Coffee and Endothelial Function: A Coffee Paradox?
Abstract
1. Introduction
2. Role of Endothelial Function in Atherosclerosis
3. Endothelial Function Test
4. Effects of Coffee and Caffeine on Endothelial Function
4.1. Coffee
4.2. Caffeine
4.3. Tea
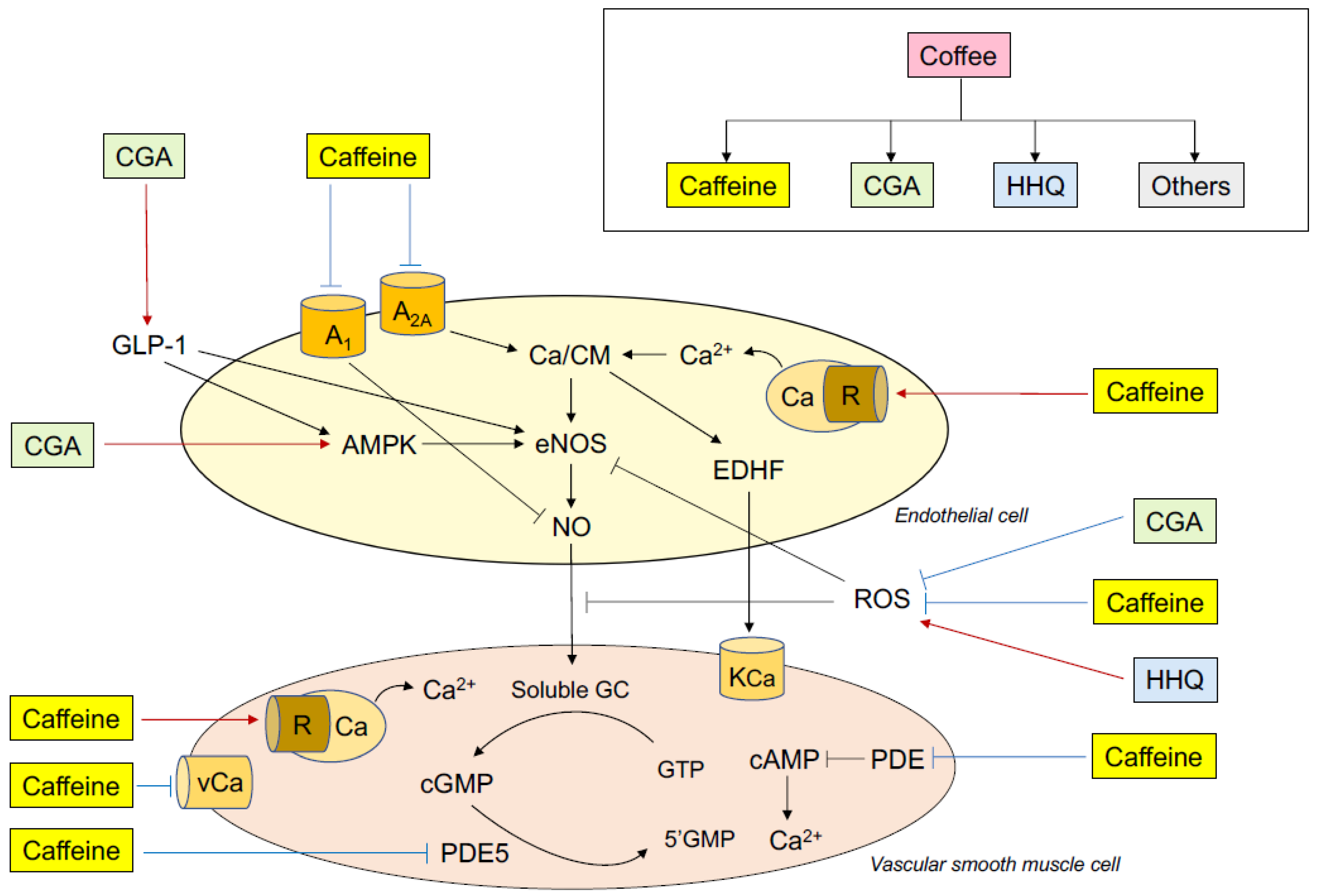
5. Mechanisms of the Effects of Coffee and Caffeine on Endothelial Function
5.1. Coffee
5.2. Caffeine
5.3. Tea
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Keefe, J.H.; DiNicolantonio, J.J.; Lavie, C.J. Coffee for Cardioprotection and Longevity. Prog. Cardiovasc. Dis. 2018, 61, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Do Coffee Polyphenols Have a Preventive Action on Metabolic Syndrome Associated Endothelial Dysfunctions? An Assessment of the Current Evidence. Antioxidants 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.; Tunstall-Pedoe, H. Coffee and tea consumption in the Scottish Heart Healthy Study follow up: Conflicting relations with coronary risk factors, coronary disease, and all cause mortality. J. Epidemiol. Community Health 1999, 53, 481–487. [Google Scholar] [CrossRef]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of Coffee Drinking with Total and Cause-Specific Mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Feskens, E.J. Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2002, 360, 1477–1478. [Google Scholar] [CrossRef]
- Hino, A.; Adachi, H.; Enomoto, M.; Furuki, K.; Shigetoh, Y.; Ohtsuka, M.; Kumagae, S.; Hirai, Y.; Jalaldin, A.; Satoh, A.; et al. Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome: An epidemiological study in a general Japanese population. Diabetes Res. Clin. Pract. 2007, 76, 383–389. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Lacroix, A.Z.; Mead, L.A.; Liang, K.-Y.; Thomas, C.B.; Pearson, T.A. Coffee Consumption and the Incidence of Coronary Heart Disease. N. Engl. J. Med. 1986, 315, 977–982. [Google Scholar] [CrossRef]
- James, J.E. Is habitual caffeine use a preventable cardiovascular risk factor? Lancet 1997, 349, 279–281. [Google Scholar] [CrossRef]
- Happonen, P.; Voutilainen, S.; Salonen, J.T. Coffee drinking is dose-dependently related to the risk of acute coronary events in middle-aged men. J. Nutr. 2004, 134, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Grobbee, D.E.; Rimm, E.B.; Giovannucci, E.; Colditz, G.; Stampfer, M.; Willett, W. Coffee, Caffeine, and Cardiovascular Disease in Men. N. Engl. J. Med. 1990, 323, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Kleemola, P.; Jousilahti, P.; Pietinen, P.; Vartiainen, E.; Tuomilehto, J. Coffee Consumption and the Risk of Coronary Heart Disease and Death. Arch. Intern. Med. 2000, 160, 3393. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Kuriyama, S.; Akhter, M.; Kakizaki, M.; Nakaya, N.; Ohmori-Matsuda, K.; Shimazu, T.; Nagai, M.; Sugawara, Y.; Hozawa, A.; et al. Coffee Consumption and Mortality Due to All Causes, Cardiovascular Disease, and Cancer in Japanese Women. J. Nutr. 2010, 140, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Rundek, T.; Wright, C.B.; Elkind, M.S.V.; Sacco, R.L. Coffee and Tea Consumption Are Inversely Associated with Mortality in a Multiethnic Urban Population123. J. Nutr. 2013, 143, 1299–1308. [Google Scholar] [CrossRef][Green Version]
- De Koning Gans, J.M.; Uiterwaal, C.S.; van der Schouw, Y.T.; Boer, J.M.; Grobbee, D.E.; Verschuren, W.M.; Beulens, J.W. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1665–1671. [Google Scholar] [CrossRef]
- Bonita, J.; Mandarano, M.; Shuta, D.; Vinson, J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharmacol. Res. 2007, 55, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Pincomb, G.A.; Lovallo, W.R.; Passey, R.B.; Whitsett, T.L.; Silverstein, S.M.; Wilson, M.F. Effects of caffeine on vascular resistance, cardiac output and myocardial contractility in young men. Am. J. Cardiol. 1985, 56, 119–122. [Google Scholar] [CrossRef]
- Mahmud, A.; Feely, J. Acute effect of caffeine on arterial stiffness and aortic pressure waveform. Hypertension 2001, 38, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.; Binggeli, C.; Sudano, I.; Spieker, L.; Hänseler, E.; Ruschitzka, F.; Chaplin, W.F.; Lüscher, T.F.; Noll, G. Coffee acutely increases sympathetic nerve activity and blood pressure independently of caffeine content: Role of habitual versus nonhabitual drinking. Circulation 2002, 106, 2935–2940. [Google Scholar] [CrossRef]
