinii (Guss.) | β-pinene, (Z)-β-ocimene, and α-pinene were the major compounds | S. aureus, B. cereus, L. monocytogenes, M. flavus, P. aeruginosa, E. coli, S. Typhimurium, E. cloacae | B. cereus (MIC: 0.21 mg/mL, MBC: 0.53 mg/mL). S. Typhimurium, E. coli, P. aeruginosa (MICs: 0.23 mg/mL, MBCs: 0.47 mg/mL), S. aureus (MIC: 0.23 mg/mL, MBC: 0.47 mg/mL) |
| Utegenova et al. [70] | Ferula ovina (Boiss.) | α-pinene (47.8%), β-pinene (7.1%), sabinene (20.5%), β-phellandrene (6.5%), and trans-verbenol (7.4%) | MRSA | IC50: 19.1–22.9 μg/mL |
| Vieira et al. [71] | Eucalyptus globulus, Thymus mastichina L., Mentha pulegium L., Rosmarinus officinalis L., Calamintha nepeta, Cistus ladanifer L., Foeniculum vulgare L., Dittrichia viscosa | Lamiaceae: isopulegol, isopulegone and 1,8- Cineole; C. nepeta: pulegone; M. pulegium: β-myrcene, camphor, 1,8-cineole; R. officinalis: α-pinene, and 1,8-cineole; T. mastichina: α-terpinyl acetate; C. ladanifer: α-pinene, camphene, fenchone, bornyl acetate, and viridiflorol; E. globulus: 1,8-cineole; F. vulgare: anethol, b-myrcene and fenchone; D. viscosa: E-nerolidol and fokienol | S. aureus, B. subtilis, E. coli, P. aeruginosa | MIC: 6–25 mg/mL |
| Xu et al. [72] | Syringa yunnanensis | Eugenol (76.23%), β-caryophyllene (11.54%), caryophyllene oxide (4.29%), and eugenyl acetate (1.76%) | S. aureus | MIC: 0.625 mg/mL |
| Zhao et al. [73] | Fagopyrum esculentum, Fagopyrum tataricum, Fagopyrum Cymosum | F. esculentum: nonanoic acid (7.58%), (E)-3-hexen-1-ol (6.52%), benzothiazole (5.08%), 2-Pentadecanone (18.61%), eugenol (17.18%); F. tataricum: 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester (13.19%), and (E,E)-farnesylacetone (7.15%); F.Cymosum: eugenol (12.22%), (E)-3-hexen-1-yl acetate (8.03%), linalool oxide (7.47%), 1-hexanol (7.07%), and benzothiazole (6.72%) | A. tumefaciens, E. coli, P. lachrymans, X. vesicatoria, B. subtilis, S. aureus | MIC: 100.0–800.0 g/mL |
| Article | Plant Derived EOs | Main Components of EOs | Method | Antioxidant Effects |
|---|---|---|---|---|
| Bag et al. [74] | Bay leaf, black pepper, coriander, cumin, garlic, ginger, mustard, onion, and turmeric | Coriander and cumin seed oil, linalool, p-coumaric acid | DPPH method | Coriander 150.62 (µg/mL), cumin 163.50 (µg/mL), mustard 155.16 (µg/mL) |
| Djenane et al. [75] | Orange (Citrus sinensis L.), lemon (Citrus limonum L.), and bergamot (Citrus aurantium L.) from Algeria | Limonene (77.37%) for orange EO; linalyl acetate (37.28%), linalool (23.36%) for bergamot EO; and limonene (51.39%), β-pinene (17.04%), and γ-terpinene (13.46%) for lemon EO | Antioxidant effect in treated sardine | A reduction of 2.50 log10 CFU/g was recorded during the third day of storage |
| Ehsani et al. [76] | Melissa officinalis and Deracocephalum moldavica | M. officinalis, citronellal, thymol, and citral; D. moldavica, geraniol, geranial, geranyl acetate, and neral | DPPH, BCBT, and ABTS assay | Both EOs showed strong activity in the maintenance of β-carotene molecules, which was higher than that of ascorbic acid |
| Hu et al. [77] | Cyperus rotundus L. | α–pinene, cyperene, α–cyperone, and cyperotundone were the major compounds | DPPH and ABTS radicals | DPPH radicals were far lower than that of Trolox (13.1 μg/mL); however, ABTS radicals were significantly higher than Trolox (84.7 μg/mL) |
| Jaradat et al. [78] | Ruta chalepensis | Linalyl acetate, 𝛽-linalool, 2-undecanone, and 2-nonanone were the major compounds | DPPH method | Percentages of inhibition for R. chalepensis collected from Jerusalem, Hebron, and Jenin were 6.9 ± 0.94 µg/mL, 69.56%; 7.8 ± 1.05 µg/mL, 61.53%; and 19.9 ± 0.68 µg/mL, 24.12%, respectively |
