Evaluation of Agraz Consumption on Adipocytokines, Inflammation, and Oxidative Stress Markers in Women with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Anthropometrics: Body Weight, Height, Body Mass Index (BMI), and Waist Circumference (WC)
2.3. Blood Pressure (BP)
2.4. Blood and Urine Collection
2.5. Blood Lipids, Glucose and Insulin, and Urinary Creatinine
2.6. Index of Insulin Resistance
2.7. Inflammation Markers and Adipocytokines
2.8. Total Phenols Concentration and Antioxidant Capacity
2.9. Oxidative Stress Markers
2.10. Statistical Analysis
3. Results
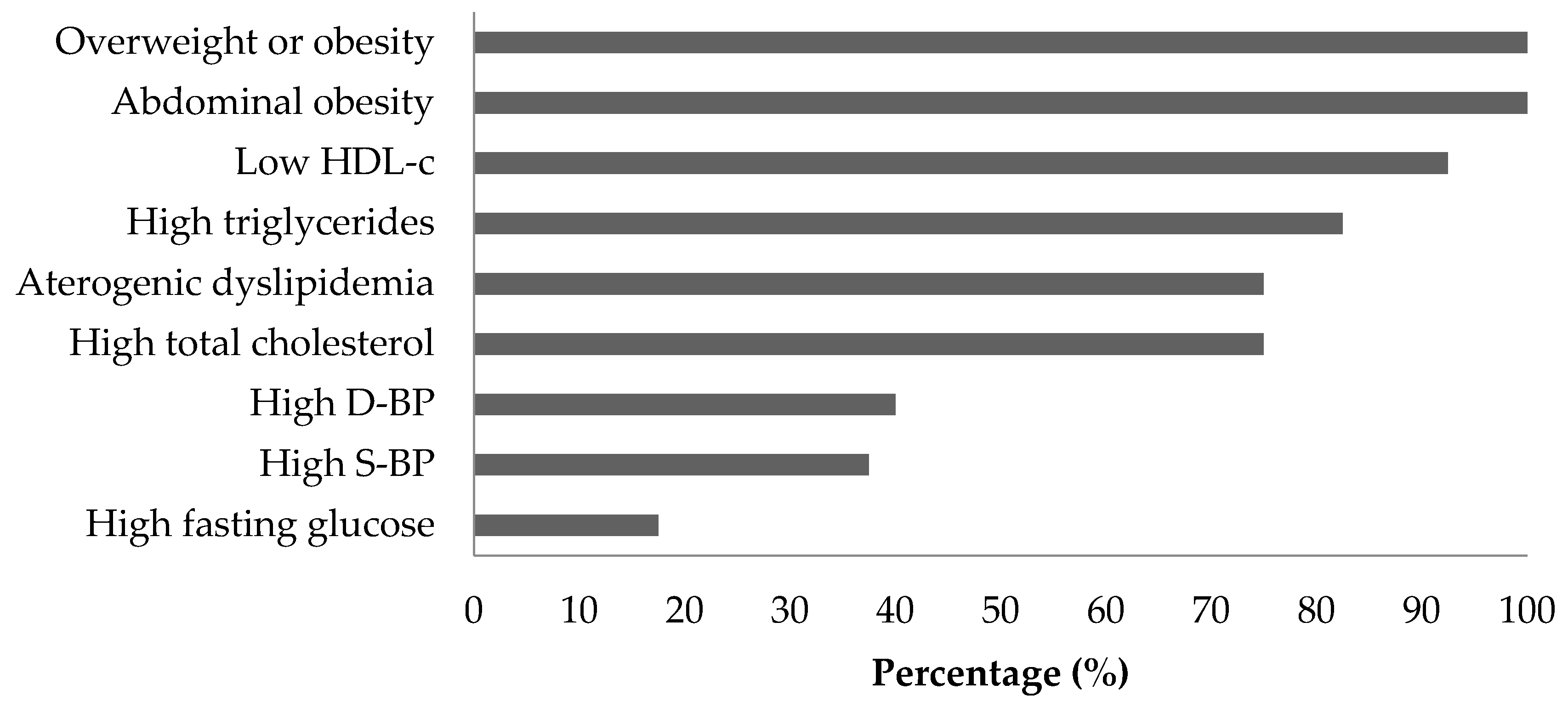
3.1. MetS Criteria and Participant Characteristics
3.2. Antioxidant Capacity and Oxidative Stress Markers
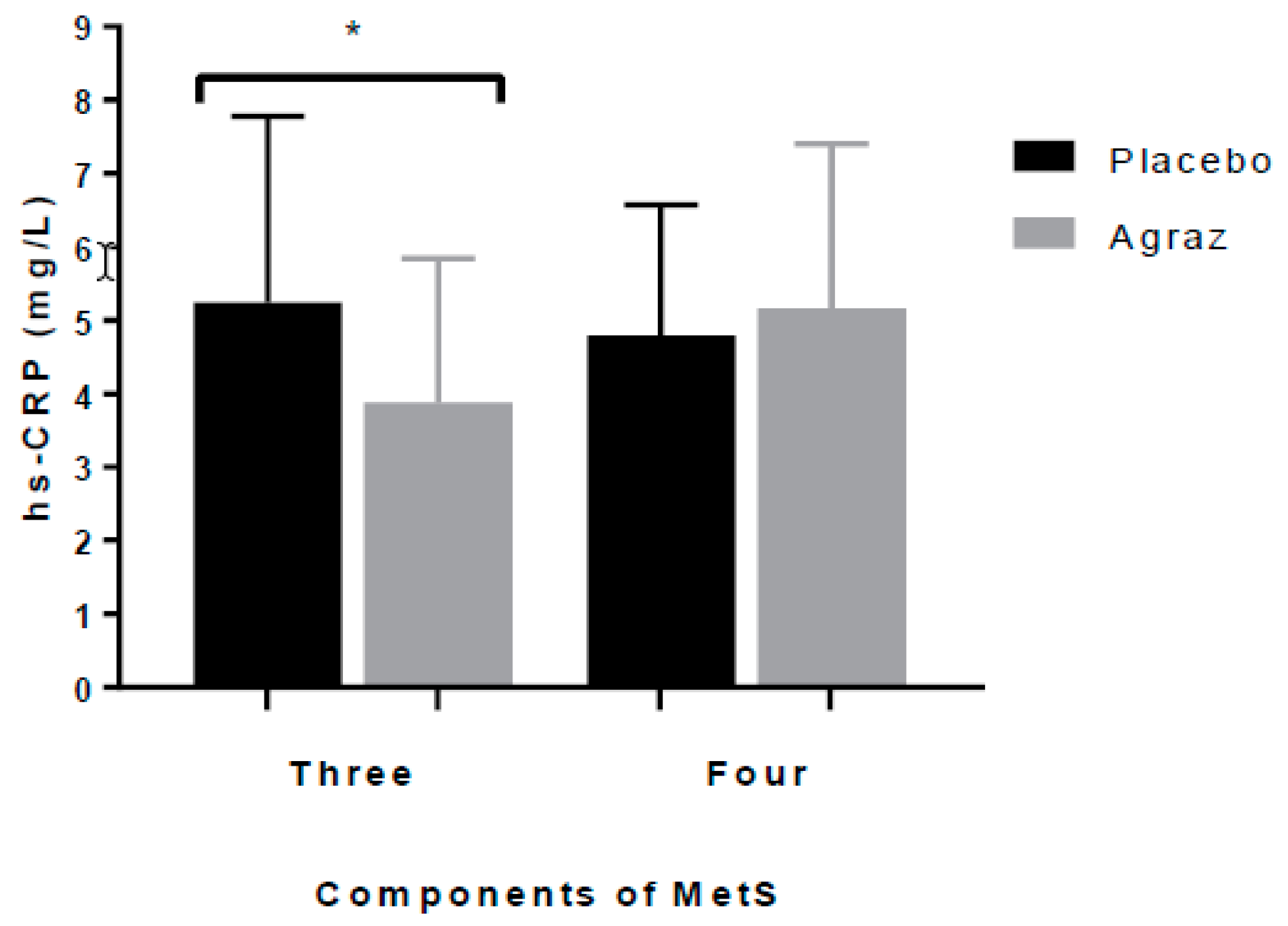
3.3. Insulin Resistance and Inflammatory Markers
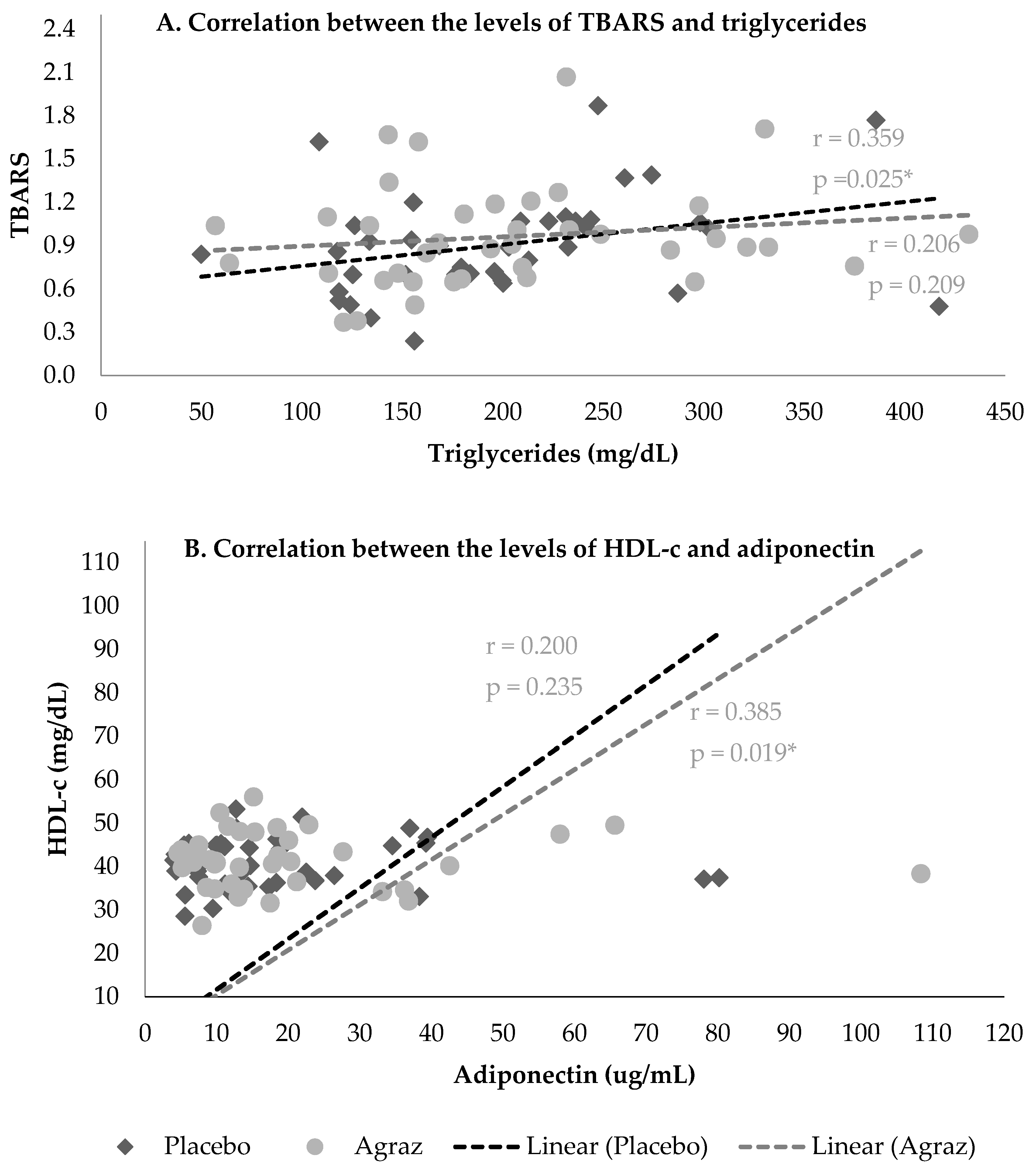
3.4. Correlations
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fanning, E.; O’Shea, D. Genetics and the metabolic syndrome. Clin. Dermatol. 2018, 36, 9–13. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Statistics and Information Systems Estimates for 2000–2015; WHO World Health Organization: Geneva, Switzerland, 2017; Available online: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (accessed on 11 May 2018).
- Gaviria Uribe, A.; Davila, C.; Correa, E.; Burgos, L.F.; Girón, B.C.; Vargas, S.L. Análisis de Situación de Salud (ASIS) Colombia, 2016. Minist Salud y Protección Soc. 2016, 1–163. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/PSP/asis-colombia-2016.pdf (accessed on 11 May 2018).