- Hartley, T.R.; Lovallo, W.R.; Whitsett, T.L. Cardiovascular effects of caffeine in men and women. Am. J. Cardiol. 2004, 93, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; He, J.; Whelton, P.K.; Suh, I.; Klag, M.J. The effect of chronic coffee drinking on blood pressure a meta-analysis of controlled clinical trials. Hypertension 1999, 33, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, M.; Uiterwaal, C.S.P.M.; Arends, L.R.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Blood pressure response to chronic intake of coffee and caffeine: A meta-analysis of randomized controlled trials. J. Hypertens. 2005, 23, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. The pathogenesis of atherosclerosis. N. Engl. J. Med. 1986, 314, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Oxidative stress and endothelial function in cardiovascular diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E., Jr.; Epstein, S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990, 323, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Matsuura, H.; Oshima, T.; Chayama, K. Endothelial function and oxidative stress in renovascular hypertension. N. Engl. J. Med. 2002, 346, 1954–1962. [Google Scholar] [CrossRef]
- Drexler, H.; Horning, B. Endothelial dysfunction in human disease. J. Mol. Cell Cardiol. 1999, 31, 51–60. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Kurisu, S.; Yoshimizu, A.; Sasaki, N.; Matsuura, H.; Kajiyama, G.; Oshima, T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: Role of endothelium-derived nitric oxide. Circulation 1999, 100, 1194–1202. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Li, T.Y.; Mukamal, K.J.; Hu, F.B.; van Dam, R.M. Coffee consumption and mortality in women with cardiovascular disease. Am. J. Clin. Nutr. 2011, 94, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Jokura, H.; Suzuki, A.; Tokimitsu, I.; Ohishi, M.; Komai, N.; Rakugi, H.; Ogihara, T. Green coffee bean extract improves human vasoreactivity. Hypertens. Res. 2004, 27, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Chikama, A.; Kataoka, K.; Tokimitsu, I.; Maekawa, Y.; Ohishi, M.; Rakugi, H.; Mikami, H. Effects of hydroxyhydroquinone-reduced coffee on vasoreactivity and blood pressure. Hypertens. Res. 2009, 32, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Verga, S.; Batsis, J.A.; Tranchina, M.R.; Belmonte, S.; Mattina, A.; Re, A.; Rizzo, R.; Cerasola, G. Dose-dependent effects of decaffeinated coffee on endothelial function in healthy subjects. Eur. J. Clin. Nutr. 2009, 63, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Oikonomou, E.; Chrysohoou, C.; Tousoulis, D.; Panagiotakos, D.; Zaromitidou, M.; Zisimos, K.; Kokkou, E.; Marinos, G.; Papavassiliou, A.G.; et al. Consumption of a boiled Greek type of coffee is associated with improved endothelial function: The Ikaria Study. Vasc. Med. 2013, 18, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Sugiura, Y.; Shioya, Y.; Otsuka, K.; Katsuragi, Y.; Hashiguchi, T. Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutr. Res. 2014, 34, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Sugiura, Y.; Otsuka, K.; Katsuragi, Y.; Hashiguchi, T. Coffee bean polyphenols ameliorate postprandial endothelial dysfunction in healthy male adults. Int. J. Food Sci. Nutr. 2015, 66, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Jokura, H.; Watanabe, I.; Umeda, M.; Hase, T.; Shimotoyodome, A. Coffee polyphenol consumption improves postprandial hyperglycemia associated with impaired vascular endothelial function in healthy male adults. Nutr. Res. 2015, 35, 873–881. [Google Scholar] [CrossRef]
- Noguchi, K.; Matsuzaki, T.; Sakanashi, M.; Hamadate, N.; Uchida, T.; Kina-Tanada, M.; Kubota, H.; Nakasone, J.; Sakanashi, M.; Ueda, S.; et al. Effect of caffeine contained in a cup of coffee on microvascular function in healthy subjects. J. Pharmacol. Sci. 2015, 127, 217–222. [Google Scholar] [CrossRef]
- Mills, C.E.; Flury, A.; Marmet, C.; Poquet, L.; Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Brenner, R.; Allemann, Y.; Zimmermann, D.; et al. Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials. Clin. Nutr. 2017, 36, 1520–1529. [Google Scholar] [CrossRef]
- Boon, E.A.J.; Croft, K.D.; Shinde, S.; Hodgson, J.M.; Ward, N.C. The acute effect of coffee on endothelial function and glucose metabolism following a glucose load in healthy human volunteers. Food Funct. 2017, 8, 3366–3373. [Google Scholar] [CrossRef] [PubMed]
- Tesselaar, E.; Dernroth, D.N.; Farnebo, S. Acute effects of coffee on skin blood flow and microvascular function. Microvasc. Res. 2017, 114, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Nomura, T.; Jokura, H.; Kitamura, N.; Saiki, A.; Fujii, A. Chlorogenic acid-enriched green coffee bean extract affects arterial stiffness assessed by the cardio-ankle vascular index in healthy men: A pilot study. Int. J. Food Sci. Nutr. 2019, 70, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Maruhashi, T.; Hidaka, T.; Nakano, Y.; Kurisu, S.; Matsumoto, T.; Iwamoto, Y.; Kishimoto, S.; Matusi, S.; Hashimoto, H.; et al. Coffee with a high content of chlorogenic acids and low content of hydroxyhydroquinone improves postprandial endothelial dysfunction in patients with borderline and stage 1 hypertension. Eur. J. Nutr. 2019, 53, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.; Yates, D.H.; Thomas, P.S. Caffeine decreases exhaled nitric oxide. Thorax 2002, 57, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, C.M.; Aznaouridis, K.A.; Karatzis, E.N.; Karatzi, K.N.; Stamatelopoulos, K.S.; Vamvakou, G.; Lekakis, J.P.; Mavrikakis, M.E. Effect of coffee on endothelial function in healthy subjects: The role of caffeine. Clin. Sci. 2005, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Verga, S.; Batsis, J.A.; Donatelli, M.; Tranchina, M.R.; Belmonte, S.; Mattina, A.; Re, A.; Cerasola, G. Acute effects of coffee on endothelial function in healthy subjects. Eur. J. Clin. Nutr. 2010, 64, 483–489. [Google Scholar] [CrossRef]
