ATR-FTIR-MIR Spectrometry and Pattern Recognition of Bioactive Volatiles in Oily versus Microencapsulated Food Supplements: Authenticity, Quality, and Stability
Abstract
:1. Introduction
2. Results
2.1. ATR-FTIR-MIR Spectra of the Oily Formulations
2.2. Comparative FTIR Spectra of Different Microencapsulated Products
2.3. Multivariate Analysis of Microencapsulated Products
2.4. One-Way ANOVA Univariate Analysis
2.5. Recognition Patterns and Stability of Microencapsulated Products
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. EOs and Oily Formulations
4.1.2. Microencapsulated Powders Obtained from Biomicin Brand Products
4.2. FTIR Analysis
4.3. Chemometrics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability: Samples of the compounds are not available from the authors. |
References
- Johnson, S.A. Medicinal Essential Oils: The Science and Practice of Evidence-Based Essential Oil Therapy; Lightning Source Inc.: La Vergne, TN, USA, 2017; 1164p, ISBN 9780997548709. [Google Scholar]
- Fisher, C.; Scott, T.R. Food Flavours: Biology and Chemistry; Royal Society of Chemistry: London, UK, 2007; p. 176. ISBN 9781847550866. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Rivera Calo, J.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems, a review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Athar, T.; Al-Faraidy, A.A.; Almuhaiza, M.S. Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian Pac. J. Trop. Biomed. 2017, 7, 147–150. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the Volatile Composition of Essential Oils of Some Lamiaceae Spices and the Antimicrobial and Antioxidant Activities of the Entire Oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed.; European Medicines Agency, Council of Europe: Amsterdam, The Netherlands, 2020; 1250p.
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Wesołowska, A.; Jadczak, D. Comparison of the Chemical Composition of Essential Oils Isolated from Two Thyme (Thymus vulgaris L.) Cultivars. Not. Bot. Horti Agrobo. 2019, 47, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.Y.; Liu, W.H.; Lv, G.Y.; Zhou, X. Analysis of essential oils of Origanum vulgare from six production areas of China and Pakistan. Rev. Bras. Farmacogn. 