| Kazemi et al. [79] | Achillea millefolium L., Anethum graveolens L., and Carum copticum L. | A. millefolium, thymol, carvacrol, borneol, and limonene; A. graveolens, thymol, limonene, α-pinene; and C. copticum, thymol, sabinene, and borneol | DPPH, FRAP, and BCBT assays | A. millefolium had the highest antioxidant activity in all conducted assays |
| Marin et al. [80] | Foeniculum vulgare, Petroselium crispum, and Lavandula officinalis | L. officinalis, linalool, and linalyl acetate; F. Vulgare, limonene, anethole, and fenchone; P. crispum, myristicin, α-pinene, and β-pinene | DPPH and FRAP assays | P. crispum presented the best antioxidant profile, given its highest % of inhibition of DPPH radical (64.28%) and FRAP (0.93 mmol/L Trolox) |
| Marrelli et al. [81] | Six different populations of Origanum heracleoticum L. | Limonene, carvacrol-methyl-ether, and carvacrol were the major compounds | DPPH and BCBT assays | Samples showed a modest DPPH value of 320.9 μg/mL, and BCBT of 4.68 μg/mL. |
| Okoh et al. [82] | Dennettia tripetala G. Baker | 2-Methylphenyl formate, α–terpinene, and caryophyllene were the major compounds | DPPH, ABTS, nitric oxide, and lipid peroxyl | The EOs demonstrated strong ability in ABTS, lipid peroxide, and nitric oxide radical assays in a concentration-dependent manner |
| Okoh et al. [51] | Jatropha gossypifolia L. | Phytol, germacrene D, 𝛼-copaene, 𝛼-terpinene, and limonene were the major compounds | DPPH, ABTS, nitric oxide, and lipid peroxyl | The stem showed that the antiradical strength was superior to leaf EO |
| Okoh et al. [50] | Peperomia pellucida (L.) Kunth | Linalool, d-limonene, β-caryophyllene, and linalyl acetate were the major compounds | DPPH, ABTS, nitric oxide, and lipid peroxyl | The EOs demonstrated strong ability in DPPH, ABTS, nitric oxide and lipid peroxyl assays in a concentration-dependent manner |
| Ouedrhiri et al. [83] | Ormenis mixta and Pelargonium asperum | P. asperum, citronellol, citronellyl formate, and geraniol; O. mixta, germacrene, 1,8 cineol, and cis-methyl isoeugenol | DPPH method | O. mixta exhibited an important antioxidant activity, which was significantly higher than that exhibited by P. asperum |
| Pirbalouti et al. [84] | Ferulago angulata | α-pinene, and cis-β-ocimene were the major compounds | DPPH method | The highest antioxidant activity was obtained from the oil of the Kallar population (488 µg/mL) and butylhydroxyanisole as a positive control (321 µg/mL) |
| Poaty et al. [85] | Balsam fir, black spruce, white spruce, tamarack, jack pine, eastern white cedar, and Labrador tea EOs | α–pinene, β-pinene, δ-3-carene, and limonene were the major compounds. α–thujone was the main compound in white cedar | DPPH, FRAP assays | DPPH (concentration providing 50% inhibition ≥7 mg/mL) |
| Semeniuc et al. [86] | Parsley, lovage, basil, and thyme EOs | β-myrcene, β-phellandrene, γ-terpinene, and α-pinene were the major compounds | TEAC assay | The highest antioxidant capacity was found in thyme oil |
| Shakeri et al. [87] | Glycyrrhiza triphylla Fisch. and C.A.Mey | β-caryophyllene, limonene, and myrcene were the major compounds | The DPPH, and β-Carotene/linoleic acid assay | The oil was considerably active in the DPPH assay (100.40 ± 0.03 µg/mL) |
| Sharafati Chaleshtori et al. [61] | Bunium persicum, Eucalyptus globulus, and rose water | B. persicum, β-pinene (11.72%), p-cymene (15.47%), gterpinene (18.32%), cumin aldehyde (38.4%), p-mentha-1,3-dien-7-al (5.37%), and p-mentha1,4-dien-7-al (2.86%); E. globulus, limonene (9.4%) and 1,8-cineole (70.3%); rose water, linalool (6.6%), terpineol (3.3%), carvone (0.31%), citronellol (6.85%), trans-geraniol (7.11%), phenylethanol (66.84%), eugenol (4.53%), cytronellol, hydroxyl (1.15%), and geranic acid (1.2%) | FRAP | Bunium persicum EO showed the greatest antioxidant activity |