- Molnar, J.; Somberg, J.C. Evaluation of the Effects of Different Energy Drinks and Coffee on Endothelial Function. Am. J. Cardiol. 2016, 116, 1457–1460. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzmán, O.J.; Muñoz-Durango, K. Coffee Consumption Increases the Antioxidant Capacity of Plasma and Has No Effect on the Lipid Profile or Vascular Function in Healthy Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef]
- Zimmermann, D.; Poquet, L.; Hodgson, J.M.; Woodman, R.J.; Actis-Goretta, L.; Puddey, I.B.; Ward, N.C.; Leveques, A.; Croft, K.D. Acute effects of chlorogenic acids on endothelial function and blood pressure in healthy men and women. Food Funct. 2016, 7, 2197–2203. [Google Scholar]
- Vallance, P.; Collier, J.; Moncada, S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 1989, 2, 997–1000. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. Endothelium and control of vascular function: State of the art lecture. Hypertension 1989, 13, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Anggard, E.E.; Botting, R.M. Regulatory functions of the vascular endothelium. N. Engl. J. Med. 1990, 323, 27–36. [Google Scholar] [PubMed]
- Linder, L.; Kiowski, W.; Bühler, F.R.; Lüscher, T.F. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo: Blunted response in essential hypertension. Circulation 1990, 81, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Treasure, C.B.; Klein, J.L.; Vita, J.A.; Manoukian, S.V.; Renwick, G.H.; Selwyn, A.P.; Ganz, P.; Alexander, R.W.; Vita, J. Hypertension and left ventricular hypertrophy are associated with impaired endothelium-mediated relaxation in human coronary resistance vessels. Circulation 1993, 87, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Raij, L. Nitric oxide and the kidney. Circulation 1993, 87 (Suppl. V), V26–V29. [Google Scholar]
- Higashi, Y.; Oshima, T.; Ozono, R.; Matsuura, H.; Kajiyama, G. Aging and severity of hypertension attenuate endothelialum-dependent renal vascular relaxation in humans. Hypertension 1997, 30, 252–258. [Google Scholar] [CrossRef]
- Creager, M.A.; Cooke, J.P.; Mendelsohn, M.E.; Gallagher, S.J.; Coleman, S.M.; Loscalzo, J.; Dzau, V.J. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J. Clin. Investig. 1990, 86, 228–234. [Google Scholar] [CrossRef]
- Gilligan, D.M.; Sack, M.N.; Guetta, V.; Casino, P.R.; Quyyumi, A.A.; Rader, D.J.; Panza, J.A.; Cannon, R.O. Effect of antioxidant vitamins on low density lipoprotein oxidation and impaired endothelium-dependent vasodilation in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 1994, 24, 1611–1617. [Google Scholar] [CrossRef][Green Version]
- Ting, H.H.; Timimi, F.K.; Boles, K.S.; Creager, S.J.; Ganz, P.; Creager, M.A. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1996, 97, 22–28. [Google Scholar] [CrossRef]
- Levine, G.N.; Frei, B.; Koulouris, S.N.; Gerhard, M.D.; Keaney, J.F.; Vita, J.A. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 1996, 93, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Zeiher, A.M.; Drexler, H.; Saurbier, B.; Just, H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J. Clin. Investig. 1993, 92, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Treasure, C.B.; Klein, J.L.; Stillabower, M.E.; Kosinski, A.S.; Boccuzzi, S.J.; Cedarholm, J.C.; Weintraub, W.S.; Talley, J.D.; Zhang, J.; Alexander, R.W. Beneficial Effects of Cholesterol-Lowering Therapy on the Coronary Endothelium in Patients with Coronary Artery Disease. N. Engl. J. Med. 1995, 332, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Hidaka, T.; Kihara, Y.; et al. Relationship between flow-mediated vasodilatation and cardiovascular risk factors in a large community-based study. Heart 2013, 99, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Al Suwaidi, J.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R.; Lerman, A.; Suwaidi, J.A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef]
- Ceravolo, R.; Pujia, A.; Schillaci, G.; Verdecchia, P.; Perticone, F. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001, 104, 191–196. [Google Scholar] [CrossRef]
- Kajikawa, M.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Matsumoto, T.; Oda, N.; Hidaka, T.; Kihara, Y.; Chayama, K.; Goto, C.; et al. A combination of flow-mediated vasodilation combined with nitroglycerine-induced vasodilation is more useful for prediction of cardiovascular events. Hypertension 2016, 67, 1045–1052. [Google Scholar] [CrossRef]