2014, 24, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Kula, J.; Majda, T.; Stoyanova, A.; Georgiev, E. Chemical composition of Origanum vulgare L. essential oil from Bulgaria. J. Essent. Oil Bear. Plant. 2007, 10, 215–220. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcãoa, S.; Bacémb, I.; Igrejasb, G.; Rodriguesc, P.J.; Vilas-Boasa, M.; Amarala, J.S. Chemical composition and antimicrobial activity of hydrodistilled oil from juniper berries. Ind. Crops Prod. 2018, 124, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Salamon, I.; Petruska, P. Quality of Juniper Essential Oil (Oleum Juniperi) în the South Slovakia and it’s Curative and Industrial Utilization. Indian J. Pharmaceut. Educ. Res. 2017, 51, S479–S482. [Google Scholar] [CrossRef] [Green Version]
- Raymond, C.A.; Davies, N.W.; Larkman, T. GC-MS method validation and levels of methyl eugenol in a diverse range of tea tree (Melaleuca alternifolia) oils. Anal. Bioanal. Chem. 2017, 409, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Mateua, D.; Largo-Arangoa, C.D.; Larkmanb, T.; Garriguesa, S.; De La Guardia, M. Fast authentication of tea tree oil through spectroscopy. Talanta 2018, 189, 404–410. [Google Scholar] [CrossRef]
- Oliva, A.; Costantini, S.; De Angelis, M. High Potency of Melaleuca alternifolia Essential Oil against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boughendjioua, H. Essential Oil Composition of Syzygium aromaticum (L.). IRJPMS 2018, 11, 26–28. [Google Scholar]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Ben Kahla-Nakbi, A.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil (Syzigium aromaticum L. Myrtaceae): A short review. Phytotherapy Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Wicochea Rodriguez, J.D.; Peyron, S.; Rigou, P.; Chalier, P. Rapid quantification of clove (Syzygium aromaticum) and spearmint (Mentha spicata) essential oils encapsulated in a complex organic matrix using an ATR-FTIR spectroscopic method. PLoS ONE 2018, 13, e0207401. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.F.M.; Queiroz Monte, F.; Gomes De Lemos, J.; Garcia Do Nascimento, T.L.P.G.; De Medeiros Costa, A.K.; Mota De Paiva, L.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, M.; Rasul, A.; Waqas, M.K.; Saadullah, M.; Aslam, N.; Abbas, G.; Latif, S.; Afzal, H.; Inam, S.; Shah, P.A. Formulation and Evaluation of a Clove Oil-Encapsulated Nanofiber Formulation for Effective Wound-Healing. Molecules 2021, 26, 2491. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, Y.; Zhu, Z.; Chen, X.; He, F. Chemical components of volatile oil from Cinnamomum jensenianum and Mazz leaf in Yongzhou and its antibacterial and antioxidant properties. Trop. J. Pharmaceut. Res. 2018, 17, 1839–1845. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Rao, L. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011, 51, 547–562. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, Z.; Li, P.; Zhao, Z.; Chen-Zhou, J. Tissue-specific chemical profiling and quantitative analysis of bioactive components of Cinnamomum cassia by combining laser-microdissection with UPLC-Q/TOF–MS. Chem. Cent. J. 2018, 12, 71–78. [Google Scholar] [CrossRef]
- Brodowska, K.M.; Brodowska, A.J.; Śmigielski, K.; Łodyga-Chruścińska, E. Antioxidant profile of essential oils and extracts of cinnamon bark (Cinnamomum cassia). Eur. J. Biol. Res. 2016, 6, 310–316. [Google Scholar] [CrossRef]
- Fabiana, C.A.; Solarte, A.L.; Tarradas, C.; Luque, I.; Maldonado, A.; Galán-Relaño, Á.; Huerta, B. Antimicrobial activity of selected essential oils against Streptococcus suis isolated from pigs. Microbiol. Open 2018, 7, e613. [Google Scholar] [CrossRef] [Green Version]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Socaciu, C.; Trif, M.; Baciu, A.; Diehl, H.A. Microcapsules Made of Seabuckthorn Biocomposite Fractions Inserted in Natural Matrices (Alginates and Pectins). Anim. Sci. Biotechnol. 2009, 66, 374–379. [Google Scholar]
- Vasisht, N. Selection of Materials for Microencapsulation, Chapter 16. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 173–180. [Google Scholar] [CrossRef]
- Campos Toledo Hijo, A.A.; Gomes Da Costa, J.M.; Silva, E.K.; Machado Azevedo, V.; Yoshida, M.I.; Borges, S.V. Understanding the Influence of Encapsulating Matrix on the Physical and Thermal Properties of Oregano Essential Oil Powder. Int. J. Hortic. Agric. 2017, 1–8. Available online: www.symbiosispublishing.com (accessed on 10 July 2021). [CrossRef]
- Marquez-Gomez, M.; Galicia-García, T.; Arquez-Mel Endez, R.M.; Ruiz-Gutierrez, M.; Quintero-Ramos, A. Spray-dried microencapsulation of orange essential oil using modified rice starch as wall material. J. Food Process. Preserv. 2018, 42, e13428. [Google Scholar] [CrossRef]