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Pinzón, J.B.; Serrano, N.C.; Díaz, L.A.; Mantilla, G.; Velasco, H.M.; Martínez, L.X.; Millán, P.A.; Acevedo, S.M.; Moreno, D. Impacto de las nuevas definiciones en la prevalencia del síndrome metabólico en una población adulta de Bucaramanga, Colombia. Biomédica 2007, 27, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paternina-Caicedo, Á.J.; Alcalá-Cerra, G.; Paillier-Gonzales, J.; Romero-Zarante, Á.M.; Alvis-Guzmán, N. Concordancia de tres definiciones de síndrome metabólico en pacientes hipertensos. Rev. Salud Públ. 2009, 11, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Rincon-Peña, O.; Gamarra-Hernandez, G.; Jerez-Rodriguez, H.V.-C.L. Valoración del riesgo cardiovascular global y prevalencia de síndrome metabólico en trabajadores de la salud del Hospital Universitario Ramón González Valencia. Acta Med. Colomb. 2004, 29, 312–321. [Google Scholar]
- Davila, E.P.; Quintero, M.A.; Orrego, M.L.; Ford, E.S.; Walke, H.; Arenas, M.M.; Pratt, M. Prevalence and risk factors for metabolic syndrome in Medellin and surrounding municipalities, Colombia, 2008–2010. Prev. Med. (Baltim). 2013, 56, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Luna-Luna, M.; Medina-Urrutia, A.; Vargas-Alarcón, G.; Coss-Rovirosa, F.; Vargas-Barrón, J.; Pérez-Méndez, Ó. Adipose Tissue in Metabolic Syndrome: Onset and Progression of Atherosclerosis. Arch. Med. Res. 2015, 46, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurti, S.P.; Emerson, S.R.; Rosenkranz, S.K.; Teeman, C.S.; Emerson, E.M.; Cull, B.J.; Smith, J.R.; Harms, C.A. Post-prandial systemic 8-isoprostane increases after consumption of moderate and high-fat meals in insufficiently active males. Nutr. Res. 2017, 39, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Burd, R.M.; Patel, K.; Barnard, A.; Lunec, J.; Hutchinson, P.E. Induction and excretion of ultraviolet-induced 8-oxo-2′-deoxyguanosine and thymine dimers in vivo: Implications for PUVA. J. Investig. Dermatol. 2001, 116, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Ouchi, N.; Ohashi, K.; Murohara, T. The role of adipokines in cardiovascular disease. J. Cardiol. 2017, 70, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Mantzoros, C.S. From leptin to other adipokines in health and disease: Facts and expectations at the beginning of the 21st century. Metabolism 2015, 64, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Leal, V.O.; Mafra, D. Adipokines in obesity. Clin. Chim. Acta 2013, 419, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhuo, X.; Bardenheier, B.; Rolka, D.B.; Gregg, W.E.; Hong, Y.; Wang, G.; Albright, A.; Zhang, P. Cost-effectiveness of the 2014 U.S. Preventive Services Task Force (USPSTF) Recommendations for Intensive Behavioral Counseling Interventions for Adults With Cardiovascular Risk Factors. Diabetes Care 2017, 40, dc161186. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013; Available online: http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf (accessed on 25 May 2018).