- Gokce, N.; Keaney, J.F.; Hunter, L.M.; Watkins, M.T.; Nedeljkovic, Z.S.; Menzoian, J.O.; Vita, J.A. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J. Am. Coll. Cardiol. 2003, 41, 1769–1775. [Google Scholar] [CrossRef]
- Fischer, D.; Rossa, S.; Landmesser, U.; Spiekermann, S.; Engberding, N.; Hornig, B.; Drexler, H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur. Heart J. 2004, 26, 65–69. [Google Scholar] [CrossRef]
- Huang, A.L.; Silver, A.E.; Shvenke, E.; Schopfer, D.W.; Jahangir, E.; Titas, M.A.; Shpilman, A.; Menzoian, J.O.; Watkins, M.T.; Raffetto, J.D.; et al. Predictive Value of Reactive Hyperemia for Cardiovascular Events in Patients With Peripheral Arterial Disease Undergoing Vascular Surgery. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2113–2119. [Google Scholar] [CrossRef]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrington, D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Delles, C.; Schneider, M.P.; John, S.; Gekle, M.; Schmieder, R.E. Angiotensin converting enzyme inhibition and angiotensin II AT1-receptor blockade reduce the levels of asymmetrical N(G), N(G)-dimethylarginine in human essential hypertension. Am. J. Hypertens. 2002, 15, 590–593. [Google Scholar] [CrossRef][Green Version]
- Ludmer, P.L.; Selwyn, A.P.; Shook, T.L.; Wayne, R.R.; Mudge, G.H.; Alexander, R.W.; Ganz, P. Paradoxical Vasoconstriction Induced by Acetylcholine in Atherosclerotic Coronary Arteries. N. Engl. J. Med. 1986, 315, 1046–1051. [Google Scholar] [CrossRef]
- Morimoto, H.; Kajikawa, M.; Oda, N.; Idei, N.; Hirono, H.; Hida, E.; Maruhashi, T.; Iwamoto, Y.; Kishimoto, S.; Matsui, S.; et al. Endothelial function measured by enclosed zone flow-mediated vasodilation is an independent predictor of cardiovascular events. J. Am. Heart Assoc. 2016, 5, e004385. [Google Scholar] [CrossRef]
- Umemura, T.; Ueda, K.; Nishioka, K.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Jitsuiki, D.; Soga, J.; Goto, C.; Chayama, K.; et al. Effects of Acute Administration of Caffeine on Vascular Function. Am. J. Cardiol. 2006, 98, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M.; Shalmon, G.; Scheinowitz, M.; Koren-Morag, N.; Feinberg, M.S.; Harats, D.; Sela, B.A.; Sharabi, Y.; Chouraqui, P. Impact of Acute Caffeine Ingestion on Endothelial Function in Subjects With and Without Coronary Artery Disease. Am. J. Cardiol. 2011, 107, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Vlachopoulos, C.; Aznaouridis, K.; Baou, K.; Vasiliadou, C.; Pietri, P.; Xaplanteris, P.; Stefanadi, E.; Stefanadis, C. The acute effect of green tea consumption on endothelial function in healthy individuals. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 300–305. [Google Scholar] [CrossRef]
- Duffy, S.J.; Keaney, J.F., Jr.; Holbrook, M.; Gokce, N.; Swerdloff, P.L.; Frei, B.; Vita, J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 2001, 104, 151–156. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Puddey, I.B.; Burke, V.; Watts, G.F.; Beilin, L.J. Regular ingestion of black tea improves brachial artery vasodilator function. Clin. Sci. 2002, 102, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Burke, V.; Puddey, I.B. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J. Hypertens. 2005, 23, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Mulder, T.P.; Draijer, R.; Desideri, G.; Molhuizen, H.O.; Ferri, C. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J. Hypertens. 2009, 27, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Rauhut, F.; Hofer, C.; Gwosc, S.; Müller, E.; Praeger, D.; Zimmermann, B.F.; Wernecke, K.-D.; Baumann, G.; Stangl, K.; et al. Tea-induced improvement of endothelial function in humans: No role for epigallocatechin gallate (EGCG). Sci. Rep. 2017, 7, 2279. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Draijer, R.; Schalkwijk, C.; Desideri, G.; D’Angeli, A.; Francavilla, S.; Mulder, T.; Ferri, C. Black Tea Increases Circulating Endothelial Progenitor Cells and Improves Flow Mediated Dilatation Counteracting Deleterious Effects from a Fat Load in Hypertensive Patients: A Randomized Controlled Study. Nutrient 2016, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.F.; Rich, L.; Koch, H.; Croft, K.D.; Ferruzzi, M.G.; Kay, C.D.; Hodgson, J.M.; Ward, N.C. Effect of adding milk to black tea on vascular function in healthy men and women: A randomised controlled crossover trial. Food Funct. 2018, 9, 6307–6314. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Lang, R.; Hofmann, T. Quantitative Precursor Studies on Di- and Trihydroxybenzene Formation during Coffee Roasting Using “In Bean” Model Experiments and Stable Isotope Dilution Analysis. J. Agric. Food Chem. 2006, 54, 10086–10091. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Fujii, A.; Jokura, H.; Tokimitsu, I.; Hase, T.; Saito, I. Hydroxyhydroquinone Interferes With the Chlorogenic Acid-induced Restoration of Endothelial Function in Spontaneously Hypertensive Rats. Am. J. Hypertens. 2008, 21, 23–27. [Google Scholar] [CrossRef]