- De Barros, F.R.V.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; El-Ghorab, A.H.; Hussein, A.M.S.; El-Massry, K.F.; Lotfy, S.N.; Sayed Ahmed, M.Y.; Soliman, T.N. Correlation between chemical composition and radical scavenging activity of 10 commercial essential oils: Impact of microencapsulation on functional properties of essential oils. Arab. J. Chem. 2020, 13, 6815–6827. [Google Scholar] [CrossRef]
- Manaf, M.A.; Subuki, I.; Jai, J.; Raslan, R.; Mustapa, A.N. Encapsulation of Volatile Citronella Essential Oil by Coacervation: Efficiency and Release Study, 3RD International conference on global sustainability and chemical engineering (icgsce) book series. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012072. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Medina, D.I.; Narayanan, J.; Parra-Saldívar, R.; Iqbal, H.M.N. Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities. Molecules 2021, 26, 2880. [Google Scholar] [CrossRef]
- Bonda, A.F.; Regis, L.; Giovannelli, L.; Segale, L. Alginate/maltodextrin and alginate/shellac gum core-shell capsules for the encapsulation of peppermint essential oil. Int. J. Biol. Macromolecules 2020, 162, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Paredes Juárez, G.A.; Spasojevic, M.; Faas, M.M.; De Eos, P. Immunological and Technical Considerations in Application of Alginate-Based Microencapsulation Systems. Front. Bioeng. Biotechnol. 2014, 2, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdemir, N.; Bayrak, A.; Tat, T.; Altay, F.; Kiralan, M.; Kurt, A. Microencapsulation of basil essential oil: Utilization of gum arabic/whey protein isolate/maltodextrin combinations for encapsulation efficiency and in vitro release. Food Meas. 2021, 15, 1865–1876. [Google Scholar] [CrossRef]
- Campelo, P.H.; Sanches, E.A.; De Barros Fernandes, R.V.; Botrel, D.A.; Borges, S.V. Stability of lime essential oil microparticles produced with protein-carbohydrate blends. Food Res. Int. 2018, 105, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Sahlan, M.; Fadhan, A.M.; Pratami, D.K.; Wijanarko, A.; Lischer, K.; Hermansyah, H.; Mahira, K.F. Encapsulation of Agarwood Essential Oil with Maltodextrin and Gum Arabic. Int. J. Technol. 2019, 10, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Hofman, D.L.; Van Buul, V.J.; Brouns, F.J.P.H. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef]
