Effects of a Fruit and Vegetable-Based Nutraceutical on Biomarkers of Inflammation and Oxidative Status in the Plasma of a Healthy Population: A Placebo-Controlled, Double-Blind, and Randomized Clinical Trial
Abstract
:1. Introduction
2. Results
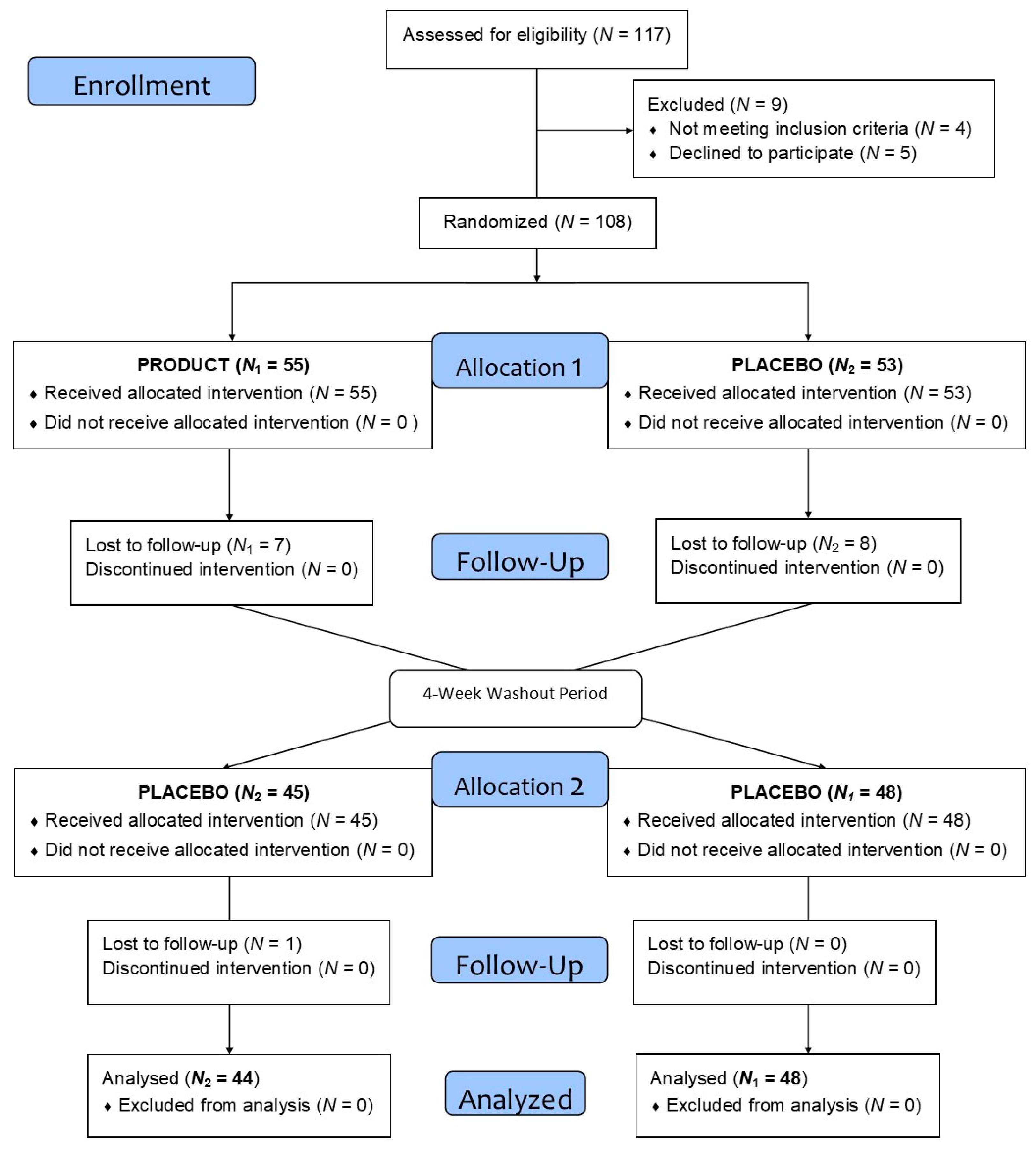
2.1. Study Population
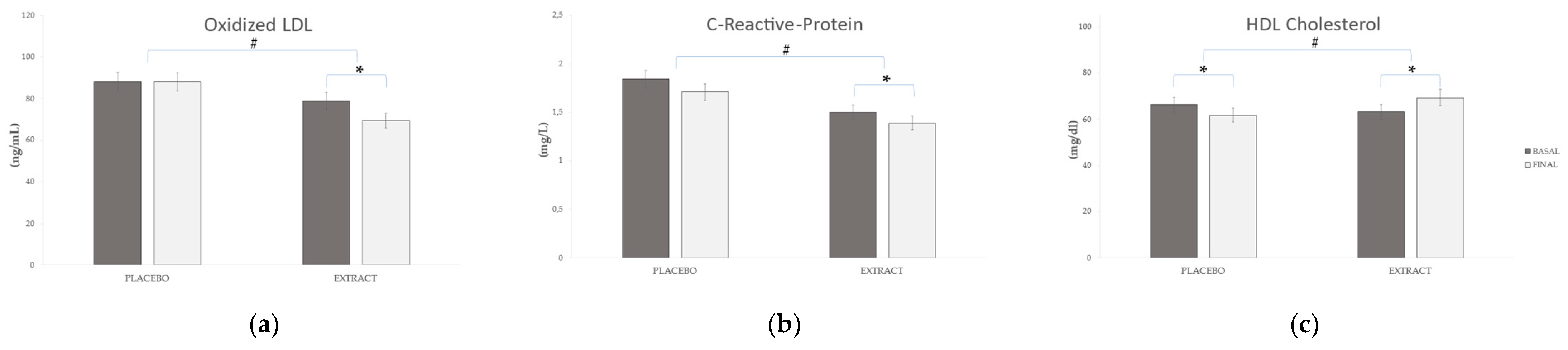
2.2. Plasmatic Biomarkers
3. Discussion
4. Materials and Methods
4.1. Trial Design
4.2. Participants
4.3. Test Supplement
4.4. Study Variables
Blood Sample Measurements
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; López-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Blomhoff, R.; Andersen, L.F.; Iversen, P.O.; Johansson, L.; Smeland, S. Kostråd for å fremme folkehelsen og forebygge kroniske sykdommer. Metodol. Vitensk. kunnskapsgrunnlag 2011. Available online: https://www.helsedirektoratet.no/rapporter/kostrad-for-a-fremme-folkehelsen-og-forebygge-kroniske-sykdommer-metodologi-og-vitenskapelig-kunnskapsgrunnlag/Kostr%C3%A5d%20for%20%C3%A5%20fremme%20folkehelsen%20og%20forebygge%20kroniske%20sykdommer%20%E2%80%93%20metodologi%20og%20vitenskapelig%20kunnskapsgrunnlag.pdf/_/attachment/inline/2a6293e0-169e-41bd-a872-f3952dbb22c2:0d09926111d614e6059e804b7f9b21c17bd0c1cd/Kostr%C3%A5d%20for%20%C3%A5%20fremme%20folkehelsen%20og%20forebygge%20kroniske%20sykdommer%20%E2%80%93%20metodologi%20og%20vitenskapelig%20kunnskapsgrunnlag.pdf (accessed on 11 January 2021).