- Chong, M.F.-F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104 (Suppl. S3), S28–S39. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends Food Sci. Technol. 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kortesniemi, M. Clinical evidence on potential health benefits of berries. Curr. Opin. Food Sci. 2015, 2, 36–42. [Google Scholar] [CrossRef]
- Del Bo’, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite profiling of polyphenols in Vaccinium berries and determination of their chemopreventive properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Isabel, C.Z.V.; Susana, O.; Mariacutea, E.M.; Arley, D.Z.Z.; Benjamiacuten, R. Cytotoxic effect and antioxidant activity of Andean berry (Vaccinium meridionale Sw) wine. J. Med. Plants Res. 2016, 10, 402–408. [Google Scholar] [CrossRef]
- Agudelo, C.D.; Luzardo-Ocampo, I.; Campos-Vega, R.; Loarca-Piña, G.; Maldonado, M. Bioaccessibility during in vitro digestion and antiproliferative effect of bioactive compounds from Andean berry (Vaccinium meridionale Swartz) juice. J. Agric. Food Chem. 2018, 66, 7358–7366. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Klimis-Zacas, D.; Del Bo, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopera, Y.E.; Fantinelli, J.; Arbelaez, L.; Ez, L.F.; Rojano, B.; Schinella, G.; Mosca, S. Antioxidant Activity and Cardioprotective Effect of a Nonalcoholic Extract of Vaccinium meridionale Swartz during Ischemia-Reperfusion in Rats. Evid.-Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Li, K.; Jing, H.; Zheng, L. In Vivo Therapeutic Effect of Vaccinium Meridionale Swartz in Ischemia-Reperfusion Induced Male Albino Rats. J. Food Sci. 2018, 83, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape Polyphenols Exert a Cardioprotective Effect in Pre- and Postmenopausal Women by Lowering Plasma Lipids and Reducing Oxidative Stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape Polyphenols Reduce Blood Pressure and Increase Flow-Mediated Vasodilation in Men with Metabolic Syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero-Quiroz, J.; Galv-Pérez, Y.; Galeano-Vásquez, S.; Marín-Echeisverri, C.; Franco-Escobar, C.; Ciro-Gómez, G.; Núñez-Rangel, V.; Aristizábal-Rivera, J.C.; Barona-Acevedo, J. Physico-chemical characterization and antioxidant capacity of the Colombian berry (Vaccinium meridionale Swartz) with a high-polyphenol content: Potential effects in people with metabolic syndrome. Food Sci. Technol. 2018. under review. [Google Scholar]
- Monsalve, J.M.; Zapata, L.I.G. Development of questionnaire to assess food intake in the University of Antioquia, Colombia. [“Diseño de un cuestionario de frecuencia para evaluar ingesta alimentaria en la Universidad de Antioquia, Colombia.]. Nutr. Hosp. 2011, 26, 1333–1344. [Google Scholar]
- World Health Organization. Global Database on Body Mass Index—World Health Organization; World Health Organization: Geneva, Switzerland, 2006; Available online: http://www.assessmentpsychology.com/icbmi.htm (accessed on 30 August 2018).
- Knopfholz, J.; Disserol, C.C.D.; Pierin, A.J.; Schirr, F.L.; Streisky, L.; Takito, L.L.; Massucheto Ledesma, P.; Faria-Neto, J.R.; Olandoski, M.; da Cunha, C.L.; et al. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterology 2014, 2014, 11–13. [Google Scholar]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med. J. Islam. Repub. Iran. 2015, 29, 627–635. [Google Scholar]