- Siccama, J.W.; Pegiou, E.; Zhang, L.; Mumm, R.; Hall, R.D.; Boom, R.M.; Schutyser, M.A.I. Maltodextrin improves physical properties and volatile compound retention of spray-dried asparagus concentrate. LWT 2021, 142, 111058. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Diaz, A.; Valcarcel, M.; Ayora-Canada, M.J. Two-dimensional correlation spectroscopy and multivariate curve resolution for the study of lipid oxidation in edible oils monitored by FTIR and FT-Raman. Anal. Chim. Acta 2007, 593, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-W. Infrared Spectroscopy for Food Quality Analysis and Control; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Franca, A.S.; Nollet, L.M.L. Spectroscopic Methods in Food Analysis; CRC Press: New York, NY, USA, 2017; p. 664. [Google Scholar]
- Rodriguez-Saona, L.E.; Allen Dorf, M.E. Use of FTIR for Rapid Authentication and Detection of Adulteration of Food. Ann. Rev. Food Sci. Technol. 2011, 2, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W. Mid-infrared (MIR) Spectroscopy for Quality Analysis of Liquid Foods. Food Eng. Rev. 2019, 11, 142–158. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F.; Ruckebusch, C.; Vitale, R. Chemometric Strategies for Spectroscopy-Based Food Authentication. Appl. Sci. 2020, 10, 6544. [Google Scholar] [CrossRef]
- Cebi, N.; Taylan, O.; Abusurrah, M.; Sagdic, O. Detection of Orange Essential Oil, Isopropyl Myristate, and Benzyl Alcohol in Lemon Essential Oil by FTIR Spectroscopy Combined with Chemometrics. Foods 2021, 10, 27. [Google Scholar] [CrossRef]
- Bachion de Santana, F.; Borges Neto, W.; Poppi, R.J. Random forest as one-class classifier and infrared spectroscopy for food adulteration detection. Food Chem. 2019, 293, 323–332. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573–574, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Taylan, O.; Cebi, N.; Sagdic, O. Rapid Screening of Mentha spicata Essential Oil and L-Menthol in Mentha piperita Essential Oil by ATR-FTIR Spectroscopy Coupled with Multivariate Analyses. Foods 2021, 10, 202. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Huyan, Z.; Kou, Y.; Xu, L.; Yu, X.; Gao, J.-M. Application of Fourier transform infrared spectroscopy for the quality and safety analysis of fats and oils: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3597–3611. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, L.; Giuliani, A.; Maggio, R.M.; Bendini, A.; Toschi, T.G.; Cichell, A. Rapid FTIR determination of water, phenolics and antioxidant activity of olive oil. Eur. J. Lipid Sci. Technol. 2010, 112, 1150–1157. [Google Scholar] [CrossRef]