- Benton, D.; Young, H.A. Role of fruit juice in achieving the 5-a-day recommendation for fruit and vegetable intake. Nutr. Rev. 2019, 77, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Pagán, J.A. Social Norms and the Consumption of Fruits and Vegetables across New York City Neighborhoods. J. Urban Health 2016, 93, 244–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Quiñones, M.; Miguel, M.; Aleixandre, A. The polyphenols, naturally occurring compounds with beneficial effects on cardiovascular disease. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Li, S.; Zhang, Y.; Xu, X.; Chen, Y.; Li, H. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Risk assessment for peri-and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015, 13, 4246. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G.; Afanas’ Ev, I.B. Antioxidant and chelating properties of flavonoids. Adv. Pharmacol. 1996, 38, 151–163. [Google Scholar]
- Dorjgochoo, T.; Gao, Y.-T.; Chow, W.-H.; Shu, X.; Yang, G.; Cai, Q.; Rothman, N.; Cai, H.; Li, H.; Deng, X.; et al. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am. J. Clin. Nutr. 2012, 96, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; D’Ardes, D.; Davì, G. Oxidative stress in chronic vascular disease: From prediction to prevention. Vascul. Pharmacol. 2015, 74, 23–37. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. US department of agriculture and US department of health and human services, dietary guidelines for Americans, 2010. Washington, DC: US government printing office, January 2011. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroni, L.; Bonetto, C.; Rizzo, G.; Bertola, C.; Caberlotto, L.; Bazzerla, G. Association Between Cognitive Impairment and Vitamin B12, Folate, and Homocysteine Status in Elderly Adults: A Retrospective Study. J. Alzheimers. Dis. 2019, 70, 443–453. [Google Scholar] [CrossRef]
- Samman, S.; Sivarajah, G.; Man, J.C.; Ahmad, Z.I.; Petocz, P.; Caterson, I.D. A mixed fruit and vegetable concentrate increases plasma antioxidant vitamins and folate and lowers plasma homocysteine in men. J. Nutr. 2003, 133, 2188–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.; Li, Y.; Zhang, W.; Zhao, Y.; Niu, X.; Guo, J. The relationship between cognitive impairment and homocysteine in a B12 and folate deficient population in China: A cross-sectional study. Medicine 2019, 98, e17970. [Google Scholar] [CrossRef]
- Lin, F.; Pei, L.; Zhang, Q.; Han, W.; Jiang, S.; Lin, Y.; Dong, B.; Cui, L.; Li, M. Ox-LDL induces endothelial cell apoptosis and macrophage migration by regulating caveolin-1 phosphorylation. J. Cell. Physiol. 2018, 233, 6683–6692. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Albers, R.; Antoine, J.-M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, A.; Paur, I.; Bøhn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.F.L.; Santos, M.C.; Ginabreda, M.G.; Fortunato, R.S.; Carvalho, D.P.d.; Freitas Ferreira, A.C. Flavonoid rutin increases thyroid iodide uptake in rats. PLoS ONE 2013, 8, e73908. [Google Scholar] [CrossRef] [Green Version]
- Mancini, A.; Martorana, G.E.; Magini, M.; Festa, R.; Raimondo, S.; Silvestrini, A.; Nicolotti, N.; Mordente, A.; Mele, M.C.; Miggiano, G.A.D.; et al. Oxidative stress and metabolic syndrome: Effects of a natural antioxidants enriched diet on insulin resistance. Clin. Nutr. ESPEN 2015, 10, e52–e60. [Google Scholar] [CrossRef]