- Dobiasova, M.; Frohlich, J. The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: Changes during lipanor therapy. Vnitr. Lek. 2000, 46, 152–156. [Google Scholar] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessiong Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Maiani, G.; Ferro-Luzzi, A. Alcohol-Free Red Wine Enhances Plasma Antioxidant Capacity in Humans. J. Nutr. 1998, 128, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chrzczanowicz, J.; Gawron, A.; Zwolinska, A.; De Graft-Johnson, J.; Krajewski, W.; Krol, M.; Markowski, J.; Kostka, T.; Nowak, D. Simple method for determining human serum 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity—Possible application in clinical studies on dietary antioxidants. Clin. Chem. Lab. Med. 2008, 46, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Shooter, L.A.; Henson, D.A.; Utter, A.C.; Milne, G.; McAnulty, S.R. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl. Physiol. Nutr. MeTable 2011, 36, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Gaw, A.; Scherbakova, O.; Ford, I.; O’Reilly, D.S.J.; Haffner, S.M.; Isles, C.; Macfarlane, P.W.; Packard, C.J.; Cobbe, S.M.; et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003, 108, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Buring, J.E.; Cook, N.R.; Rifai, N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14,719 initially healthy American women. Circulation 2003, 107, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Duffey, K.J.; Sutherland, L.A. Adult consumers of cranberry juice cocktail have lower C-reactive protein levels compared with nonconsumers. Nutr. Res. 2015, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Lee, W.S.; Shin, S.C.; Kim, G.Y.; Choi, B.T.; Choi, Y.H. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-κB and Akt/MAPKs signaling pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Paur, I.; Bohn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-kB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Hakimi, M.; Asgary, S.; Ghanadian, S.M.; Keshvari, M.; Sarrafzadegan, N. Evaluation of the effects of Vaccinium arctostaphylos L. Fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: A randomized, double-blind, placebo-controlled clinical trial. Evid. Based Complement. Alternat. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Kojadinovic, M.; Arsic, A.; Debeljak-Martacic, J.; Konic-Ristic, A.; Kardum, N.; Popovic, T.; Glibetic, M.D. Consumption of pomegranate juice decreases blood lipid peroxidation and levels of arachidonic acid in women with metabolic syndrome. J. Sci. Food Agric. 2016, 97, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Kunz, C.; Herrmann, J.; Borsch, C.H.; Abel, G.; Fröhling, B.; Dietrich, H.; Rudloff, S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. Br. J. Nutr. 2014, 112, 925–936. [Google Scholar] [PubMed]
- Carlos, A.G.M.; Clara, O.O.; Nelly, S.M.; Clara, M.C.; Mario, L.A.; Paula, G.G.; Ana, M.M. Actividad antioxidante e inhibición de la peroxidación lipídica de extractos de frutos de mortiño (Vaccinium meridionale SW). Bol. Latinoam y del Caribe Plantas Med. y Aromat. 2009, 8, 519–528. [Google Scholar]
- Bravo, K.; Alzate, F.; Osorio, E. Fruits of selected wild and cultivated Andean plants as sources of potential compounds with antioxidant and anti-aging activity. Ind. Crops Prod. 2016, 85, 341–352. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kihara, S.; Funahashi, T.; Matsuzawa, Y.; Libby, P. Adiponectin: A key adipocytokine in metabolic syndrome. Clin. Sci. 2006, 110, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kihara, S.; Ouchi, N.; Nishida, M.; Arita, Y.; Kumada, M.; Ohashi, K.; Sakai, N.; Shimomura, I.; Kobayashi, H.; et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 2002, 106, 2767–2770. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Kihara, S.; Arita, Y.; Nishida, M. Lipid Accumulation and Class A Scavenger Receptor. Circulation 2001, 103, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Kazumi, T.; Kawaguchi, A.; Hirano, T.; Yoshino, G. Serum Adiponectin Is Associated with High-Density Lipoprotein Cholesterol, Triglycerides, and Low-Density Lipoprotein Particle Size in Young Healthy Men. Metabolism 2004, 53, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Kamigaki, M.; Sakaue, S.; Tsujino, I.; Ohira, H.; Ikeda, D.; Itoh, N.; Ishimaru, S.; Ohtsuka, Y.; Nishimura, M. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2006, 339, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detopoulou, P.; Panagiotakos, D.B.; Chrysohoou, C.; Fragopoulou, E.; Nomikos, T.; Antonopoulou, S.; Pitsavos, C.; Stefanadis, C. Dietary antioxidant capacity and concentration of adiponectin in apparently healthy adults: The ATTICA study. Eur. J. Clin. Nutr. 2010, 64, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Bourguignon, C.M.; Vincent, K.R.; Weltman, A.L.; Bryant, M.; Taylor, A.G. Antioxidant supplementation lowers exercise-induced oxidative stress in young overweight adults 1565. Obesity (Silver Spring) 2006, 14, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Park, P.J.; Shin, H.J.; Kim, Y.-K.; Shin, D.W.; Shin, E.S.; Lee, H.H.; Lee, B.G.; Baik, J.H.; Lee, T.R. (−)-Catechin suppresses expression of Kruppel-like factor 7 and increases expression and secretion of adiponectin protein in 3T3-L1 cells. AJP Endocrinol. Metab. 2006, 292, E1166–E1172. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J. Anthocyanin supplementation improves serum LDL-and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Barona, J.; Blesso, C.N.; Andersen, C.J.; Lee, J.; Fernandez, M.L. Grape Consumption Increases Anti-Inflammatory Markers and Upregulates Peripheral Nitric Oxide Synthase in the Absence of Dyslipidemias in Men with Metabolic Syndrome. Nutrients 2012, 2, 1945–1957. [Google Scholar] [CrossRef] [PubMed]
- Mulero, J.; Bernabé, J.; Cerdá, B.; García-Viguera, C.; Moreno, D.A.; Albaladejo, M.D.; Avilés, F.; Parra, S.; Abellán, J.; Zafrilla, P. Variations on cardiovascular risk factors in metabolic syndrome after consume of a citrus-based juice. Clin Nutr. 2012, 31, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Simão, T.N.C.; Lozovoy, M.A.B.; Simão, A.N.C.; Oliveira, S.R.; Venturini, D.; Morimoto, H.K.; Miglioranza, L.H.; Dichi, I. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br. J. Nutr. 2013, 110, 1885–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhang, J.; Zhang, W.; Ni, Z. Association of adiponectin with peripheral arterial disease and mortality in nondiabetic hemodialysis patients: Long-term follow-up data of 7 years. J. Res. Med. Sci. 2016, 21, 50. [Google Scholar] [PubMed]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit lipid metabolism and modulate leptin and adiponectin secretion in 3T3-L1 adipocytes. Food Chem. 2015, 185, 383–388. [Google Scholar] [CrossRef] [PubMed]
| Nutrient/Compound | Freeze-Dried Agraz | Nectar (Reconstituted from Freeze-Dried Agraz in 200 mL) | Placebo (200 mL) |
|---|---|---|---|
| Macronutrients | |||
| Calories per dose (Kcal) | ND | 26 ± 0.18 | 21.86 ± 0.21 |
| Total carbohydrate (%) | 78.85 ± 0.19 | 2.08 ± 0.19 | 2.50 ± 0.19 |
| Protein (%) | 1.39 ± 0.25 | 0.05 ± 0.01 | 0.21 ±0.05 |
| Phytochemicals | |||
| Total Phenols (mg GAE/g of freeze-dried or L) | 139.29 ± 5.69 | 1027.97 ± 41.99 | 31.91 ± 3.15 |
| Anthocyanins (mg CE/g) a | 4.66 | ND | ND |
| Anthocyanins (mg/L) b | ND | 75.65 | ND |
| Variables | Placebo | Agraz | Δ (Agraz-Placebo) | p | ||
|---|---|---|---|---|---|---|
| n | Mean or Median ± SD or IQR (p25-p75) | n | Mean or Median ± SD or IQR (p25-p75) | Mean or Median ± SD or IQR (p25-p75) | ||
| Weight (Kg) a | 40 | 76.6 ± 11.6 | 40 | 76.6 ± 11.6 | −0.05 ± 0.9 | 0.756 |