- Socaciu, C.; Ranga, F.; Fetea, F.; Leopold, D.; Dulf, F.; Parlog, R. Complementary advanced techniques applied for plant and food Authentication. Czech. J. Food Sci. 2009, 27, S70–S75. [Google Scholar] [CrossRef] [Green Version]
- Socaciu, C.; Fetea, F.; Ranga, F.; Bunea, A.; Dulf, F.; Socaci, S.; Pintea, A. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Coupled with Chemometrics, to Control the Botanical Authenticity and Quality of Cold-Pressed Functional Oils Commercialized in Romania. Appl. Sci. 2020, 10, 8695. [Google Scholar] [CrossRef]
- Popa, R.M.; Socaci, S.; Farcas, A.; Socaciu, C. Comparative Authenticity Signatures of Six Essential Oils Used as Food Flavors: A Gas chromatography—Mass Spectrometry Approach. Food Sci. Technol. 2021, 78, 88–100. [Google Scholar]
- Schädle, T.; Pejcic, B.; Mizaikoff, B. Monitoring dissolved carbon dioxide and methane in brine environments at high pressure using IR-ATR spectroscopy. Anal. Methods 2016, 8, 756–762. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
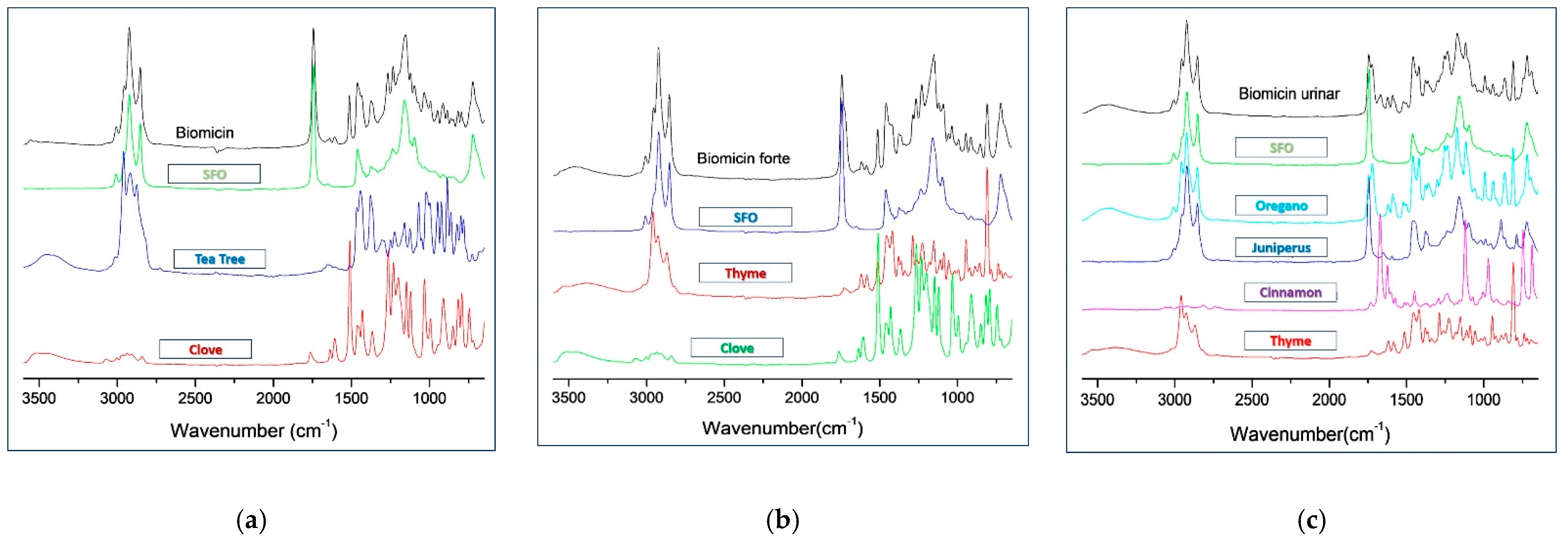
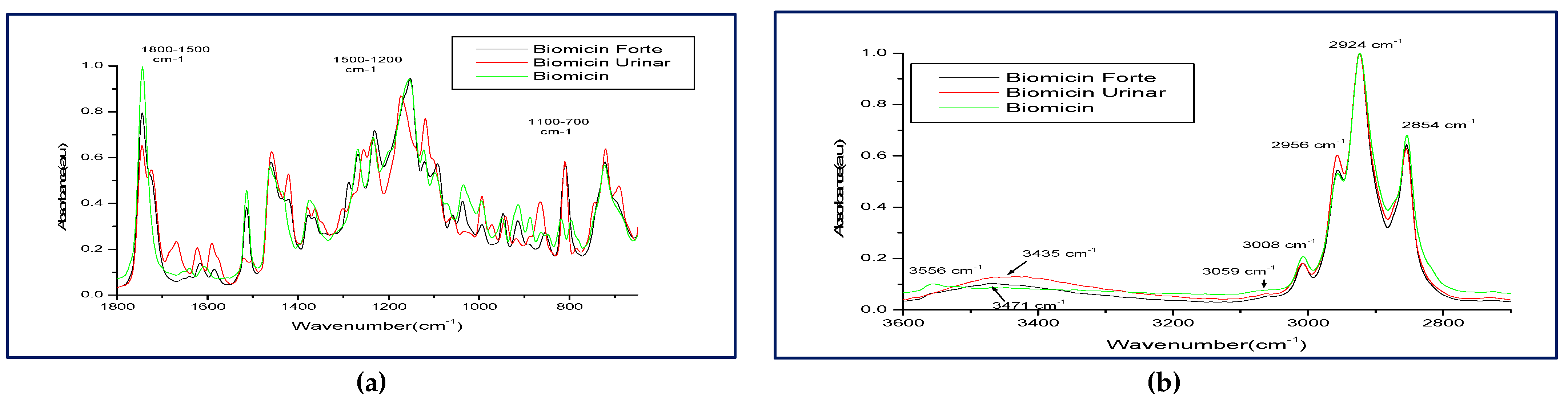
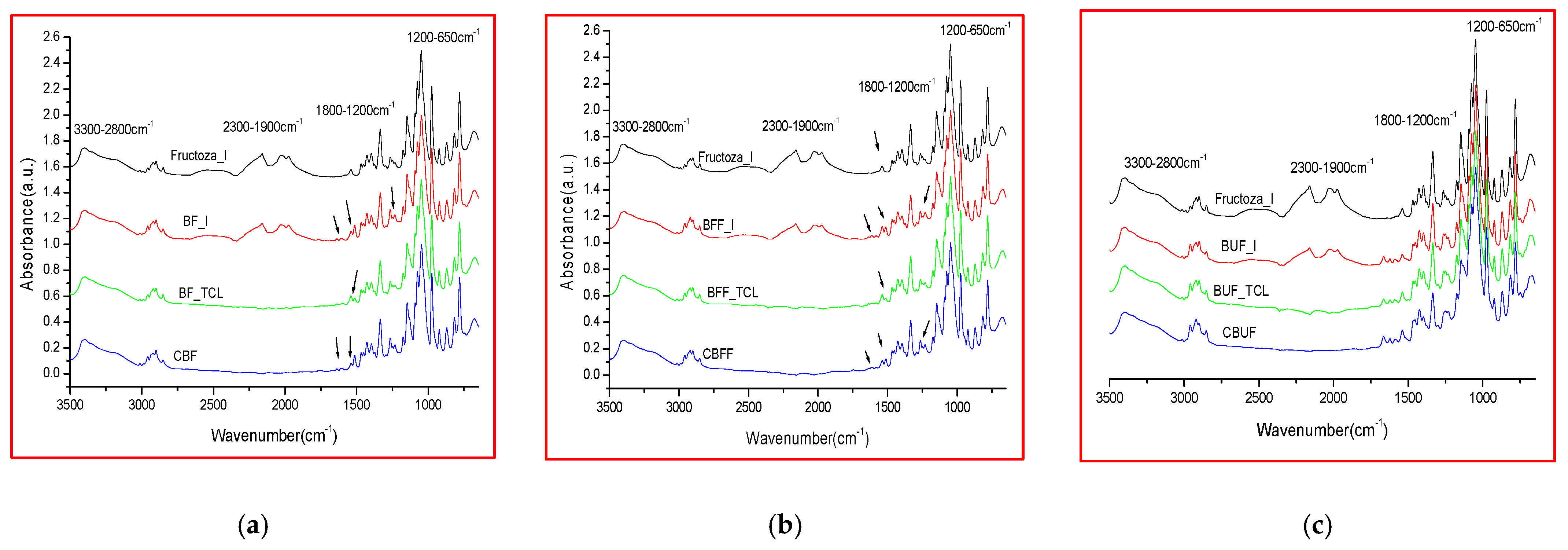
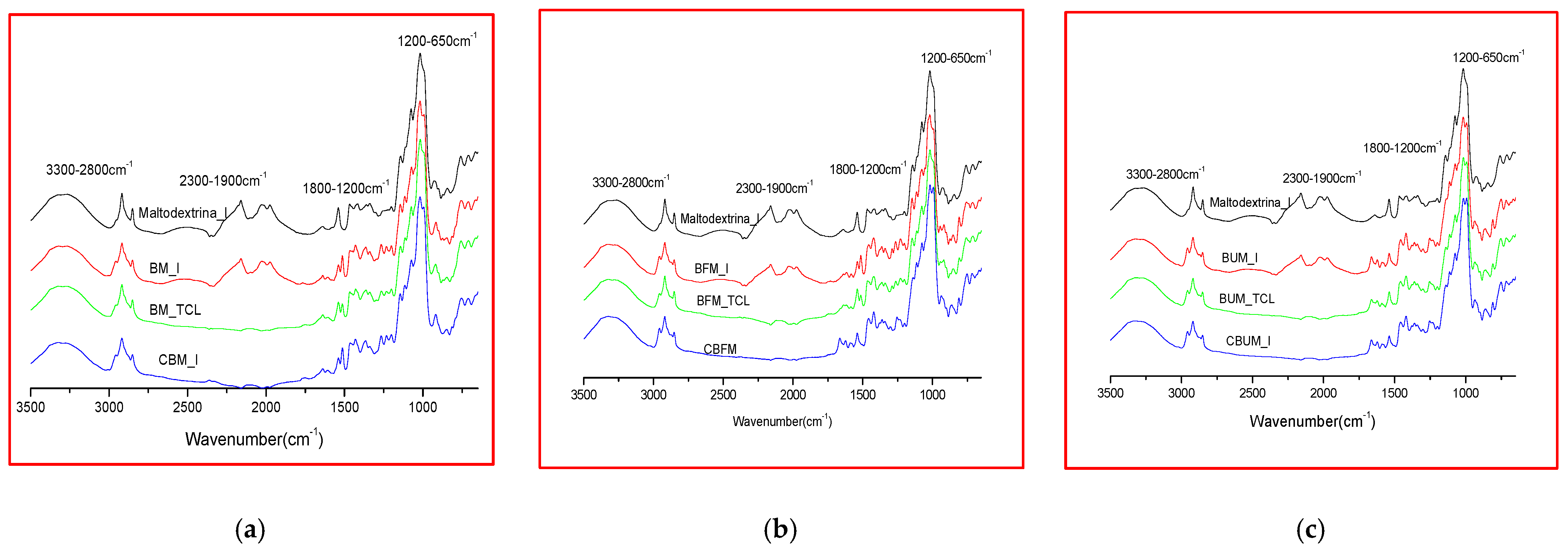
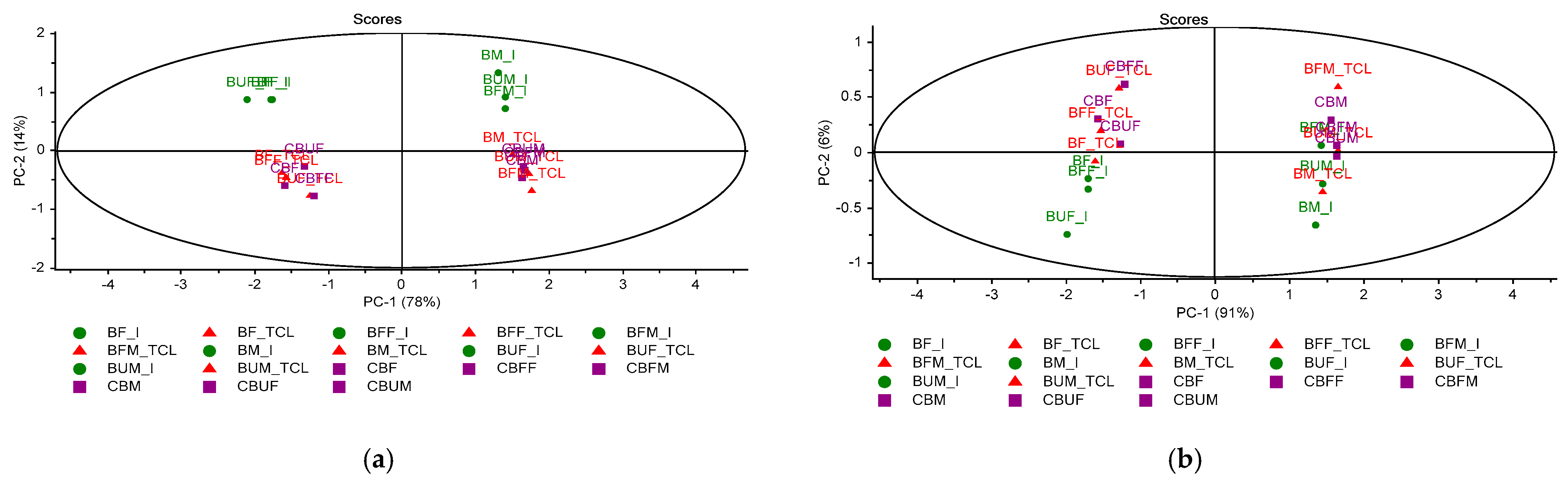
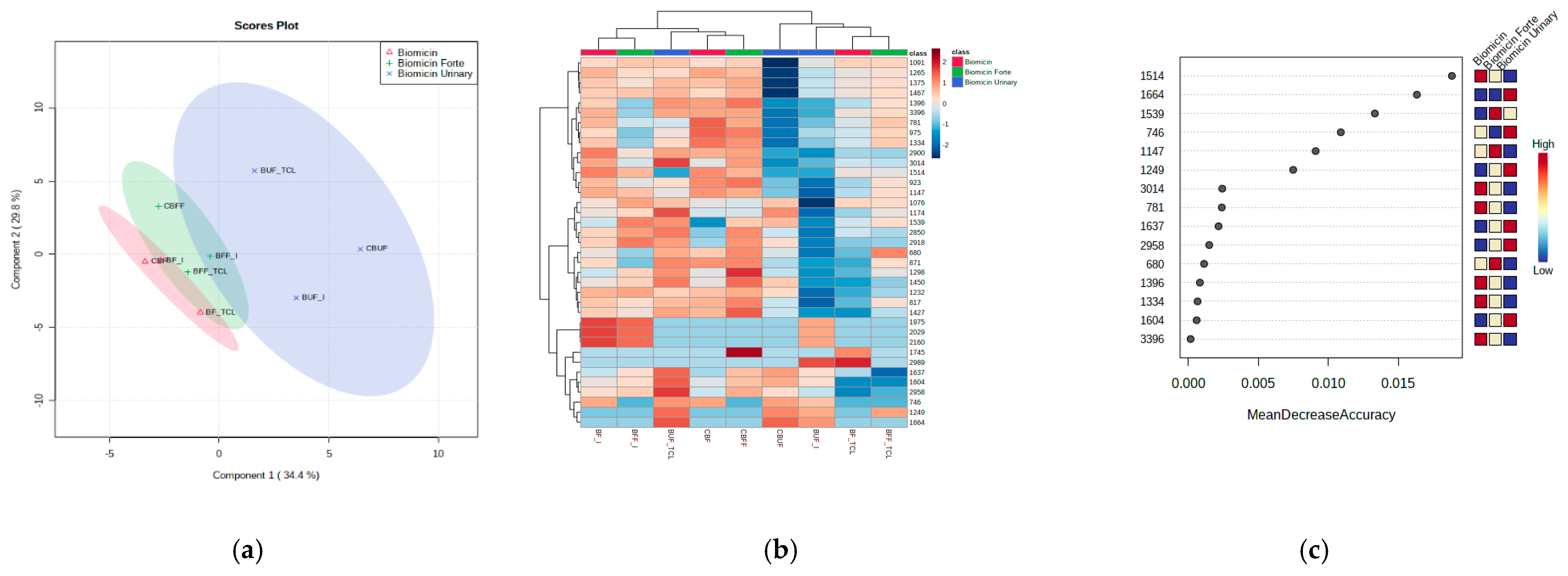
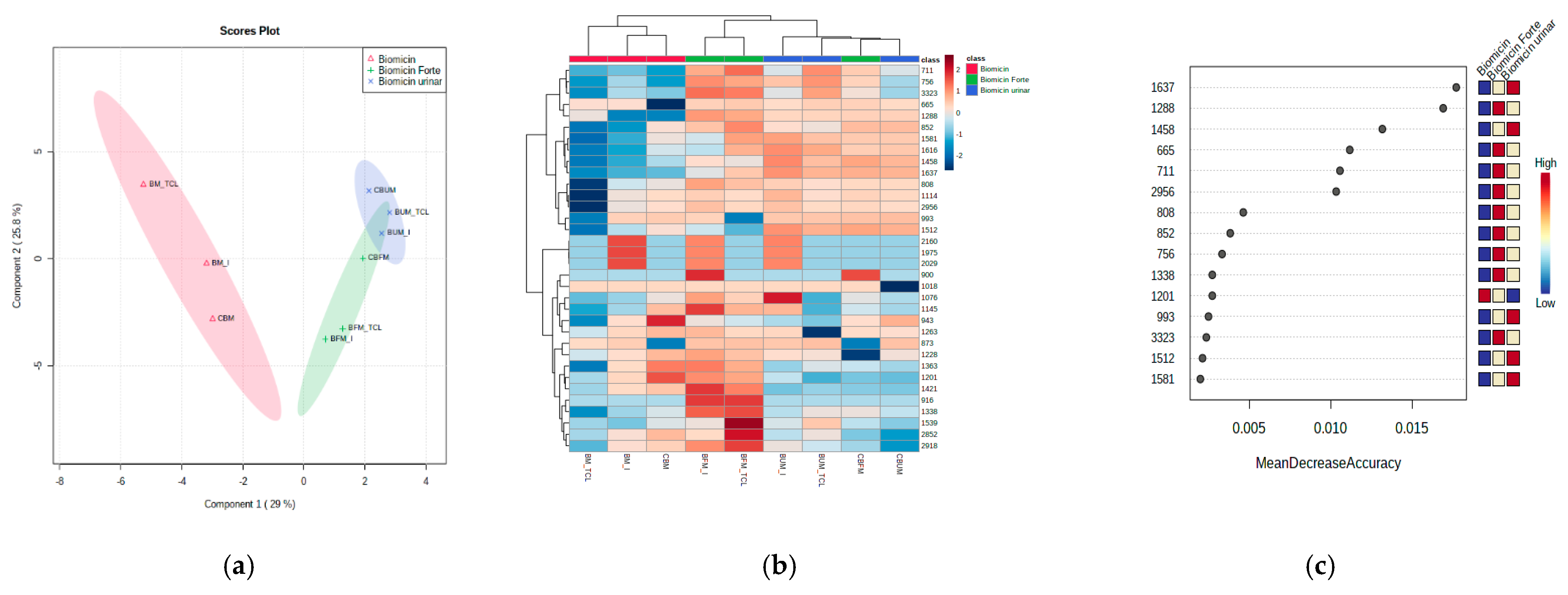
| Range (cm−1) | Biomicin | Biomicin Forte | Biomicin Urinary | |||
|---|---|---|---|---|---|---|
| WN (cm−1) | Intensity | WN (cm−1) | Intensity | WN (cm−1) | Intensity | |
| 650–1155 cm−1 | 723 | 0.567 | 721 | 0.581 | 719 | 0.638 |
| 744 | 0.385 | - | - | 744 | 0.405 | |
| 796 | 0.326 | - | - | 785 | 0.203 | |
| 817 | 0.332 | 810 | 0.576 | 810 | 0.585 | |
| 848 | 0.265 | - | - | - | - | |
| 862 | 0.273 | 856 | 0.271 | 864 | 0.407 | |
| 887 | 0.339 | 891 | 0.223 | 887 | 0.257 | |
| 914 | 0.394 | 914 | 0.324 | 918 | 0.246 | |
| 948 | 0.337 | 947 | 0.356 | 941 | 0.344 | |
| - | - | - | - | 972 | 0.307 | |
| 995 | 0.411 | 993 | 0.307 | 993 | 0.432 | |