| Smeriglio et al. [64] | Pistacia vera L. | 4-carene, α-pinene, and δ-3-carene were the major compounds | Determination of total phenolic compounds, DPPH, TEAC, FRAP, chelating capacity on Fe2+, BCBT assays, superoxide anion (O2−) scavenging assay and hydroxyl radical (−OH) scavenging assay | The Pistacia vera L. variety Bronte showed a strong iron-chelating activity and was found to be markedly active against hydroxyl radical, while little effect was found against the DPPH method |
| Snoussi et al. [88] | Mentha spicata | Limonene, 1,8-cineole, and carvone were the major compounds | DPPH method, reducing power, chelating power, and BCBT assays | DPPH IC50 3.08 ± 0.07, reducing power EC50, 2.49 ± 0.07, chelating power IC50, 6.33 ± 0.12, and BCBT 6.4 ± 0.07 |
| Salem et al. [59] | Cupressus macrocarpa and Corymbia citriodora | Terpinen-4-ol (23.7%), α-phellandrene (19.2%), α-citronellol (17.3%), and citronellal were the major constituents of C. macrocarpa, and α-citronellal (56%), α-citronellol (14.7%), citronellol acetate (12.3%), isopulegol, and eucalyptol were the primary constituents of C. citriodora | Standard butylhydroxytoluene | C. citriodora was higher than that of the positive control but lower than that of the standard, butylhydroxytoluene |
| Zhao et al. [73] | Fagopyrum esculentum, Fagopyrum tataricum, and Fagopyrum Cymosum | F. esculentum: Nonanoic acid (7.58%), (E)-3-hexen-1-ol (6.52%), benzothiazole (5.08%), 2-Pentadecanone (18.61%), and eugenol (17.18%); F. tataricum: 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester (13.19%) and (E,E)-farnesylacetone (7.15%); F.Cymosum: Eugenol (12.22%), (E)-3-hexen-1-yl acetate (8.03%), linalool oxide (7.47%), 1-hexanol (7.07%), and benzothiazole (6.72%) | DPPH and BCBT assays | Three EOs have a similar antioxidant capacity in both evaluated methods |
| Author | Cell Line | Plant Derived EOs | EOs Concentration | Main Components of EOs | Exposure Time | LPS Stimulation | Main Results |
|---|---|---|---|---|---|---|---|
| Chen et al. [31] | THP-1 human monocyte/macrophage cell line | Kanuka and manuka | 0.1–0.5–1–5–10% | 48 h | yes (20 µg/mL) | EOs have no major toxic side effects on THP-1 cells. Kanuka and manuka EOs reduced the LPS-induced TNF-α secretion but have no effect on IL-4 secretion. Kanuka and manuka EO have no effect on unstimulated THP-1 cells. | |
| Chen et al. [89] | C57BL/6 mouse bone marrow-derived dendritic cells (DCs) | Litsea cubea L. | 1–2–4 × 105- and 5 × 104-fold dilution | Terpene aldehydes (75.09%) were the most abundant compounds | Cytotoxicity assay: 24 h; TNF-α assay: 6 h; IL-12 assay: 12 h | yes (100 ng/mL) | A slight cytotoxic effect was observed at 5 × 104 -fold diluted EO. Release of TNF-α and IL-12 by LPS-induced DCs were inhibited by EO in a dose-dependent fashion (IC50 of 1 × 105- and 2 × 105-fold dilution for TNF-α and IL-12, respectively). |
| Chen et al. [22] | Murine macrophage RAW264.7 cells | Artemisia argyi | 270, 90, 30, and 10 µg/mL) | Cineole, camphor, (−)-borneol, and α-(−)-thujone were the major compounds | 16 h | yes (1 µg/mL) | In LPS-induced cells, the EOs inhibited the release of NO, PGE2, and ROS and TNF-α, IL-6, IFN-β and MCP-1. In addition, EOs downregulate the gene and protein expression of iNOS and COX-2 without affecting its enzymatic activity. EOs also inhibited the phosphorylation of JAK2 and STAT1/3 but did not affect the MAPK and NF-κB cascades. |