- Giuliani, C.; Noguchi, Y.; Harii, N.; Napolitano, G.; Tatone, D.; Bucci, I.; Piantelli, M.; Monaco, F.; Kohn, L.D. The flavonoid quercetin regulates growth and gene expression in rat FRTL-5 thyroid cells. Endocrinology 2008, 149, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, K.; Sakakibara, H. High content of dopamine, a strong antioxidant, in cavendish banana. J. Agric. Food Chem. 2000, 48, 844–848. [Google Scholar] [CrossRef]
- Parmar, H.S.; Kar, A. Protective role of Mangifera indica, Cucumis melo and Citrullus vulgaris peel extracts in chemically induced hypothyroidism. Chem. Biol. Interact. 2009, 177, 254–258. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ichiyanagi, T.; Komiyama, T.; Sato, S.; Konishi, T. Effects of anthocyanins on psychological stress-induced oxidative stress and neurotransmitter status. J. Agric. Food Chem. 2008, 56, 7545–7550. [Google Scholar] [CrossRef]
- Graumann, R.; Paris, I.; Martinez-Alvarado, P.; Rumanque, P.; Perez-Pastene, C.; Cardenas, S.P.; Marin, P.; Diaz-Grez, F.; Caviedes, R.; Caviedes, P. Oxidation of dopamine to aminochrome as a mechanism for neurodegeneration of dopaminergic systems in Parkinson’s disease. Possible neuroprotective role of DT-diaphorase. Pol. J. Pharmacol. 2002, 54, 573–580. [Google Scholar] [PubMed]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Cognitive Impairment and Dementia: Meta-Analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl(3)-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, F.; Xiang, T.; Wang, W.; Ma, C.; Yang, C.; Chen, H.; Xiang, H. Influence of food groups on plasma total homocysteine for specific MTHFR C677T genotypes in Chinese population. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Upadya, H.; Prabhu, S.; Prasad, A.; Subramanian, D.; Gupta, S.; Goel, A. A randomized, double blind, placebo controlled, multicenter clinical trial to assess the efficacy and safety of Emblica officinalis extract in patients with dyslipidemia. BMC Complement. Altern. Med. 2019, 19, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, N.; Baba, S.; Yasuda, A.; Iwamoto, T.; Kamiyama, M.; Takizawa, T.; Itakura, H.; Kondo, K. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic. Res. 2001, 34, 93–99. [Google Scholar] [CrossRef]
- Martini, D.; Rossi, S.; Biasini, B.; Zavaroni, I.; Bedogni, G.; Musci, M.; Pruneti, C.; Passeri, G.; Ventura, M.; Di Nuzzo, S.; et al. Claimed effects, outcome variables and methods of measurement for health claims proposed under European Community Regulation 1924/2006 in the framework of protection against oxidative damage and cardiovascular health. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health: (Revision 1). EFSA J. Eur. Food Saf. Auth. 2018, 16, e05136. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, A.; Anggård, E.E.; Carrier, M.J. Oxidized LDL-mediated monocyte adhesion to endothelial cells does not involve NFkappaB. Biochem. Biophys. Res. Commun. 2001, 284, 239–244. [Google Scholar] [CrossRef]
- Quinn, M.T.; Parthasarathy, S.; Fong, L.G.; Steinberg, D. Oxidatively modified low density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. USA 1987, 84, 2995–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endemann, G.; Stanton, L.W.; Madden, K.S.; Bryant, C.M.; White, R.T.; Protter, A.A. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993, 268, 11811–11816. [Google Scholar] [CrossRef]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediators Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, D.; Witztum, J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Singh, U.; Jialal, I. The evolving role of C-reactive protein in atherothrombosis. Clin. Chem. 2009, 55, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, D.; de Lacerda, A.P. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev. Port. Cardiol. 2012, 31, 733–745. [Google Scholar] [CrossRef]