- Jiang, R.; Hodgson, J.M.; Mas, E.; Croft, K.D.; Ward, N.C.; Information, P.E.K.F.C. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J. Nutr. Biochem. 2016, 27, 53–60. [Google Scholar] [CrossRef]
- Ponka, P. Cell biology of heme. Am. J. Med. Sci. 1999, 318, 241–256. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.; Glazer, A.; Ames, B. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Handa, H.; Nishizawa, M. Induction of cellular antioxidative stress genes through heterodimeric transcription factor Nrf2/small Maf by antirheumatic gold(I) compounds. J. Biol. Chem. 2001, 276, 34074–34081. [Google Scholar] [CrossRef] [PubMed]
- Boettler, U.; Sommerfeld, K.; Volz, N.; Pahlke, G.; Teller, N.; Somoza, V.; Lang, R.; Hofmann, T.; Marko, D. Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J. Nutr. Biochem. 2011, 22, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Li, X.; Makimoto, M.; Kato, T.; Kikugawa, K. Identification of hydroxyhydroquinone in coffee as a generator of reactive oxygen species that break DNA single strands. Mutat. Res. 1998, 419, 43–51. [Google Scholar] [CrossRef]
- Halliwell, B.; Long, L.H.; Yee, T.P.; Lim, S.; Kelly, R. Establishing biomarkers of oxidative stress: The measurement of hydrogen peroxide in human urine. Curr. Med. Chem. 2004, 11, 1085–1092. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Ullrich, V.; Mülsch, A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1551–1557. [Google Scholar]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty years of saying NO: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef]
- Zampelas, A.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Stefanadis, C. Associations between coffee consumption and inflammatory markers in healthy persons: The ATTICA study. Am. J. Clin. Nutr. 2004, 80, 862–867. [Google Scholar] [CrossRef]
- Gomez-Ruiz, J.A.; Leake, D.S.; Ames, J.M. In Vitro Antioxidant Activity of Coffee Compounds and Their Metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Ohie, T.; Yonekawa, Y.; Yonemoto, K.; Aizawa, H.; Mori, Y.; Watanabe, M.; Takeuchi, M.; Hasegawa, M.; Taguchi, C.; et al. Coffee and Green Tea As a Large Source of Antioxidant Polyphenols in the Japanese Population. J. Agric. Food Chem. 2009, 57, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, K.; Vallance, P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation 1997, 96, 3042–3047. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Spencer, E.A.; Thompson, M.J.; Heneghan, C.J. The effect of chlorogenic acid on blood pressure: A systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2015, 29, 77–81. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chikama, A.; Mori, K.; Watanabe, T.; Shioya, Y.; Katsuragi, Y.; Tokimitsu, I. Hydroxyhydroquinone-free coffee: A double-blind, randomized controlled dose–response study of blood pressure. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 408–414. [Google Scholar] [CrossRef]
- Mesas, A.E.; Leon-Muñoz, L.M.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1113–1126. [Google Scholar] [CrossRef]
- Ding, M.; Satija, A.; Bhupathiraju, S.N.; Hu, Y.; Sun, Q.; Han, J.; Lopez-Garcia, E.; Willett, W.; van Dam, R.M.; Hu, F.B. Association of coffee consumption with total and cause-specific mortality in 3 large prospective cohorts. Circulation 2015, 132, 2305–2315. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK—Sensing energy while talking to other signaling pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef]
- Shirwany, N.A.; Zou, M.H. AMPK: A cellular metabolic and redox sensor. A minireview. Front. Biosci. 2014, 19, 447–474. [Google Scholar] [CrossRef]
- Nagata, D.; Hirata, Y. The role of AMP-activated protein kinase in the cardiovascular system. Hypertens. Res. 2010, 33, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D.; Carlson, M. THE AMP-ACTIVATED/SNF1 PROTEIN KINASE SUBFAMILY: Metabolic Sensors of the Eukaryotic Cell? Annu. Rev. Biochem. 1998, 67, 821–855. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-L.; Hung, C.-H.; Chan, S.-H.; Hsieh, P.-L.; Ou, H.-C.; Cheng, Y.-H.; Chu, P.-M.; Tsai, K.; Hung, C.; Chan, S.; et al. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic Acid Stimulates Glucose Transport in Skeletal Muscle via AMPK Activation: A Contributor to the Beneficial Effects of Coffee on Diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.; Wei, Q.; Xia, Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: Co-commitment and interplay of Akt and AMPK. J. Biol. Chem. 2008, 283, 25256–25263. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Fujii, Y.; Osaki, N.; Hase, T.; Shimotoyodome, A. Ingestion of coffee polyphenols increases postprandial release of the active glucagon-like peptide-1 (GLP-1(7–36)) amide in C57BL/6J mice. J. Nutr. Sci. 2015, 4, e9. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol. Sin. 2012, 33, 75–78. [Google Scholar] [CrossRef]