| Cheng et al. [90] | Murine macrophage RAW264.7 cell | Oregano (Origanum vulgare L.) | ≤10 μg/mL | Carvacrol and thymol were the major compounds | 12 h | yes (1 µg/mL) | Low dose of EOs (1.25–20 μg/mL) did not produce any toxicity. In LPS-induced RAW264.7 cells, pretreatment with the EOs reduced the expression and secretion of IL-1β, IL-6, and TNF-α. Inhibition of LPS-induced MAPK, PKB, and NF-κB was also observed. The EOs also inhibited the LPS-induced elevation of NADPH oxidase and oxidative stress |
| Krifa et al. [91] | Splenocyte suspension from Balb/c mice; Murine melanoma B16F10 cell line | Pituranthos tortuosus | Splenocyte suspension: 1.25, 2.5, 5, and 10 μg/mL. B16F10 cell line: 25, 50, 100, 200, and 400 μg/mL. | Sabinene, α-pinene, limonene, and terpinen-4-ol were the major compounds | 48 h | yes (5 μg/mL) | EOs treatment was able to promote LPS-stimulated splenocyte proliferation. In B16F10 cells, incubation with the EOs inhibited cell proliferation in a dose- and time-dependent fashion (IC50: 80 μg/mL). In addition, EOs treatment was also able to increase the number of apoptotic cells. |
| Ma et al. [92] | L02 cell line; Human lung adenocarcinoma A549 cell line; Murine macrophage RAW264.7 cell | Cirsium japonicum DC | 25, 50, 100, and 200 μg/mL | Flavonoids, saponins, polysaccharides EO, coumarin, and alkaloids | 24 h | yes (1 µg/mL) | EOs have no major toxic side effects on L02 cells, and even promoted cell proliferation. In the A549 cell line, EOs promote the proliferation of cancer cells. NO production was inhibited in LPS-induced RAW264.7 cells treated with EOs at 50 and 100 μg/mL. In addition, EOs treatment reduces the secretion of IL-6, but has no effect on TNF-α gene expression. Furthermore, EOs decreased lipid accumulation in ox-LDL-induced RAW264.7 cell, and decreased the secretion of IL-6. |
| Marelli et al. [81] | Murine macrophage RAW264.7 cells | Origanum heracleticum L. | 25–1000 μg/mL | Limonene, carvacrol-methyl-ether, and carvacrol were the major compounds | 24 h | yes (1 µg/mL) | In LPS-stimulated RAW264.7 cells, all EOs from Origanun heracleticum L. showed anti-inflammatory activity by means of its capacity to decrease the NO production. |
| Özek et al. [93] | Human blood isolated neutrophils from healthy donors; bone marrow leukocytes isolated from Balb/c mice | Ferula iliensis | 1% | (E)-Propenyl sec butyl disulfide, (Z)-Propenyl sec butyl disulfide, and 10-Epi-g-eudesmol were the major compounds | Ca2+ flux assay: 0.06 h; ROS production: 0.5 h | no | EOs activated human neutrophil Ca2+ flux; this activation was dose-dependently inhibited by capsazepine, a TRPV1 channel antagonist. This effect was confirmed on TRPV1 channel-transfected HEK293 cells. Furthermore, EOs also activated SOD-inhibitable ROS production in both human neutrophils and mouse bone marrow phagocytes. |
| Park et al. [94] | Murine macrophage RAW264.7 cells | Chamaecyparis obtusa | 1, 10, 50, and 100 μg/mL | α-terpinyl acetate, β-phellandrene, β-myrcene, limonene, bornyl acetate, γ-terpinene, β-thujaplicin, and α-terpineol | 1 h | yes (1 µg/mL) | In LPS-stimulated cells, EOs treatment reduced nitric oxide, TNF-α, and IL-6 production, and inhibited iNOS and COX-2 expression. |
| Puskárova et al. [57] | human embryo lung HEL12469 cells | Origanum vulgare; Thymus vulgaris; Salvia sclarea; Lavandula angustifolia; Eugenia caryophyllata; and Thuja plicata | 0.0025–1.0 μL/mL | - | 24 h | no | EOs present toxic side effects at higher concentrations. Treatment with EOs did not induce any significant increase in DNA strand breaks; only Thuja plicata EO (0.2 μL/mL) showed a negative effect on DNA single-strand breaks in HEL 1269 cells. |