- Koenig, W.; Sund, M.; Frohlich, M.; Fischer, H.-G.; Löwel, H.; Döring, A.; Hutchinson, W.L.; Pepys, M.B. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: Results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984. Circulation 1999, 99, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Danesh, J.; Pepys, M.B. C-reactive protein in healthy and in sick populations. Eur. Heart J. 2000, 21, 1564–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandraki, K.; Piperi, C.; Kalofoutis, C.; Singh, J.; Alaveras, A.; Kalofoutis, A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann. N. Y. Acad. Sci. 2006, 1084, 89–117. [Google Scholar] [CrossRef]
- He, F.J.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J. Hum. Hypertens. 2007, 21, 717–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.-P.; Sinaiko, A.R. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J. Am. Diet. Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprecht, M.; Oettl, K.; Schwaberger, G.; Hofmann, P.; Greilberger, J.F. Several Indicators of Oxidative Stress, Immunity, and Illness Improved in Trained Men Consuming an Encapsulated Juice Powder Concentrate for 28 Weeks. J. Nutr. 2007, 137, 2737–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, E.J.; Baines, K.J.; Berthon, B.S.; Wood, L.G. Effects of an Encapsulated Fruit and Vegetable Juice Concentrate on Obesity-Induced Systemic Inflammation: A Randomised Controlled Trial. Nutrients 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprecht, M.; Obermayer, G.; Steinbauer, K.; Cvirn, G.; Hofmann, L.; Ledinski, G.; Greilberger, J.F.; Hallstroem, S. Supplementation with a juice powder concentrate and exercise decrease oxidation and inflammation, and improve the microcirculation in obese women: Randomised controlled trial data. Br. J. Nutr. 2013, 110, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novembrino, C.; Cighetti, G.; De Giuseppe, R.; Vigna, L.; de Liso, F.; Pellegatta, M.; Gregori, D.; Maiavacca, R.; Bamonti, F. Effects of encapsulated fruit and vegetable juice powder concentrates on oxidative status in heavy smokers. J. Am. Coll. Nutr. 2011, 30, 49–56. [Google Scholar] [CrossRef]
- Beltrán-Debón, R.; Rodríguez-Gallego, E.; Fernández-Arroyo, S.; Senan-Campos, O.; Massucci, F.A.; Hernández-Aguilera, A.; Sales-Pardo, M.; Guimerà, R.; Camps, J.; Menendez, J.A.; et al. The acute impact of polyphenols from Hibiscus sabdariffa in metabolic homeostasis: An approach combining metabolomics and gene-expression analyses. Food Funct. 2015, 6, 2957–2966. [Google Scholar] [CrossRef]
- Tsang, C.; Hodgson, L.; Bussu, A.; Farhat, G.; Al-Dujaili, E. Effect of Polyphenol-Rich Dark Chocolate on Salivary Cortisol and Mood in Adults. Antioxidants 2019, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Allgrove, J.; Farrell, E.; Gleeson, M.; Williamson, G.; Cooper, K. Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Michels, N.; Sioen, I.; Braet, C.; Huybrechts, I.; Vanaelst, B.; Wolters, M.; De Henauw, S. Relation between salivary cortisol as stress biomarker and dietary pattern in children. Psychoneuroendocrinology 2013, 38, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, K.A.; Chirouzes, D.M.; Ceddia, M.A. Supplementation with a polyphenolic blend improves post-exercise strength recovery and muscle soreness. Food Nutr. Res. 2015, 59, 30034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarubbo, F.