- Ban, K.; Noyan-Ashraf, M.H.; Hoefer, J.; Bolz, S.-S.; Drucker, D.J.; Husain, M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008, 117, 2340–2350. [Google Scholar] [CrossRef]
- Wei, R.; Ma, S.; Wang, C.; Ke, J.; Yang, J.; Li, W.; Liu, Y.; Hou, W.; Feng, X.; Wang, G.; et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E947–E957. [Google Scholar] [CrossRef] [PubMed]
- Nyström, T.; Gutniak, M.K.; Zhang, Q.; Zhang, F.; Holst, J.J.; Ahren, B.; Sjöholm, Å. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Metab. 2004, 287, E1209–E1215. [Google Scholar]
- Clifford, M.N.; Wight, J. The measurement of feruloylquinic acids and caffeoylquinic acids in coffee beans. Development of the technique and its preliminary application to green coffee beans. J. Sci. Food Agric. 1976, 27, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; De Paulis, T.; Moreira, D.P.; Trugo, L.C.; Martin, P.R. Chlorogenic Acids and Lactones in Regular and Water-Decaffeinated Arabica Coffees. J. Agric. Food Chem. 2006, 54, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H.; Qiu, M.; Hu, G. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef]
- Clarke, R.J. Coffee: Green Coffee/Roast and Ground. In Encyclopedia of Food Science and Nutrition, 2nd ed.; Caballero, B., Trugo, L.C., Finglas, P., Eds.; Academic Press: Oxford, UK, 2003; Volume 3. [Google Scholar]
- Díaz-Rubio, M.E.; Saura-Calixto, F. Dietary fiber in brewed coffee. J. Agric. Food Chem. 2007, 55, 1999–2003. [Google Scholar] [CrossRef]
- Sudano, I.; Spieker, L.; Binggeli, C.; Ruschitzka, F.; Luscher, T.F.; Noll, G.; Corti, R. Coffee blunts mental stress-induced blood pressure increase in habitual but not in nonhabitual coffee drinkers. Hypertension 2005, 46, 521–526. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Persson, C.G.A. Xanthine derivatives as adenosine receptor antagonists. Eur. J. Pharmacol. 1982, 81, 673–676. [Google Scholar] [CrossRef]
- Smits, P.; Lenders, J.W.M.; Thien, T. Caffeine and theophylline attenuate adenosine-induced vasodilation in humans. Clin. Pharmacol. Ther. 1990, 48, 410–418. [Google Scholar] [CrossRef]
- Tucker, A.L.; Linden, J. Cloned receptors and cardiovascular responses to adenosine. Cardiovasc. Res. 1993, 27, 62–67. [Google Scholar] [CrossRef]
- Li, J.-M.; Fenton, R.A.; Wheeler, H.; Powell, C.C.; Peyton, B.D.; Cutler, B.S.; Dobson, J.G., Jr. Adenosine A2aReceptors Increase Arterial Endothelial Cell Nitric Oxide. J. Surg. Res. 1998, 80, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.; Williams, S.B.; Lipson, D.E.; Banitt, P.; Rongen, G.A.; Creager, M.A. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation 1995, 92, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Headrick, J.P.; Ashton, K.J.; Rose’Meyer, R.B.; Peart, J.N. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Closs, E.I.; Pollock, J.S.; Nakane, M.; Schwarz, P.; Gath, I.; Kleinert, H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 1994, 23, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Busse, R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Hatano, Y.; Mizumoto, K.; Yoshiyama, T.; Yamamoto, M.; Iranami, H. Endothelium-dependent and -independent vasodilation of isolated rat aorta induced by caffeine. Am. J. Physiol. Circ. Physiol. 1995, 269, H1679–H1684. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.D.; Francis, S.H. Pharmacology of phosphodiesterase-5 inhibitors. Int. J. Clin. Pract. 2002, 56, 453–459. [Google Scholar]
- Bredt, D.S.; Snyder, S.H. Nitric Oxide: A Physiologic Messenger Molecule. Annu. Rev. Biochem. 1994, 63, 175–195. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Chen, Y.; Sun, Z.; Wang, R.; Dai, Y. Effect of Caffeine on Erectile Function via Up-Regulating Cavernous Cyclic Guanosine Monophosphate in Diabetic Rats. J. Androl. 2008, 29, 586–591. [Google Scholar] [CrossRef]
- Watanabe, C.; Yamamoto, H.; Hirano, K.; Kobayashi, S.; Kanaide, H. Mechanisms of caffeine-induced contraction and relaxation of rat aortic smooth muscle. J. Physiol. 1992, 456, 193–213. [Google Scholar] [CrossRef]
- Ozaki, H.; Kasai, H.; Hori, M.; Sato, K.; Ishihara, H.; Karaki, H. Direct inhibition of chicken gizzard smooth muscle contractile apparatus by caffeine. Naunyn Schmiedebergs Arch. Pharmacol. 1990, 341, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.G.; Weston, A.H. Endothelium-derived hyperpolarizing factor: A new endogenous inhibitor from the vascular endothelium. Trends Pharmacol. Sci. 1988, 9, 272–274. [Google Scholar] [CrossRef]