| Smeriglio et al. [64] | Human blood isolated lymphocytes from healthy donors | Pistacia vera L. | 20, 17.5, 15, 12.5, 10, 7.5, 5, and 1 μg/mL | 4-Carene, α-pinene, and δ-3-carene were the major compounds | 24 h | no | EOs did not show any cytotoxic effects. In tert-butyl hydroperoxide-treated lymphocytes, incubation with EOs (20–12.5 μg/mL) significantly increased cell viability. |
| Touihri et al. [68] | Human colonic adenocarcinoma HT29-D4 and Caco-2 cell lines | Allium roseum L. | 10, 20, 30, and 40 μg/mL | Methyl methanethiosulfinate, 3-vinyl-1,2-dithiacyclohex-5-ene, and diallyl trisulfide were the major compounds | Cytotoxicity assay: 5 h; Proliferation assay: 72 h | no | EOs did not show cytotoxic effects. Antiproliferative assay depicted that the number of cells was reduced by the incubation of HT29-D4 and Caco-2 cells with EOs in a dose-dependent fashion. |
| Ušjak et al. [69] | Human cervix Hela cell; human colon carcinoma LS174 cell; non-small cell lung carcinoma A549; human normal fetal lung fibroblast MRC-5 cell | Heracleum pyrenaicum subsp. orsinii | 12.5, 25, 50, 100, and 200 μg/mL | β-pinene, (Z)-β-ocimene, and α-pinene were the major compounds | 72 h | no | The cytotoxic effect of EOs was prominent against HeLa, LS174, and A549 cell lines. EOs did not show toxicity side effects against normal MRC-5 cell (IC50 >200 μg/mL). |
| Wang et al. [95] | Murine macrophage RAW264.7 cells | Trachydium roylei | 1.25, 2.5, 5.0, 10, and 20 mg/mL | β–phellandrene, myristicin, and elemicine were the major compounds | 1 h | yes (100 ng/mL) | In LPS-stimulated RAW264.7 cells, only a high concentration of EOs (40 mg/mL) showed a negative effect on cell viability. In addition, incubation with EOs inhibited the production of TNF-α, IL-1β, and IL-6, whereas it increased the release of IL-10. EOs also inhibited the secretion of NO and PGE2. |
| Author | Animal | Plant Derived EOs | EOs Concentration | Main Components of EOs | EOs Administration | Treatment Duration | Main Results | |
|---|---|---|---|---|---|---|---|---|
| Adaszynska-Skwirzynska et al. [96] | Broiler chickens | Lavandula angustifolia | 0.4 mL/L | Linalool and linalool acetate were the major compounds (>80%) | Drinking water (6 h/day) | From 1 to 42 d of age and from 22 to 42 d of age | Broiler chickens treated with EO weighed an average of 6.35% more than those in the control group. No differences were found in feed and water intake, survival rate, or biochemical parameters. EOs intake also has an impact on ileum gastrointestinal microbiota (pathogenic microorganisms decreased, while the number of probiotic bacteria increased). | |
| Altop et al. [97] | Broiler chickens | Liquidambar | 0.0405, 0.0811, and 0.1622 g/kg | γ-Terpinen, terpinen-4-ol, and α-terpinene were the major compounds | Basal diet supplemented (ad libitum) | 42 d | Treatment with EOs, mainly at 0.0811 g/kg concentration, improved growth performance and carcass traits while reducing blood cholesterol levels and E. coli counts. | |
| Cetin et al. [98] | Broiler chickens | Origanum sp, Rosmarinus officinalis L and Foeniculum vulgare L. | Individual EO: 100 mg/kg. EO mixture: 100, 200 and 400 mg/kg | Rosemary oil, 1,8-cineol, α-pinene, and camphene; oregano oil, carvacrol; and fennel oil, trans-anethole, and fenchone | Basal diet supplemented (ad libitum) | 42 d | Dietary supplementation increased the body weight of broilers at 7, 14, and 21 d of age. The blend of EO at 400 mg/kg significantly increased Lactobacillus spp. in feces, and also exhibited stronger antibacterial activity against coliform bacteria. | |
| Chen et al [89] | C57BL/6 mouse | Litsea cubea L. | 50- and 100-fold diluted | Terpene aldehydes (75.09%) were the most abundant compounds | Abdomens were painted | 5 d | Treatment with EO showed an inhibitory effect on contact hypersensitivity response. | |