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic silymarin, quercetin and naringenin treatments increase monoamines synthesis and hippocampal Sirt1 levels improving cognition in aged rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Rebas, E.; Rzajew, J.; Radzik, T.; Zylinska, L. Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways. Curr. Neuropharmacol. 2020, 18, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Uchida, S.; Yamada, K.; Ano, Y. Anxiolytic effects of theaflavins via dopaminergic activation in the frontal cortex. Biosci. Biotechnol. Biochem. 2019, 83, 1157–1162. [Google Scholar] [CrossRef]
- Kehr, J.; Yoshitake, S.; Ijiri, S.; Koch, E.; Nöldner, M.; Yoshitake, T. Ginkgo biloba leaf extract (EGb 761®) and its specific acylated flavonol constituents increase dopamine and acetylcholine levels in the rat medial prefrontal cortex: Possible implications for the cognitive enhancing properties of EGb 761®. Int. Psychogeriatr. 2012, 24 (Suppl. 1), S25–S34. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Robbins, T.W. Noradrenergic modulation of cognition: Therapeutic implications. J. Psychopharmacol. 2013, 27, 694–718. [Google Scholar] [CrossRef]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Carrillo, J.Á.; Arcusa, R.; Zafrilla, M.P.; Marhuenda, J. Effects of Fruit and Vegetable-Based Nutraceutical on Cognitive Function in a Healthy Population: Placebo-Controlled, Double-Blind, and Randomized Clinical Trial. Antioxidants 2021, 10, 116. [Google Scholar] [CrossRef]
- Marino, M.; Del Bo’, C.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly)Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Gladine, C.; Combe, N.; Vaysse, C.; Pereira, B.; Huertas, A.; Salvati, S.; Rossignol-Castera, A.; Cano, N.; Chardigny, J.-M. Optimized rapeseed oil enriched with healthy micronutrients: A relevant nutritional approach to prevent cardiovascular diseases. Results of the Optim’Oils randomized intervention trial. J. Nutr. Biochem. 2013, 24, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.-I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victoria-Montesinos, D.; Abellán Ruiz, M.S.; Luque Rubia, A.J.; Guillén Martínez, D.; Pérez-Piñero, S.; Sánchez Macarro, M.; García-Muñoz, A.M.; Cánovas García, F.; Castillo Sánchez, J.; López-Román, F.J. Effectiveness of Consumption of a Combination of Citrus Fruit Flavonoids and Olive Leaf Polyphenols to Reduce Oxidation of Low-Density Lipoprotein in Treatment-Naïve Cardiovascular Risk Subjects: A Randomized Double-Blind Controlled Study. Antioxidants 2021, 10, 589. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Santos-Beneit, G.; Bodega, P.; Pocock, S.; Mattei, J.; Peñalvo, J.L. Validation of a questionnaire to measure overall mediterranean lifestyle habits for research application: The mediterranean lifestyle index (medlife). Nutr. Hosp. 2015, 32, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Calani, L.; Cossu, M.; Mena, P.; Sayegh, M.; Ray, S.; Del Rio, D. (Poly) phenolic characterization of three food supplements containing 36 different fruits, vegetables and berries. PharmaNutrition 2015, 3, 11–19. [Google Scholar] [CrossRef]