| Body Mass Index (Kg/mt2) b | 40 | 29.8 (4.26) | 40 | 29.9 (3.87) | −0.11 (0.64) | 0.975 |
| Waist circumference (cm) b | 40 | 98.7 (9.43) | 40 | 98.5 (10.23) | −0.2 (3.87) | 0.185 |
| Systolic blood pressure (mm Hg) a | 40 | 115.4 ± 12.5 | 40 | 116.0 ± 11.8 | 0.5 ± 8.6 | 0.692 |
| Diastolic blood pressure (mm Hg) a | 40 | 74.8 ± 8.0 | 40 | 75.1 ± 9.5 | 0.3 ± 5.2 | 0.73 |
| Fasting glucose (mg/dL) a | 40 | 96.8 ± 8.1 | 40 | 95.9 ± 8.0 | −0.9 ± 0.3 | 0.291 |
| Triglycerides (mg/dL) b | 39 | 197.61 (104.59) | 39 | 193.82 (105.32) | −9.79 (110.53) | 0.759 |
| Total cholesterol (mg/dL) a | 40 | 219.6 ± 43.3 | 40 | 216.1 ± 45.1 | −3.5 ± 38.7 | 0.574 |
| HDL cholesterol (mg/dL) a | 40 | 41.6 ± 6.4 | 40 | 41.7 ± 6.8 | 0.9 ± 4.4 | 0.905 |
| LDL cholesterol (mg/dL) a | 36 | 137.9 ± 39.3 | 36 | 132.2 ± 42.1 | −5.7 ± 35.1 | 0.339 |
| Non-HDL cholesterol (mg/dL) a | 39 | 177.3 ± 42.3 | 39 | 174.5 ± 44.3 | −2.8 ± 38.2 | 0.649 |
| TG/HDL-c index b | 39 | 4.45 (2.81) | 39 | 4.84 (3.55) | −0.23 (3.57) | 0.606 |
| TC/HDL-c index a | 40 | 5.35 ± 1.2 | 40 | 5.27 ± 1.2 | −0.09 ± 0.9 | 0.565 |
| LDL-c/HDL-c index a | 36 | 3.29 ± 0.96 | 36 | 3.17 ± 0.95 | −0.12 ± 0.8 | 0.357 |
| Variables | Placebo | Agraz | Δ (Agraz-Placebo) | p | ||
|---|---|---|---|---|---|---|
| n | Mean or Median ± SD or IQR (p25-p75) | n | Mean or Median ± SD or IQR (p25-p75) | Mean or Median ± SD or IQR (p25-p75) | ||
| DPPH (% Scavenging effect) a | 40 | 10.55 ± 6.19 | 40 | 12.63 ± 7.47 | 2.08 ± 5.75 | 0.028 * |
| Total phenols mgGA/L b | 40 | 297.29 (57.29) | 40 | 331.88 (56.04) | 7.92 (72.70) | 0.279 |
| TBARS b | 40 | 0.89 (0.39) | 40 | 0.92 (0.45) | 0.04 (0.42) | 0.402 |
| F2-Isoprostanes (ng/mg creatinine) b | 35 | 2.86 (3.97) | 35 | 3.03 (3.41) | 0.11 (3.39) | 0.863 |
| OHdG (ng/mg creatinine) a | 35 | 1.97 ± 0.66 | 35 | 1.66 ± 0.5 | −0.27 ± 0.72 | 0.041 * |
| Variables | Placebo | Agraz | Δ (Agraz-Placebo) | p | ||
|---|---|---|---|---|---|---|
| n | Mean or Median ± SD or IQR (p25-p75) | n | Mean or Median ± SD or IQR (p25-p75) | Mean or Median ± SD or IQR (p25-p75) | ||
| Insulin (mUI/L) b | 39 | 16.34 (13.03) | 39 | 15.0 (14.01) | 0.26 (4.91) | 0.922 |
| HOMA 2 index b | 38 | 2.33 (1.83) | 40 | 2.21 (1.8) | 0.02 (0.66) | 0.577 |
| QUICKI index a | 39 | 0.314 ± 0.024 | 39 | 0.315 ± 0.024 | 0.0 ± 0.012 | 0.714 |
| hs-CRP (mg/L) b | 37 | 4.8 (2.81) | 37 | 3.75 (2.80) | −0.54 (2.5) | 0.103 |
| Adiponectin (ug/mL) b | 37 | 12.75 (14.53) | 37 | 13.23 (11.38) | 0.89 (4.43) | 0.225 |
| Resistin (ng/mL) a | 38 | 31.96 ± 7.77 | 38 | 33.84 ± 10.1 | 1.88 ± 9.45 | 0.229 |
| Leptin (ng/mL) a | 38 | 3.58 ± 1.52 | 38 | 3.58 ± 1.66 | 0.025 ± 0.76 | 0.986 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Moncada, J.; Marín-Echeverri, C.; Galvis-Pérez, Y.; Ciro-Gómez, G.; Aristizábal, J.C.; Blesso, C.N.; Fernandez, M.L.; Barona-Acevedo, J. Evaluation of Agraz Consumption on Adipocytokines, Inflammation, and Oxidative Stress Markers in Women with Metabolic Syndrome. Nutrients 2018, 10, 1639. https://doi.org/10.3390/nu10111639
Espinosa-Moncada J, Marín-Echeverri C, Galvis-Pérez Y, Ciro-Gómez G, Aristizábal JC, Blesso CN, Fernandez ML, Barona-Acevedo J. Evaluation of Agraz Consumption on Adipocytokines, Inflammation, and Oxidative Stress Markers in Women with Metabolic Syndrome. Nutrients. 2018; 10(11):1639. https://doi.org/10.3390/nu10111639
Chicago/Turabian StyleEspinosa-Moncada, Juliana, Catalina Marín-Echeverri, Yeisson Galvis-Pérez, Gelmy Ciro-Gómez, Juan C. Aristizábal, Christopher N. Blesso, Maria Luz Fernandez, and Jacqueline Barona-Acevedo. 2018. "Evaluation of Agraz Consumption on Adipocytokines, Inflammation, and Oxidative Stress Markers in Women with Metabolic Syndrome" Nutrients 10, no. 11: 1639. https://doi.org/10.3390/nu10111639