| Chen et al. [22] | C57BL/6 mouse | Artemisia argyi | 750, 250, and 83 mg/kg | Cineole, camphor, (−)-borneol, and α-(−)-thujone were the major compounds | Oral administration | 30 minutes before 12-O-tetradeconoylphorbol-13-acetate application | Oral administration of the EO significantly attenuated TPA-induced mouse ear edema and decreased the protein level of COX-2 | |
| Gomes Cairo et al. [99] | Weaned pigs | Schinus terebinthifolius Raddi | 0.5, 1.0, and 1.5 g/kg | 𝛿-3-carene, 𝛼-phellandrene, limonene, and 𝛼-pinene were the major compounds | microencapsulated product | 14 d | EO treatment modulated the gastrointestinal microbiota by increasing Lactobacillus and reducing enterobacteria counts. Growth performance was not affected by EO treatment, although EO (1.5 g/kg) can reduce diarrhea incidence. | |
| Li et al. [100] | Weaned piglets | Carvacrol and thymol | Carvacol: 62.5 mg/kg; Thymol: 7.5 mg/kg | N-(2-hydroethyl)-iminodiacetic acid 2 | Basal diet supplemented (ad libitum) | 30 d | EO treatment significantly increased the relative abundance of Bacillli, Lactobacillales, Streptocpccaceae and Veillonellaceae in colonic samples. Metabolomics analysis showed that protein biosynthesis, amino acid metabolism, and lipid metabolisms were enriched in the EO group. | |
| Park et al. [94] | Carrageenan-induced paw edema model (C57BL/6) and thioglycollate-induced peritonitis model (C57BL/6) | Chamaecyparis obtusa | 5 and 10 mg/kg | α-terpinyl acetate, β-phellandrene, β-myrcene, limonene, bornyl acetate, γ-terpinene, β-thujaplicin, and α-terpineol | Intraperitoneal administration | 1 h prior to inflammation-induced treatment | EO treatment reduced the levels of IL-6 and IL-1β in paw homogenates and in peritoneal fluid. In thioglycollate-induced peritonitis levels of TNF-α in peritoneal fluid. | |
| Sutili et al. [101] | Silver catfish | Hesperozygis ringes, Ocimun gratissimum, and Ocimun americanum | Hesperozygis ringes: 20 and 40 mg/L; Ocimun gratissimun: 5 and 10 mg/L; Ocimum americanun: 10 and 20 mg/L | H. ringens, pulegone; O. gratissimum, eugenol; O. americanum, 1·8-cineole, β-linalool, eugenol, and camphor | Daily bath for | 1 h during 5 d | Fish exposed to EOs showed significant lower hematocrit values and higher complement system activity and plasma cortisol levels. There was no significant difference in the survival of fish challenged with Aeromonas hydrophila. | |
| Yang et al. [23] | Weaned piglets | Mixture of EOs and organic acids: cinnamaldehyde (15%), thymol (5%), citric acid (10%), sorbic acid (10%), malic acid (6.5%) and fumaric acid (13.5%) | 1 kg/ton | Basal diet supplemented (ad libitum) | 28 d | Diet supplementation with the mixture improved the final body weight and average daily gain, increased the concentration of serum complement 4, and enhanced the isovaleric acid fecal concentration. Regarding the gastrointestinal microbiota composition in fecal samples, the mixture treatment increased the beta diversity. | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. https://doi.org/10.3390/nu11112786
Valdivieso-Ugarte M, Gomez-Llorente C, Plaza-Díaz J, Gil Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients. 2019; 11(11):2786. https://doi.org/10.3390/nu11112786
Chicago/Turabian StyleValdivieso-Ugarte, Magdalena, Carolina Gomez-Llorente, Julio Plaza-Díaz, and Ángel Gil. 2019. "Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review" Nutrients 11, no. 11: 2786. https://doi.org/10.3390/nu11112786
APA StyleValdivieso-Ugarte, M., Gomez-Llorente, C., Plaza-Díaz, J., & Gil, Á. (2019). Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients, 11(11), 2786. https://doi.org/10.3390/nu11112786