- Srinivasan, V.S. Bioavailability of nutrients: A practical approach to in vitro demonstration of the availability of nutrients in multivitamin-mineral combination products. J. Nutr. 2001, 131, 1349S–1350S. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.-E.; Lamprecht, M.; et al. Absorption Profile of (Poly)Phenolic Compounds after Consumption of Three Food Supplements Containing 36 Different Fruits, Vegetables, and Berries. Nutrients 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]

| Total | N1 | N2 | |
|---|---|---|---|
| N | 92 | 48 | 44 |
| Men | 45 (48.91%) | 20 (44.44%) | 25 (55.56%) |
| Women | 47 (51.09%) | 28 (59.57%) | 19 (40.43%) |
| Age (years) | 34 ± 11 | 33 ± 10 | 36 ± 12 |
| Weight (kg) | 73.10 ± 14.29 | 70.68 ± 13.88 | 75.68 ± 14.44 |
| Height (m) | 1.72 ± 9 | 1.71 ± 9 | 1.73 ± 9 |
| BMI (kg/m2) | 24.40 ± 3.43 | 23.87 ± 3.42 | 24.99 ± 3.38 |
| Baseline | Final | p-Value | ||
|---|---|---|---|---|
| TNF-α (pg/mL) | Placebo | 5.69 ± 1.02 | 5.30 ± 0.87 * | 0.609 |
| Extract | 5.82 ± 1.14 | 5.22 ± 1.21 ** | ||
| sTNFR1 (ng/mL) | Placebo | 1.43 ± 0.32 | 1.43 ± 0.33 | 0.089 |
| Extract | 1.49 ± 0.37 | 1.36 ± 0.26 ** | ||
| Homocysteine (mcm/L) | Placebo | 11.75 ± 2.21 | 11.07 ± 2.92 * | 0.053 |
| Extract | 11.65 ± 2.3 | 10.33 ± 2.27 ** | ||
| Vitamin B12 (pg/mL) | Placebo | 309.11 ± 85.51 | 314.99 ± 82.20 | 0.205 |
| Extract | 308.41 ± 123.51 | 333.45 ± 115.00 | ||
| Vitamin E (mcg/mL) | Placebo | 13.20 ± 3.33 | 13.21 ± 3.53 | 0.974 |
| Extract | 13.15 ± 3.68 | 13.20 ± 3.37 | ||
| Cortisol (mcg/L) | Placebo | 14.32 ± 3.22 | 13.92 ± 3.72 | 0.93 |
| Extract | 13.42 ± 2.07 | 13.87 ± 2.03 | ||
| Adrenaline (pg/mL) | Placebo | 22.05 ± 8.03 | 23.50 ± 6.98 | 0.7 |
| Extract | 22.67 ± 8.61 | 23.91 ± 6.97 | ||
| Norepinephrine (pg/mL) | Placebo | 361.86 ± 50.58 | 367.06 ± 52.28 | 0.322 |
| Extract | 343.64 ± 46.38 | 382.85 ± 43.88 ** | ||
| T3 (mcg/L) | Placebo | 1.13 ± 0.23 | 1.07 ± 0.18 ** | 0.195 |
| Extract | 1.1 ± 0.23 | 1.03 ± 0.16 ** | ||
| T4 (mcg/L) | Placebo | 7.64 ± 1.31 | 8.21 ± 1.31 ** | 0.781 |
| Extract | 8.74 ± 0.75 | 8.27 ± 1.03 | ||
| TSH (mU/L) | Placebo | 2.28 ± 0.51 | 2.27 ± 0.53 | 0.654 |
| Extract | 2.23 ± 0.61 | 2.20 ± 0.51 | ||
| Total cholesterol (mg/dL) | Placebo | 184.71 ± 40.21 | 188.78 ± 39.07 | 0.773 |
| Extract | 184.06 ± 36.30 | 187.13 ± 32.07 | ||
| Triglycerides (mg/dL) | Placebo | 74.07 ± 26.92 | 73.94 ± 29.27 | 0.178 |
| Extract | 69.79 ± 24.93 | 68.39 ± 21.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcusa, R.; Carrillo, J.Á.; Xandri-Martínez, R.; Cerdá, B.; Villaño, D.; Marhuenda, J.; Zafrilla, M.P. Effects of a Fruit and Vegetable-Based Nutraceutical on Biomarkers of Inflammation and Oxidative Status in the Plasma of a Healthy Population: A Placebo-Controlled, Double-Blind, and Randomized Clinical Trial. Molecules 2021, 26, 3604. https://doi.org/10.3390/molecules26123604
Arcusa R, Carrillo JÁ, Xandri-Martínez R, Cerdá B, Villaño D, Marhuenda J, Zafrilla MP. Effects of a Fruit and Vegetable-Based Nutraceutical on Biomarkers of Inflammation and Oxidative Status in the Plasma of a Healthy Population: A Placebo-Controlled, Double-Blind, and Randomized Clinical Trial. Molecules. 2021; 26(12):3604. https://doi.org/10.3390/molecules26123604
Chicago/Turabian StyleArcusa, Raúl, Juan Ángel Carrillo, Raquel Xandri-Martínez, Begoña Cerdá, Débora Villaño, Javier Marhuenda, and María Pilar Zafrilla. 2021. "Effects of a Fruit and Vegetable-Based Nutraceutical on Biomarkers of Inflammation and Oxidative Status in the Plasma of a Healthy Population: A Placebo-Controlled, Double-Blind, and Randomized Clinical Trial" Molecules 26, no. 12: 3604. https://doi.org/10.3390/molecules26123604