- Rusko, J.; Slooten, G.; Adams, D. Caffeine-evoked, calcium-sensitive membrane currents in rabbit aortic endothelial cells. Br. J. Pharmacol. 1995, 115, 133–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.C.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Zholos, A.V.; Baidan, L.V.; Shuba, M.F.; Zholos, A. The inhibitory action of caffeine on calcium currents in isolated intestinal smooth muscle cells. Pflügers Arch. 1991, 419, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Metro, D.; Cernaro, V.; Santoro, D.; Papa, M.; Buemi, M.; Benvenga, S.; Manasseri, L. Beneficial effects of oral pure caffeine on oxidative stress. J. Clin. Transl. Endocrinol. 2017, 10, 22–27. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Salva, T.J.; Bragagnolo, N. Influence of coffee genotype on bioactive compounds and the in vitro capacity to scavenge reactiveoxygen and nitrogen species. J. Agric. Food Chem. 2015, 63, 4815–4826. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.O., 3rd. Role of nitric oxide in cardiovascular disease: Focus on the endothelium. Clin. Chem. 1998, 44, 1809–1819. [Google Scholar]
- Amer, M.G.; Mazen, N.F.; Mohamed, A.M. Caffeine intake decreases oxidative stress and inflammatory biomarkers in experimental liver diseases induced by thioacetamide: Biochemical and histological study. Int. J. Immunopathol. Pharmacol. 2017, 30, 13–24. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 2003, 9, BR325–BR330. [Google Scholar]
- Devasagayam, T.; Kamat, J.; Mohan, H.; Kesavan, P. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. Biophys. Acta 1996, 1282, 63–70. [Google Scholar] [CrossRef]
- Teekachunhatean, S.; Tosri, N.; Sangdee, C.; Wongpoomchai, R.; Ruangyuttikarn, W.; Puaninta, C.; Srichairatanakool, S. Antioxidant effects after coffee enema or oral coffee consumption in healthy Thai male volunteers. Hum. Exp. Toxicol. 2012, 31, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.S.; de Cássia, G.; de Lima, R.; Elekofehinti, O.O.; Ogunbolude, Y.; Duarte, A.E.; Rocha, J.B.T.; de Menezes, I.R.A.; Barros, L.M.; Tsopmo, A.; et al. Caffeine-supplemented diet modulates oxidative stress markers and improves locomotor behavior in the lobster cockroach Nauphoeta cinerea. Chem. Biol. Interact. 2018, 282, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Karatzis, E.; Papaioannou, T.G.; Aznaouridis, K.; Karatzi, K.; Stamatelopoulos, K.; Zampelas, A.; Papamichael, C.; Lekakis, J.; Mavrikakis, M. Acute effects of caffeine on blood pressure and wave reflections in healthy subjects: Should we consider monitoring central blood pressure? Int. J. Cardiol. 2005, 98, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Hartley, T.R.; Sung, B.H.; Pincomb, G.A.; Whitsett, T.L.; Wilson, M.F.; Lovallo, W.R. Hypertension risk status and effect of caffeine on blood pressure. Hypertension 2000, 36, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.F.; Matthews, K.A. Interaction between autonomic nervous system activity and endothelial function: A model for the development of cardio vascular disease. Psychosom. Med. 2004, 66, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Seydoux, J.; Girardier, L. Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: Adenosine antagonism or phosphodiesterase inhibition? Metabolism 1992, 41, 1233–1241. [Google Scholar] [CrossRef]
- Harpaz, E.; Tamir, S.; Weinstein, A. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Belza, A.; Toubro, S.; Astrup, A. The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur. J. Clin. Nutr. 2009, 63, 57–64. [Google Scholar] [CrossRef]
- Robertson, D.; Frölich, J.C.; Carr, R.K.; Watson, J.T.; Hollifield, J.W.; Shand, D.G.; Oates, J.A. Effects of Caffeine on Plasma Renin Activity, Catecholamines and Blood Pressure. N. Engl. J. Med. 1978, 298, 181–186. [Google Scholar] [CrossRef]
- Waring, W.S.; Goudsmit, J.; Marwick, J.; Webb, D.J.; Maxwell, S.R. Acute caffeine intake influences central more than peripheral blood pressure in young adults. Am. J. Hypertens. 2003, 16, 919–924. [Google Scholar] [CrossRef]
- Sondermeijer, H.P.; Van Marle, A.G.; Kamen, P.; Krum, H. Acute effects of caffeine on heart rate variability. Am. J. Cardiol. 2002, 90, 906–907. [Google Scholar] [CrossRef]
| Study (ref.) | Coffee (Contents)/Caffeine | Participants (Number) | Follow-Up Period | Endothelial Function Test | Results |
|---|---|---|---|---|---|
| [45] | Coffee (caffeine 100 mg)/Caffeine 200 mg | Healthy subjects (n = 12; 4 men, 8 women) | 1 h | Exhaled NO levels | Both coffee and caffeine deceased exhaled NO levels. |
| [80] | Black tea (caffeine 200 mg) | CAD patients (n = 66) | 2 h, 4 weeks | FMD | Black tea improved FMD in brachial artery, while an equivalent dose of caffeine did not alter FMD. Both black tea and caffeine did not alter NID. |
| [81] | Black tea (caffeine ≈250 mg) | Hyperlipidemia patients (n = 21) | 4 weeks | FMD | Black tea improved FMD and NID in brachial artery. |
| [82] | Black tea (caffeine ≈50 mg) | CAD patients (n = 20) | 4 h | FMD | Black tea improved FMD and NID in brachial artery after meal loading, while black tea alone did not alter both FMD and NID. |
| [46] | Coffee (caffeine 80 mg) | Healthy young adults (n = 17; 9 men, 11 women) | 2 h | FMD | Caffeinated coffee decreased FMD in brachial artery, while decaffeinated coffee did not alter FMD. |
| [77] | Caffeine 300 mg | Healthy young men (n = 20) | 1 h | ACh-induced vasodilation | Caffeine augmented ACh-induced vasodilation in forearm tissue and did not alter SNP-induced vasodilation. |