| 1033 | 0.481 | 1035 | 0.409 | 1031 | 0.279 | |
| 1070 | 0.399 | 1058 | 0.349 | 1058 | 0.34 | |
| 1097 | 0.534 | 1091 | 0.573 | - | - | |
| 1124 | 0.633 | 1120 | 0.582 | 1118 | 0.771 | |
| 1153 | 0.941 | 1151 | 0.947 | 1172 | 0.87 | |
| 1199 | 0.628 | - | - | - | - | |
| 1200–1500 cm−1 | 1234 | 0.687 | 1230 | 0.718 | 1234 | 0.68 |
| 1267 | 0.637 | 1267 | 0.615 | 1255 | 0.636 | |
| - | - | 1288 | 0.493 | 1269 | 0.445 | |
| - | - | - | - | 1301 | 0.377 | |
| - | - | 1363 | 0.339 | 1361 | 0.377 | |
| 1375 | 0.414 | 1377 | 0.35 | 1379 | 0.381 | |
| - | - | 1421 | 0.417 | 1421 | 0.529 | |
| 1462 | 0.560 | 1460 | 0.581 | 1458 | 0.627 | |
| - | - | - | - | 1502 | 0.149 | |
| 1500–1800 cm−1 | 1514 | 0.458 | 1514 | 0.382 | 1519 | 0.161 |
| 1608 | 0.125 | 1585 | 0.112 | 1591 | 0.226 | |
| - | - | 1616 | 0.138 | 1622 | 0.206 | |
| 1641 | 0.116 | 1639 | 0.081 | 1668 | 0.234 | |
| - | - | - | - | 1724 | 0.546 | |
| 1743 | 0.996 | 1743 | 0.795 | 1745 | 0.652 | |
| 2800–3600 cm−1 | 2854 | 0.680 | 2854 | 0.644 | 2854 | 0.629 |
| 2924 | 1 | 2924 | 1 | 2924 | 1 | |
| 2953 | 0.522 | 2956 | 0.544 | 2956 | 0.604 | |
| 3007 | 0.207 | 3007 | 0.181 | 3008 | 0.181 | |
| Microencapsulated Products | Fructose | Maltodextrin | ||
|---|---|---|---|---|
| WN I and C | WN TCL (% TCL/I) | WN I and C | WN TCL (% TCL/I) | |
| Biomicin | 746 | - | 813 | - |
| - | - | 993 | - | |
| - | - | 1114 | - | |
| - | - | 1232 | 1232 (+14%) | |
| - | - | 1265 | 1265 (+9.1%) | |
| - | - | - | 1296 (0) | |
| 1514 | 1512 (−4%) | 1514 | 1514 (+10%) | |
| 1604 | 1604 (−8%) | 1606 | 1606 (+17%) | |
| 1637 | 1637 (−11.6%) | - | - | |
| 1745 | 1745 (0) | 1766 | 1749 (+10%) | |
| - | - | 2956 | 2956 (+8%) | |
| Biomicin Forte | - | - | 808 | 808 (−14.6%) |
| - | - | 900 | - | |
| - | - | 916 | 916 (0) | |
| - | - | 993 | - | |
| - | - | 1114 | 1114 (−4.2%) | |
| - | - | 1228 | 1228 (−5.1%) | |
| - | - | 1263 | 1263 (−9.4%) | |
| - | - | 1288 | 1288 (−5.1%) | |
| 1514 | 1514 (0) | 1512 | 1512 (−14.1%) | |
| - | - | 1539 | 1539 (+27%) | |
| 1614 | - | 1616 | 1616 (+13.8%) | |
| - | - | 2956 | 2956 (-6.7%) | |
| Biomicin urinary | 748 | 750 (+17%) | - | - |
| 810 | 810 (−6.5%) | |||
| - | - | 993 | 993 (+0.8%) | |
| - | - | 1112 | 1112 (−12.6%) | |
| - | - | 1232 | 1232 (−0.8%) | |
| - | - | 1253 | - | |
| - | - | 1379 | 1379 (+3%) | |