| [31] | Coffee (caffeine 83–373 mg) | Healthy women (n = 730), diabetic women (n = 663) | Cross-sectional | Chemical biomarkers | Coffee consumption is inversely correlated with markers of endothelial dysfunction. |
| [83] | Black tea (caffeine 100 mg) | Healthy men (n = 19) | 1 week | FMD | Black tea dose-dependently augmented FMD in brachial artery. |
| [32] | Coffee (CGA 140 mg) | Healthy men (n = 20) | 3 months, 4 months | RH ratio | Coffee polyphenols improved RH ratio in forearm tissue after glucose loading. |
| [33] | Coffee (CGA 134 mg–300 mg, caffeine 59–70 mg, HHQ 0.03–0.12 mg) | Subjects with CV risk factors (n = 21) | 8 weeks | FMD | Coffee improved FMD in brachial artery. |
| [79] | Green tea (caffeine 125 mg)/Caffeine 125 mg | Healthy subjects (n = 14) | 2 h | FMD | Green tea augmented FMD in brachial artery, while an equivalent dose of caffeine did not alter FMD. |
| [84] | Black tea (caffeine 125 mg) | Healthy women (n = 16) | 2 h | FMD | Black tea augmented FMD in brachial artery and did not alter NID. |
| [34] | Coffee (2 cups of decaffeinated) | Healthy subjects (n = 15; 8 men, 7 women) | 1 h | FMD | Decaffeinated coffee increased FMD in brachial artery. |
| [47] | Coffee (Italian espresso 25 mL) | Healthy subjects (n = 20; 10 men, 10 women) | 5–7 days | FMD | Caffeinated coffee decreased FMD in brachial artery, while decaffeinated coffee did not alter FMD. |
| [78] | Caffeine 200 mg | Subjects without CVD (n = 40) and with CVD (n = 40) | 1 h | FMD | Caffeine increased FMD in brachial artery and did not alter NID. |
| [35] | Coffee (boiled Greek coffee, caffeine 56–126 mg) | Elderly subjects (n = 142) | Cross-sectional | FMD | Greek type of coffee had higher increased FMD in brachial artery compared to other groups. |
| [36] | Coffee (CGA) | Healthy men (n = 15) | 1.5 h | RH index | Coffee polyphenols improved RH index in finger tips after glucose loading. |
| [37] | Coffee (CGA) | Healthy men (n = 13) | 2 h | FMD | Coffee polyphenols improved FMD in brachial artery after meal loading. |
| [38] | Coffee (CGA 355 mg, caffeine 54.7 mg) | Healthy men (n = 19) | 3 h | FMD | Coffee polyphenols improved FMD in brachial artery after meal loading. |
| [39] | Coffee (caffeine 54.5 mg) | Healthy subjects (n = 27; 13 men, 14 women) | 75 min | RH flow | Caffeinated coffee augmented reactive hyperemia of finger blood flow, while decaffeinated coffee did not alter reactive hyperemia of finger blood flow. |
| [48] | Coffee (caffeine 240 mg) | Healthy subjects (n = 19; 11 men, 8 women) | 4 h | RH index | Coffee did not alter RH index in finger tips. |
| [49] | Coffee (CGA 420–780 mg, caffeine 193 mg) | Healthy subjects (n = 74; 37 men, 37 women) | 1 h, 8 weeks | FMD | Coffees containing CGA did not alter FMD in brachial artery. |
| [50] | Coffee (CGA 450–900 mg) | Healthy subjects (n = 16; 6 men, 10 women) | 1 h, 4 h | FMD | Coffees containing CGA did not alter FMD in brachial artery. |
| [40] | Coffee (CGA 89–310 mg, caffeine 110 mg) | Healthy men (n = 15) | 5 h | FMD | Coffees containing CGA increased FMD in brachial artery. |
| [85] | Black tea (caffeine 37.3 mg) | Hypertension (n = 19; 7 men, 12 women) | 8 days | FMD | Black tea improved FMD in brachial artery and increased circulating progenitor cells. |
| [41] | Coffee (CGA 300 mg) | Healthy men (n = 12) | 2 h | FMD | Caffeinated coffee containing CGA increased FMD in brachial artery, while decaffeinated coffee containing CGA did not alter FMD. |
| [42] | Coffee (caffeine 78 mg) | Healthy subjects (n = 16; 8 men, 8 women) | 1.5 h | ACh-induced vasodilation | Caffeinated coffee augmented ACh-induced vasodilation in the forearm skin, while decaffeinated coffee did not alter ACh-induced vasodilation. |
| [43] | Coffee (CGA-enriched green coffee bean) | Healthy men (n = 16) | 2 weeks | FMD | CGA-enriched green coffee bean increased FMD in brachial artery. |
| [86] | Black tea (3 Lipton tea bags) | Healthy young adults (n = 17, 7 men, 10 women) | 4 weeks | FMD | Black tea augmented FMD in brachial artery. |
| [44] | Coffee (CGA 373–412 mg, caffeine 59–75 mg, HHQ 0.10–0.76 mg) | Stage 1 hypertension (n = 37; 26 men, 11 women) | 1 week | FMD | Caffeinated coffee containing high content of CGA and low content of HHQ improved FMD in brachial artery after meal loading. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashi, Y. Coffee and Endothelial Function: A Coffee Paradox? Nutrients 2019, 11, 2104. https://doi.org/10.3390/nu11092104
Higashi Y. Coffee and Endothelial Function: A Coffee Paradox? Nutrients. 2019; 11(9):2104. https://doi.org/10.3390/nu11092104
Chicago/Turabian StyleHigashi, Yukihito. 2019. "Coffee and Endothelial Function: A Coffee Paradox?" Nutrients 11, no. 9: 2104. https://doi.org/10.3390/nu11092104
APA StyleHigashi, Y. (2019). Coffee and Endothelial Function: A Coffee Paradox? Nutrients, 11(9), 2104. https://doi.org/10.3390/nu11092104