| 1589 | 1589 (+29%) | 1589 | 1589 (−4%) | |
| 1622 | 1622 (+29%) | 1622 | 1622 (−2.5%) | |
| 1664 | 1664 (+20%) | - | - | |
| 2956 | 2956 (−6.7%) | |||
| Sample Codes | I | TCL | C |
|---|---|---|---|
| Fructose | Fructose_I | Fructose_TCL | - |
| Biomicin in Fructose (BF) | BF_I | BF_TCL | CBF |
| Biomicin forte in Fructose (BFF) | BFF_I | BFF_TCL | CBFF |
| Biomicin urinary in Fructose (BUF) | BUF_I | BUF_TCL | CBUF |
| Maltodextrin | Maltodextrin_I | Maltodextrin_TCL | - |
| Biomicin in maltodextrin (BM) | BM_I | BM_TCL | CBM |
| Biomicin forte in maltodextrin (BFM) | BFM_I | BFM_TCL | CBFM |
| Biomicin urinary in maltodextrin (BUM) | BUM_I | BUM_TCL | CBUM |
| Range (cm−1) | Functional Group | Mode of Vibration |
|---|---|---|
| 3029–2989 | =C–H (trans and cis) | Stretching |
| 1745 | -C=O | Stretching |
| 1635–1650 | RHC=CH2 | Deformation |
| 1375, 1450 | =CH2 | Deformation in plane |
| 1380, 1458 | -CH2−, dimethyl | Stretching; bending |
| 1147–1159 | -C–O, C-OH | Stretching, bending |
| 850–920 | =CH2 | Out of plane deformation |
| 842–862 | Isopropyl group | Out of plane deformation |
| 810 | -C–H; -HC=CH− (cis) | Bending (out of plane) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, R.M.; Fetea, F.; Socaciu, C. ATR-FTIR-MIR Spectrometry and Pattern Recognition of Bioactive Volatiles in Oily versus Microencapsulated Food Supplements: Authenticity, Quality, and Stability. Molecules 2021, 26, 4837. https://doi.org/10.3390/molecules26164837
Popa RM, Fetea F, Socaciu C. ATR-FTIR-MIR Spectrometry and Pattern Recognition of Bioactive Volatiles in Oily versus Microencapsulated Food Supplements: Authenticity, Quality, and Stability. Molecules. 2021; 26(16):4837. https://doi.org/10.3390/molecules26164837
Chicago/Turabian StylePopa, Ramona Maria, Florinela Fetea, and Carmen Socaciu. 2021. "ATR-FTIR-MIR Spectrometry and Pattern Recognition of Bioactive Volatiles in Oily versus Microencapsulated Food Supplements: Authenticity, Quality, and Stability" Molecules 26, no. 16: 4837. https://doi.org/10.3390/molecules26164837
APA StylePopa, R. M., Fetea, F., & Socaciu, C. (2021). ATR-FTIR-MIR Spectrometry and Pattern Recognition of Bioactive Volatiles in Oily versus Microencapsulated Food Supplements: Authenticity, Quality, and Stability. Molecules, 26(16), 4837. https://doi.org/10.3390/molecules26164837







